Abstract
The potential for genetic discovery in human DNA sequencing studies is greatly diminished if DNA samples from a cohort are mislabeled, swapped, or contaminated or if they include unintended individuals. Unfortunately, the potential for such errors is significant since DNA samples are often manipulated by several protocols, labs, or scientists in the process of sequencing. We have developed a software package, peddy, to identify and facilitate the remediation of such errors via interactive visualizations and reports comparing the stated sex, relatedness, and ancestry to what is inferred from the individual genotypes derived from whole-genome (WGS) or whole-exome (WES) sequencing. Peddy predicts a sample’s ancestry using a machine learning model trained on individuals of diverse ancestries from the 1000 Genomes Project reference panel. Peddy facilitates both automated and interactive, visual detection of sample swaps, poor sequencing quality, and other indicators of sample problems that, if left undetected, would inhibit discovery.
Keywords: pedigree, quality control, VCF, genetic variation, sample mixup, QC
Introduction
Human DNA sequencing studies frequently involve the handling of DNA samples and associated manifests by multiple laboratories and individuals. Both WES and WGS protocols involve multiple DNA manipulations prior to sequencing. Each new procedure or handling is another opportunity for sample mix-ups, contamination, or mislabeling. Even a single DNA mix-up has the potential to destroy discovery and diagnostic power. For example, undetected DNA swaps in family studies of human disease (e.g., an unaffected father is swapped with his affected son) will prevent a genetic diagnosis and yield misleading candidate variants. Even without sample errors, the sample manifest (e.g., PED file1), which contains vital information about the relatedness of individuals within a cohort, may include sample-naming errors or swaps. In our experience, familial relationships are often transcribed manually from pedigree diagrams drawn from the researcher. In addition, large studies may have unknowingly recruited the same subject who is unintentionally represented multiple times in a study. Such errors can easily go unnoticed without careful review.
Therefore, a critical aspect of quality control is assuring that each sequenced DNA sample originated from the expected individual. Unfortunately, these sample-level quality-control problems are not detected by existing tools that leverage raw sequence data (e.g., FastQC) or sequence alignments (e.g., bam.iobio,2 samtools3). While tools such as PLINK1 and KING4 can detect sex and pedigree errors, they solely produce text output that requires further manual inspection of custom scripts to detect sample issues. Other tools5, 6 are able to infer pedigree structure from sample genotype data, but they are cumbersome for identifying and resolving sample swaps. To address the need for robust, rapid, and automated detection of problems with sample DNA fidelity, we have developed peddy, a software package that evaluates the correspondence between the stated sexes, relationships, and ancestries in a pedigree file1 and those inferred from the genotypes in the VCF file7 resulting from human WES or WGS studies. Peddy is fast and user friendly: a single command executes a variety of sample analyses directly on a VCF and an associated PED file. The resulting interactive web page and comma-separated (CSV) files convey the results of each quality-control test for each sample, as well as indications of which samples are likely to have been swapped or mislabeled or have poor DNA quality.
Material and Methods
Overview of Quality-Control Measurements
Peddy interrogates the genotypes reported in a VCF file to identify potential sample quality problems based on four primary statistics. First, each sample’s stated sex is compared to the genotypes observed on the X chromosome. Second, it compares the degree of relatedness observed between each pair of samples to the expected relatedness measure based on what is stated in the PED file. Third, sample quality is assessed by the count, sequencing depth, and ratio of sequence alignments for each allele at sites where an individual is heterozygous. The variance in these measurements facilitates the detection of DNA contamination, unexpected diversity, and insufficient sequencing depth. Finally, the ancestry of each sample is predicted using a support vector machine (SVM) trained on individuals of diverse ancestry from the 1000 Genomes Project.
Sampling Selected Polymorphic Sites to Increase Speed
Each of these quality-control statistics can be computationally expensive, especially for WGS studies, as standard methods examine every polymorphic locus observed in a study (tens of millions for WGS). Relatedness statistics are especially onerous as they require the comparison of each sample to all other samples at each polymorphic site. We have identified a subset of bi-allelic, single-nucleotide polymorphisms (SNPs) from the 1000 Genomes Project (1000G) that mitigate the computational burden incurred in calculating these tests, while maintaining high accuracy (Figure 1). Our goal was to identify a subset of markers that are informative for measuring relatedness and predicting ancestry across diverse ancestries. With these goals in mind, the subset of markers chosen were required to have: a reported allele frequency greater than 0.04 in each of European, African, American, and South-East Asian populations; a lack of deviation from Hardy-Weinberg equilibrium (HWE; p value exceeding > 0.04); and a called (i.e., not unknown) genotype for at least 2,500 of the 2,504 samples in the 1000G. Finally, we required that each locus is common to all exome capture platforms used in the 1000 Genomes Project, so that the set of chosen markers is informative for sample quality measurements in both WGS and WES studies. These criteria resulted in a final set of 23,556 bi-allelic SNPs that are the basis of each statistical measure that peddy conducts. In order to quickly interrogate this subset of markers from whole-exome and whole-genome studies, peddy performs a tabix8 genome interval query for each of the sites using cyvcf2, a python wrapper of htslib we have developed for programmatic exploration and processing of VCF files.
