Abstract
In rhesus macaques, classic systemic infection, characterized by persistent viremia and seroconversion, occurred after multiple low-dose (103 50% tissue culture infective doses) intravaginal (IVAG) inoculations with simian immunodeficiency virus (SIV) strain SIVmac251. Monkeys developed classic SIV infections after a variable number of low-dose IVAG exposures to SIVmac251. Once established, the systemic infection was identical to SIV infection following high-dose IVAG SIV inoculation. However, occult systemic infection characterized by transient cell-associated or cell-free viremia consistently occurred early in the series of multiple vaginal SIV exposures. Further, antiviral cellular immune responses were present prior to the establishment of a classic systemic infection in the low-dose vaginal SIV transmission model.
The goal of this study was to determine if simian immunodeficiency virus (SIV) can be reliably transmitted to rhesus monkeys by multiple intravaginal (IVAG) inoculations with a low-dose cell-free SIV inoculum. Eight adult, multiparous female rhesus macaques (Macaca mulatta) seronegative for human immunodeficiency virus type 2 (HIV-2), SIV, type D retrovirus, and simian T-cell lymphotropic virus type 1 and housed in accordance with American Association for Accreditation of Laboratory Animal Care standards were used. When necessary, animals were anesthetized with ketamine hydrochloride (10 mg/kg; Parke-Davis, Morris Plains, N.J.) or 0.7 mg of tiletamine HCl and zolazepan (Telazol; Fort Dodge Animal Health, Fort Dodge, Iowa) per kg injected intramuscularly. A cell-free stock of SIVmac251 (UCD-2/02) produced by short-term expansion of a previous virus stock (SIVmac251 UCD-2/00) in staphylococcus enterotoxin A (SEA)-stimulated rhesus monkey peripheral blood mononuclear cells (PBMC) was used for these studies. This SIVmac251 stock contains approximately 109 viral RNA (vRNA) copies/ml and 105 50% tissue culture infection doses (TCID50)/ml when the titer is determined on CEMX174 cells. For IVAG inoculation, the stock was diluted 100-fold to produce an inoculum containing 103 TCID50/ml, and 1 ml was introduced into the vaginal canal with a needleless 1-ml tuberculin syringe. The animals were inoculated twice in 1 day with a 4-h interval between inoculations. This SIV inoculation series was performed weekly for 13 weeks or until systemic SIV infection was detected. Blood samples were collected prior to each inoculation, and blood and cervicovaginal secretions (CVS) were collected 3 days after inoculation, by previously published methods (8).
Systemic SIV infection following multiple low-dose IVAG SIVmac251 inoculations.
A monkey is defined as systemically infected from the day that the first virus isolation or SIV gag-positive PBMC sample in a series of three consecutive positive samples was collected. On the basis of these criteria, two animals (27361 and 29029) were systemically SIV infected by 2 and 3 weeks postinoculation (p.i.), respectively; five monkeys were systemically infected by 5 to 7 weeks p.i.; and one animal (26513) was not infected until 8 weeks p.i. A SIV gag-specific nested PCR was carried out on all PBMC samples by a previously published technique (13). The PBMC of two animals were SIV DNA (vDNA) positive at 11 days p.i. (4 days after the second inoculation set). PBMC from two animals were consistently vDNA positive from the initial positive sample through the conclusion of the study (Table 1). Initially, PBMC from six of the eight animals were intermittently vDNA positive, but by 8 weeks p.i., PBMC from all of the animals were consistently positive (Table 1). To determine when infectious virus was readily detectable, SIV was isolated by coculturing PBMC with CEMx174 cells as previously described (7). Once the first SIV isolation-positive PBMC sample from an animal was detected, most of the subsequent PBMC samples from that animal were also virus isolation positive (Table 1). In all eight monkeys, the first virus isolation-positive PBMC sample was collected after the first vDNA PCR-positive sample (Table 1).
TABLE 1.
