Abstract
Further advances are required in understanding protection from AIDS by T-cell immunity. We analyzed a set of multigenic simian/human immunodeficiency virus (SHIV) DNA and fowlpox virus priming and boosting vaccines for immunogenicity and protective efficacy in outbred pigtail macaques. The number of vaccinations required, the effect of DNA vaccination alone, and the effect of cytokine (gamma interferon) coexpression by the fowlpox virus boost was also studied. A coordinated induction of high levels of broadly reactive CD4 and CD8 T-cell immune responses was induced by sequential DNA and fowlpox virus vaccination. The immunogenicity of regimens utilizing fowlpox virus coexpressing gamma interferon, a single DNA priming vaccination, or DNA vaccines alone was inferior. Significant control of a virulent SHIV challenge was observed despite a loss of SHIV-specific proliferating T cells. The outcome of challenge with virulent SHIVmn229 correlated with vaccine immunogenicity except that DNA vaccination alone primed for protection almost as effectively as the DNA/fowlpox virus regimen despite negligible immunogenicity by standard assays. These studies suggest that priming of immunity with DNA and fowlpox virus vaccines could delay AIDS in humans.
In the absence of technologies to reliably induce broadly neutralizing antibodies, protection against human immunodeficiency virus (HIV) relies on the clearance of already infected cells by T-cell immunity (27). Heterologous priming and boosting HIV vaccine strategies involving priming by DNA vaccination and boosting with recombinant attenuated pox virus vectors (such as fowlpox virus) encoding common HIV or simian immunodeficiency virus (SIV) antigens reliably induce high levels of T-cell immune responses in outbred nonhuman primates (4, 17, 20, 22). Vaccine-induced simian/human immunodeficiency virus (SHIV)-specific T-cell responses correlate with partial protection from virulent SHIV infection (9). The optimal use of these vaccines to protect humans from HIV-1 remains to be defined.
There are outstanding scientific and practical issues with this vaccination approach. Although large peak CD8 T-cell responses are likely to be helpful, other qualities of T-cell immunity, such as the breadth, durability, presence, and timing of CD4 T helper responses and recall immune response to infection may be equally important (19). Unadjuvanted DNA vaccination of outbred primates is often poorly immunogenic as measured by current technologies, despite clearly priming for a rapid expansion of T-cell immunity following recombinant pox virus boosting (4, 22). Interestingly, pox virus vaccination alone has, despite inducing lower levels of immune responses, been reported to provide nearly similar immunity to DNA/pox virus priming/boosting regimens in a rhesus/SHIV model (5).
High rates of viral mutation during early replication strongly select for T-cell escape mutants and long-term protection from lentiviral disease may be difficult to achieve with current, primarily T-cell-based vaccines (8, 37). A potential problem associated with nonsterilizing protection from lentiviral disease is the dysfunction and infection of virus-specific CD4 T-cell responses associated with HIV-1 infection (6, 7, 15). Only certain subgroups of HIV-1-infected subjects, long-term nonprogressors and those treated with antiretroviral therapy early in infection, have preserved CD4 T-cell immune responses to HIV-1 (16, 32). If CD4 T-cell dysfunction follows the blunted burst of replication occurring in infected subjects with prior T-cell immunity, this could abolish long-term protection from disease.
Rhesus macaques selected for MamuA*01 major histocompatibility complex class I allele expression control virulent SHIV89.6P infection following priming/boosting and other vaccination strategies (4, 9, 34). Protection is much poorer when either SIVmac239 is used as the challenge virus or MamuA*01-negative macaques are studied (38). We previously reported a virulent mucosal HIV-1IIIB-based SHIVmn229 infection model of outbred pigtail macaques (14). A codon-optimized SIVgag DNA vaccine (previously shown to assist protection of MamuA*01-positive rhesus macaques from SHIV89.6P disease) (9) and virus-like-particle vaccines demonstrated poor protective immunity against a SHIVmn229 challenge in outbred pigtail macaques (14). The SHIVmn229/pigtail macaque model could provide a rigorous alternative model to study protective immunity of DNA and pox virus vaccines.
A disadvantage of priming/boosting vaccination strategies in the field is the requirement for multiple different vaccinations. Whether one DNA vaccination, in the absence of readily detectable immune responses, sufficiently primes specific T-cell immune responses for augmentation by fowlpox virus boosting and protection against a virulent challenge in nonhuman primates is not clear. In efforts to improve and further understand the limitations of DNA/poxvirus priming/boosting regimens, we studied multigenic SHIV DNA and fowlpox virus vaccines for immunogenicity and protective efficacy in pigtail macaques. Comparator regimens included DNA vaccination alone, a fowlpox virus booster vaccine coexpressing gamma interferon (IFN-γ), and a DNA/fowlpox virus regimen with a single DNA priming.
MATERIALS AND METHODS
Monkeys.
Juvenile Macaca nemestrina monkeys were free from HIV-1, SIV, and simian retrovirus infection, housed under physical containment level 3 conditions, and anaesthetized with ketamine (10 mg/kg intramuscularly) prior to procedures. All experiments were performed according to National Institutes of Health guidelines on the care and use of laboratory animals and were approved by the University of Melbourne and CSIRO Livestock Industries Animal Experimentation and Ethics committees.
DNA vaccinations.
The DNA vaccine strain, pHIS-SHIV-B, encoded full-length unmutated SIVmac239 Gag and Pol, HIV-1AD8, Tat, Rev, and Vpu, and the 5′ third of HIV-1AD8 Env. Genes were inserted into vector pHIS-64 (Coley Pharmaceutical Group, Wellesley, Mass.) behind the human cytomegalovirus immediate-early promoter. Plasmid vector pHIS-64 has kanamycin resistance, the bovine growth hormone poly(A) termination signal, and 64 CpG motifs in addition to those naturally present that are primate optimized. Empty vector plasmid DNA, pHIS, served as a control vaccine. Plasmid pHIS-SHIV-B for immunization was prepared by Qiagen (Hilden, Germany), control DNA vaccine pHIS was prepared with the EndoFree Plasmid Giga kit (Qiagen). Plasmid DNA in normal saline was injected intramuscularly at 1 mg/ml at weeks 0 and 4 (Table 1).
TABLE 1.
Vaccine and challenge regimens
| Vaccine regimen | No. of monkeys | Immunization regimen
|
|||
|---|---|---|---|---|---|
| Wk 0 | Wk 4 | Wk 8 | Wk 18 | ||
| Control | 6 | Control | Control | Control | SHIV challenge |
| 2DNA/FPV | 6 | DNA | DNA | FPV | |
| 1DNA/FPV | 6 | DNA | FPV | ||
| 3DNA | 6 | DNA | DNA | DNA | |
| 2DNA/FPV-IFN-γ | 6 | DNA | DNA | FPV-IFN-γ | |
Recombinant fowlpox virus vaccines.