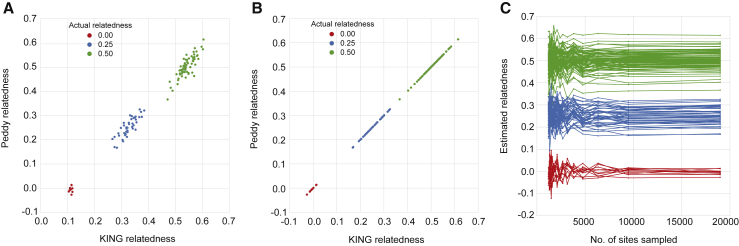
Figure 1.
Validation and Convergence of Sampling Method
A comparison of the relatedness coefficient estimated by KING (KING estimates kinship which is 0.5 ∗ relatedness) compared to that from peddy, using genotype data from the CEPH1463 pedigree (A). A similar comparison when the relatedness estimate is restricted to the subset of 23,556 sites used by peddy (B). Convergence of peddy’s relatedness estimate as a function of the number of sites sampled (C). The three clusters of converging lines reflect the estimated relatedness among pairs of individuals with an actual relatedness of 0.0, 0.25, and 0.5, respectively. The estimated relatedness rapidly stabilizes to the actual relatedness statistic when at least 5,000 markers are used.
To validate the accuracy of our sampling approach, we used the lllumina Platinum Genomes VCF for the 17-member CEPH pedigree 14639 to compare peddy’s coefficient of relatedness statistic to the kinship estimate of KING, which uses the full set of variants observed. Peddy’s coefficient of relatedness is well correlated with KING’s kinship coefficient, and peddy provides a better calibrated estimate for unrelated samples from the same family, where we expect the coefficient of relatedness to be approximately 0 (Figure 1A). In our experience, KING generally gives an accurate estimate of kinship, but in this case, it overestimates the relatedness especially for unrelated samples; it has been shown10 that kinship estimates are more accurate when only sites with less variability in allele frequency among populations are used. When KING is restricted to our subset of 23,556 sites, KING’s estimates are better calibrated (Figure 1B). Furthermore, peddy’s estimate of relatedness rapidly converges for the CEPH1463 pedigree as the number of sites sampled increases to our full set of 23,556 sites, demonstrating the accuracy and predictive power of the subset of markers we have chosen (Figure 1C). For example, after sampling 10,000 sites, the relatedness estimate converges, implying that full set of ∼23,000 markers is conservatively large. However, this larger subset is necessary to maintain accuracy across datasets of diverse quality, as the number of informative sites could be reduced owing to low coverage, quality, or exome capture failures. We emphasize that the user may also specify their own selection of sites, thereby making peddy usable for any genome or build, and allowing it to be applied to studies based upon genotyping arrays or customized research scenarios.
Pre-processing Steps
In addition to quality controls that compare attributes inferred from the genotypes to what is reported in a pedigree file, peddy also performs several internal consistency checks directly on the pedigree file. For example, the pedigree file may report an individual as the maternal parent of another individual, but may also report this individual to have either unknown or male sex. Furthermore, cases often arise in which individuals are listed as parents, yet the parental identifiers are not present in the PED file. Peddy automatically reports these and similar inconsistencies to the user. When provided with a relevant VCF file, peddy also reports samples that are present in the VCF but not in the PED, and vice versa.