Virologic analysis of peripheral blood samples from monkeys inoculated IVAG multiple times with a low dose of SIVmac251
| Animal and assay | Result at following time (wk) after first inoculation:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13c | 14 | |
| 29029 | |||||||||||||||
| vDNAa | − | + | − | + | + | + | + | + | + | + | + | + | + | + | + |
| Virus isolation | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + |
| vRNAb | − | − | 5.0 | 7.4 | 7.3 | 6.8 | 6.6 | 6.3 | 6.4 | 6.5 | 6.6 | 6.7 | 6.8 | 6.8 | 7.0 |
| 27361 | |||||||||||||||
| vDNA | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Virus isolation | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + |
| vRNA | − | − | 5.7 | 6.6 | 7.0 | 6.2 | 6.1 | 5.6 | 5.7 | 5.6 | 5.8 | 5.6 | 5.3 | 5.4 | 5.0 |
| 25479 | |||||||||||||||
| vDNA | − | − | − | + | − | + | + | + | − | + | + | + | + | − | + |
| Virus isolation | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + |
| vRNA | − | − | − | − | − | 6.4 | 8.3 | 7.8 | 7.8 | 7.9 | 7.7 | 7.8 | 7.8 | 8.0 | 8.3 |
| 29459 | |||||||||||||||
| vDNA | − | − | − | + | − | − | + | + | + | + | + | + | + | + | + |
| Virus isolation | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + |
| vRNA | − | − | − | − | − | − | 2.1 | 4.9 | 8.1 | 7.5 | 7.3 | 7.3 | 7.5 | 7.7 | 7.9 |
| 25948 | |||||||||||||||
| vDNA | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + |
| Virus isolation | − | − | − | − | − | + | + | + | + | + | + | + | + | + | − |
| vRNA | − | − | − | − | − | 5.3 | 7.2 | 6.4 | 5.2 | 4.8 | 4.7 | 4.3 | 4.2 | 4.3 | 4.5 |
| 25908 | |||||||||||||||
| vDNA | − | − | − | − | + | − | − | + | + | + | + | + | + | + | + |
| Virus isolation | − | − | − | − | − | − | − | − | + | + | + | + | + | + | − |
| vRNA | − | − | − | − | − | − | − | 4.5 | 7.6 | 6.0 | 5.8 | 5.7 | 5.3 | 5.3 | 5.3 |
| 26811 | |||||||||||||||
| vDNA | − | − | − | + | − | − | − | + | − | + | − | + | + | + | + |
| Virus isolation | − | − | − | − | − | − | − | + | + | + | + | + | + | + | + |
| vRNA | − | − | − | − | − | 2.3 | 3.1 | 7.4 | 7.0 | 6.5 | 5.9 | 6.2 | 5.9 | 6.1 | 6.2 |
| 26513 | |||||||||||||||
| vDNA | − | + | − | − | − | − | − | − | + | + | + | + | + | + | + |
| Virus isolation | − | − | − | − | − | − | − | − | − | − | + | + | + | + | + |
| vRNA | − | − | − | − | 2.9 | − | − | − | − | 4.5 | 7.1 | 6.9 | 6.0 | 5.8 | 5.8 |
SIVgag nested PCR assay. + indicates vDNA detected in PBMC (106 PBMC tested at each time point).
Plasma vRNA level (log10 copies per milliliter).
Final inoculation in series.
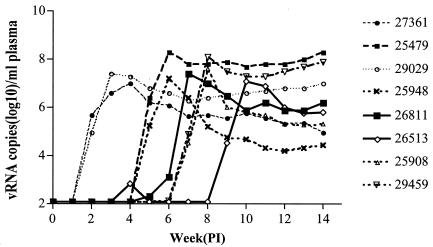
Plasma SIV vRNA levels were determined by Bayer Diagnostics Inc. (Berkeley, Calif.) with an SIV-specific branched DNA amplification assay (4). The lower limit of detection of the assay was 125 copies of SIV RNA per ml of plasma. For most of the animals, the peak plasma vRNA levels (7.0 to 8.3 log10 copies/ml) occurred 1 or 2 weeks after the first vRNA-positive plasma sample was collected (Table 1 and Fig. 1). After the acute peak phase viremia, plasma vRNA levels decreased to a variable extent but remained high (Fig. 1). In six of the eight animals, plasma vRNA became detectable after vDNA was detected in PBMC samples, but in two of the animals, vRNA and vDNA were initially found in the same blood samples. Compared to the isolation of infectious virus, plasma vRNA was detected earlier in six of the eight animals and simultaneously in two of the eight monkeys (Table 1).