Construction of the fowlpox virus vaccines has been described (11). Briefly, FPVgag/pol, expressing SIV Gag and Pol, was constructed by inserting the promoter-SIV gag/pol PCR amplicon into pKG10a for insertion into FPV-M3 at the F6,7,9 site. PCR primers for the fowlpox virus early/late promoter and an early transcription terminator were used for insertion into fowlpox virus vector FPV-M3. HIV-193TH254 env (mutated to remove the middle third) was similarly amplified with fowlpox virus promoter and terminator sequences and inserted into the plasmid vector pCH34 for construction of a recombinant fowlpox virus with HIV Env expressed from the REV insertion site. Human IFN-γ was inserted into FPV-SIVgag/pol under the control of the fowlpox virus early/late promoter immediately downstream of the fowlpox virus thymidine kinase gene to construct vaccine FPV-SIVgag/pol-IFN-γ, coexpressing human IFN-γ. Recombinants were selected on the basis of coexpression of the Escherichia coli gpt gene and plaque purified on the basis of coexpression of the E. coli β-galactosidase gene. Fowlpox virus vaccines were prepared in saline, and 5 × 107 PFU was injected intramuscularly at week 8 (Table 1).
IFN-γ Elispot assay.
Enumeration of antigen-specific IFN-γ-secreting cells was assessed with a monkey IFN-γ Elispot commercial kit (U-CyTech, Utrecht, The Netherlands) as previously described (14). Briefly, freshly isolated peripheral blood mononuclear cells (PBMC) were stimulated with aldrithiol-2-inactivated whole SIVmne or control microvesicles (5 μg/ml, kindly provided by Jeff Lifson, AIDS Vaccine Program, National Cancer Institute) or SIVmac239 Gag and Pol overlapping 15-mer peptide pools (1 μg/ml/peptide, supplied by the National Institutes of Health AIDS Research and Reference Reagent Program) for 18 h. Cells were washed twice, transferred to anti-IFN-γ monoclonal antibody-coated flat-bottomed 96-well plates containing antigen, and incubated for a further 5 h. Cells were lysed, and plates were washed prior to incubation with biotinylated anti-IFN-γ polyclonal rabbit antibody, followed by incubation with gold-labeled anti-biotin immunoglobulin G antibody. IFN-γ spots were developed and analyzed by an automated reader (AID, Strassberg, Germany). Results were normalized to antigen-specific IFN-γ-secreting precursor frequency per 106 PBMC.
Intracellular IFN-γ staining.
Induction of antigen-specific intracellular IFN-γ expression in CD8+ or CD3+CD4+ T lymphocytes was assessed by flow cytometry as previously described (14, 25). Briefly, 200 μl of whole blood was incubated with 1 μg/ml of overlapping 15-mer peptide pools in dimethyl sulfoxide or dimethyl sulfoxide alone and the costimulatory antibodies CD28 and CD49d (BD Biosciences Pharmingen, San Diego, Calif.) for 7 h. Brefeldin A (10 μg/ml; Sigma) was included during the last 5 h of the incubation. Anti-CD3-phycoerythrin, anti-CD4-fluorescein isothiocyanate, and anti-CD8-peridinin chlorophyll protein (BD) antibodies were added to each well and incubated for 30 min. Red blood cells were lysed (fluorescence-activated cell sorting lysing solution; BD) and washed with phosphate-buffered saline, and the remaining cells were permeabilized (fluorescence-activated cell sorting permeabilizing solution 2; BD). Permeabilized cells were then incubated with anti-human IFN-γ-allophycocyanin antibody (BD) prior to fixing with formaldehyde and acquisition (FACScan; BD). Acquisition data were analyzed with CellQuest (BD). The percentage of antigen-specific gated lymphocytes expressing IFN-γ was assessed in both CD3+CD4+ and CD3+CD8+ lymphocyte subsets.
Lymphoproliferative responses.
Lymphoproliferative responses were assessed by the standard [3H]thymidine incorporation assay to 1 μg of SIV Gag peptide pool per ml, 10 μg of inactivated SIV per ml, or control antigens as described (22) and also phenotyped by 5-(and 6)carboxyfluorescein diacetate,succinimidyl ester (CFSE) proliferation studies. PBMC were stained with the fluorescent dye CFSE (5 μM; Molecular Probes, Eugene, Oreg.) prior to culturing for 6 days with the SIVgag peptide pool or inactivated SIV as above. Cells were washed in phosphate-buffered saline and phenotyped with monoclonal antibodies: phycoerythrin-conjugated anti-human CD3 (clone SP34; BD), peridinin chlorophyll protein-conjugated anti-human CD4 (clone L200; BD), and allophycocyanin-conjugated anti-human CD8 (clone Leu-2a; BD). Cells were formaldehyde fixed before fluorescence-activated cell sorting analysis. Proliferating CD4 or CD8 T cells were expressed as the percentage of CD3+CD4+ or CD3+CD8+ cells emitting CFSE of a lower intensity than the CFSE intensity of the nondividing T-lymphocyte subset.
SHIV challenge of macaques.
To assess vaccine efficacy, all macaques were inoculated atraumatically intrarectally with SHIVmn229 at 5 × 104 50% tissue culture infectious doses (TCID50)/ml in 0.5-ml doses over 2 days (total, 105 TCID50/ml) as previously described (14). SHIV viremia was quantified by reverse transcriptase real-time PCR, and depletion of peripheral CD4 T cells was quantified by flow cytometry as described (14).
To further define the monkey infectious dose of the SHIVmn229 challenge stock used (described in reference 14), the stock was further diluted and used for intrarectal inoculation of M. nemestrina monkeys at doses of 2 × 102 and 2 × 103 TCID50. The two macaques used for each dilution of the stock became infected, as evidenced by high viral load (mean peak SHIV plasma RNA for the four animals, 7.9 log10 copies/ml; range, 7.6 to 8.7) and low peripheral CD4 T cells by week 5 after inoculation (mean, 0.7% CD4 lymphocytes; range, 0.3 to 1.2%). The administered dose of 105 TCID50 therefore represented a monkey infectious dose of ≥500 TCID50.
TZM-bl luciferase reporter gene assay for neutralizing antibodies.
Neutralization was measured as a function of reductions in luciferase reporter gene expression after a single round of virus infection in TZM-bl cells as described (26). TZM-bl cells were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program, as contributed by John Kappes and Xiaoyun Wu. These cells are engineered to express CD4 and CCR5 (28) and contain integrated reporter genes for firefly luciferase and E. coli β-galactosidase under control of an HIV-1 long terminal repeat (39). Briefly, cell-free SHIVmn229 (200 TCID50, amplified on human PBMC) was incubated with serial dilutions of test samples in triplicate in a total volume of 150 μl for 1 h at 37°C in 96-well flat-bottomed culture plates. Freshly trypsinized cells (10,000 cells in 100 μl of growth medium containing 75 μg of DEAE dextran per ml and 2.5 μM indinavir) were added to each well. One set of control wells received cells and virus (virus control), and another set received cells only (background control). After a 48-h incubation, 100 μl of cells was transferred to 96-well black solid plates (Costar) for measurements of luminescence with Bright Glo substrate solution as described by the supplier (Promega). Neutralization titers are the dilution at which relative luminescence units were reduced by 50% compared to virus control wells after subtraction of background relative luminescence units.
Power and statistical considerations.
The primary endpoints, set prior to starting the study, were differences in outcome of SHIV challenge (peak and set point SHIV viremia and set point peripheral CD4 T-cell levels) between groups. Based on previous studies with this challenge stock (14), six macaques per group powered the study (80%, 2α = 0.05) to detect 0.5 log10 differences in both peak (week 2) and set point (mean, of weeks 5 to 11) plasma SHIV RNA and 5% set point CD4 T lymphocytes between groups. Comparison of viral load at set point utilized a time-weighted area-under-the-curve analysis. Week 11 was chosen as the end of the set point time period based on previous studies (14), as all macaques were likely to be alive and contributing data points up to that time, but at least some control macaques were likely to be euthanized shortly thereafter. Statistical comparisons of secondary endpoints of immunogenicity between vaccine groups utilized a t test of pairwise comparisons without compensation for the multiple analyses performed.