Measures of Relatedness
Peddy calculates the coefficient of relatedness from the genotypes observed for each pair of samples using the method described by Manichaikul et al.4 and implemented in the KING software package. We have modified the KING algorithm to use the geometric mean instead of using different formulas for sample pairs from the same family versus those from different families. We have chosen this modification because large pedigrees often contain many unrelated individuals (i.e., via marriage); our results are less affected by this since we’ve chosen sites that are in HWE but we also find that our results more closely match the expected relatedness (Figure 1A; Figure S1 further explores the effects of this change). Our modified formula for the coefficient of relatedness is:
where i and j represent the indices for each individual, Heti is the count of sites where individual i is heterozygous, Hetj is the count of sites where individual j is heterozygous, Heti,j is the count of sites where individuals i and j are both heterozygous, and NIBS0 is the count of IBS0 sites observed between individuals i and j. The algorithmic change with respect to KING’s estimator is that instead of dividing by their sum or by twice the lower number (for the robust estimator), we divide by half of their geometric mean. Otherwise, our relatedness metric is identical to KING’s robust method. The relatedness calculation tests each sample against each other sample so it has an inherent O(n2) complexity, but the computational cost is mitigated by examining only the subset of sites previously described. Furthermore, while the majority of the peddy codebase is written in Python, this relatedness calculation is written in C for optimal performance. Lastly, we parallelize the computation among as many processes as are requested by the user. The speed improvement relative to the number of cores scales well, especially as the number of samples increases.
As with KING, our relatedness estimate depends upon the “IBS0” statistic, which represents the number of sites at which a pair of individuals shares 0 alleles (e.g., individual i is A/A and individual j is G/G). The IBS0 statistic is particularly informative when differentiating between parent-offspring and sibling-sibling pairs, as each relationship has an expected coefficient of relatedness of 0.5. In contrast, the IBS0 statistic should be near 0 for parent-offspring pairs since this should happen only in cases of a Mendelian violation (e.g., a de novo germline mutation), whereas there should be many such sites observed between siblings (e.g., in cases where both parents are heterozygous and the siblings inherit different alleles from each parent). We also report an “IBS2” statistic, which reflects the number of sites at which both individuals share the same genotype and thus share two alleles. In practice, plotting IBS0 versus IBS2 gives the best visual separation of unrelated samples and grouping of related samples. Nonetheless, we also provide plots of relatedness, since it is a more intuitive metric.
After calculating these statistics, we perform multiple sanity checks based upon the relationships reported in the pedigree file. We ensure that a pair reported as parent-child has an IBS0 statistic close to zero and alert the user to situations when a pair of individuals has a low IBS0 but is not listed as a parent-offspring pair. Lastly, we report cases in which two individuals are either more or less related than what is stated in the pedigree file. Pairs of samples with a rate of IBS0 less than our empirically derived cutoff of 0.012 are called as parent-child pairs by their genotypes; if they are not indicated as such in the PED file, an error is reported. Sample-pairs with extremely high (i.e., ∼1) levels of relatedness are reported as duplicates; this can occur in the case of monozygotic twins, multiple tissues from the same sample, or actual sample duplicates. The information in the reports is also apparent in the interactive plot, where points are colored by the relationship defined in the PED file and positioned according to the inferred relatedness. A blue point (e.g., indicating an unrelated pair) inside a cluster of green triangles (indicating sibling-sibling pairs) is evidence of a sample swap involving at least one of those samples (e.g., Figure 2C). All pairwise IBS and relatedness values are reported so the user can, for example, utilize the relatedness matrix in downstream analyses.
Figure 2.
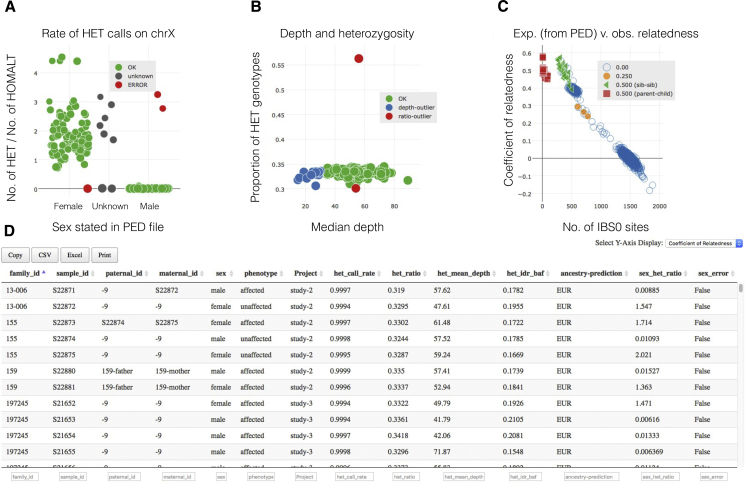
Interactive Website for Identifying and Resolving Sample Mix-ups and Quality Issues
The sex check (A), heterozygosity (B), relatedness (C), and ancestry (not shown; see Figure 4B) plots are interlinked such that clicking in a single point in one plot will highlight all points germane to the selected individual in the other plots. Moreover, the sample information table (D) can be sorted, filtered, and selected to focus the visualization and interpretation to desired subsets of individuals or families.