FIG. 1.
Plasma SIV vRNA levels during multiple IVAG inoculation series with a low dose of SIVmac251. Week 13 was the end of the inoculation series. PI, postinfection.
Monkey 26513 had a unique pattern of viremia characterized by a period of transient detectable plasma vRNA and PBMC vDNA prior to the establishment of a classic systemic infection. After two sets of IVAG inoculations, vDNA was detected in the PBMC collected at day 11 p.i. and PBMC from day 14 to day 52 p.i. were vDNA negative (Table 1). At week 4 p.i., vRNA was detected at a low level (731 copies/ml) in plasma, but plasma vRNA was undetectable again until week 8 p.i. Beginning at 9 weeks p.i., plasma samples were consistently vRNA positive with a typical acute peak and a lower set point vRNA pattern (Table 1).
Anti-SIV immune responses in multiple low-dose IVAG-inoculated rhesus monkeys.
T-cell proliferation in response to aldithriol-2-inactivated SIVmac239 was measured in PBMC from fresh blood samples as previously described (1, 10). SIV-specific T-cell proliferative responses were found in the PBMC of all eight animals during the study, and T-cell proliferation was the first detectable SIV-specific immune response in four of the eight study animals (Table 2). In six of the eight monkeys, anti-SIV T-cell proliferative responses were detected after the first vDNA-positive PBMC sample was detected. Once detected, SIV-specific T-cell proliferative responses were intermittently detected in all of the animals. In one animal (25948), the first T-cell proliferation-positive PBMC sample was collected before vRNA or vDNA was detected in blood. In five of the eight monkeys, SIV-specific T-cell proliferation was detectable before, or coincident with, the detection of SIV-specific antibodies (Table 2).
TABLE 2.
Anti-SIV immune responses in peripheral blood and CVS from monkeys IVAG inoculated multiple times with a low dose of SIVmac251
| Animal and assay | Result at following time (wk) after first inoculation:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| 29029 | |||||||||||||||
| T-cell proliferationa | − | −d | − | − | − | 4.6 | 2.1 | − | − | − | − | 8.1 | 4.5 | 15.5 | 4.8 |
| ELISPOTb | − | − | − | − | − | − | − | − | |||||||
| Plasma IgGc | − | − | − | 4,000 | 10,000 | 20,000 | 200,000 | 200,000 | 400,000 | 200,000 | |||||
| CVS IgGc | − | − | − | − | − | 80 | 80 | 160 | 160 | 320 | |||||
| CVS IgAc | − | − | − | 2 | 2 | 2 | 4 | 2 | 2 | 2 | |||||
| 27361 | |||||||||||||||
| T-cell proliferation | − | − | −d | − | − | − | − | − | − | − | 165 | − | 11.3 | 4.5 | 39.3 |
| ELISPOT | − | − | − | 50 | 220 | 70 | 175 | 125 | |||||||
| Plasma IgG | − | − | − | − | − | 1,000 | 4,000 | 20,000 | 200,000 | 200,000 | 800,000 | 320,000 | |||
| CVS IgG | − | − | − | − | − | 80 | 160 | 160 | 160 | 320 | |||||
| CVS IgA | − | − | − | − | − | 4 | 4 | 4 | 4 | 4 | |||||
| 25479 | |||||||||||||||
| T-cell proliferation | − | − | − | 9.9d | 2.9 | − | 2.5 | − | − | − | − | 3.1 | 2.5 | − | − |
| ELISPOT | − | − | − | − | − | − | − | − | |||||||
| Plasma IgG | − | − | − | − | − | − | − | − | − | − | 100 | 100 | |||
| CVS IgG | − | − | − | − | − | − | − | − | − | ||||||
