Abstract
We show that E6 proteins from benign human papillomavirus type 1 (HPV1) and oncogenic HPV16 have the ability to alter the regulation of the G1/S transition of the cell cycle in primary human fibroblasts. Overexpression of both viral proteins induces cellular proliferation, retinoblastoma (pRb) phosphorylation, and accumulation of products of genes that are negatively regulated by pRb, such as p16INK4a, CDC2, E2F-1, and cyclin A. Hyperphosphorylated forms of pRb are present in E6-expressing cells even in the presence of ectopic levels of p16INK4a. The E6 proteins strongly increased the cyclin A/cyclin-dependent kinase 2 (CDK2) activity, which is involved in pRb phosphorylation. In addition, mRNA and protein levels of the CDK2 inhibitor p21WAF1/CIP1 were strongly down-regulated in cells expressing E6 proteins. The down-regulation of the p21WAF1/CIP1 gene appears to be independent of p53 inactivation, since HPV1 E6 and an HPV16 E6 mutant unable to target p53 were fully competent in decreasing p21WAF1/CIP1 levels. E6 from HPV1 and HPV16 also enabled cells to overcome the G1 arrest imposed by oncogenic ras. Immunofluorescence staining of cells coexpressing ras and E6 from either HPV16 or HPV1 revealed that antiproliferative (p16INK4a) and proliferative (Ki67) markers were coexpressed in the same cells. Together, these data underline a novel activity of E6 that is not mediated by inactivation of p53.
Human papillomaviruses (HPVs) infect keratinocytes in the basal layer of stratified epithelia at a variety of anatomical sites (26). On the basis of their tissue tropism, the HPVs can be subdivided into cutaneous and mucosal types, which infect the skin and the mucosae, respectively. All HPV types are able to induce hyperproliferative benign lesions. Indeed, HPV-induced proliferation is an absolute requirement for completion of the viral life cycle. In addition, certain mucosal types, termed high risk, can promote transformation of a benign lesion into a malignant one (26). HPV16 is the high-risk type most frequently found in premalignant and malignant cervical lesions worldwide (2). Several studies have demonstrated that two viral early proteins, E6 and E7, play a key role in the induction of benign and malignant lesions (23) by associating with several cellular factors and altering their function (11, 13). The best-characterized property of HPV16 E6 is its binding to the p53 tumor suppressor, leading to degradation of the cellular protein via the ubiquitin pathway (20). The role of p53 is to safeguard the integrity of the genome by inducing cell cycle arrest or apoptosis in the presence of DNA damage. Therefore, its inactivation by the E6 protein leads to chromosomal instability and increases the probability of an HPV-infected cell evolving towards malignancy. Several studies have shown that HPV16 E6 is able to associate with other cellular proteins (11), indicating its involvement in additional pathways. Experiments with transgenic mice have shown that E6 can induce epithelial hyperplasia (14, 19). It has recently been reported that E6 protein from nononcogenic and oncogenic HPV types alters the regulation of the G1/S restriction point and induces S-phase progression in immortalized rodent cells (NIH 3T3) in the presence of antiproliferative stimuli such as high levels of the cyclin-dependent kinase (CDK) inhibitors p16INK4a and p27KIP1 (10). This E6 activity appears to be associated with pRb phosphorylation, since HPV16 E6 did not promote G1/S transition in the presence of high levels of a pRb mutant lacking all phosphorylation sites (11). However, the precise mechanism underlying this activity remains to be characterized.
In this study, we have further investigated the effect of HPV16 E6 and HPV1 E6 in altering cell cycle control in human primary oral fibroblasts (POFs) in which all cell cycle regulatory pathways are unaltered. We show that in these cells, HPV1 and HPV16 E6 proteins induce cellular proliferation, pRb phosphorylation, and accumulation of products of genes that are negatively regulated by pRb, such as p16INK4a, CDC2, E2F-1, and cyclin A. Consistent with the hyperphosphorylated state of pRb, cyclin A/CDK2 activity is highly elevated in cells expressing either of the two E6 proteins. We also show that the E6 proteins induce strong down-regulation of the p21WAF1/CIP1 gene. Overexpression of p21WAF1/CIP1 decreases the E6-induced proliferation, indicating that down-regulation of the endogenous p21WAF1/CIP1 gene observed in E6-expressing cells is a key mechanism for cell cycle deregulation. Interestingly, all these events appear to be independent of p53 inactivation.
MATERIALS AND METHODS
Retroviral expression vectors.
The E6 genes were cloned in retroviral vectors pBabe-puro or pBabe-neo (12). The H-rasV12 pBabe-puro construct has been previously described (1), while the pBabe-puro/p16INK4a construct was kindly provided by Bruno Amati (European Institute of Oncology, Milan, Italy).
Cell culture and retroviral infections.