Ancestry Prediction
The observed ancestry composition of a cohort is a valuable metric to identify unexpected individuals or potential mix-ups in genetic studies. Peddy leverages the known ancestry of 2,504 individuals collected from diverse world populations as part of the 1000 Genomes Project (a 10-Mb file of genotypes of these 2,504 individuals is distributed with peddy) to predict the ancestry of each individual in a study cohort. For each analysis, peddy conducts a dimensionality reduction using randomized PCA.11 It then trains an SVM on the first four principal components identified from the genotypes of each 1000 Genomes (1000G) sample, while using their known ancestries as training labels. Peddy does this training for each cohort since we include only sites that are present above a certain allele frequency and “call rate” (i.e., the fraction of individuals with predicted genotypes at a given site) among the individuals in a given study. The randomized PCA runs on the 2,504 samples in approximately 4 s, making the cost of the retraining negligible.
We decided on four principal components and an SVM penalty (C) of 2 using 20-fold cross-validation on the actual 1000 Genomes data, while retaining 70% and 30% of samples for training and testing in each set, respectively. Once the SVM is trained on the 1000G samples, the resulting classifier can be applied to the individuals in the study. A study cohort’s individuals are first projected onto the principal components identified from the 1000G samples; the SVM is then used to predict the ancestry. This information is reported in the text output and in an interactive plot, where the 1000G samples cluster by “super-population”12 and the predicted ancestry of the individuals in the cohort is displayed on top of the 1000G background samples (e.g., Figure 5B). Samples with an SVM prediction probability for a particular ancestry greater than 0.65 are assigned to that ancestry, while the remaining samples are classified as unknown. This entire process takes only a few seconds once the genotypes for each site have been collected.
Figure 5.
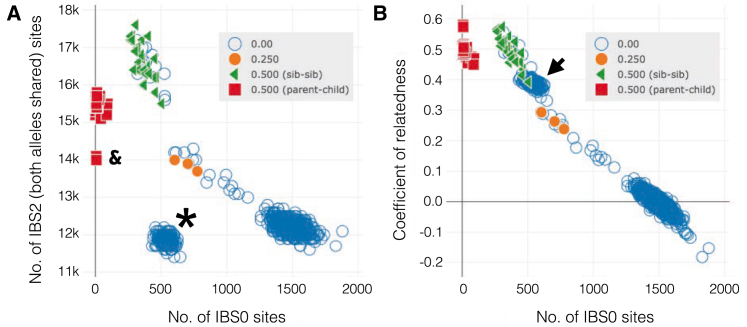
Relatedness with IBS2 or CoR
We compare plots with IBS2 (A) or the coefficient of relatedness (B) for the same data. The coefficient of relatedness provides an intuitive metric with which to validate that, for example, siblings have a CoR of 0.5 and unrelated pairs have a CoR of around 0. However, IBS2 often provides better visual separation of clusters even with lower-quality data. The cluster of blue points with an IBS0 around 500 and IBS2 around 12K are clearly unrelated in the IBS2 plot (A), but in the relatedness plot, they appear to cluster almost with the cluster of sibling-sibling pairs (green triangles). This blue cluster is all from a single sample with a high rate of heterozygote calls that skews the relatedness calculation.
Sex Prediction
By tracking the genotypes observed for each individual outside of the pseudo-autosomal region of the X chromosome, we are able to derive an accurate prediction of an individual’s sex. Since males typically have only one X chromosome, they should have zero true heterozygote calls in the X chromosome, while females should have a mixture of heterozygous and homozygous genotypes similar to that found in autosomes. We have found that an informative measure is the ratio of heterozygous to homozygous genotypes. Comparing this ratio to the sex stated in the pedigree file provides another statistic to detect either mix-ups or sex labeling errors in the pedigree file. In cases where the ratio has an intermediate value between the values observed for males and females, it can also be used as an indicator of lower coverage (e.g., a female with a lower heterozygote to homozygote ratio) or individuals with rare sex chromosome disorders such as Klinefelter syndrome.13
Detection of Poor Sequencing Quality, Consanguinity, or Contamination by Inspecting the Properties of Heterozygous Genotypes
Examining the heterozygous genotypes and the underlying sequence alignments observed for each individual at each of the 23,556 sampled sites allows peddy to detect problems with DNA quality and purity and unexpectedly high levels of homozygosity/heterozygosity. Samples with high rates of heterozygosity or a higher inter-decile range of alternate allele ratios (see Figure S2) may be contaminated. Samples with low rates of heterozygosity (and reasonable coverage) could be checked for consanguinity. The data files and visualizations that are generated based upon heterozygous genotypes also report mean observed depth for each sample, since the number of heterozygous genotype calls will increase with sequencing depth owing to increased detection power. Finally, peddy also reports the per-individual “call rate,” which reflects the proportion of sampled sites with a known genotype, as an indication of the overall quality of an individual’s genotype predictions.