| CVS IgA | − | − | − | − | − | − | − | − | − | − | |||||
| 29459 | |||||||||||||||
| T-cell proliferation | − | − | − | −d | − | − | 8.5 | − | − | − | − | − | − | 11.1 | 3.2 |
| ELISPOT | − | − | − | − | − | − | − | − | |||||||
| Plasma IgG | − | − | − | − | − | − | − | − | 200 | 4,000 | 4,000 | 100 | 2,000 | ||
| CVS IgG | − | − | − | − | − | − | − | − | − | − | |||||
| CVS IgA | − | − | − | − | − | − | − | − | − | 1 | |||||
| 25948 | |||||||||||||||
| T-cell proliferation | − | − | − | 2.7 | − | −d | 2.6 | 6.5 | 3.9 | 3.0 | 46.0 | 20.1 | 26.7 | 37.5 | 35.9 |
| ELISPOT | − | − | 70 | − | − | − | 225 | 170 | |||||||
| Plasma IgG | − | − | − | − | − | 100 | 2,000 | 2,000 | 16,000 | 16,000 | 160,000 | ||||
| CVS IgG | − | − | − | − | − | 2 | 2 | 32 | 40 | 320 | |||||
| CVS IgA | − | − | − | − | 1 | 1 | 1 | 2 | 2 | 2 | |||||
| 25908 | |||||||||||||||
| T-cell proliferation | − | − | − | − | −d | 2.1 | 4.2 | 3.1 | − | − | − | − | 2.3 | 4.5 | 3.6 |
| ELISPOT | − | − | 130 | − | − | − | − | − | |||||||
| Plasma IgG | − | − | − | − | − | − | 200 | 4,000 | 10,000 | 100,000 | 160,000 | ||||
| CVS IgG | − | − | − | − | − | − | 8 | 10 | 10 | ||||||
| CVS IgA | − | − | − | − | − | − | − | 2 | 2 | 2 | |||||
| 26811 | |||||||||||||||
| T-cell proliferation | − | − | − | −d | − | − | − | − | − | − | 9.0 | − | − | 2.1 | − |
| ELISPOT | − | − | 95 | − | 40 | − | − | − | |||||||
| Plasma IgG | − | − | − | − | − | − | 100 | 200 | 400 | 400 | 8,000 | ||||
| CVS IgG | − | − | − | − | − | − | − | − | 2 | 20 | |||||
| CVS IgA | − | − | − | − | − | − | − | − | − | − | |||||
| 26513 | |||||||||||||||
| T-cell proliferation | − | −d | 20.9 | 2.8 | 2.6 | 3.7 | − | − | − | − | 2.7 | − | − | 2.6 | |
| ELISPOT | − | − | − | − | − | − | 110 | − | |||||||
| Plasma IgG | − | − | − | − | − | − | − | − | 100 | 400 | 80,000 | ||||
| CVS IgG | − | − | − | − | − | − | − | − | − | − | |||||
| CVS IgA | − | − | − | − | − | − | − | − | − | − | |||||
AT-2 SIV T-cell proliferation assay results are shown as a stimulation index. A minus sign indicates a stimulation index of ≤2.
SIV gag-specific IFN-γ ELISPOT assay results are shown as the number of spot-forming cells per 106 PBMC. The number of background spots in medium-only wells was subtracted from the number of spots in peptide-stimulated wells.
Detergent-disrupted SIV ELISA results are shown as the reciprocal of the highest positive dilution that was above the cutoff.
First PBMC sample in an animal that was vDNA positive by nested PCR for SIVgag.
Gamma interferon (IFN-γ)-secreting T cells were detected in the PBMC of five of the eight monkeys in this study with an IFN-γ monkey cytokine ELISPOT kit (U-CyTech, Utrecht, The Netherlands) and in vitro stimulation with a pool of 20-mer peptides, with a 10-amino-acid overlap, that span the entire p27 Gag region of SIVmac239 (Table 2) (1, 17). At 4 weeks p.i., PBMC of three animals were positive for SIV-specific IFN-γ-secreting cells (Table 2). IFN-γ-secreting T cells were intermittently detected in the PBMC of three of the five animals. However, two animals (27361 and 25948) consistently had SIV-specific IFN-γ-secreting T cells in PBMC and they also had relatively low set point plasma vRNA levels.