NIH 3T3 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% calf serum. Phoenix cells and human POFs were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. POFs were purified from oral mucosa as previously described (21, 22). High-titer retroviral supernatants were generated by transient transfection of Phoenix cells (amphotropic viruses) (16) and used to infect POFs as previously described (1). After infection, POFs were selected with 0.5 mg of G418 (for 5 to 6 days) or 0.5 μg of puromycin (for 4 to 5 days). Coexpression of the viral genes and p16INK4a or ras was achieved by consecutive retroviral infections (5, 18).
Population doublings.
Cells were cultured in flasks (7.5 × 10 cm) and trypsinized when they reached approximately 80% confluence (3 × 106 to 4 × 106 cells/ml). Population doublings were calculated with the number of passages and the split ratio taken into consideration. P values of the slopes for the differences between the growth curves were determined by t test.
Cell extract preparation.
Total cellular extractions were performed by lysing the cells in lysis buffer (20 mM Tris-HCl [pH 8], 200 mM NaCl, 0.5% Nonidet P-40, 1 mM EDTA, 10 mM NaF, 0.1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin/ml, 1 μg of aprotinin/ml) for 20 min at 4°C. After centrifugation (12 000 × g, 5 min), the supernatant was collected and used for immunoblot analysis.
Determination of CDK-associated activities.
The activities of CDK4 and CDK6 were determined using a mixture of CDK4 and CDK6 monoclonal antibodies (kindly provided by Michele Pagano, New York University Cancer Institute, New York, N.Y.). The complexes were immunoprecipitated from 1 mg of cellular extracts. After immunoprecipitation, the kinase activity was measured according to a previously described method (6) with bacterial glutathione S-transferase-pRb fusion protein as the substrate. For determination of cyclin A-associated activity, the cyclin A/CDK2 complex was immunoprecipitated from 1 mg of various cellular extracts. The kinase activity was determined as described previously (10), using histone H1 as the substrate.
Immunoblot analysis and antibodies.
Cell extracts were fractionated by electrophoresis on a sodium dodecyl sulfate (SDS)-polyacrylamide gel. Proteins were transferred onto a Polyscreen polyvinylidene difluoride (PVDF) membrane (NEN Life Sciences) in a Trans-Blot semidry electrophoretic transfer cell (Bio-Rad) (130 mA, 1 h 30 min).
Immunoblot analyses were performed using the following antibodies: anti-β-tubulin (TUB2.1; dilution, 1:1,000; Sigma), anti-p53 (dilution, 1:1,000; DAKO), anti-p16INK4a (DCS-50; dilution, 1:250; NovoCastra), anti-pRb (554136; dilution, 1:1,000; Pharmingen), anti-E2F-1 (C-20; dilution, 1:1,000; Santa Cruz Biotechnology), anti-cyclin A (H-432; dilution, 1:1,000; Santa Cruz Biotechnology), anti-CDC2 (17; dilution, 1:1,000; Santa Cruz Biotechnology), and anti-p21WAF1/CIP1 (AB-1; dilution, 1:1,000; Calbiochem).
Immunofluorescence.
Immunofluorescence staining was performed as previously described (24), with primary antibodies anti-Ki67 rabbit (dilution, 1:100; DAKO) and anti-p16INK4a mouse (F-12; dilution, 1:100; Santa Cruz Biotechnology).
Northern blotting.
Total RNA was prepared using TRIZOL reagent (Life Technologies) by following the manufacturer's instructions and loaded onto a formaldehyde agarose gel, blotted on a nylon membrane (Hybond-N; Amersham Pharmacia Biosciences), and incubated with a 32P-labeled p21 DNA gene fragment. After being washed, the membrane was exposed to Kodak film for 12 h at −80°C.
Reverse transcription-PCR analysis.
Total RNA was extracted from mammalian cells with the RNeasy kit (Stratagene), and cDNA was synthesized with a First Strand cDNA synthesis kit (MBI Fermentas) by use of 1 μg of total RNA, a random hexamer primer, and Moloney murine leukemia virus reverse transcriptase. The following primers were used in the PCR to determine the expression of HPV16 E6, HPV1 E6, and Gap-DH genes: 5′-GTT TCA GGA CCC ACA GGA GC-3′ and 5′-GCA TAA ATC CCG AAA AGC AAA G-3′, which amplify a 151-bp fragment of the HPV16 E6 gene; 5′-AAT TCA TGG CGA CAC CAA-3′ and 5′-TTT GCA ATA AAG GTC TGT-3′, which amplify a 290-bp fragment of the HPV1 E6 gene; and 5′-AAG GTG GTG AAG CAG GCG T-3′ and 5′-GAG GAG TGG GTG TCG CTG TT-3′, which amplify a 110-bp fragment of the Gap-DH gene.
BrdU incorporation.
