Abstract
The immune mechanisms associated with the evolution from latent to clinically active mycobacterial coinfection in human immunodeficiency virus type 1 (HIV-1)-infected humans remain poorly understood. Previous work has demonstrated that macaques infected with simian immunodeficiency virus (SIVmac) can develop persistent Mycobacterium bovis BCG coinfection and a fatal SIV-related tuberculosis-like disease by 4 months after BCG inoculation. In the present study, SIVmac-infected monkeys that developed clinically quiescent mycobacterial infection after BCG inoculation were followed prospectively for the reactivation of the BCG and the development of SIV-related tuberculosis-like disease. The development of clinically latent BCG coinfection in these SIVmac-infected monkeys was characterized by a change from high to undetectable levels of bacterial organisms, with or without measurable BCG mRNA expression in lymph node cells. The reactivation of clinically latent BCG coinfection and development of SIV-related tuberculosis-like disease were then observed in these SIVmac-BCG-coinfected monkeys during a 21-month period of follow-up. The reactivation of SIV-related tuberculosis-like disease in these animals coincided with a severe depletion of CD4 T cells and a loss of BCG-specific T-cell responses. Interestingly, bacterial superantigen challenge of the SIVmac-BCG-coinfected monkeys resulted in an up-regulation of clinically latent BCG coinfection, suggesting that infection with superantigen-producing microbes may increase the susceptibility of individuals to the reactivation of AIDS-related mycobacterial coinfection. Thus, reactivation of latent mycobacterial infections in HIV-1-infected individuals may result from a loss of T-cell immunity or from a superimposed further compromise of the immune system.
It is estimated that one-third of the world's population is latently infected with Mycobacterium tuberculosis. Approximately 5 to 10% of the latently infected individuals may subsequently develop active tuberculosis during their lifetimes (21). The immune system can effectively control an M. tuberculosis infection but not eradicate the latent bacilli. The capacity of M. tuberculosis to survive immune elimination and reactivate suggests that both host and microbial factors play a role in maintaining latent infection with M. tuberculosis. While gene deletion studies have shown that some M. tuberculosis-encoded metabolic enzymes are associated with persistence of the bacilli (6, 16, 20, 29), host factors that prevent reactivation of latent M. tuberculosis infection are poorly characterized (5, 13). Studies with mice suggest that immune mechanisms for controlling primary M. tuberculosis infection may be different from those for maintaining chronic (latent) infection of the bacilli. CD4+ T cells are required to control primary or persistent M. tuberculosis infection in mice, whereas the role of these cells in controlling reactivation of chronic (latent) infection has not been demonstrated (5, 17, 18). The depletion of CD8+ T cells induces reactivation of chronic (latent) M. tuberculosis infection in mice, although there appears to be only a limited role of these cells in controlling an active M. tuberculosis infection (28). Recent studies suggest that interleukin-10 may play a role in reactivation of chronic M. tuberculosis infection in mice (9, 27). It is likely that early granulomatous responses and the competence of both the innate and acquired immune systems determine the fate of latent M. tuberculosis infection.
Clinical studies have demonstrated that human immunodeficiency virus type 1 (HIV-1) infection is one of the most important risk factors for susceptibility to tuberculosis and reactivation tuberculosis. Purified protein derivative (PPD)-positive persons not infected with HIV-1 have a 10% lifetime risk of developing active tuberculosis, whereas coinfection with M. tuberculosis and HIV-1 is associated with a 5 to15% yearly risk of active tuberculosis (21). The specific cause of M. tuberculosis reactivation is unknown in the vast majority of cases, and it is clinically difficult to determine whether a case of tuberculous disease results from reactivation of latent bacilli or from a new infection (2). While HIV-1-induced immune suppression and loss of CD4 T cells likely account for an increased risk for developing tuberculosis, in-depth studies of the evolution of latent M. tuberculosis infection and reactivation/relapse of HIV-related tuberculosis have not been reported (1, 8, 21, 25). It is not known the extent to which T-cell immunodeficiency or immune suppression can trigger the reactivation of latent mycobacterial coinfection in HIV-1-infected individuals. Useful animal models for HIV-1 and latent M. tuberculosis infections should facilitate our definition of the immune mechanisms responsible for the reactivation of M. tuberculosis and relapse of tuberculosis.
We have recently demonstrated that macaque models of simian immunodeficiency virus of macaques (SIVmac)-Mycobacterium bovis BCG coinfection are useful for studying immune aspects and disease consequences of AIDS virus-related mycobacterial coinfection (23). We have shown that inoculation of SIVmac-infected macaques with a large dose of BCG can result in the development of active BCG coinfection and SIVmac-related tuberculosis-like disease within 1 to 4 months after BCG inoculation (23). This SIVmac-related tuberculosis-like disease is characterized clinically by persistent diarrhea, anorexia, and weight loss and, pathologically, by BCG dissemination and granulomas in multiple organs (23). The resultant BCG-induced disease was associated with progressive BCG coinfection as well as increased depletion and suppression of antigen-specific CD4 T cells (23). While these observations indicate that the competence of CD4 T cells is important for controlling active BCG coinfection, little is known about whether and how a given latent BCG coinfection can reactivate and evolve to mycobacterial disease in the setting of advanced SIVmac infection. This issue is relevant to understanding whether and how a latent infection with weakly virulent mycobacteria reactivates and causes disease in HIV-1-infected individuals. In the present studies, we prospectively evaluated clinically latent BCG infection in SIVmac-BCG-coinfected macaques and examined whether progression of T-cell deficiency or a superimposed compromise of the immune system by exposure to bacterial superantigen could cause reactivation of latent BCG coinfection and BCG-induced disease.
MATERIALS AND METHODS
Animals and virus.
Nine rhesus (Macaca mulatta) and three pigtailed (Macaca nemestrina; monkeys 267, 263, and 269) macaques, 2 to 8 years of age, were used in these studies. These animals were maintained in accordance with the guidelines of the Committee on Animals for the Harvard Medical School and the “Guide for the Care and Use of Laboratory Animals” (17a). Macaques were inoculated intravenously with 106 50% tissue culture infective doses of SIVmac 251, as described previously (23).