With a previously described detergent-disrupted SIV enzyme-linked immunosorbent assay (ELISA) (8), anti-SIV immunoglobulin G (IgG) antibodies were found in the plasma of SIV-inoculated monkeys as early as 5 weeks p.i. and in all eight animals by 13 weeks p.i. Plasma anti-SIV IgG antibodies were first detected in samples collected 2 to 8 weeks after the first positive plasma vRNA sample, or 1 to 8 weeks after the first virus isolation-positive sample. Monkey 25479 had a delayed plasma anti-SIV IgG response first detected at week 13 p.i. and rapidly progressed to AIDS. On the basis of the detergent-disrupted SIV ELISA (8), four monkeys had both IgA and IgG SIV antibodies in CVS, one monkey had only IgG and another had only IgA anti-SIV antibodies in CVS samples, and two monkeys were negative (Table 2). Anti-SIV CVS antibodies were detected after the plasma anti-SIV antibody response in four of six monkeys tested.
Anti-SIV antibodies were detected in CVS before plasma anti-SIV antibodies or virologic evidence of systemic SIV infection was detected in monkey 25948. This monkey also had anti-SIV-specific T-cell proliferation and IFN-γ-secreting cells in PBMC before vDNA, vRNA, or infectious virus was detected in the blood. It is noteworthy that the viral load of this monkey was relatively low throughout the 14 weeks of this study.
Repeated low-dose IVAG SIV inoculation reliably infected rhesus monkeys, and the resulting systemic infection was similar to the classic systemic SIV infection that follows high-dose IVAG SIV inoculation. However, six of the eight monkeys exposed to the low-dose SIV IVAG inoculation series had a period of transient viremia (plasma vRNA or PBMC vDNA) before the onset of a classic systemic infection. While occult SIV infection has been described after a single low-dose IVAG SIV inoculation (12), high-dose IVAG inoculation is generally characterized by a classic systemic infection (9, 12). Thus, the biology of acute SIV infection in rhesus monkeys after multiple IVAG inoculations with 103 TCID50 is distinctly different from acute infection after IVAG inoculation with 105 TCID50 of the same stock. As we have demonstrated here, following low-dose IVAG SIV inoculation, occult or cryptic systemic infection typically precedes the onset of a classic systemic infection with persistent viremia and consistently detectable antiviral immune responses, while transient viremia is rarely seen after high-dose IVAG SIV inoculation.
While other investigators have reported mucosal SIV transmission with doses of SIV that are apparently lower than those used here, comparison of the infectious titers in the virus stocks used by different laboratories is difficult because the TCID50 assays use different reagents or protocols. For example, in our original studies of vaginal SIV transmission, we used human PBMC as the target cells for the TCID50 assay and found that 0.1 TCID50 of this stock reliably infected rhesus monkeys by intravenous inoculation (11). Thus, our previous system grossly underestimated the amount of infectious SIV in our stocks, and in general, in vitro titration of primate lentiviruses underestimates the amount of infectious virus in a stock. Our current system for in vitro titration uses CEMx174 cells as target cells, and the TCID50 of the SIVmac251 stock used in this study is essentially equivalent to the minimum dose of the stock needed to infect animals by intravenous inoculation (data not shown). In addition to the TCID50, it will simplify comparison of studies if RNA levels, p27 levels, and the minimal intravenous infectious dose of a stock are provided. The wide range of viral loads that have been found in human semen makes rational modeling of an infectious HIV dose impossible (reviewed in reference 3). Further, although infectious virus has been isolated from seminal cells and HIV RNA levels in seminal plasma (undetectable to 107 copies/ml) have been characterized (reviewed in reference 3), quantitation of infectious, cell-free virus in seminal plasma is not possible because seminal plasma produces apoptosis in PBMC cultures (14). Thus, the amount of infectious, cell-free HIV in semen remains unknown.
In both low-dose and high-dose SIV IVAG models, plasma anti-SIV antibodies are found only after the onset of persistent systemic infection and peripheral anti-SIV antibodies are detected a few weeks after the onset of persistent systemic infection (6, 9, 12, 18). Anti-SIV T-cell responses developed before anti-SIV antibodies were detected, and anti-SIV antibodies in CVS were generally detected after SIV antibodies were found in plasma. A unique finding in the low-dose IVAG SIV injection-treated monkeys was that the earliest T-cell responses were detected during the occult stage of infection.