Cells were grown on cover slides under appropriate conditions and labeled with 5′-bromo-2-deoxyuridine (BrdU) for the desired time. Cells were fixed in 70% ethanol, and BrdU incorporation was determined with an in situ cell proliferation kit (Roche).
DNA microarrays.
Briefly, total RNA was extracted from the two cell cultures, i.e., the reference culture containing the empty vector and the test culture expressing HPV16 E6, by use of TRIZOL reagent (Life Technologies) and by following the manufacturer's protocol. The DNA microarray procedure was performed as previously described (3). Briefly, cDNA was synthesized in the presence of fluorescent nucleotides (Cye3 for the reference and Cye5 for the test) and simultaneously hybridized to the glass chip. Finally, the array was scanned, and the image was analyzed.
RESULTS
HPV1 E6 and HPV16 E6 induce proliferation of primary fibroblasts.
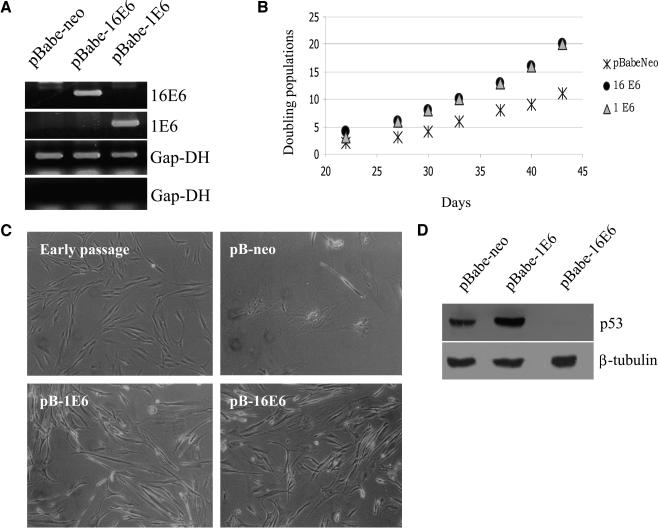
It has previously been shown that HPV16 E6 is able to promote G1/S transition in immortalized rodent fibroblasts (10). To analyze this activity of E6 in the context of intact cell cycle regulatory pathways, we determined the effect of E6 expression in human POFs. These cells can survive in culture for several passages, but their growth capacity decreases after approximately 1 month. POFs were infected with recombinant retroviruses expressing E6 from the high-risk HPV16 or the benign-type HPV1. The expression of the viral gene in the different cultures is shown in Fig. 1A. After neomycin selection, the different cell populations showed dissimilar growth behaviors (Fig. 1B). Cells expressing either HPV1 E6 or HPV16 E6 proliferated faster than control cells containing the empty vector. Microphotographs (Fig. 1C) reveal that the morphologies of these cells were also very different. HPV1 E6- and HPV16 E6-expressing cells appeared smaller and more refractive than the control cells containing the empty vector. Cells expressing HPV1 E6 behaved similarly to those expressing high-risk HPV16 E6. HPV1 E6 does not induce degradation of the tumor suppressor p53 (Fig. 1D) (4). Thus, the E6-induced proliferation in primary human cells is not a consequence of p53 degradation.
FIG. 1.
HPV16 E6 and HPV1 E6 induce proliferation of POFs. (A) Determination of mRNA levels of HPV16 E6 and HPV1 E6 by reverse transcription-PCR. Total RNA was extracted from cells, and cDNA was generated. The cDNA obtained from all the cells infected with different retroviruses, including the empty vector retroviruses, was probed with all the different primers. As an internal control, the Gap-DH cDNA was also amplified (third panel). cDNA produced with the omission of the reverse transcriptase was probed with Gap-DH primers to confirm the absence of genomic DNA contamination (fourth panel). The products were separated on 2% agarose gels and visualized by staining with ethidium bromide. (B) Growth profiles of POFs expressing the viral proteins. After selection, the growth of the different cell populations was monitored for more than 40 days. Differences between the growth curves are statistically significant (pBabe-neo versus HPV16 E6, P = 0.001; pBabe-neo versus HPV1 E6, P < 0.0010). (C) Morphologies of POFs expressing HPV16 E6 or HPV1 E6. The different cell populations were photographed at day 40 after selection. Original magnification, ×10 (all photographs). A photograph of early-passage POFs (approximately three doubling times) is included for comparison. (D) Determination of p53 levels in POFs expressing HPV16 E6 or HPV1 E6. Total protein extracts (100 μg) from cell populations expressing the different viral proteins indicated were applied to an SDS-10% polyacrylamide gel, transferred onto a PVDF membrane, and incubated with an anti-p53 or β-tubulin antibody. The β-tubulin signal was used as a loading control.
HPV16 E6 or HPV1 E6 expression in primary fibroblasts leads to accumulation of p16INK4a.