RESULTS
T-cell immunogenicity.
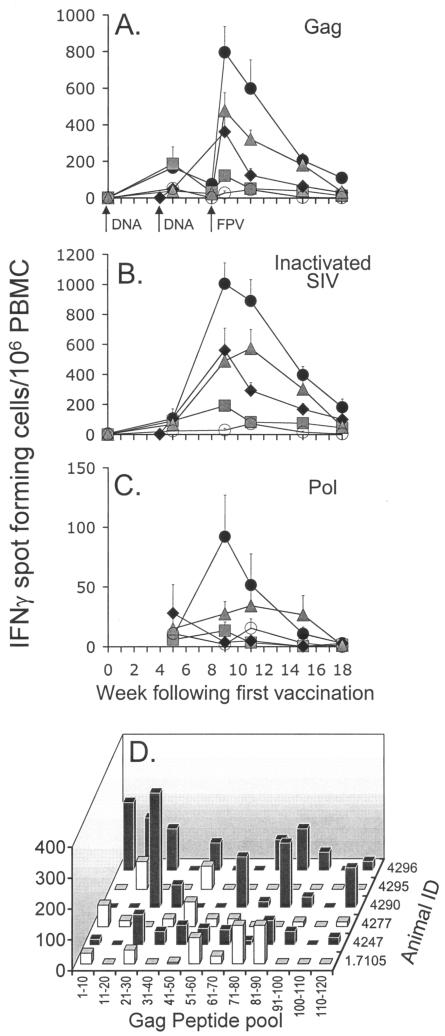
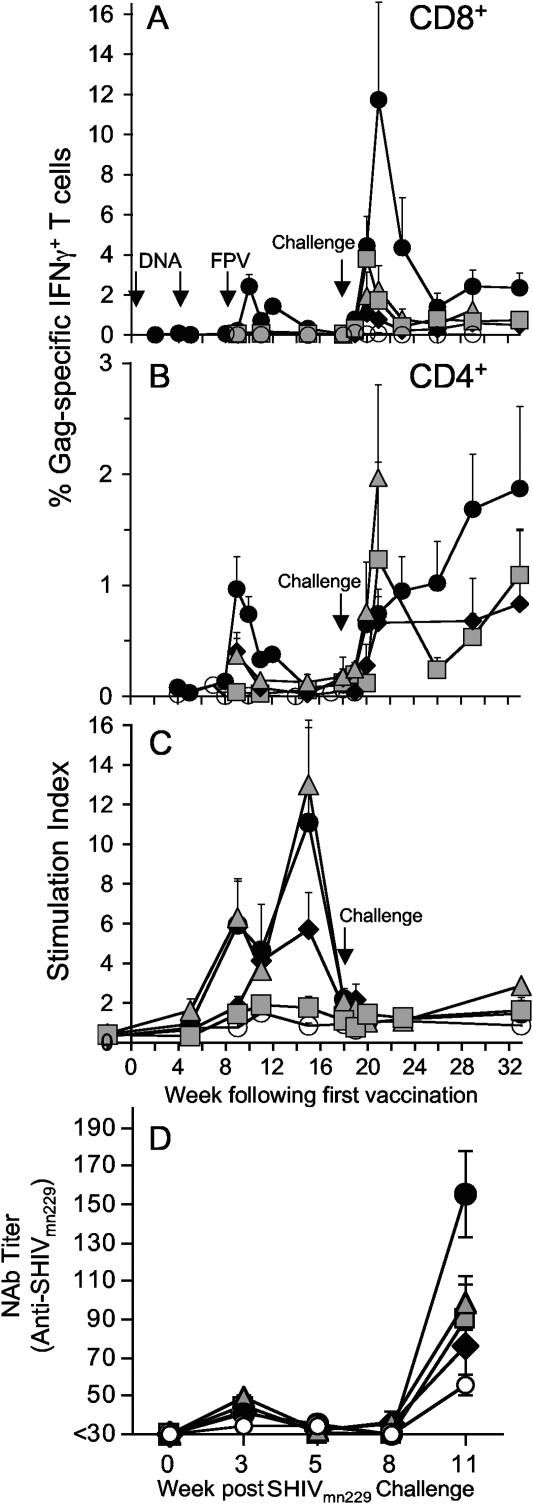
The kinetics, magnitude, and phenotype of the cellular response induced by vaccination was quantified by antigen-specific T-cell expression of IFN-γ by Elispot (Fig. 1) and intracellular IFN-γ staining (Fig. 2) assays. The 2DNA/FPV vaccine regimen was the most immunogenic, inducing a mean peak response to SIV Gag peptides of 797 spot-forming cells/106 PBMC (range, 455 to 1345) 1 week after the fowlpox virus booster was administered (Fig. 1A). Similarly, the mean response to whole inactivated SIV peaked at 1,005 spot-forming cells/106 PBMC (range, 595 to 1400) at week 9, demonstrating vigorous recognition of processed viral particles (Fig. 1B). Despite the small number of IFN-γ-secreting T cells after DNA vaccination (mean response to Gag peptides at week 8, 75 spot-forming cells/106 PBMC; range, 25 to 215), a rapid rise within 1 week of the fowlpox virus booster indicated that the DNA vaccine induced effective priming of the cellular response. All other vaccine groups also exhibited significantly elevated SIV Gag responses relative to controls 1 week after the final vaccination (P < 0.05).
FIG. 1.
Time course of mean cellular immune responses and standard error, as determined by IFN-γ Elispot in groups of six macaques immunized with • 2DNA/FPV,  2DNA/FPV-IFN-γ, ⧫ 1DNA/FPV,
2DNA/FPV-IFN-γ, ⧫ 1DNA/FPV,  3DNA, and ○ control vaccines in response to activation with (A) SIV Gag peptide pool, (B) aldrithiol-2-inactivated SIV, and (C) polymerase peptide pool. The breadth of the SIV Gag 125-peptide pool response at week 9 was further characterized for the six 2DNA/FPV-vaccinated macaques by (D) assessing responses in sequential 10-peptide pools.
3DNA, and ○ control vaccines in response to activation with (A) SIV Gag peptide pool, (B) aldrithiol-2-inactivated SIV, and (C) polymerase peptide pool. The breadth of the SIV Gag 125-peptide pool response at week 9 was further characterized for the six 2DNA/FPV-vaccinated macaques by (D) assessing responses in sequential 10-peptide pools.
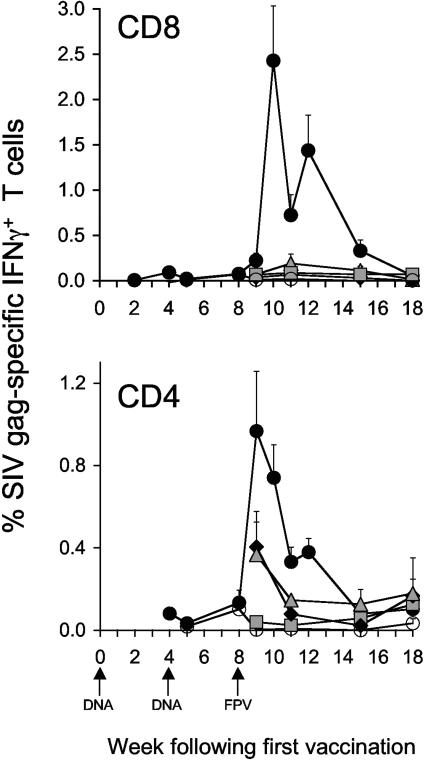
FIG. 2.