User Interaction
Peddy provides text-based reports of the above metrics for each individual, enabling automated detection of problematic individuals via simple scripts. It also provides an interactive web page (Figure 2) allowing the user to identify potential problems by clicking on points in each plot to inspect which individuals are outliers for each statistic. Each plot is linked so that when an individual point is selected in one plot, the same individual is highlighted in the other plots while also displaying the relevant values of each statistic for that individual. Furthermore, the relatedness plot displays all pairwise measurements comparing the selected individual to other individuals. Lastly, the web page provides an interactive HTML table allowing the user to filter, sort, and select the displayed individuals via attributes provided in the input pedigree file and by the statistics computed by peddy. In the relatedness plot, the user can contrast the position of each point, determined by the genotypes, with the color, determined by the relation defined in the pedigree file. Together, these interactive features provide the researcher with a powerful and efficient means of identifying problematic individuals and possible mix ups and for correcting potential errors in the input pedigree file.
Results
Overview
Given a VCF file and associated PED file describing the expected relationships and sex of the individuals in a sequencing study, peddy automatically conducts all of the tests described using the subset of 23,556 informative SNPs, thereby allowing the rapid detection of possible issues with individual samples. To demonstrate peddy’s utility for detecting sample issues, we will first demonstrate a contrived example from a small pedigree, followed by a real example derived from a large WGS cohort collected as part of an ongoing study at the University of Utah. In each case, we will identify discrepancies between what is reported in the pedigree file and what is inferred from the genotypes. Peddy’s unique functionality and analytical power comes from displaying and integrating all of the sample quality control checks together. This allows one to, for example, note that an individual who appears to be less related than expected to her parents actually has a higher rate of heterozygosity, indicating poor sample quality possibly owing to contamination (which artificially increases heterozygosity and consequently decreases apparent relatedness). For this reason, we present the results on peddy’s interactive web page.
An Intentionally Injected Error in the CEPH1463 Pedigree File
To demonstrate the functionality in peddy, we injected an error into the pedigree file describing a family quartet that is a subset of the CEPH1463 17-member pedigree. We intentionally forced a sample swap between the mother (NA12877) and her son (NA12880) by switching the identifiers in the PED file. This change is analogous to a situation where the DNA samples were swapped during sequencing. Inspecting the plot resulting from the sex check, we note that one individual stated to be a male (NA12880) and one stated to be a female (NA12877) appear to have the opposite sex indicated by their genotypes (Figure 3A). We can further dissect the potential problem by inspecting the relatedness plot generated by peddy (Figure 3B). Because we have four samples, each sample will be involved in a pairing with the three other samples.
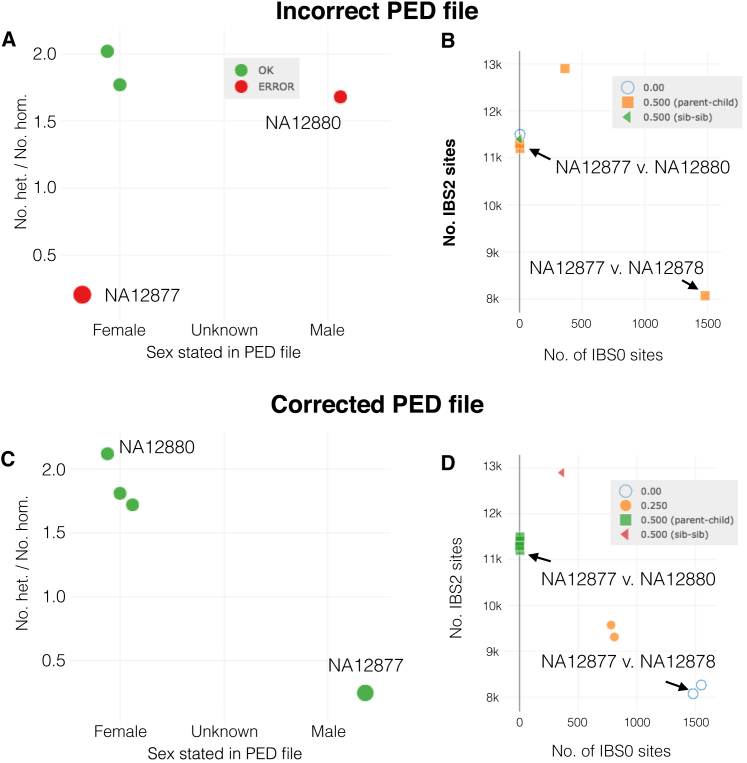
Figure 3.