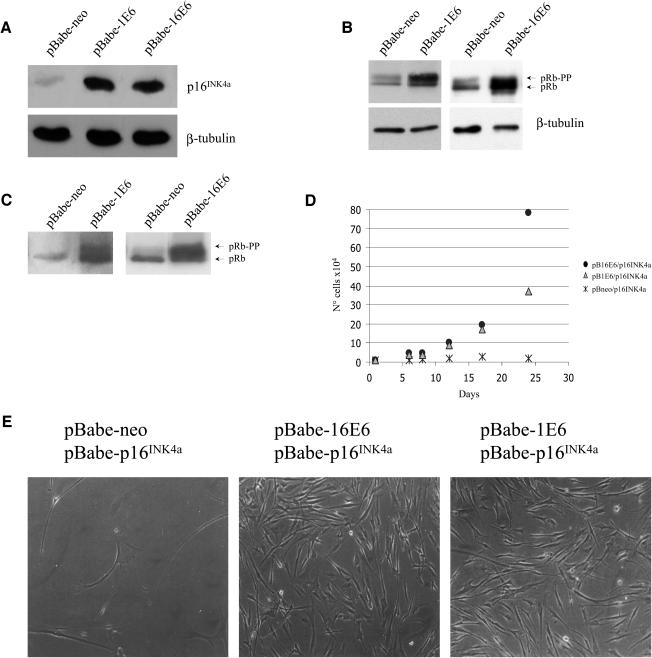
Inactivation of pRb leads to deregulation of the G1/S transition, induction of proliferation, and accumulation of the CDK inhibitor p16INK4a (7). The increase in p16INK4a levels is part of a safety mechanism which is activated in an attempt to arrest abnormal proliferation. We observed that in highly proliferative E6-expressing cells, the levels of p16INK4a were higher than in the control culture (Fig. 2A), indicating that the pRb pathway is altered in these cells. In fact, cells infected with HPV1 or HPV16 E6 recombinant retroviruses have higher levels of both forms of pRb (hyperphosphorylated and hypophosphorylated) than cells infected with empty retroviruses (Fig. 2B). Interestingly, both E6 proteins promoted pRb phosphorylation even in the presence of high exogenous levels of p16INK4a (Fig. 2C). In agreement with the phosphorylation state of pRb, cells overexpressing E6 and p16INK4a proliferate at a higher rate than control cells (p16INK4a only) (Fig. 2D). However, after several passages, HPV1 E6 appeared to be less efficient than HPV16 E6 in blocking the inhibitory functions of p16INK4a (Fig. 2D and data not shown). The morphologies of the different cell populations are shown in Fig. 2E. Cells expressing only p16INK4a were very few and became flat and enlarged, characteristics of arrested cells. In contrast, cells coexpressing HPV16 E6 or HPV1 E6 with p16INK4a were small and refractive and had a much higher density.
FIG. 2.
pRb is hyperphosphorylated in the presence of high levels of p16INK4a in E6-expressing cells. (A) Determination of p16INK4a levels in POFs expressing HPV16 E6 or HPV1 E6. Total protein extracts (100 μg) of cells expressing the different viral proteins as indicated were applied to an SDS-15% polyacrylamide gel, transferred onto a PVDF membrane, and incubated with a p16INK4a or β-tubulin antibody. The β-tubulin signal was used as a loading control. (B) Phosphorylation state of pRb in E6-expressing cells. Total protein extracts (100 μg) of the different cells indicated were loaded onto an SDS-6% polyacrylamide gel. To obtain separation of the different pRb phosphorylated forms, electrophoresis was performed until the 75-kDa marker reached the bottom of the gel. Proteins were transferred onto a PVDF membrane and incubated with pRb antibody. As a loading control, protein extracts of the same amount (100 μg) were loaded on an SDS-12% polyacrylamide gel, processed as described for panel A, and incubated with β-tubulin antibody. (C) Overexpression of p16INK4a does not prevent pRb phosphorylation in E6-expressing cells. POFs were doubly infected with recombinant retroviruses as described previously (10), and Western blot analysis was performed as described for panel B. (D) Growth profile of POFs coexpressing HPV1 or HPV16 E6 and p16INK4a. The growth of the different cell populations was monitored for about 1 month. Day 0 represents the first day after completion of antibiotic selection. Differences between the growth curves are statistically significant (pBabe-neo versus HPV16 E6, P = 0.022; pBabe-neo versus HPV1 E6, P < 0.078). (E) Microphotographs (magnifications, ×10) of the different cell populations indicated were taken 25 days after selection.
Together, these data show that E6 promotes cellular proliferation and pRb phosphorylation in the presence of high levels of the CDK inhibitor p16INK4a.