Immunogenicity of vaccine regimens as shown by flow cytometric analysis of intracellular IFN-γ staining. Time course of mean CD8+IFN-γ+ and CD4+IFN-γ+ Gag-specific T cells (+ standard error of the mean) in groups of macaques immunized with • 2DNA/FPV,  2DNA/FPV-IFN-γ, ⧫ 1DNA/FPV,
2DNA/FPV-IFN-γ, ⧫ 1DNA/FPV,  3DNA, and ○ control vaccines.
3DNA, and ○ control vaccines.
Modification of the 2DNA/FPV vaccination regimen induced lower numbers of antigen-specific cells. Coexpression of IFN-γ from the fowlpox virus construct had the undesired effect of reducing the peak number of IFN-γ-secreting T cells in response to inactivated SIV and Gag peptides by 51% (P = 0.01) and 40% (P = 0.13), respectively. Similarly, administration of a single DNA vaccination prior to the fowlpox virus booster reduced the response to inactivated SIV and SIV Gag relative to the 2DNA/FPV regimen by 44% (P = 0. 055) and 54% (P = 0.11), respectively. Three DNA vaccinations alone induced much lower levels of IFN-γ-expressing cells (122 and 192 cells/106 PBMC to Gag peptides and whole SIV, respectively; both, P < 0.01 compared to 2DNA/FPV).
Responses to the polymerase region of SIV were substantially lower than observed for the other antigens (Fig. 1C), presumably reflecting the lower level of polymerase expression from the vaccines. The only vaccine group to exhibit a polymerase response above background levels was the 2DNA/FPV regimen for a period of 3 weeks after the booster (P < 0.05 at week 9).
The Gag response in 2DNA/FPV vaccine recipients was further characterized at peak response for the breadth of the response (Fig. 1D). Stimulation with pools of 10 SIV Gag peptides showed that the response across the Gag region was broad in most animals with responses of >50 spots/106 PBMC generated to an average of four pools (range, two to seven pools).
The immunogenicity of all vaccine regimens was phenotyped by intracellular IFN-γ staining for IFN-γ-expressing CD4 and CD8 T cells and the kinetic pattern of CD4 and CD8 T-cell responses was studied weekly in the 2DNA/FPV group early following the fowlpox virus boost (Fig. 2). Peak numbers of Gag-specific CD4 T cells occurred 1 week after the fowlpox virus booster. CD4 T-cell responses were highest in the 2DNA/FPV-vaccinated group (1.0% of CD3+CD4+ lymphocytes; range, 0.2 to 2.1%) and reduced in other vaccine groups.
The frequency of Gag-specific CD8 T cells was higher than Gag-specific CD4 T cells, peaking at 2.4% (range, 0.3 to 4.7%) of all CD8 T cells in the 2DNA/FPV-vaccinated group. The peak SIV Gag-specific CD8 T-cell response in these animals occurred 1 week later than the peak CD4 T-cell response. All other comparator vaccine regimens generated substantially lower CD8 T cells expressing IFN-γ. The difference in magnitude of Gag-specific CD8 T-cell responses between groups detected by intracellular IFN-γ staining was exaggerated compared with CD4 T-cell responses by intracellular IFN-γ staining and T-cell immunogenicity by IFN-γ Elispot.
Lymphoproliferation studies.
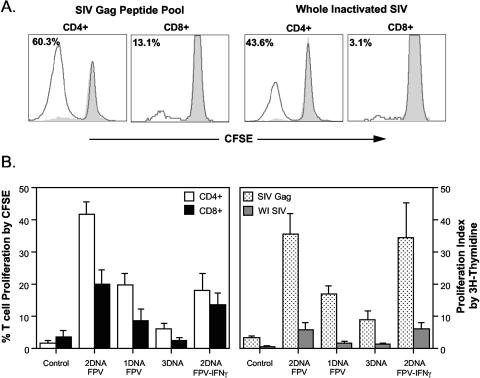
To assess the proliferative capacity of SIV-specific CD4 and CD8 T cells, lymphoproliferative responses were analyzed with both the [3H]thymidine incorporation assay and a CFSE proliferation assay. A 6-day culture of CFSE-stained PBMC isolated from a macaque immunized with the 2DNA/FPV vaccine regimen 2 weeks after fowlpox virus boosting had a large proportion of CD4 T cells proliferating in response to both SIV Gag peptides and inactivated SIV stimulation (Fig. 3). Although responses were lower, CD8 T cells also proliferated to the SIV antigens.
FIG. 3.
Lymphoproliferative responses postimmunization. (A) CFSE-labeled PBMC obtained 2 weeks following fowlpox virus boosting were incubated with either SIV Gag peptides or inactivated SIV and the corresponding controls (dimethyl sulfoxide and microvesicles, respectively). Proliferating PBMC were phenotyped, and the CD4 and CD8 proliferative responses were detected in one macaque immunized with the SHIV vaccines are shown. The shaded peak (grey) shows the background proliferation to control antigens, and the solid line shows the proliferating cells. The percentage of cells proliferating is indicated in each histogram. (B) Mean (+ standard error) proliferation responses are shown for each group postimmunization with the fowlpox virus vaccines. Proliferation responses for CD4 and CD8 T cells are shown for CFSE-labeled PBMC in response to SIV Gag peptides (mean of four macaques per group, left panel) and the standard [3H]thymidine incorporation method to both SIV Gag overlapping peptides and whole inactivated SIV (mean of six macaques per group, right panel).
When analyzed across the different vaccine regimens, proliferative SIV Gag-specific CD4 T cells early after fowlpox virus boosting were greatest in macaques immunized with the 2DNA/FPV regimen, with lower responses in the 1DNA/FPV and 2DNA/FPV-IFN-γ regimens and negligible Gag-specific proliferative responses in the 3DNA regimen (Fig. 3), similar to the pattern of T-cell immune responses observed by IFN-γ Elispot and intracellular IFN-γ staining. A similar pattern of proliferative responses to both SIV Gag peptides and inactivated SIV was observed by the [3H]thymidine incorporation, with the exception that the responses in the 2DNA/FPV-IFN-γ immunized group were equivalent to those of the 2DNA/FPV group.
SHIV challenge.