Using Peddy to Visualize a Manufactured Error in a CEPH Pedigree
Two individuals (red) where the sex stated in the PED file does not match that inferred from the rate of heterozygous calls on the non-pseudo autosomal region of the X chromosome are shown in (A). In the relatedness plot (B), we can see that the swap has caused unexpected relationships (or lack thereof) for both individuals. In (C) and (D), these errors have been resolved by switching the names of the sample in the PED.
In turn, this means that the two sample errors we have created will be propagated as errors in the observed relatedness with all other individuals. The simplest error to follow is one where the pedigree file states a parent-child relationship, yet the genotypes do not support such a relationship. For example, the number of IBS0 sites observed between individual NA12877 and NA12878 is much higher than expected (that is, near 0) for a true parent-child relationship. Since Figure 3A indicates a sex swap, we can further infer from the relatedness information that NA12877 and NA12880 had been swapped. When we rerun peddy with a corrected pedigree file reflecting this knowledge, we observe the expected sex and relatedness (Figures 3C and 3D). In our experience, such errors are simple to resolve with peddy, since most studies deal with smaller families, and the interactive web page allows us to filter to specific families. Since each peddy run completes in seconds, these samples errors can be fixed iteratively by retesting with peddy after each correction, in order to resolve all potential errors in a stepwise fashion.
Unexpected Heterozygosity Rates and Leveraging Ancestry Predictions
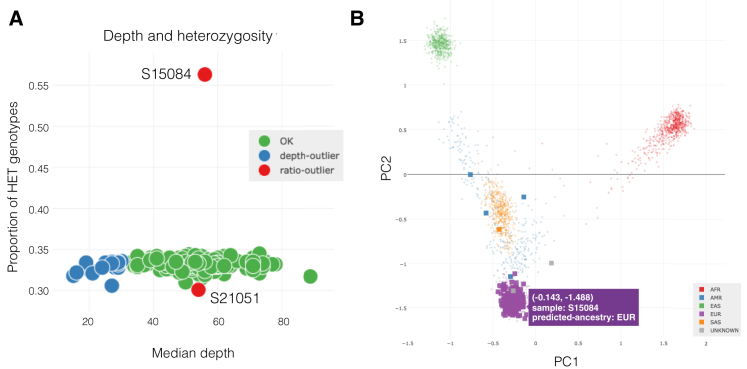
As part of a large, ongoing study of rare, familial diseases, we have sequenced the genomes of multiple families at the University of Utah. All analyses presented were in accordance with the ethical standards on human experimentation (both institutional and national) and proper informed consent was obtained. We applied peddy to a subset of 225 individuals from this study in an effort to further demonstrate the types of errors that it can detect. Although we are not able to share the data due to HIPAA constraints, we use this cohort to demonstrate peddy’s utility in a large study. First, the plot of heterozygous genotypes reveals a single individual (S15084) with a substantially higher rate of heterozygote calls than any other individual (Figure 4A). While a small increase in the rate of heterozygosity could suggest a different ancestry, in this case, the observed level is extreme. In addition, we can use the PCA ancestry plot to confirm that individual S15084 is predicted to be of European ancestry and clusters with many other individuals of the same ancestry (Figure 4B). Therefore, we conclude that this DNA sample may be the result of contamination with one or more other samples, and we may therefore remove this sample from downstream analyses. Similarly, individual S21051 is also an outlier exhibiting a lower rate of heterozygosity. Such reduced heterozygosity could be caused by either extremely low average sequencing coverage (thereby minimizing the power to detect heterozygous genotypes) or consanguinity. Since this plot also reveals that individual S21051’s mean sequencing depth observed across all heterozygous genotypes is 49.1, it is possible that the lack of heterozygosity arises from consanguinity. Therefore, we may want to follow up on this individual and family by talking with the researcher or examining the relatedness that peddy reports for the parents.
Figure 4.
Depth, Heterozygosity, and Ancestry
(A) Outlier individuals with unexpectedly high and low proportions of heterozygous (HET) genotypes.
(B) A PCA analysis is conducted and an SVM trained on the 1000 Genomes samples (small background points) is used to predict the ancestry of each of the individuals in a study (large square points).