M. bovis BCG coinfection.
M. bovis BCG (Pasteur strain) was used for BCG coinfections as described previously (23). For all BCG infections, macaques were inoculated intravenously with 108 CFU of BCG. Two groups of SIVmac-infected macaques were included to study the reactivation of SIV-related tuberculosis (Table 1). The first group consisted of six SIVmac-infected macaques: monkeys 259, 276, 278, 269, 263, and 267 (monkeys 263 and 267 received simultaneous SIVmac-BCG coinfection). These animals had plasma SIV RNA levels of >105 copies/ml following infection. Five of the six macaques developed a BCG-associated clinical syndrome within 2 months of BCG reinfection or simultaneous SIV-BCG coinfection. This syndrome was controlled by the restoration of effective antimycobacterial immunity during antiretroviral treatment (reference 22 and see below). The resolution of BCG disease in these SIVmac-infected macaques provides a useful setting in which to assess BCG latency, reactivation of BCG coinfection, and the eventual relapse of SIV-related tuberculosis-like disease during progression of SIV infection. The second group of two SIVmac-infected monkeys (monkeys 336 and 349) had plasma SIV RNA levels below 104 copies/ml prior to BCG coinfection. Our previous studies have shown that SIVmac-infected macaques with low viral loads are not likely to develop fatal SIV-related tuberculosis-like disease after BCG coinfection (23). As controls, naïve macaques and animals previously infected with a nonpathogenic simian HIV (SHIV) were infected with BCG and assessed for BCG latency and reactivation of BCG-induced disease. Monkeys that became moribund and died from SIV-related tuberculosis-like disease were subjected to necropsy and histology studies of mycobacterium-related lesions in the lungs and other organs. Tissue sections were formalin fixed and stained by routine hematoxylin and eosin procedures. The Ziehl-Neelsen method was used for staining acid-fast bacilli in the lesions. Southern blot and sequencing analyses confirmed that acid-fast bacilli in lesions were BCG but not M. tuberculosis or Mycobacterium avium.
TABLE 1.
Clinical follow-up after BCG infections in SIVmac-infected macaques
| Infection group and monkey | Time points for clinical events (mod)
|
|||||
|---|---|---|---|---|---|---|
| SIV → BCG infection
|
Primary SIV-BCG-related diseasea | Antiretroviral treatment | SEB injection | Relapse of SIV-BCG-related diseaseb | ||
| SIV | BCG | |||||
| BCG→SIV→BCG | ||||||
| 259 | −2 | 0 | 0.5 | 1, 12 | 12 | 14 |
| 276 | −2 | 0 | 1 | 1.5, 12 | 12 | 17 |
| 278 | −2 | 0 | 0.5 | 1, 12 | 12 | 17 |
| 269 | −3 | 0 | NAe | 8.5 | 9 | |
| Simultaneous SIV-BCG | ||||||
| 263 | 0 | 0 | 1 | 1 | 15 | |
| 267 | 0 | 0 | 1 | 1 | 7 | |
| Chronic SIV→BCG | ||||||
| 336 | −6 | 0 | NA | 6 | 6 | NA (18) |
| 349 | −6 | 0 | NA | 6 | 6 | NA (18) |
| Controlsc | ||||||
| 545 | −9 | 0 | NA | NA (28) | ||
| 540 | −9 | 0 | NA | NA (18) | ||
| 185 | 0 | NA | 6 | NA (20) | ||
| 213 | 0 | NA | 12 | NA (28) | ||
Occurrence of diarrhea, anorexia, and weight loss, which resolved as a result of immune restoration by a 2-month antiretroviral treatment.
Reoccurrence of diarrhea, anorexia, and weight loss. Monkeys 259, 276, and 278 received a first BCG inoculation 2 months before SIV infection and, after BCG coinfection (second BCG inoculation), received two short-term antiretroviral treatments, the first for controlling SIV-BCG-related disease and the second for studying mechanisms of SEB-induced up-regulation of BCG. Monkey 269 received a first BCG inoculation 3 months before SIV infection. The time points in parentheses in the last column represent the study end month, when necropsy was performed for all monkeys except monkey 213. Macaques 336 and 349 still survived at the end of the studies and maintained strong PPD-specific T-cell responses.
Controls 540 and 545 were coinfected with nonpathogenic SHIV and BCG. Animals 185 and 213 were infected with BCG only. Monkey 185 developed fatal adult respiratory distress syndrome-like illness due to a hypersensitivity reaction to BCG 1 day after the second BCG inoculation. Monkeys 545 and 213 were reinfected with BCG 6 months after the first BCG inoculation; the time points shown for these two animals were those after the first BCG inoculation.
mo, months after BCG coinfection or BCG infection.
NA, clinical BCG disease not apparent.
SEB inoculation.
SIVmac-infected and -uninfected monkeys were injected by the intramuscular route with staphylococcal enterotoxin B (SEB) (Toxin Technology, Sarasota, Fla.) at a dose of 0.3 μg/kg (3, 11). Following SEB inoculation, the collection of blood samples and lymph node biopsies were done as previously described (3, 11).
Antiretroviral treatment of SIVmac-BCG-coinfected macaques.
Short-term antiretroviral therapy was employed to facilitate studies of clinically latent BCG coinfection. Antiretroviral treatment was introduced to restore protective immunity against mycobacterial disease at the onset of the SIV-BCG-related clinical syndrome during acute BCG infection. Five of eight SIVmac-BCG-coinfected monkeys were treated in this setting for 2 months with antiretroviral drugs (Table 1). The antiretroviral drug regimen was comprised of tenofovir (also termed PMPA) and a protease inhibitor, indinovir (Merck, Inc.), or tenofovir alone (22). The antiretroviral treatment reduced plasma SIV RNA levels, restored antimycobacterial immunity, and effectively treated the SIV-BCG-related clinical syndrome (22). Antiretroviral treatment was also used to determine if the increase in BCG replication after SEB superantigen challenge was dependent upon an increase in SIV viral loads in SIVmac-BCG-coinfected monkeys. The antiretroviral regimens were as follows: zidovudine, 8 mg/kg/day; lamivudine, 6 mg/kg/day; 141w94 (a protease inhibitor), 40 mg/kg/day. The three drugs were administered orally in pediatric syrup (Glaxo Wellcome, Research Triangle Park, N.C.) twice a day for 4 weeks, beginning 1 week before SEB superantigen challenge.
Immune flow cytometry analyses of T cells.