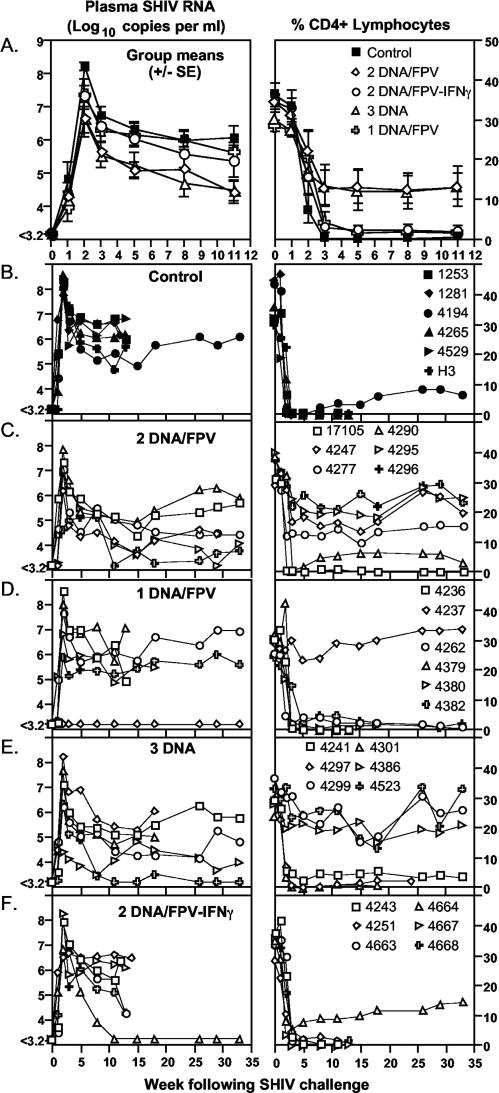
The pathogenic SHIVmn229 challenge stock was inoculated intrarectally (105 TCID50 or ≥500 monkey infectious doses) into all 30 macaques 10 weeks after the last vaccination. CD4 T cells and plasma SHIV RNA were followed for 33 weeks, unless macaques were euthanized to avoid AIDS-related illnesses (Fig. 4). The six control macaques receiving DNA and fowlpox virus vaccines not expressing SHIV antigens had high peak levels of SHIV RNA (mean, 8.2 log10 copies/ml; Table 2) following inoculation and at set point (weeks 5 to 11; mean, 6.2 log10 copies/ml; Fig. 4B). All six control macaques lost peripheral CD4 T cells precipitously, declining to a mean of 0.43% of total lymphocytes 3 weeks after challenge, and five of the six macaques were euthanized within 15 weeks of inoculation with incipient AIDS.
FIG. 4.
Outcome of SHIVmn229 challenge. Each macaque was challenged intrarectally with a monkey infectious dose of ≥500 SHIVmn229 10 weeks post-final immunizations. Blood samples were analyzed for plasma SHIV RNA by real-time PCR and for CD4 T-cell loss. (A) Mean (±standard error of the mean) plasma SHIV RNA and CD4 T cells for each vaccine group. (B to F) Plasma SHIV RNA and CD4 T-cell responses are shown for individual macaques grouped for each vaccine regimen.
TABLE 2.
Outcome of SHIVmn229 viral challenge
| Group | SHIVmn229 viral load
|
% CD4+ CD3+ T cells at set point
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak viral load (log10 copies/ml)
|
Set pointa viral load (log10 copies/ml)
|
||||||||||||
| Mean | Range | Pb vs. controls | Mean | Range |
P
|
Mean | Range |
P
|
|||||
| Vs. controls | Vs. 2DNA/FPV | Vs. 3DNA | Vs. controls | Vs. 2DNA/FPV | Vs. 3DNA | ||||||||
| Control | 8.2 | 7.8-8.6 | 6.2 | 5.4-6.7 | 0.5 | 0.05-2.0 | |||||||
| 2DNA/FPV | 6.7 | 5.1-7.9 | 0.004 | 4.9 | 4.4-5.4 | 0.002 | 12.3 | 0.7-21.5 | 0.006 | ||||
| 3DNA | 6.7 | 4.7-8.2 | 0.016 | 4.8 | 3.8-6.0 | 0.011 | 0.785 | 11.4 | 0.6-22.6 | 0.033 | 0.92 | ||
| 1DNA/FPV | 7.4 | 5.9-8.6 | 0.09 | 5.95 | 5.3-6.6 | 0.565 | 0.006 | 0.031 | 1.8 | 0.1-4.2 | 0.154 | 0.023 | 0.082 |
| 2DNA/FPV-IFN-γ | 7.4 | 6.6-8.2 | 0.02 | 5.6 | 4.0-6.5 | 0.224 | 0.107 | 0.139 | 2.3 | 0.1-8.7 | 0.216 | 0.021 | 0.073 |
Set point values taken from weeks 5, 8, and 11 postchallenge.
P represents the P value for pairwise comparisons between the group noted and the other vaccine groups.
All 24 vaccinated macaques became infected except one in the 1DNA/FPV group (animal 4237). Peak SHIV viral load in the infected macaques at 2 weeks following inoculation was lowest in the groups vaccinated with the 2DNA/FPV and 3DNA regimens (means, 6.7 log10 copies/ml), being significantly lower than controls (Fig. 4A, Table 2). The peak viral load in the 2DNA/FPV-IFN-γ regimen (mean, 7.3 log10 copies/ml; Table 2) was also lower than controls, and the peak viral load in the 1DNA/FPV regimen, excluding the outlier animal 4237 that did not become infected, was not significantly different from controls (mean, 7.4 log10 copies/ml). There were no significant differences in viral load between the vaccinated groups.
Set point viral load was defined prior to challenge as the mean viral load over weeks 5 to 11 following challenge, when viral load is relatively stable and all animals appear healthy. Set point SHIV viral load was lowest in the groups vaccinated with the 2DNA/FPV and 3DNA regimens (4.9 and 4.8 log10 copies/ml, respectively), being significantly lower than controls and the 1DNA/FPV regimen (6.2 and 6.0 log10 copies/ml, respectively; Fig. 4A, Table 2). The set point viral load in the 2DNA/FPV-IFN-γ regimen (5.6 log10 copies/ml) was not significantly different from that of controls.
There was significant retention of peripheral CD4 T cells in the groups immunized with the 2DNA/FPV regimen at set point levels between weeks 5 and 11 (mean, 12.3% CD4 T lymphocytes) compared to controls (0.5%), the 1DNA/FPV-vaccinated group (1.8%), and the 2DNA/FPV-IFN-γ-vaccinated group (2.3%; Fig. 4A, Table 2). The 3DNA-vaccinated group also showed significant retention of CD4 T lymphocytes (11.7%) compared to controls (Table 2).
The animals were followed for 33 weeks following SHIV challenge (Fig. 4B to F). Five animals in the 2DNA/FPV-IFN-γ group, four in the 1DNA/FPV group, two in the 3DNA group, and none in the 2DNA/FPV group were euthanized with incipient AIDS within 18 weeks after SHIV challenge. Only 2 of the 29 infected macaques controlled viremia to below detectable levels (one in each of the 3DNA and 2DNA/FPV-IFN-γ groups), with all the remaining macaques experiencing ongoing viremia. Peripheral CD4 T cells were retained (>10% of lymphocytes) at the end of the study in four of the 2DNA/FPV-immunized animals, three of the 3DNA-immunized animals, and only one each of the 1DNA/FPV- and 2DNA/FPV-IFN-γ-immunized animals.