In one animal, local anti-SIV antibodies and systemic T-cell responses were detectable before a classic systemic infection was established and another monkey had anti-SIV antibodies in CVS several weeks before anti-SIV antibodies were detected in plasma. It seems likely that in these two cases infection was established in lymphoid tissues and transient viremia occurred prior to the onset of these immune responses but the sampling schedule or the amount of sample material assayed did not permit detection of the occult infection. In any case, there was no evidence that these immune responses conferred protection from subsequent inoculations or that they cleared the occult infections. In fact, there are no credible reports of a lentiviral infection being cleared in any host by any means, although the infections may become undetectable in peripheral blood with standard assays and thus remain hidden.
In a condition that is very similar to the occult infection in IVAG low-dose SIV-inoculated monkeys, HIV-specific systemic cellular and local immune responses have been detected in seronegative individuals repeatedly exposed to HIV (2, 5, 15, 16), and HIV DNA is present at very low levels in PBMC of repeatedly exposed (19), persistently seronegative individuals. Thus, occult HIV infections that produce transient periods of viremia that are undetectable by routine clinical tests probably occur much more frequently than is generally appreciated. The most likely explanation for the antiviral T-cell responses reported in HIV-exposed seronegative people and the results obtained in the primate model and reported here is that, following mucosal HIV exposure, a low-level infection is established in lymphoid tissues and virus replicates sufficiently to produce transient viremia and T-cell immunity but not to the level required to produce a clinical HIV infection. Because of the design of the study, we were unable to determine if the animals with occult infections would have progressed to classic infection and simian AIDS if the inoculation series had been stopped when the transient viremia was detected. The difference between the early stages of infection after high- and low-dose vaginal SIV transmission likely represents the spectrum of infections that occur because of heterosexual HIV transmission, and this information may be useful in developing vaccines or other strategies to control HIV transmission.
Acknowledgments
We thank the Immunology Core Laboratory and Primate Services Unit at the CNPRC and Lara Compton, Ding Lu, Blia Vang, Kristen Bost, and Rino Dizon for excellent technical assistance and Julian Bess, Jr., AIDS Vaccine Program, SAIC Frederick, Inc., National Cancer Institute—Frederick, Frederick, Md.
This work was supported by Public Health Service grants U51RR00169 from the National Center for Research Resources and P01 AI48484 and R01 AI51596 from the National Institute of Allergy and Infectious Diseases. Aldithriol-2-inactivated SIV was provided by the AIDS Vaccine Program, SAIC Frederick, Inc.
REFERENCES
- 1.Abel, K., L. Compton, T. Rourke, D. Montefiori, D. Lu, K. Rothaeusler, L. Fritts, K. Bost, and C. J. Miller. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J. Virol. 77:3099-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyrer, C., A. W. Artenstein, S. Rugpao, H. Stephens, T. C. VanCott, M. L. Robb, M. Rinkaew, D. L. Birx, C. Khamboonruang, P. A. Zimmerman, K. E. Nelson, and C. Natpratan. 1999. Epidemiologic and biologic characterization of a cohort of human immunodeficiency virus type 1 highly exposed, persistently seronegative female sex workers in northern Thailand. J. Infect. Dis. 179:59-67. [DOI] [PubMed] [Google Scholar]
- 3.Coombs, R. W., P. S. Reichelderfer, and A. L. Landay. 2003. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS 17:455-480. [DOI] [PubMed] [Google Scholar]
- 4.Dailey, P. J., M. Zamroud, R. Kelso, J. Kolberg, and M. Urdea. 1995. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected rhesus macaques using a branched DNA (bDNA) signal amplification assay. J. Med. Primatol. 24:209. [Google Scholar]