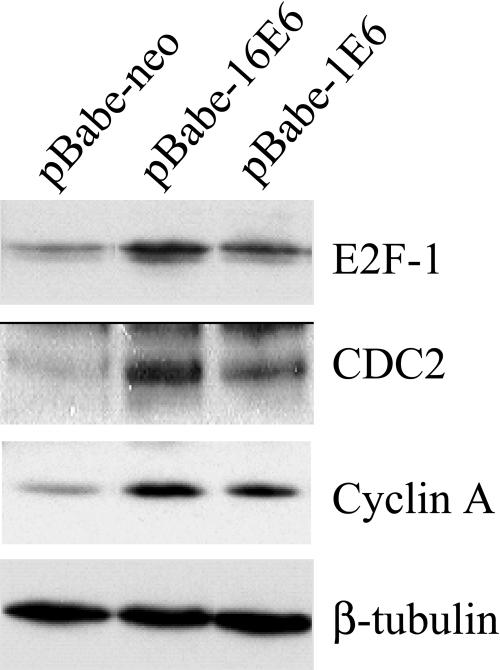
Products of genes that are negatively regulated by pRb are up-regulated in E6-expressing cells.
In quiescent cells, the hypophosphorylated form of pRb associates with the transcription factor E2F, blocking its activity. Hyperphosphorylation of pRb results in the disruption of the complex and the release of active E2F that in turn promotes the expression of genes involved in S-phase progression. To further confirm that the inhibitor functions of pRb are lost in E6-expressing cells, we determined by immunoblotting the levels of several gene products negatively regulated by pRb. As shown in Fig. 3, the levels of CDC2, cyclin A, and E2F-1 were elevated in POFs infected with the HPV1 E6 or HPV16 E6 recombinant retroviruses. The difference in levels of these cellular proteins in control and E6-expressing cells was even more evident in serum-deprived medium (data not shown). These data are in agreement with previous findings, which showed that E6 activates the cyclin A promoter in serum-deprived NIH 3T3 cells (10). In summary, these findings provide further evidence for functional inactivation of the pRb pathway in E6-expressing cells.
FIG. 3.
Levels of proteins encoded by E2F-regulated genes are elevated in E6-expressing cells. Total protein extracts (100 μg) from cell populations expressing the different viral proteins indicated were applied to an SDS-10% polyacrylamide gel, transferred onto a PVDF membrane, and incubated with an anti-E2F-1, -CDC2, -cyclin A, or -β-tubulin antibody. The β-tubulin signal was used as a loading control.
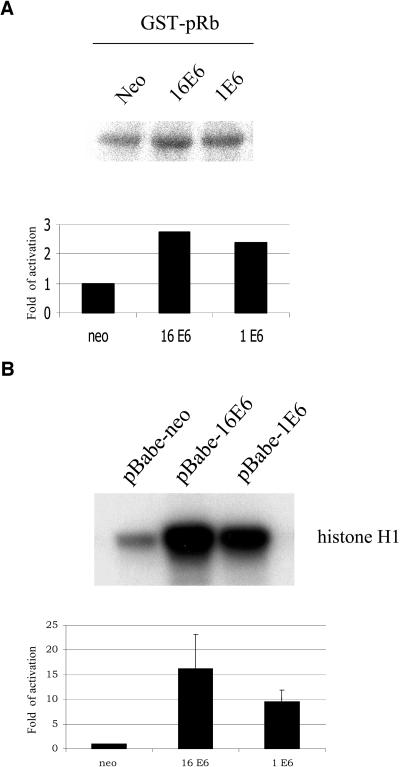
CDK-associated activities are elevated in E6-expressing cells.
Phosphorylation of pRb is initiated in G1 phase by active CDK4 and CDK6 associated with the D cyclins (1, 2, and 3) and is continued in the subsequent phases of the cell cycle by additional CDK complexes, such as cyclin A/CDK2. Therefore, we next checked whether the increase in pRb phosphorylation observed in E6-expressing cells corresponds to an activation of CDK complexes. We initially determined the activity of cyclin D/CDK4 and cyclin D/CDK6 complexes. For this purpose, we immunoprecipitated the CDK complexes by use of a mixture of CDK4- and CDK6-specific antibodies and determined their kinase activities by using bacterial recombinant pRb as the substrate. We observed in independent experiments that the cyclin D/CDK4 and cyclin D/CDK6 complexes were weakly activated in cells expressing HPV1 E6 and HPV16 E6 in comparison to activation in the control cells. A representative experiment is shown in Fig. 4A. Next, we immunoprecipitated the cyclin A/CDK2 complex by use of a cyclin A antibody and determined its activity by using histone H1 as the substrate. As shown in Fig. 4B, high levels of cyclin A/CDK2 activity were detected in POFs expressing the E6 proteins. Similar results were obtained by immunoprecipitation of the kinase complex with a CDK2 antibody (data not shown). Thus, the hyperphosphorylation of pRb in E6-expressing cells correlates with activation of the CDK complexes that are directly involved in this event.