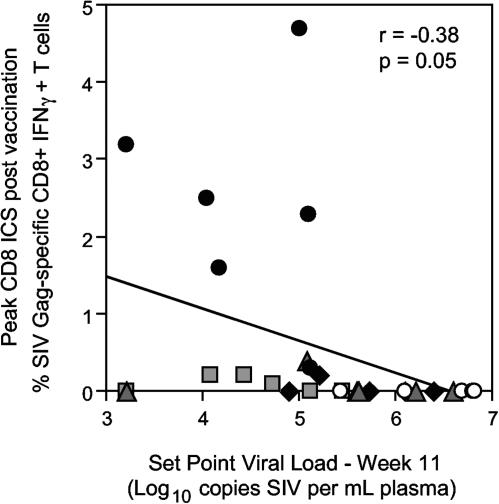
The partial protection in the group receiving the 3DNA vaccines in the absence of significant T-cell immunity as a group was interesting, and we therefore attempted to correlate the outcome of challenge with prechallenge T-cell immune responses across all animals in all groups. There was a weak but significant correlation between peak CD8 T-cell responses to SIV Gag as measured by intracellular IFN-γ staining with set point viral load (r = −0.38, P = 0.05, Fig. 5) and CD4 T-cell count (r = 0.47, P = 0.01). However, reductions in peak viral load did not correlate with any T-cell immunity parameter studied, and SIV Gag-specific CD4 T-cell responses by intracellular IFN-γ staining or SIV-specific immunity as measured by Elispot did not correlate with outcome of challenge (not shown). The three animals in the 3DNA immunization group, which effectively controlled the SHIVmn229 challenge, did not have high levels of T-cell immunity (Fig. 5, grey squares).
FIG. 5.
Correlation between CD8 T immune responses induced by immunization and outcome of challenge—set point viral load at week 11. The 30 individual macaques are from the five vaccination regimens are indicated by the symbols: • 2DNA/FPV,  2DNA/FPV-IFN-γ, ⧫ 1DNA/FPV,
2DNA/FPV-IFN-γ, ⧫ 1DNA/FPV,  3DNA, and ○ controls.
3DNA, and ○ controls.
Immune responses following challenge.
T-cell immunity was analyzed following SHIV challenge by intracellular IFN-γ staining and proliferation studies (Fig. 6). SIV Gag-specific CD8 T cells expressing IFN-γ were dramatically boosted after challenge (Fig. 6A). Mean Gag-specific CD8 T cells peaked 3 weeks following challenge at 11.7% (range, 3.0 to 34.7%) of all CD8 T cells in these outbred pigtail macaques immunized with the 2DNA/FPV regimen. The other vaccine groups had lower mean postchallenge CD8 T-cell responses, with peak responses at week 2 following challenge numbering 3.8% (range, 0.1% to 9.5%) in the 3DNA group, 1.1% (range, 0% to 4.0%) responses in the 1DNA/FPV group, and 2.0% (range, 0% to 6.2%) in the 2DNA/FPV-IFN-γ-immunized animals.
FIG. 6.
Immune responses detected post-SHIVmn229 intrarectal challenge. A time course of the mean (+ standard error of the mean) cellular immune responses, prechallenge (weeks 0 to 18) and postchallenge (weeks 19 to 33), is shown. (A) Percentage of Gag-specific CD8. (B) CD4 T-cell population expressing IFN-γ by intracellular IFN-γ staining. (C) Lymphoproliferation in response to stimulation with inactivated SIV. (D) The plasma neutralizing antibody titer to SHIVmn229 on TZM-bl cells following challenge. Groups: • 2DNA/FPV,  2DNA/FPV-IFN-γ, ⧫ 1DNA/FPV,
2DNA/FPV-IFN-γ, ⧫ 1DNA/FPV,  3DNA, and ○ control.
3DNA, and ○ control.
A substantial postchallenge increase in IFN-γ-expressing Gag-specific CD4 T cells was also observed in some vaccinated macaques after SHIV challenge, despite at least some depletion of total CD4 T cells in almost all animals (Fig. 6B). In the 2DNA/FPV group, a gradual rise in the proportion of SHIV-specific CD4 T cells expressing IFN-γ occurred, reaching a mean of 1.9% (range, 0.2% to 3.8%) by 15 weeks after challenge. Data were not available for macaques in other groups at time points when there were too few macaques (less than three out of six) with sufficient peripheral CD4 T cells (>1%) to estimate the mean number of SHIV-specific CD4 T cells. Despite these limitations, a significant proportion of CD4 T cells was expressing IFN-γ in response to Gag stimulation in the 3DNA group, the 1DNA/FPV group, and (at 1 week after challenge) the 2DNA/FPV-IFN-γ group.
In contrast to the large anamnestic SHIV-specific CD4 and CD8 T cells expressing IFN-γ in response to SHIV challenge, there was a nearly complete lack of SHIV-specific proliferating T cells (Fig. 6C). Proliferating T cells were readily detected up to 5 weeks after the last vaccination in the 2DNA/FPV, 1DNA/FPV, and 2DNA/FPV-IFN-γ groups, but no significant rise in SHIV-specific proliferating T cells was observed out to 15 weeks after challenge. This lack of anamnestic proliferation response to virus challenge was observed with both inactivated SIV as a stimulating antigen (Fig. 6C) and SIV Gag peptides (not shown).
Since the partial control of SHIV89.6P viremia in previous DNA and pox virus vaccine studies has been associated with a rapid rise in neutralizing antibodies (4), we assessed neutralizing antibody responses to SHIVmn229 following challenge. There were no neutralizing antibodies to SHIVmn229 at the time of challenge (consistent with the divergent and mutated env genes in the vaccines) and no significant neutralizing antibody responses developed prior to week 11 postchallenge (Fig. 6D). At week 11 (when all animals were still alive), higher neutralizing antibody responses were detected in the 2DNA/FPV group compared to controls or the other vaccine groups.
DISCUSSION
This study demonstrated high levels of CD4 and CD8 T-cell immunity in outbred pigtail macaques immunized with DNA and FPV vaccines. Partial protective immunity was observed following a very high dose, virulent SHIVmn229 mucosal challenge. Comparator regimens that used a single DNA priming vaccination or coexpressed IFN-γ with the fowlpox virus boost were less immunogenic and less protective. A vaccine regimen that utilized only the DNA vaccine demonstrated reasonable protective efficacy despite being far less immunogenic than the DNA/fowlpox virus regimens.
The protection observed in the DNA vaccination alone group is intriguing. Little or no SHIV-specific CD4 or CD8 T-cell response was induced by DNA vaccination alone by IFN-γ Elispot, intracellular cytokine staining, or proliferation studies. Although there was a weak but significant inverse correlation across all animals between set point viral load and SIV Gag-specific CD8+ T cells, this correlation was primarily driven by high levels of immunity in animals receiving the 2DNA/FPV vaccines. Levels of T-cell immunity were low in animals receiving 3DNA immunizations that controlled the SHIVmn229 challenge. Following challenge, a sharp rise in CD8 and CD4 T-cell responses was observed in the 3DNA vaccination group, parallel to but lower than those observed in the 2DNA/FPV group. Mean viral load and CD4 counts were indistinguishable from the 2DNA/FPV group for the first 11 weeks postchallenge, which were the primary endpoints. These results suggest that the “priming” effect of the DNA vaccine alone may be sufficient to recall effective immune responses following challenge. The priming of immunity by the DNA vaccine may have been assisted by the multiple immunostimulatory CpG motifs added to the vector and expression of a large proportion of SHIV antigens. These results raise questions about whether the expansion (and contraction) of T cells following the boosting poxvirus vaccination is either required or useful for long-term protective immunity (10). Technologies to more readily measure and characterize this priming immune response following DNA vaccination could facilitate a better understanding of protective T-cell immunity.