To measure changes in numbers of CD4 T cells, three-color analyses of CD3, CD4, and CD8 T cells in whole blood were performed on an XL flow cytometer (Coulter, Hialeah, Fla.). Absolute numbers of T-cell populations in the blood were calculated based on the flow cytometric data and complete blood count analyses. Complete blood counts were performed on a Coulter T 540 hematologic analyzer. To examine SEB-induced expansions of reactive Vβ-expressing T cells, three-color staining of whole blood and cells in lymph nodes was performed using fluorescein isothiocyanate (FITC)-conjugated anti-human Vβ3 or Vβ19 antibodies and phycoerythrin (PE)-conjugated anti-CD3 and Cy5-conjugated anti-CD8 antibodies (11). The following anti-human monoclonal antibodies that cross-react with corresponding macaque antigens were used: PE-conjugated anti-rhesus monkey CD3 (FN18; Biosource, Camarillo, Calif.), PE-conjugated anti-human CD4 (Ortho Diagnostic Systems, Raritan, N.J.), Cy5-conjugated anti-human CD8 (Dako Corporation, Carpinteria, Calif.), FITC-conjugated anti-human Vβ3 and FITC-conjugated anti-human Vβ5 (Endogen, Woburn, Mass.), and FITC-conjugated Vβ19 (Coulter).
Proliferation assays.
Conventional proliferation assays were carried out as described previously (23). Briefly, unfractionated peripheral blood lymphocytes (PBL) (1 × 105 cells per well) were cultured in triplicate in 96-well plates in the presence of BCG PPD (5 μg/ml), concanavalin A (ConA) (5 μg/ml), or medium alone. Five days later, cells were pulsed with [3H]thymidine at 1.0 μCi per well, and uptake was measured 8 h later using a 1450 Microbeta scintillation counter (Wallac, Gaithersburg, Md.). The stimulation index was defined as the ratio of the mean counts per minute of PPD- or ConA-stimulated wells relative to the mean counts per minute of control wells (medium alone).
Quantitative measurement of plasma SIV RNA.
Both real-time quantitative PCR and quantitative competitive PCR (QC-PCR) were employed, as previously described (32), to measure levels of plasma SIV RNA. Briefly, viral RNA in plasma was extracted following the instructions of the RNA extraction kit from QIAGEN (Valencia, Calif.), and expression levels were measured using real-time quantitative PCR as previously described (31). The primers used for quantitation of SIVmac gag expression were as follows: forward primer, TGT CAA AAA ATA CTT TCG GTC TTA GC; reverse primer, GAT GAC GCA GAC AGT ATT ATA AAG GC; TaqMan probe, 6FAM-CCA TTA GTG CCA ACA GGC TCA GAA AAT TTA AAA-MGBNFQ. Real-time quantitative PCR was performed following instructions from the manufacturer as previously described (24). For QC-PCR, the extracted RNA was aliquoted into six different tubes, each of which contained defined copies of SIVmac gag competitor RNA. The RNA mixtures were reverse transcribed to cDNA and competitively amplified by a 35-cycle PCR using a pair of SIVmac gag-specific primers (26). The amplified PCR products containing wild-type and competitor were separated on 2% agarose gels and measured for their densities in a GS 700 Imaging Densitometer (Bio-Rad). Quantitation was achieved by data analysis using Molecular Analyst system software (Bio-Rad). The intra- and interassay coefficients of variation using this protocol were less than 20%. The sensitivity of real-time quantitative PCR or QC-PCR was 4 × 102 RNA copies in 1 ml of plasma.
Measurement of mycobacterial burdens.
Quantitation of BCG infection was accomplished by measuring bacterial colony counts and levels of BCG Ag85B mRNA expression (24). Viable BCG colony counts in the lymph nodes were determined by the quantitation of BCG CFU in cell lysates from 106 lymph node cells from SIVmac-BCG-coinfected macaques. Cell pellets prepared from 106 lymph node cells were lysed with 10% saponin to release intracellular BCG. Fivefold dilutions of the lysate were plated in duplicate on Middlebrook 7H10 agar plates (Difco) (24). The CFU were counted after a 3-week incubation at 37°C. For measuring levels of BCG mRNA expression, BCG mRNA was extracted by TRIzol-based methods from lymph node cells, as previously described (24). The extracted RNA was reverse transcribed to cDNA. The synthesized cDNAs were used as templates to quantitate the expression of BCG Ag85B mRNA, using real-time quantitative PCR in a TaqMan system on an ABI 7700 system (PE Biosystems, Foster City, Calif.). Standard procedures of real-time PCR quantitation were followed based on the instructions of the operation manuals and technical support from ABI. To normalize the expression of Ag85B RNA in the cells, β actin mRNA was also quantitated as previously described (24). The expression levels were expressed as mean copy numbers of Ag85B RNA in 106 β-actin molecules. The detection limits for BCG CFU and Ag85B mRNA were determined by using 106 lymph node cells. The normalization of bacterial loads in tissues gave rise to an estimate of 1 to 3 BCG CFU and 10 copies of Ag85B mRNA in approximately 10 mg of lymph node tissues.
Statistical analysis.
Student's t test and nonparametric tests, as described previously (23), were employed to examine whether any differences in BCG loads or reactivation of BCG coinfection and in PPD-specific T-cell responses were statistically significant.
RESULTS
Clinically latent BCG coinfection was identified prior to the relapse of SIV-related tuberculosis-like disease in SIVmac-BCG-coinfected monkeys.
To demonstrate the reactivation of BCG-induced disease in SIVmac-infected monkeys, it was first important to characterize the evolution of latent BCG coinfection following the resolution of active mycobacterial infection. SIVmac-infected macaques that were primarily infected or reinfected with BCG were included in these studies (Table 1). During early BCG coinfection, some of these monkeys developed exacerbated SIV disease and a BCG-related clinical syndrome characterized by diarrhea, anorexia, altered consciousness, and weight loss, with pathological evidence of infiltration of histiocytes in the biopsies of colonic mucosae or lymph nodes (Table 1 and data not shown). This clinical syndrome was resolved after the control of SIV replication and reconstitution of antimycobacterial immunity during antiviral treatment with tenofovir and protease inhibitors (22).
After the resolution of early BCG coinfection and BCG disease, the SIVmac-BCG-coinfected monkeys were assessed for latency and reactivation of BCG infection and for clinical progression of the coinfection in the absence of antiretroviral treatment (Table 1). The clinical quiescence was characterized by an absence of signs of illness (Table 1). Following this quiescent phase, which lasted up to 17 months, these animals again developed a clinical syndrome characterized by diarrhea, anorexia, and wasting (Table 1 and Fig. 1).
FIG. 1.