FIG. 4.
Activities of CDK complexes are higher in E6-expressing cells than control cells. Cyclin D/CDK4 or cyclin D/CDK6 (A) and cyclin A/CDK2 (B) complexes were immunoprecipitated, and kinase activities were determined as described in Materials and Methods. Autoradiographs of the CDK reactions are shown in the upper panels. The intensities of the bands were determined by Phosphorimager scanning (ImageQuant software; Molecular Dynamics). The glutathione S-transferase (GST)-pRb-kinase activities and the histone H1-kinase activities in E6-expressing cells were normalized to the activities in control cells. The data shown in panel B are the averages of the results of two independent experiments.
HPV1 and HPV16 E6 down-regulate the p21WAF1/CIP1 gene.
Several lines of evidence indicate that the E6 protein interferes with the cellular transcription machinery. For instance, HPV16 E6 associates with the transcriptional coactivator p300/CBP, repressing its activity (15, 25). Therefore, it is possible that the activity of E6 in cell cycle deregulation is mediated by alteration in the expression of specific cellular genes. To evaluate this hypothesis, we compared the gene expression profiles of POFs expressing or not expressing HPV16 E6 by use of a cDNA microarray. We used a chip in which 13,348 human genes were spotted at least two times (F. Diehl and J. Hoheisel, unpublished data). Analysis of the data revealed several genes to be down- or up-regulated in HPV16 E6-expressing cells (I. Malanchi et al., unpublished data). Interestingly, two genes involved in cell cycle regulation, the cyclin D2 and p21WAF1/CIP1 genes, were found to be differentially expressed.
The cyclin D2 gene was weakly up-regulated (approximately 1.5- to 2-fold). However, we did not observe any increase in levels of cyclin D2 mRNA or protein by Northern or Western blot analyses, respectively (data not shown).
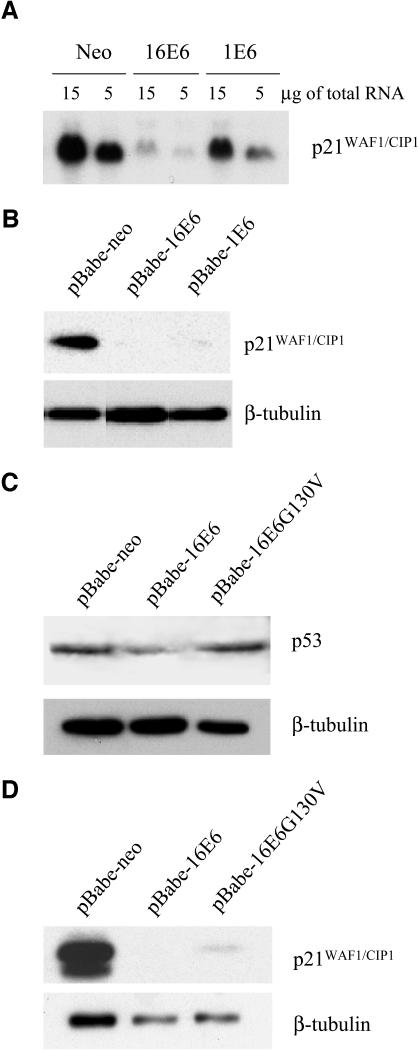
The p21WAF1/CIP1 gene was found to be strongly down-regulated in HPV16 E6-expressing cells. The p21WAF1/CIP1 gene is positively regulated by p53, and thus its down-regulation may be due to an indirect effect of E6 via inactivation of p53. Northern and Western blot analyses showed that HPV1 E6 also down-regulates p21WAF1/CIP1, although less efficiently than HPV16 E6 does (Fig. 5A and B). Since HPV1 E6 is not able to promote p53 degradation (Fig. 1D) (4), this activity of E6 appears to be partly independent of the p53 pathway. To corroborate these data, we analyzed the ability of an HPV16 E6 mutant (G130V) which is not able to target p53 (9) (Fig. 5C) to induce p21WAF1/CIP1 down-regulation. Figure 5D shows that this HPV16 E6 mutant induced decreases in p21WAF1/CIP1 levels, further supporting the existence of a p53-independent mechanism of down-regulation of p21WAF1/CIP1 by E6.
FIG. 5.