The poorer SHIV-specific immunity in the DNA/fowlpox virus group coexpressing IFN-γ confirmed data in macaques with similar HIV-1 vaccines (Dale et al., submitted for publication). Not only were T-cell responses significantly lower in animals vaccinated with fowlpox virus coexpressing IFN-γ, but a nearly complete loss of protective efficacy was observed. We hypothesize that the expression of IFN-γ may downregulate fowlpox virus vector expression of the inserted SHIV genes. This is supported by findings in a previous study where altered anti-fowlpox virus antibody responses were observed after boosting with cytokine coexpressing fowlpox virus vaccines (Dale et al., submitted).
Several phenotypic features of the T-cell responses induced by these DNA and fowlpox virus vaccines were of interest. Serial weekly measurements of SHIV-specific CD4 and CD8 T-cell responses by intracellular IFN-γ staining demonstrated a coordinated early induction of CD4 responses of lower magnitude 1 week following the boost and then later induction of CD8 responses of higher magnitude 2 weeks following the boost. Multiple regions of SIV Gag were recognized by the T-cell responses induced as detected by both IFN-γ Elispot (Fig. 1D) and intracellular IFN-γ staining assays (not shown). Additional analyses of the T-cell responses induced by these vaccines are ongoing, including epitope mapping, analyzing memory markers, defining additional cytokines secreted by specific T cells, and examining the in vivo cytotoxic potential of the immunity induced.
The vaccinations did not induce significant envelope-specific T-cell responses (not shown) likely because of the truncated and heterologous Env proteins expressed by these DNA and fowlpox virus vaccines. SIV Gag-specific antibody responses were induced by the vaccines, but no significant antibody response to envelope was detected prechallenge (unpublished data; Dale et al., submitted) and no significant neutralizing antibody responses were detected at the time of challenge. Improving expression of whole Env antigens may also facilitate at least narrowly directed neutralizing antibody responses. Responses to polymerase were lower than to Gag, consistent with lower expression of polymerase by the vaccines (Purcell et al., unpublished data). Improvement in expression of polymerase proteins could potentially improve the breadth of immune responses induced by these vaccines.
Several aspects differentiated this study from previous DNA prime/poxvirus boost macaque studies (2, 4, 17, 18, 22, 23, 29, 31, 33). First, we studied outbred, unselected pigtail macaques that were highly susceptible to SHIV infection. Second, we used a pathogenic, high-dose, intrarectal SHIVmn229 challenge and demonstrated partial protection. In contrast to SHIV89.6P challenge of vaccinated MamuA*01-positive rhesus macaques (4, 9, 34), few (3 out of 24) vaccinated pigtail macaques challenged with SHIVmn229 completely controlled viremia. Homologous neutralizing antibody responses to SHIVmn229 were not rapidly (≤8 weeks) generated following challenge, which is also in contrast to SHIV89.6P challenge studies. The slow generation of neutralizing antibody to SHIVmn229 is consistent with the long (30 to 70 weeks) initial in vivo passage of SHIVHXB2 through two pigtail macaques, the heterologous nature of the neutralization determinants in the vaccines, and the previously described resistance to neutralizing antibody of pathogenic SHIVs derived from SHIVHXB2 (1, 12, 13, 21, 35, 36, 41). The late generation of neutralizing antibody (11 weeks postchallenge) did not completely control SHIVmn229 viremia in these animals, potentially due to evolution of neutralizing antibody escape variants over time, as described in humans with HIV-1 infection (30, 40). Ongoing viral replication observed following the acute infection is also highly likely to select for T-cell escape variants and eventual loss of viral control in this model, as observed in SIVmac239/rhesus macaque studies (38).
Following SHIV challenge, SHIV-specific IFN-γ-secreting CD4 T-cell responses were detected in animals with partial control of viremia. Interestingly, few or no SHIV-specific T cells could be detected by proliferation studies, suggesting that SHIV-specific CD4 T cells were dysfunctional. In vitro SHIV infection in the culture could have killed proliferating T cells, although additional experiments with antiretroviral drug treatment in vitro did not rescue T-cell proliferative capacity (not shown). A proliferative defect in HIV-1-specific T cells is common in humans chronically infected with HIV-1, and HIV-1-specific CD4 T cells appear to be preferential targets for infection and elimination by HIV-1 (15). The long-term effectiveness of HIV-1 vaccines that induce nonsterilizing immunity could be limited by infection-induced dysfunction of HIV-specific T cells (10).
These studies have been used to guide subtype B-based HIV-1 DNA and fowlpox virus clinical vaccine studies in Australia that use the 2DNA/FPV vaccine regimen. The SHIV DNA vaccine component studied herein expressed six SHIV genes, while the fowlpox virus SHIV vaccines expressed only three, and one of these (Env) was derived from a different HIV-1 strain. Recent studies suggest including additional shared SHIV genes, in particular that for the envelope, can facilitate the induction of broadly reactive T-cell immunity (3, 24). In order to evaluate whether improved DNA/FPV vaccine combinations can generate broader immunity, our group has now constructed single recombinant FPV for both HIV-1 (based on HIV-1 subtype A/E) and SHIV that express nearly identical large amounts of antigenic material. Plans are under way in 2004 to evaluate an HIV-1 A/E DNA/fowlpox virus combination in clinical trials in Thailand.
Acknowledgments
This work was supported by the National Institutes of Health HIV Vaccine Design and Development Team, award N01-AI-05395, and the Australian Commonwealth Department of Health and Aging.
We thank all members of the Australian Thai HIV Vaccine Consortium for their support and guidance. We thank Richard Sydenham, Andrew Sydenham, Leah Protyniak, and Kim Szalnowski for providing excellent animal care.
REFERENCES
- 1.Agy, M. B., J. L. Thompson, E. M. Coon, L. Kuller, D. M. Anderson, S. L. Hu, and W. R. Morton. 1997. Enhanced pathogenicity of SHIV HXBc2 following whole blood passage in Macaca nemestrina. Conf. Adv. AIDS Vaccine Dev., poster 27.