Evolution of clinically latent BCG coinfection in SIVmac-infected macaques. Changes in bacterial colony counts (top) and levels of BCG Ag85B mRNA expression (bottom) in lymph node cells of the coinfected monkeys. All data were generated from superficial lymph nodes except for those for end time points for monkeys 276 and 278, for which data were derived from spleen cells. The last data points correspond to the time at which animals were moribund or dead due to the terminal stage of SIV-related tuberculosis-like disease. BCG CFU were measured by using lysates of 106 cells from lymph nodes or spleens obtained from macaques after BCG coinfection, whereas levels of BCG mRNA were determined as copy numbers in 106 β-actin molecules. Higher levels of mRNA transcripts in comparison with CFU might reflect the sensitive nature of real-time PCR quantitation of mRNA expression. The BCG CFU loads in monkeys might be underestimated due to using a limited amount of tissues for CFU measurement and due to the difficulty in isolating or growing intracellularly adapted BCG under these culture conditions. Monkeys 259 and 278 exhibited detectable BCG mRNA before BCG coinfection (day 0 for the second BCG inoculation) because SIVmac infection resulted in a delayed clearance of BCG after the first BCG inoculation (2 months before SIV infection). Monkeys 259, 276, 278, and 269 were injected with a single dose of SEB 0.5 to 5 months prior to death, whereas monkeys 263 and 267 did not receive SEB injection. CFU data for monkey 269 were not shown since CFU measurements of viable BCG could not be done at some time points due to insufficient available cells. However, viable BCG bacilli were isolated from mesentery lymph node cells (20 CFU/106cells) at the final point. Monkeys 336 and 349, with low viral loads, survived the coinfection during an 18-month follow-up despite receiving an SEB injection 6 months after BCG coinfection. A P value of <0.001 was obtained when nonparametric statistical analyses were performed to evaluate any differences in BCG CFU or Ag85B mRNA at final time points versus those obtained 4 months after the last BCG inoculation for the control group and the SIVmac-BCG-coinfected group.
To characterize the latent BCG coinfection, the macaques were assessed for a change in detectable levels of BCG organisms in lymph node cells. Expression levels of BCG Ag85B mRNA in tissue cells were also measured using real-time quantitative PCR (24). For 4 to 10 months after the resolution of active BCG coinfection, viable BCG bacilli were undetectable in the lymph node cells obtained from these SIVmac-BCG-coinfected monkeys (Fig. 1, top). Undetectable levels of BCG Ag85B mRNA expression were also documented at some time points during this period of clinical quiescence (Fig. 1, bottom). Interestingly, a notable increase in bacterial organisms and BCG Ag85B mRNA expression was noted in lymph node cells of some monkeys as they developed the clinical syndrome of SIVmac-related BCG disease (Fig. 1, bottom). The results of the present studies therefore demonstrated that latent BCG coinfection can develop and reactivate in SIVmac-infected macaques.
Reactivation of BCG resulted in the development of SIV-related tuberculosis-like disease.
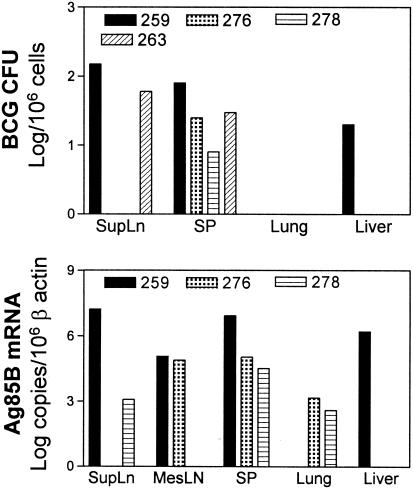
We then characterized the pathological changes seen in these SIVmac-BCG-coinfected monkeys with active disease. Complete necropsies demonstrated the gross and histological findings of BCG-related inflammation (Table 2). Evidence of chronic diarrhea and weight loss was seen in these macaques. The diarrhea and weight loss appeared to be related to BCG coinfection, since granulomatous inflammation or acid-fast bacilli were identified in intestinal mucosae and other organs of these animals and there was no gross or histological evidence indicating the presence of other opportunistic infections (Table 2). Evidence of BCG-related granulomatous inflammation was mainly found in lymphoid tissues, such as spleen and mesenteric lymph nodes, of the SIVmac-BCG-coinfected monkeys (Fig. 2). The lesions varied in degree from severe disseminated granulomas to the multifocal infiltration of epithelioid cells or modified histiocytes. Interestingly, macaque 259 even developed central caseous necrosis and some mineralization within the granulomas, pathological lesions resembling what is seen in patients with tuberculosis (Fig. 2a and b). Consistent with the presence of these BCG-related lesions, high levels of BCG Ag85B mRNA expression and high BCG bacterial counts were documented in the cells isolated from lymphoid tissues (Fig. 3). In contrast, the control normal or SHIV-infected macaques showed no evidence of wasting or BCG-related lesions in examined tissues. In the two SIVmac-infected macaques (monkeys 336 and 349) that survived BCG coinfection, no pathological lesions were seen in the biopsies collected from the colon, bone marrow, and lymph nodes. These results therefore provided pathological evidence that BCG reactivation can result in a relapse of SIV-related tuberculosis-like disease in SIVmac-infected macaques.
TABLE 2.
Progression of latent BCG infection during advanced SIVmac infection can result in development of SIV-related BCG disease
| Infaction group and monkey | Follow-up (mo)a | Pathology at necropsy |
|---|---|---|
| BCG→SIV→BCG | ||
| 259 | 14 | Granulomatous inflammation in spleen and lymph nodes; severe and multifocal or coalescing granulomas with central caseous necrosis; some mineralization at the center of caseous areas |
| 276 | 17 | Evidence of diarrhea; weight loss; spleenitis with BCG-related histiocytes infiltrated in white pulps; foci of histiocytes in gut-associated lymph nodes |
| 278 | 17 | Weight loss; signs of diarrhea; colonitis with histiocytes and hemorrhage; acid-fast bacili in spleen and colon |
| 269 | 9 | Evidence of diarrhea; BCG-related colonitis with hemorrhage; acid-fast bacilli in colon; pleural fibrosis |
| Simultaneous SIV/BCG | ||
| 263 | 15 | Severe multifocal granulomas in spleen and lymph nodes; pleuralitis and adhesions between right lung, thoracic wall, and pericardium with fibrosis in the adhesions |
| 267 | 7 | No apparent lesions associated with BCG infection; no evidence of other opportunistic infections |
| Chronic SIV→BCG | ||
| 336 | 18 | Alive, no evidence of BCG-related illness or lesions in biopsies |
| 349 | 18 | Alive, no evidence of BCG-related illness or lesions in biopsies |
| Controls | ||
| 545 | 28 | No apparent lesions associated with BCG infection |
| 540 | 18 | No apparent lesions associated with BCG infection |
| 185 | 20 | No apparent lesions associated with BCG infection |
| 213 | 28 | No apparent lesions in biopsies |
The duration from the BCG coinfection through necropsy or the end points of studies. Macaques 336 and 349 maintained strong PPD-specific T-cell responses at the end points of the studies. All controls except monkey 213 were sacrificed for necropsy analyses. Monkey 185 was necropsied because it developed fatal adult respiratory distress syndrome-like illness due to a hypersensitivity reaction to BCG 1 day after the second BCG inoculation.