- 5.Kaul, R., S. L. Rowland-Jones, J. Kimani, K. Fowke, T. Dong, P. Kiama, J. Rutherford, E. Njagi, F. Mwangi, T. Rostron, J. Onyango, J. Oyugi, K. S. MacDonald, J. J. Bwayo, and F. A. Plummer. 2001. New insights into HIV-1 specific cytotoxic T-lymphocyte responses in exposed, persistently seronegative Kenyan sex workers. Immunol. Lett. 79:3-13. [DOI] [PubMed] [Google Scholar]
- 6. Knipe, M. D., P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus. 2001. Fields virology, 4 ed., 2:. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 7.Lohman, B. L., J. Higgins, M. L. Marthas, P. A. Marx, and N. C. Pedersen. 1991. Development of simian immunodeficiency virus isolation, titration, and neutralization assays which use whole blood from rhesus monkeys and an antigen capture enzyme-linked immunosorbent assay. J. Clin. Microbiol. 29:2187-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu, X., H. Kiyono, D. Lu, S. Kawabata, J. Torten, S. Srinivasan, P. J. Dailey, J. R. McGhee, T. Lehner, and C. J. Miller. 1998. Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS 12:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marthas, M. L., D. Lu, M. C. Penedo, A. G. Hendrickx, and C. J. Miller. 2001. Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses. AIDS Res. Hum. Retrovir. 17:1455-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McChesney, M. B., J. R. Collins, D. Lu, X. Lu, J. Torten, R. L. Ashley, M. W. Cloyd, and C. J. Miller. 1998. Occult systemic infection and persistent simian immunodeficiency virus (SIV)-specific CD4+-T-cell proliferative responses in rhesus macaques that were transiently viremic after intravaginal inoculation of SIV. J. Virol. 72:10029-10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller, C. J., N. J. Alexander, S. Sutjipto, A. A. Lackner, A. G. Hendrickx, A. Gettie, L. J. Lowenstine, M. Jennings, and P. A. Marx. 1989. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J. Virol. 63:4277-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller, C. J., M. Marthas, J. Torten, N. J. Alexander, J. P. Moore, G. F. Doncel, and A. G. Hendrickx. 1994. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J. Virol. 68:6391-6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller, C. J., M. B. McChesney, X. Lü, P. J. Dailey, C. Chutkowski, D. Lu, P. Brosio, B. Roberts, and Y. Lu. 1997. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J. Virol. 71:1911-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto, M., R. Byrn, R. C. Eyre, T. Mullen, P. Church, and A. A. Kiessling. 2002. Seminal plasma induces programmed cell death in cultured peripheral blood mononuclear cells. AIDS Res. Hum. Retrovir. 18:797-803. [DOI] [PubMed] [Google Scholar]
- 15.Promadej, N., C. Costello, M. M. Wernett, P. S. Kulkarni, V. A. Robison, K. E. Nelson, T. W. Hodge, V. Suriyanon, A. Duerr, and J. M. McNicholl. 2003. Broad human immunodeficiency virus (HIV)-specific T cell responses to conserved HIV proteins in HIV-seronegative women highly exposed to a single HIV-infected partner. J. Infect. Dis. 187:1053-1063. [DOI] [PubMed] [Google Scholar]
- 16.Schmechel, S. C., N. Russell, F. Hladik, J. Lang, A. Wilson, R. Ha, A. Desbien, and M. J. McElrath. 2001. Immune defence against HIV-1 infection in HIV-1-exposed seronegative persons. Immunol. Lett. 79:21-27. [DOI] [PubMed] [Google Scholar]
- 17.Van Rompay, K. K. A., J. L. Greenier, K. S. Cole, P. Earl, B. Moss, J. D. Steckbeck, B. Pahar, T. Rourke, R. C. Montelaro, D. R. Canfield, R. P. Tarara, C. Miller, M. B. McChesney, and M. L. Marthas. 2003. Immunization of newborn rhesus macaques with simian immunodeficiency virus (SIV) vaccines prolongs survival after oral challenge with virulent SIVmac251. J. Virol. 77:179-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams, S. B., T. P. Flanigan, S. Cu-Uvin, K. Mayer, P. Williams, C. A. Ettore, A. W. Artenstein, A. Duerr, and T. C. VanCott. 2002. Human immunodeficiency virus (HIV)-specific antibody in cervicovaginal lavage specimens obtained from women infected with HIV type 1. Clin. Infect. Dis. 35:611-617. [DOI] [PubMed] [Google Scholar]
- 19.Zhu, T., L. Corey, Y. Hwangbo, J. M. Lee, G. H. Learn, J. I. Mullins, and M. J. McElrath. 2003. Persistence of extraordinarily low levels of genetically homogeneous human immunodeficiency virus type 1 in exposed seronegative individuals. J. Virol. 77:6108-6116. [DOI] [PMC free article] [PubMed] [Google Scholar]