- 2.Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. [DOI] [PubMed] [Google Scholar]
- 3.Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 5.Amara, R. R., F. Villinger, S. I. Staprans, J. D. Altman, D. C. Montefiori, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 76:7625-7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appay, V., and S. L. Rowland-Jones. 2002. Premature ageing of the immune system: the cause of AIDS? Trends Immunol. 23:580-585. [DOI] [PubMed] [Google Scholar]
- 8.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 9.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 10.Bellier, B., V. Thomas-Vaslin, M. F. Saron, and D. Klatzmann. 2003. Turning immunological memory into amnesia by depletion of dividing T cells. Proc. Natl. Acad. Sci. USA 100:15017-15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle, D. B., M. Anderson, R. Amos, R. Voysey, and B. E. H. Coupar. 2004. Construction of recombinant fowlpox viruses carrying multiple vaccine antigens and immunomodulatory molecules. BioTechniques 37:104-111. [DOI] [PubMed] [Google Scholar]
- 12.Cayabyab, M., G. B. Karlsson, B. A. Etemad-Moghadam, W. Hofmann, T. Steenbeke, M. Halloran, J. W. Fanton, M. K. Axthelm, N. L. Letvin, and J. G. Sodroski. 1999. Changes in human immunodeficiency virus type 1 envelope glycoproteins responsible for the pathogenicity of a multiply passaged simian-human immunodeficiency virus (SHIV-HXBc2). J. Virol. 73:976-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford, J. M., P. L. Earl, B. Moss, K. A. Reimann, M. S. Wyand, K. H. Manson, M. Bilska, J. T. Zhou, C. D. Pauza, P. W. Parren, D. R. Burton, J. G. Sodroski, N. L. Letvin, and D. C. Montefiori. 1999. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J. Virol. 73:10199-10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dale, C. J., X. S. Liu, R. De Rose, D. F. Purcell, J. Anderson, Y. Xu, G. R. Leggatt, I. H. Frazer, and S. J. Kent. 2002. Chimeric human papilloma virus-simian/human immunodeficiency virus virus-like-particle vaccines: immunogenicity and protective efficacy in macaques. Virology 301:176-187. [DOI] [PubMed] [Google Scholar]
- 15.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95-98. [DOI] [PubMed] [Google Scholar]
- 16.Dyer, W. B., A. F. Geczy, S. J. Kent, L. B. McIntyre, S. A. Blasdall, J. C. Learmont, and J. S. Sullivan. 1997. Lymphoproliferative immune function in the Sydney Blood Bank Cohort, infected with natural nef/long terminal repeat mutants, and in other long-term survivors of transfusion-acquired HIV-1 infection. AIDS 11:1565-1574. [DOI] [PubMed] [Google Scholar]
- 17.Hanke, T., T. J. Blanchard, J. Schneider, C. M. Hannan, M. Becker, S. C. Gilbert, A. V. Hill, G. L. Smith, and A. McMichael. 1998. Enhancement of MHC class I-restricted peptide-specific T-cell induction by a DNA prime/MVA boost vaccination regime. Vaccine 16:439-445. [DOI] [PubMed] [Google Scholar]
- 18.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 73:7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heeney, J. L. 2002. The critical role of CD4(+) T-cell help in immunity to HIV. Vaccine 20:1961-1963. [DOI] [PubMed] [Google Scholar]
- 20.Horton, H., T. U. Vogel, D. K. Carter, K. Vielhuber, D. H. Fuller, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, D. C. Montefiori, V. Erfle, R. C. Desrosiers, N. Wilson, L. J. Picker, S. M. Wolinsky, C. Wang, D. B. Allison, and D. I. Watkins. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 76:7187-7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu, S. L., J. E. Klaniecki, B. M. Travis, T. Wrey, S. Pennathur, D. Montefiori, J. L. Thompson, M. B. Agy, L. Kuller, and W. R. Morton. 1997. Immunization with HIV-1 gp160 by the “prime and boost” regimen protects macaques against SHIV HXBc2 challenge, p. 291-298. In F. Brown, H. Chanock, and E. Norrby (ed.), Vaccines 97. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Kent, S. J., A. Zhao, S. J. Best, J. D. Chandler, D. B. Boyle, and I. A. Ramshaw. 1998. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J. Virol. 72:10180-10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leong, K. H., A. J. Ramsay, D. B. Boyle, and I. A. Ramshaw. 1994. Selective induction of immune responses by cytokines coexpressed in recombinant fowlpox virus. J. Virol. 68:8125-8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letvin, N. L., Y. Huang, B. K. Chakrabarti, L. Xu, M. S. Seaman, K. Beaudry, B. Korioth-Schmitz, F. Yu, D. Rohne, K. L. Martin, A. Miura, W. P. Kong, Z. Y. Yang, R. S. Gelman, O. G. Golubeva, D. C. Montefiori, J. R. Mascola, and G. J. Nabel. 2004. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J. Virol. 78:7490-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maecker, H. T., H. S. Dunn, M. A. Suni, E. Khatamzas, C. J. Pitcher, T. Bunde, N. Persaud, W. Trigona, T. M. Fu, E. Sinclair, B. M. Bredt, J. M. McCune, V. C. Maino, F. Kern, and L. J. Picker. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods 255:27-40. [DOI] [PubMed] [Google Scholar]
- 26.Montefiori, D. C. Evaluating neutralizing antibodies against HIV, SIV and SHIV in a luciferase reporter gene assay. Curr. Prot. Immunol., in press. [DOI] [PubMed]
- 27.Nabel, G. J. 2001. Challenges and opportunities for development of an AIDS vaccine. Nature 410:1002-1007. [DOI] [PubMed] [Google Scholar]
- 28.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsay, A. J., K. H. Leong, and I. A. Ramshaw. 1997. DNA vaccination against virus infection and enhancement of antiviral immunity following consecutive immunization with DNA and viral vectors. Immunol. Cell Biol. 75:382-388. [DOI] [PubMed] [Google Scholar]
- 30.Richman, D. D., T. Wrin, S. J. Little, and C. J. Petropoulos. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. USA 100:4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson, H. L., D. C. Montefiori, R. P. Johnson, K. H. Manson, M. L. Kalish, J. D. Lifson, T. A. Rizvi, S. Lu, S. L. Hu, G. P. Mazzara, D. L. Panicali, J. G. Herndon, R. Glickman, M. A. Candido, S. L. Lydy, M. S. Wyand, and H. M. McClure. 1999. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat. Med. 5:526-534. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg, E. S., M. Altfeld, S. H. Poon, M. N. Phillips, B. M. Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D'Aquila, P. J. Goulder, and B. D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523-526. [DOI] [PubMed] [Google Scholar]
- 33.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T-cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 34.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 35.Si, Z., M. Cayabyab, and J. Sodroski. 2001. Envelope glycoprotein determinants of neutralization resistance in a simian-human immunodeficiency virus (SHIV-HXBc2P 3.2) derived by passage in monkeys. J. Virol. 75:4208-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Si, Z., P. Gorry, G. Babcock, C. M. Owens, M. Cayabyab, N. Phan, and J. Sodroski. 2004. Envelope glycoprotein determinants of increased entry in a pathogenic simian-human immunodeficiency virus (SHIV-HXBc2P 3.2) passaged in monkeys. AIDS Res. Hum. Retroviruses 20:163-173. [DOI] [PubMed] [Google Scholar]
- 37.Vogel, T. U., T. C. Friedrich, D. H. O'Connor, W. Rehrauer, E. J. Dodds, H. Hickman, W. Hildebrand, J. Sidney, A. Sette, A. Hughes, H. Horton, K. Vielhuber, R. Rudersdorf, I. P. De Souza, M. R. Reynolds, T. M. Allen, N. Wilson, and D. I. Watkins. 2002. Escape in one of two cytotoxic T-lymphocyte epitopes bound by a high-frequency major histocompatibility complex class I molecule, Mamu-A*02: a paradigm for virus evolution and persistence? J. Virol. 76:11623-11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel, T. U., M. R. Reynolds, D. H. Fuller, K. Vielhuber, T. Shipley, J. T. Fuller, K. J. Kunstman, G. Sutter, M. L. Marthas, V. Erfle, S. M. Wolinsky, C. Wang, D. B. Allison, E. W. Rud, N. Wilson, D. Montefiori, J. D. Altman, and D. I. Watkins. 2003. Multispecific vaccine-induced mucosal cytotoxic T lymphocytes reduce acute-phase viral replication but fail in long-term control of simian immunodeficiency virus SIVmac239. J. Virol. 77:13348-13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 41.Ye, Y., Z. H. Si, J. P. Moore, and J. Sodroski. 2000. Association of structural changes in the V2 and V3 loops of the gp120 envelope glycoprotein with acquisition of neutralization resistance in a simian-human immunodeficiency virus passaged in vivo. J. Virol. 74:11955-11962. [DOI] [PMC free article] [PubMed] [Google Scholar]