The p21WAF1/CIP1 gene is down-regulated in E6-expressing cells. (A) Analysis of p21WAF1/CIP1 mRNA levels by Northern blotting. Total RNA was prepared as described in Materials and Methods. Five- and 15-μg amounts of total RNA from each cell population were loaded onto a formaldehyde agarose gel, blotted onto a nylon membrane, and incubated with a 32P-labeled p21WAPI/CIPI DNA gene fragment. After being washed, the membrane was exposed to Kodak film for 12 h at −80°C. (B) p21WAF1/CIP1 levels are lower in E6-expressing cells than in the control cells. Total protein extracts (50 μg) from cell populations infected with the different recombinant retroviruses indicated were applied to an SDS-10% polyacrylamide gel, transferred onto a PVDF membrane, and incubated with an anti-p21WAF1/CIP1 or -β-tubulin antibody. (C and D) An HPV16 E6 mutant unable to target p53 efficiently decreases p21WAF1/CIP1 levels. Total protein extracts (50 μg) from the cell populations infected with the different recombinant retroviruses indicated were applied to an SDS-10% polyacrylamide gel, and Western blot analysis was performed as described for panel B with an anti-p21WAF1/CIP1, -p53, or -β-tubulin antibody.
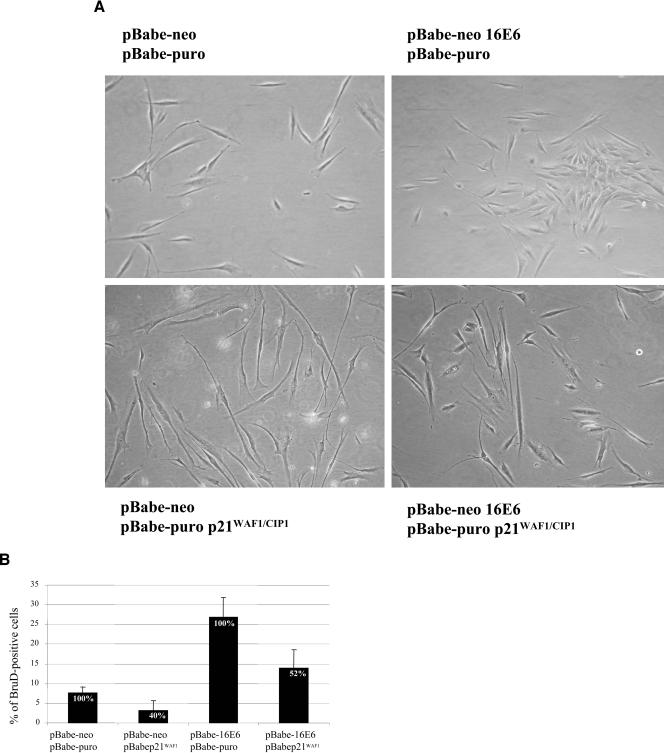
Together, these data indicate that the induction of proliferation by the E6 proteins is in part due to the down-regulation of p21WAF1/CIP1 gene expression. To further confirm our findings, we determined whether exogenous levels of p21WAF1/CIP1 lead to a reduction of the E6-induced cellular proliferation. POFs were doubly infected with recombinant retroviruses as indicated in Fig. 6. Analysis of the morphologies of the different cultures showed that cells expressing p21WAF1/CIP1 alone or together with HPV16 E6 have elongated shapes (Fig. 6A, bottom panels), a sign of low-rate proliferation. However, this phenomenon appears to be more evident in POFs infected with p21WAF1/CIP1 alone than in cells coexpressing HPV16 E6 and the cell cycle inhibitor (Fig. 6A, bottom panels). In agreement with their morphology, cells containing high exogenous levels of p21WAF1/CIP1 have a reduced proliferation in comparison to that of control cells infected with HPV16 E6 retroviruses (Fig. 6B).
FIG. 6.
High exogenous levels of p21WAF1/CIP1 lead to a reduction of proliferation in HPV16 E6-expressing cells. (A) Microphotographs (magnifications, ×6.5) of the different cell populations indicated were taken 10 days after selection. (B) Percentages of S-phase cells in different cultures. BrdU incorporation was performed as described in Materials and Methods. The percentages of BrdU-positive cells were determined for five to six randomly selected fields containing approximately 300 to 400 DAPI (4′,6′-diamidino-2-phenylindole)-stained cells. The standard deviations of the percentages of BrdU-positive cells in the different fields are indicated by error bars.
E6 of HPV1 and HPV16 cooperates with ras to induce abnormal proliferation in primary fibroblasts.
To further characterize the actions of E6 in cell cycle deregulation, we examined whether the viral protein was able to circumvent cell cycle arrest imposed by various means.