FIG. 2.
Reactivation of BCG coinfection in advanced SIVmac infection resulted in the development of SIV-related tuberculosis-like disease. Histological evaluation of those SIVmac-BCG-coinfected macaques that were euthanized with mycobacterial disease showed representative granulomatous inflammation in the lymph nodes (a and b), spleen (c), and colon mucosae (d), with granulomas present in multiple organs. Sections in panels a, b, and c were formalin fixed and stained by hematoxylin and eosin, whereas the section in panel d was stained by the Ziehl-Neelsen method for identifying acid-fast bacilli. Southern blot and sequencing analyses indicated that the acid-fast bacilli were BCG but not M. tuberculosis or M. avium. The specimens were collected at the time the animals were moribund or dead due to terminal SIVmac-BCG disease. Shown in panels a and b are necroticgranulomas and mineralization, respectively, in lymph nodes from monkey 259. Shown in panel c is a nonnecrotic granulomatous lesion in the spleen section from monkey 276. Shown in panel d is positive acid-fast staining in colon mucosae obtained from monkey 278. All the sections shown are at ×20 magnification.
FIG. 3.
Monkeys that developed SIV-related BCG disease exhibited high levels of Ag85B mRNA expression and BCG organisms in cells obtained from organs at necropsy. SupLN and MesLN represent superficial and mesenteric lymph nodes, respectively. The BCG CFU reflected an estimated number of viable BCG organisms per approximately 10 mg of an organ, whereas those of Ag85B mRNA represented the estimated level of BCG mRNA transcripts per approximately 1 mg of an organ (see Materials and Methods). The organs of the monkeys varied in sizes and weights due to differences in age, sex, body weight, and extent of inflammation of the animals. SP, spleen.
Bacterial superantigen stimulation of the immune system resulted in an activation of latent BCG infection in SIVmac-BCG-coinfected monkeys.
It is likely that the clinical latency of mycobacterial coinfection results from a balance between the capacity for microbial replication and the competence of the immune system of AIDS virus-infected individuals. One might therefore expect that immune activation might increase viral and bacterial replication and reactivate BCG-associated disease. We have previously demonstrated that a bacterial superantigen, SEB, is a potent immune and inflammatory stimulator in macaques (3, 11). The immune compromise induced by SEB challenge results in marked changes in lymphocyte turnover and SIV viral loads (3, 11). We therefore sought to examine if SEB challenge of the immune system could result in a change in BCG latency in SIVmac-BCG-coinfected monkeys. SEB-mediated immune compromise resulted in the up-regulation of BCG replication in the SIVmac-BCG-coinfected monkeys (Fig. 4). One week following SEB injection, monkeys 259 and 276 showed an increase in BCG bacterial organisms and Ag85B mRNA expression in the lymph node cells (Fig. 4a). The other two SIVmac-BCG-coinfected monkeys also showed increases in expression levels of BCG Ag85B mRNA in lymph node cells following SEB injection, although no viable BCG bacilli were isolated from these animals (Fig. 4a). In contrast, no BCG bacilli or BCG mRNA was detectable after SEB challenge in the control SIV-negative monkeys or in the SIV-infected monkeys (monkeys 336 and 349) whose viral loads were extremely low (Fig. 4a and b). Interestingly, the up-regulation of BCG coinfection appeared to be independent of increases in viral loads, since monkeys 259 and 278, whose plasma SIV RNA levels were reduced by simultaneous antiviral treatment, showed an increase in BCG bacterial organisms and/or BCG RNA expression (Fig. 4a and b). The monkeys that were susceptible to SEB-induced up-regulation of the BCG coinfection became moribund, with evidence of altered alertness, anorexia, diarrhea, or weight loss 1 week to 5 months after SEB challenge (Tables 1 and 2). These results indicated that exposure of the immune system to bacterial superantigen can result in an up-regulation of BCG coinfection in SIVmac-infected monkeys.
FIG. 4.
SEB superantigen-induced compromise of the immune system resulted in an up-regulation of clinically latent BCG coinfection. (a) SEB challenge induced increases in levels of viable bacterial organisms and Ag85B mRNA expression in cells obtained from lymph nodes of SIVmac-BCG-coinfected monkeys. (b) Changes in plasma SIV RNA and CD4+ PBL counts after SEB challenge of monkeys. (c) SEB challenge stimulated an expansion of SEB-reactive Vβ3+ and Vβ19+ CD3+ T cells in SIVmac-infected monkeys. SEB did not stimulate an expansion of control Vβ5 T cells (data not shown and reference 11). Monkeys 259, 278, and 336 were treated for 4 weeks with triple antiretroviral drugs (see Materials and Methods), which started 1 week before SEB injection. Note that monkeys 259 and 278 showed a decrease or no change in levels of plasma SIV RNA after SEB challenge. The SIVmac-BCG-coinfected monkeys 269 and 259 died 0.5 and 1.5 months, respectively, after SEB challenge due to progression of SIV-related tuberculosis-like disease. Monkeys 276 and 278 died 5 months after SEB injection. Monkeys 336 and 349 had low levels of plasma SIV RNA and survived BCG coinfection during the follow-up. A P value of <0.001 was obtained for statistical analyses of differences in BCG CFU or Ag85B mRNA before and after SEB challenge between the control group and the SIVmac-BCG-coinfected group.
Reactivation of SIV-related tuberculosis-like disease coincided with the waning of T-cell responses in advanced SIVmac infection.