Examples of Unexpected Measures of Relatedness
Peddy also produces an interactive plot of different measures of relatedness (i.e., IBS0, IBS2, coefficient of relatedness). Peddy colors each point by the stated relatedness in the pedigree file. In contrast, the location of each point in the plot is determined by the relatedness measures inferred from the genotype data for each individual in the VCF file. As expected, given that the 225 samples in Figure 5 came from many different families, most of the points on the plot reflect pairs of unrelated individuals (blue). However, we observe a large cluster of blue points with a lower-than-expected IBS0 (Figure 5A; asterisk). If we hover over those points, we see that S15084 is a member of all of those pairs. This observation is consistent with the prior observation that this individual has a much higher number of heterozygous genotypes (Figure 4A). Therefore, this outlier individual shares the alternate allele more frequently with other individuals, thereby reducing the number of IBS0 sites observed with other samples. Individual S15084 is also a member of the red cluster of points with IBS0 = 0 but below the larger red cluster (Figure 5A; ampersand). This highlights the utility of the interactive plots; a problematic sample will typically appear as an outlier in several of the plots.
In the cluster of sibling-sibling pairs indicated by green triangles, we see a number of blue circles, indicating that the pedigree file does not explicitly identify several pairs that are actually siblings. In most of these cases, inspecting the pedigree file indicates that while the siblings were sequenced, their parents were not added to the pedigree file or were left unspecified for those individuals. Similarly, in the case of the yellow circles, we find that in nearly all cases, these individuals are stated as belonging to the same family, yet the details of the family structure were not specified in the pedigree file.
The relatedness plot provides the ability to represent the y axis as either IBS2 (Figure 5A) or the coefficient of relatedness (Figure 5B). While coefficient of relatedness (CoR) is a more intuitive metric, we find that IBS2 provides greater separation, thereby allowing the researcher to identify samples issues with greater ease. For example, both sibling-sibling and parent-child relationships have the expected CoR (Figure 5B). However, the CoR plot leads us to believe that the cluster of unrelated relationships with individual S15084 exhibits a high degree of relatedness that is on par with sibling-sibling pairs (Figure 5B; arrowhead). In contrast, the IBS2 plot clearly illustrates that the relationships with individual S15084 are aberrant (Figure 5A; asterisk). This example motivates the complementary utility of both metrics for evaluating unexpected relatedness.
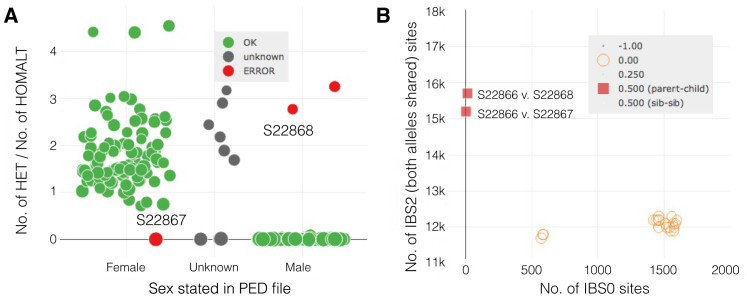
Finally, we can integrate these insights with what we can ascertain from the sex plot. We see that a number of samples didn’t have their sex specified in the pedigree file, and therefore appear in the center as gray (Figure 6A; x axis, “unknown”). In such cases, peddy will assign the sex predicted by the ratio of heterozygous to homozygous genotypes on the X chromosome. We also find an obvious swap between two parents in a trio. This results in one red point for individual S22867 and another for individual S22868, where the genotypes from the X chromosome do not match the sex reported in the pedigree file. By leveraging the interactive sample selection feature, we observe that these individuals each exhibit the expected relatedness to the child; it is only the genotypes observed on the X chromosome that provide the evidence for the clear sample swap.
Figure 6.
Sex Plot and Sample Selection
Upon observing a potential sample-swap in (A) with two members from the same family, we can leverage the table selection tool (not shown) to highlight solely the relevant family in the relatedness plot (B). In so doing, we verify that this is a husband-wife pair where both have the expected relation to their child. This allows one to infer that the husband and wife labels have been swapped.