To determine if the integrity of BCG-specific T-cell immune responses correlated with maintaining the clinical latency of BCG coinfection, we followed CD4+ PBL counts and PPD-specific proliferative T-cell responses in the SIVmac-BCG-coinfected monkeys. The SIVmac-infected monkeys that eventually developed relapses in SIV-BCG-related disease showed a marked decline of CD4+ PBL counts in the last 2 to 6 months of their disease courses (Fig. 5a). Associated with the decline of CD4+ T-cell numbers in these monkeys was a decrease in the magnitude of BCG-specific T-cell responses (Fig. 5b). The magnitude of the PPD-driven T-cell proliferation was gradually reduced with the progression of SIVmac infection, although proliferative responses were detectable in these SIVmac-infected macaques during the clinically quiescent phase of their coinfection (Fig. 5a). In some animals, a loss or low level of proliferative responses of PPD-specific CD4+ T cells was seen at the time CD4+ PBL counts declined to below 150 cells/μl. Importantly, the waning of PPD-specific T-cell responses was associated with the reactivation of BCG replication and the relapse of BCG-related illness in these monkeys (Fig. 5b and Table 2). In contrast, the SIVmac-BCG-coinfected monkeys (monkeys 336 and 349) that had low viral loads and survived BCG infection did not exhibit a marked decline of CD4+ T cells and PPD-specific proliferative responses (Fig. 5). These results suggest a role for mycobacterium-specific T cells in maintaining the latency of mycobacterial coinfection in SIVmac-infected macaques.
FIG. 5.
Reactivation of SIV-related tuberculosis-like disease was associated with an advanced deficiency of CD4 T cells and loss of BCG-specific T-cell responses. (a) Changes in CD4 PBL counts and plasma SIV RNA levels during the follow-up studies. (b) Changes in magnitudes of PPD-driven proliferative responses in PBL during the course of SIVmac-BCG coinfection. Stimulation indexes of PPD (left)- and ConA (right)-driven responses were obtained by dividing their mean values by the mean values of control medium-driven responses. Note that the absence of reactivation of BCG coinfection in monkeys 336 and 349 was associated with low levels of plasma SIV RNA and sustained levels of CD4 PBL counts and proliferative responses. A P value of <0.01 was obtained for statistical analyses of differences in PPD-specific proliferative responses at the final point versus 6 months earlier between the group with BCG disease (monkeys 259, 276, 278, and 263) and the group without BCG disease (monkeys 185, 545, 336, and 349).
DISCUSSION
We have previously demonstrated that BCG coinfection of SIVmac-infected macaques can result in a persistently active infection and induce SIV-related tuberculosis-like disease within 1 to 4 months of the coinfection. The present follow-up studies complement these previous experiments and demonstrate that progression of CD4 T-cell deficiency in SIVmac-infected monkeys is associated with reactivation of latent BCG infection. In addition, the reactivation of BCG coinfection can result in the development of tuberculosis-like disease. Thus, consequences of evolving mycobacterial infection in SIVmac-BCG-coinfected monkeys resemble those seen in M. tuberculosis coinfection of some HIV-1-infected humans. Clinical studies using PPD skin tests or DNA fingerprinting analyses of HIV-1-infected humans suggest that active tuberculosis can be caused either by endogenous reactivation of latent M. tuberculosis infection or by a new infection (4, 7, 19, 21). Moreover, a recent study has shown that latent infection can serve as a source of dissemination of M. avium complex in SIV-infected monkeys that results in disease (14). Given that HIV-1-infected humans can develop extrapulmonary tuberculosis, including lesions in lymph nodes (26), our studies of host immune responses and systemic BCG coinfection are likely relevant to HIV-1-related tuberculosis in humans.
The evolution of latency and reactivation of BCG replication appear to be consistent with the progression to clinical quiescence and then to the relapse of tuberculosis-like disease in SIVmac-BCG-coinfected monkeys. Using sensitive real-time quantitative PCR for detection of bacterial loads, we were able to detect low but measurable levels of BCG Ag85B mRNA expression after resolution of the early BCG infection, although viable BCG organisms are not readily isolated during this period of clinical latency. The difficulty in detecting viable BCG during the period of clinical quiescence may be attributed to the limited numbers of cells isolated from the biopsied lymph nodes. Changes in the biological characteristics of latent BCG organisms may also contribute to the difficulty in cultivating bacteria (30). Detectable levels of BCG Ag85B mRNA expression in the lymph nodes of these monkeys reflect the degree of clinical latency of the BCG infections, since in vitro studies have shown that BCG mRNA was detectable in viable but not dead BCG organisms (data not shown). Our present results suggest that monitoring of mycobacterial mRNA expression may be useful for following clinically latent mycobacterial coinfection in AIDS virus-infected individuals.
Various pathological lesions are evident in SIVmac-BCG-coinfected monkeys after reactivation of latent BCG coinfections. In some SIVmac-BCG-coinfected monkeys, the reactivation of the BCG infection was associated with the presence of lesions characterized by infiltration of histiocytes. In other SIVmac-BCG-coinfected monkeys, both nonnecrotic granulomas and necrotic granulomas with calcification were found at the time they developed SIV-related tuberculosis-like disease. Granulomatous lesions with caseous necrosis and mineral calcification were seen in one SIVmac-BCG-coinfected monkey who developed a typical relapse of SIV-BCG-related disease. Such lesions are similar to the granulomatous inflammation caused by M. tuberculosis in immune-competent persons and HIV-1-infected patients (26). The necrotic lesions with calcification were not identified in the tissues of the SIVmac-BCG-coinfected monkeys who died from acutely progressive tuberculosis-like disease within 4 months of BCG coinfection (23). The difference in the characteristics of these lesions may be related to the extent of SIVmac-BCG coinfection and the immune status of the monkeys at the time they develop reactivation/relapse of their BCG infection.
Our studies demonstrate the interesting finding that exposure of the immune system to SEB bacterial superantigen can result in the up-regulation of BCG coinfection. This result suggests that a compromise of the immune system caused by superantigen-producing microbes may predispose mycobacterium-infected AIDS patients with low CD4 T cell counts to reactivation of latent mycobacterial coinfection. Surprisingly, the up-regulation of BCG coinfection was independent of an increase in SIV viral loads in the monkeys. Several immune mechanisms may explain SEB superantigen-induced augmentation of BCG replication in SIVmac-BCG-coinfected monkeys. Since SEB superantigen engagement of major histocompatibility complex class II can directly stimulate monocytes/macrophages (10, 15), such a stimulation may result in an enhancement of mycobacterial replication in BCG-infected monocytes/macrophages. SEB may also enhance BCG replication in SIV-BCG-coinfected monocytes/macrophages if SEB stimulates a biological interaction between SIV and BCG in monocytes/macrophages. We cannot exclude this possibility for those SIVmac-BCG-coinfected monkeys whose plasma SIV RNA levels were reduced by antiretroviral treatment at the time of SEB superantigen exposure (Fig. 4b). SEB superantigen exposure results in the production of large amounts of various cytokines (10), and these inflammatory cytokines may up-regulate mycobacterial infection in SIVmac-BCG-coinfected monkeys. While some inflammatory cytokines may undermine the antimycobacterial immune mechanisms that are important for balancing latent BCG infection, other cytokines may be directly involved in the up-regulation of BCG coinfection. Furthermore, since SEB-mediated activation and expansion of selected Vβ-expressing T cells inevitably results in the deletion of those reactive T cells, the SEB-associated massive turnover of CD4 and CD8 T cells may result in a loss of functional mycobacterium-specific CD4 or CD8 T cells. The decrease in numbers and function of mycobacterium-specific T cells may facilitate reactivation or enhancement of latent BCG coinfection. Since the in vivo effect of SEB superantigen on the macaque immune system is prolonged (11), SEB-induced immunopathological events may accelerate the clinical progression of SIVmac-BCG coinfection and contribute to the evolution of SIV-related tuberculosis-like disease. This possibility is supported by the finding that monkeys 269 and 259 died from SIV-related tuberculosis-like disease within 6 weeks of SEB exposure.
While previous studies have demonstrated a crucial role of CD4 T cells in controlling acute BCG coinfection in SIVmac-infected monkeys, the results of the current experiments suggest that CD4 T cells are also important for containing latent BCG infection. Marked reductions in numbers of CD4 T cells and magnitudes of their proliferative responses were seen coincident with the reactivation of latent BCG infection and development of tuberculosis-like disease in the SIVmac-BCG-coinfected monkeys. In contrast, in the two SIVmac-infected monkeys who survived BCG coinfection, BCG mRNA was undetectable in lymph nodes after intravenous inoculation with large doses of BCG. Prospective studies showed that these two monkeys maintained sustained CD4 PBL counts and potent PPD-specific T-cell responses during the latent BCG infection and period of clinical quiescence. These data suggest that CD4 T cells play a role in controlling latent mycobacterial coinfection in AIDS virus-infected individuals. Such results are consistent with the clinical observations of HIV-M. tuberculosis-coinfected humans, but different from the findings reported for a T-cell-depletion study of mice (5). CD4 T-cell-mediated control of latent infection has not been demonstrated in the murine model of latent M. tuberculosis infection (28), although murine CD4 T cells have been shown to play a central role in controlling acute/persistent M. tuberculosis infections (5). Differences between species, the extent of T-cell depletion, and the nature of the infections may contribute to the different observations for mice and primates. The current studies of monkeys cannot exclude the possibility that AIDS virus infections have an impact on other immune parameters and aspects of mycobacterial biology that are involved in reactivation of latent mycobacterial infection. We have recently demonstrated that CD8 and γδ T cells have important roles in the adaptive immune responses during mycobacterial infections in monkeys (12, 24). The inhibition or dysfunction of these mycobacterium-specific T cells in advanced SIVmac infection may contribute to the reactivation or relapse of SIV-related tuberculosis-like disease.
The current studies allow us to evaluate the evolution of latency and reactivation of mycobacterial coinfection/disease in SIVmac-infected monkeys. Our findings provide experimental evidence that reactivation of latent mycobacterial coinfection or mycobacterial disease can occur as a result of a loss of T-cell immunity or from superantigen-induced compromise of immune function.
Acknowledgments
This work was supported by NIH RO1 grants HL64560 (to Z.W.C.) and RR13601 (to Z.W.C.).
We thank other members of the Chen lab for technical support.
REFERENCES
- 1.Antonucci, G., E. Girardi, M. C. Raviglione, and G. Ippolito. 1995. Risk factors for tuberculosis in HIV-infected persons. A prospective cohort study. The Gruppo Italiano di Studio Tubercolosi e AIDS (GISTA). JAMA 274:143-148. [DOI] [PubMed] [Google Scholar]
- 2.Caminero, J. A., M. J. Pena, M. I. Campos-Herrero, J. C. Rodriguez, O. Afonso, C. Martin, J. M. Pavon, M. J. Torres, M. Burgos, P. Cabrera, P. M. Small, and D. A. Enarson. 2001. Exogenous reinfection with tuberculosis on a European island with a moderate incidence of disease. Am. J. Respir. Crit. Care Med. 163:717-720. [DOI] [PubMed] [Google Scholar]
- 3.Chen, Z. W., Y. Shen, D. Zhou, M. Simon, Z. Kou, D. Lee-Parritz, L. Shen, P. Sehgal, and N. L. Letvin. 2001. In vivo T-lymphocyte activation and transient reduction of viral replication in macaques infected with simian immunodeficiency virus. J. Virol. 75:4713-4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daley, C. L., P. M. Small, G. F. Schecter, G. K. Schoolnik, R. A. McAdam, W. R. Jacobs, Jr., and P. C. Hopewell. 1992. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N. Engl. J. Med. 326:231-235. [DOI] [PubMed] [Google Scholar]