Discussion
Peddy is a powerful software package that facilitates the detection and correction of sample quality issues and mix-ups that complicate analysis and inhibit discovery. Peddy is fast; it runs in about 35 s on the complete 17-member CEPH1463 pedigree. In comparison, KING runs in 10 s after the conversion to PLINK, which requires 90 s. Furthermore, we have successfully applied peddy to multiple studies involving thousands of WGS samples. More importantly, its interactivity substantially improves upon the functionality available in previous software packages, and the text reports it produces allows for the development of simple scripts to automate sample quality-control measures. While not presented here, since peddy infers ancestry, relatedness, and sample sex, we emphasize that it may also be used to augment sample metadata with previously unknown (e.g., sex or ancestry) information. We note, however, that peddy is designed for sample quality control of WES and WGS studies. It is poorly suited to targeted gene panel studies or low (e.g., <10×) coverage sequencing, owing to the need for accurate genotypes at the subset of polymorphic sites that peddy interrogates. Nonetheless, peddy’s efficiency and flexibility allow it to be used for a broad range of studies, ranging from studies of family trios to large-scale investigations of thousands of human genomes. As such, we anticipate that peddy will be a vital tool for quality control in current and future human genome and exome studies.
Acknowledgments
Barry Moore provided valuable feedback on early versions of the software and suggested the idea of predicting an individual’s ancestry. Daniel McGoldrick and Jessica Chong provided helpful feedback and QC on the software. This research was supported by a US National Human Genome Research Institute award to A.R.Q. (NIH R01HG006693).
Published: February 9, 2017
Footnotes
Supplemental Data include two figures and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2017.01.017.
Web Resources
cyvcf2, https://github.com/brentp/cyvcf2
FastQC, http://www.bioinformatics.babraham.ac.uk/projects/fastqc
Peddy demonstration, http://peddy.readthedocs.io/en/latest/_static/ceph.html
Supplemental Data
References
- 1.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A.R., Bender D., Maller J., Sklar P., de Bakker P.I.W., Daly M.J., Sham P.C. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller C.A., Qiao Y., DiSera T., D’Astous B., Marth G.T. bam.iobio: a web-based, real-time, sequence alignment file inspector. Nat. Methods. 2014;11:1189. doi: 10.1038/nmeth.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manichaikul A., Mychaleckyj J.C., Rich S.S., Daly K., Sale M., Chen W.-M. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26:2867–2873. doi: 10.1093/bioinformatics/btq559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinrich V., Kamphans T., Mundlos S., Robinson P.N., Krawitz P.M. A likelihood ratio-based method to predict exact pedigrees for complex families from next-generation sequencing data. Bioinformatics. 2017;33:72–78. doi: 10.1093/bioinformatics/btw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staples J., Ekunwe L., Lange E., Wilson J.G., Nickerson D.A., Below J.E. PRIMUS: improving pedigree reconstruction using mitochondrial and Y haplotypes. Bioinformatics. 2016;32:596–598. doi: 10.1093/bioinformatics/btv618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danecek P., Auton A., Abecasis G., Albers C.A., Banks E., DePristo M.A., Handsaker R.E., Lunter G., Marth G.T., Sherry S.T., 1000 Genomes Project Analysis Group The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H. Tabix: fast retrieval of sequence features from generic TAB-delimited files. Bioinformatics. 2011;27:718–719. doi: 10.1093/bioinformatics/btq671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberle, M.A., Fritzilas, E., Krusche, P., Kallberg, M., Moore, B.L., Bekritsky, M.A., Iqbal, Z., Chuang, H.-Y., Humphray, S.J., Halpern, A.L., et al. (2016). A reference dataset of 5.4 million phased human variants validated by genetic inheritance from sequencing a three-generation 17-member pedigree. bioRxiv. http://biorxiv.org/lookup/doi/10.1101/055541. [DOI] [PMC free article] [PubMed]
- 10.Blue E.M., Brown L.A., Conomos M.P., Kirk J.L., Nato A.Q., Popejoy A.B., Raffa J., Ranola J., Wijsman E.M., Thornton T. Estimating relationships between phenotypes and subjects drawn from admixed families. BMC Proc. 2016;10:357–362. doi: 10.1186/s12919-016-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halko, N., Martinsson, P.-G., and Tropp, J.A. (2009). Finding structure with randomness: Probabilistic algorithms for constructing approximate matrix decompositions arXiv [math.NA]. http://arxiv.org/abs/0909.4061.
- 12.Auton A., Brooks L.D., Durbin R.M., Garrison E.P., Kang H.M., Korbel J.O., Marchini J.L., McCarthy S., McVean G.A., Abecasis G.R., 1000 Genomes Project Consortium A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boada R., Janusz J., Hutaff-Lee C., Tartaglia N. The cognitive phenotype in Klinefelter syndrome: a review of the literature including genetic and hormonal factors. Dev. Disabil. Res. Rev. 2009;15:284–294. doi: 10.1002/ddrr.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.