- 5.Flynn, J. L., and J. Chan. 2001. Tuberculosis: latency and reactivation. Infect. Immun. 69:4195-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glickman, M. S., J. S. Cox, and W. R. Jacobs, Jr. 2000. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 5:717-727. [DOI] [PubMed] [Google Scholar]
- 7.Godfrey-Faussett, P., and H. Ayles. 2003. Can we control tuberculosis in high HIV prevalence settings? Tuberculosis 83:68-76. [DOI] [PubMed] [Google Scholar]
- 8.Guelar, A., J. M. Gatell, J. Verdejo, D. Podzamczer, L. Lozano, E. Aznar, J. M. Miro, J. Mallolas, L. Zamora, J. Gonzalez, et al. 1993. A prospective study of the risk of tuberculosis among HIV-infected patients. AIDS 7:1345-1349. [DOI] [PubMed] [Google Scholar]
- 9.Jung, Y. J., L. Ryan, R. LaCourse, and R. J. North. 2003. Increased interleukin-10 expression is not responsible for failure of T helper 1 immunity to resolve airborne Mycobacterium tuberculosis infection in mice. Immunology 109:295-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotzin, B. L., D. Y. Leung, J. Kappler, and P. Marrack. 1993. Superantigens and their potential role in human disease. Adv. Immunol. 54:99-166. [DOI] [PubMed] [Google Scholar]
- 11.Kou, Z. C., M. Halloran, D. Lee-Parritz, L. Shen, M. Simon, P. K. Sehgal, Y. Shen, and Z. W. Chen. 1998. In vivo effects of a bacterial superantigen on macaque TCR repertoires. J. Immunol. 160:5170-5180. [PubMed] [Google Scholar]
- 12.Lai, X., Y. Shen, D. Zhou, P. Sehgal, L. Shen, M. Simon, L. Qiu, N. L. Letvin, and Z. W. Chen. 2003. Immune biology of macaque lymphocyte populations during mycobacterial infection. Clin. Exp. Immunol. 133:182-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manabe, Y. C., and W. R. Bishai. 2000. Latent Mycobacterium tuberculosis—persistence, patience, and winning by waiting. Nat. Med. 6:1327-1329. [DOI] [PubMed] [Google Scholar]
- 14.Maslow, J. N., I. Brar, G. Smith, G. W. Newman, R. Mehta, C. Thornton, and P. Didier. 2003. Latent infection as a source of disseminated disease caused by organisms of the Mycobacterium avium complex in simian immunodeficiency virus-infected rhesus macaques. J. Infect. Dis. 187:1748-1755. [DOI] [PubMed] [Google Scholar]
- 15.Matsuyama, S., Y. Koide, and T. O. Yoshida. 1993. HLA class II molecule-mediated signal transduction mechanism responsible for the expression of interleukin-1 beta and tumor necrosis factor-alpha genes induced by a staphylococcal superantigen. Eur. J. Immunol. 23:3194-3202. [DOI] [PubMed] [Google Scholar]
- 16.McKinney, J. D., K. Honer zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 17.Mogues, T., M. E. Goodrich, L. Ryan, R. LaCourse, and R. J. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 18.Orme, I. M. 2003. The mouse as a useful model of tuberculosis. Tuberculosis 83:112-115. [DOI] [PubMed] [Google Scholar]
- 19.Perlman, D. C., P. El-Helou, and N. Salomon. 1999. Tuberculosis in patients with human immunodeficiency virus infection. Semin. Respir. Infect. 14:344-352. [PubMed] [Google Scholar]
- 20.Ramakrishnan, L., N. A. Federspiel, and S. Falkow. 2000. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science 288:1436-1439. [DOI] [PubMed] [Google Scholar]
- 21.Selwyn, P. A., D. Hartel, V. A. Lewis, E. E. Schoenbaum, S. H. Vermund, R. S. Klein, A. T. Walker, and G. H. Friedland. 1989. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N. Engl. J. Med. 320:545-550. [DOI] [PubMed] [Google Scholar]
- 22.Shen, Y., L. Shen, P. Sehgal, D. Zhou, M. Simon, M. Miller, E. A. Enimi, B. Henckler, L. Chalifoux, N. Sehgal, M. Gastron, N. L. Letvin, and Z. W. Chen. 2001. Antiretroviral agents restore Mycobacterium-specific T-cell immune responses and facilitate controlling a fatal tuberculosis-like disease in macaques coinfected with simian immunodeficiency virus and Mycobacterium bovis BCG. J. Virol. 75:8690-8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen, Y., D. Zhou, L. Chalifoux, L. Shen, M. Simon, X. Zeng, X. Lai, Y. Li, P. Sehgal, N. L. Letvin, and Z. W. Chen. 2002. Induction of an AIDS virus-related tuberculosis-like disease in macaques: a model of simian immunodeficiency virus-mycobacterium coinfection. Infect. Immun. 70:869-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen, Y., D. Zhou, L. Qiu, X. Lai, M. Simon, L. Shen, Z. Kou, Q. Wang, L. Jiang, J. Estep, R. Hunt, M. Clagett, P. K. Sehgal, Y. Li, X. Zeng, C. T. Morita, M. B. Brenner, N. L. Letvin, and Z. W. Chen. 2002. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science 295:2255-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Small, P. M., R. W. Shafer, P. C. Hopewell, S. P. Singh, M. J. Murphy, E. Desmond, M. F. Sierra, and G. K. Schoolnik. 1993. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in patients with advanced HIV infection. N. Engl. J. Med. 328:1137-1144. [DOI] [PubMed] [Google Scholar]
- 26.Sunderam, G., R. J. McDonald, T. Maniatis, J. Oleske, R. Kapila, and L. B. Reichman. 1986. Tuberculosis as a manifestation of the acquired immunodeficiency syndrome (AIDS). JAMA 256:362-366. [PubMed] [Google Scholar]
- 27.Turner, J., M. Gonzalez-Juarrero, D. L. Ellis, R. J. Basaraba, A. Kipnis, I. M. Orme, and A. M. Cooper. 2002. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J. Immunol. 169:6343-6351. [DOI] [PubMed] [Google Scholar]
- 28.van Pinxteren, L. A., J. P. Cassidy, B. H. Smedegaard, E. M. Agger, and P. Andersen. 2000. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur. J. Immunol. 30:3689-3698. [DOI] [PubMed] [Google Scholar]
- 29.Wayne, L. G. 1994. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur. J. Clin. Microbiol. Infect. Dis. 13:908-914. [DOI] [PubMed] [Google Scholar]
- 30.Wayne, L. G., and C. D. Sohaskey. 2001. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 55:139-163. [DOI] [PubMed] [Google Scholar]
- 31.Zhou, D., X. Lai, Y. Shen, P. Sehgal, L. Shen, M. Simon, L. Qiu, D. Huang, G. Z. Du, Q. Wang, N. L. Letvin, and Z. W. Chen. 2003. Inhibition of adaptive Vγ2Vδ2+ T-cell responses during active mycobacterial coinfection of simian immunodeficiency virus SIVmac-infected monkeys. J. Virol. 77:2998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou, D., Y. Shen, L. Chalifoux, D. Lee-Parritz, M. Simon, P. K. Sehgal, L. Zheng, M. Halloran, and Z. W. Chen. 1999. Mycobacterium bovis bacille Calmette-Guerin enhances pathogenicity of simian immunodeficiency virus infection and accelerates progression to AIDS in macaques: a role of persistent T cell activation in AIDS pathogenesis. J. Immunol. 162:2204-2216. [PubMed] [Google Scholar]