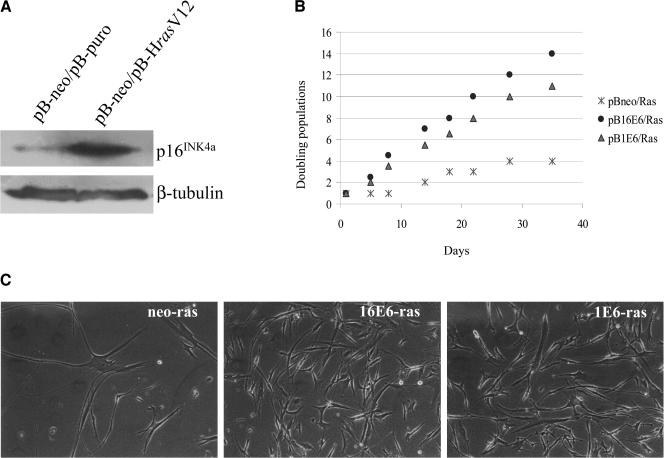
Expression of oncogenic ras (H-rasV12) alone in primary cells induces increases in p16INK4a levels, resulting in cell cycle arrest in G1 (17). In agreement with previous data, expression of oncogenic ras in POFs led to an accumulation of p16INK4a (Fig. 7A). Next, we determined whether HPV1 or HPV16 E6 could reverse the ras-induced cell cycle arrest. As shown in Fig. 7B, growth of cells coexpressing the HPV16 E6 or HPV1 E6 gene and ras followed an exponential growth curve, while control cells expressing ras alone underwent cell growth arrest. As observed for cells with ectopic levels of p16INK4a (Fig. 2D), HPV1 E6 was less efficient than HPV16 E6 in overcoming the ras-induced cell cycle arrest. In addition, the different cells presented different morphologies (Fig. 7C); ras-expressing cells became enlarged, reflecting a phenotype typical of a nonproliferative condition, while cells coexpressing HPV16 or HPV1 E6 and ras retained a morphology characteristic of proliferative cells (i.e., were small and highly refractive). The higher proliferation of the E6-containing cells corresponds to an increase of cells in S phase. Indeed, in a BrdU incorporation assay, 13% of cells in the culture expressing HPV16 E6 and ras together entered S phase, versus 1.5% of the cells in the culture expressing ras alone.
FIG. 7.
Coexpression of HPV16 E6 or HPV1 E6 and oncogenic ras in primary human fibroblasts. (A) Determination of p16INK4a levels in POFs coexpressing HPV16 E6 or HPV1 E6 and oncogenic ras. Total protein extracts (200 μg) from cells expressing ras or containing only the empty vector (as indicated) were loaded onto an SDS-15% polyacrylamide gel, transferred onto a PVDF membrane, and incubated with p16INK4a (DCS-50; dilution, 1:250; NovoCastra) or β-tubulin (TUB2.1; dilution, 1:1,000; Sigma) antibody. (B) Growth profiles of POFs coexpressing the viral proteins and oncogenic ras. The growth of the different cell populations was monitored for more than 1 month. Day 0 represents the first day after the completion of antibiotic selection. Differences between the growth curves are statistically significant (pBabe-neo versus HPV16 E6, P < 0.001; pBabe-neo versus HPV1 E6, P < 0.0010). (C) Morphologies of POFs expressing the different viral proteins with oncogenic ras. The different cell populations were photographed on day 30 after selection. Magnifications, ×10.
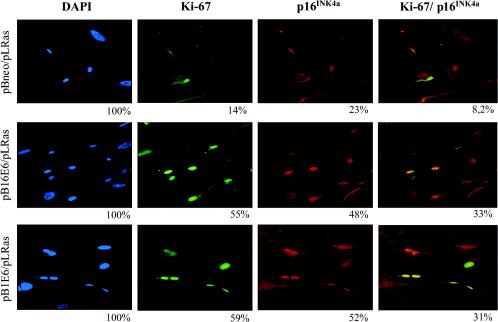
To further confirm the alteration of cell cycle regulation in E6-expressing cells, we performed an immunofluorescence assay for p16INK4a and for the proliferative marker Ki67. Higher percentages of cells staining positive for both p16INK4a and Ki67 were observed in cultures coexpressing HPV 16 or HPV1 E6 and oncogenic ras in comparison with the culture expressing ras alone (Fig. 8). Thus, the coexpression in the same cells of negative and positive proliferative markers represents a direct proof of E6-induced proliferation activity in the presence of high levels of p16INK4a.
FIG. 8.
Immunohistochemistry of POFs coexpressing the viral proteins and oncogenic ras. Cells were grown on cover slides, washed in phosphate-buffered saline, fixed in 70% ethanol, and incubated with anti-Ki67 rabbit (dilution, 1:100; DAKO) and anti-p16INK4a mouse (F-12; dilution, 1:100; Santa Cruz Biotechnology) antibodies. At the end of the procedure, an incubation of 10 min with DAPI solution in phosphate-buffered saline was performed. Photomicrographs were taken with a fluorescence microscope at a magnification of ×40. The percentages of cells positive for p16INK4a or Ki67 and with the overlapping staining were calculated, considering the DAPI-stained cells as the total number of cells on the coverslips.
Together, the data show that E6 from HPV1 and HPV16 efficiently overcomes the cell cycle arrest imposed by ras and that this activity is independent of p53 inactivation, since HPV1 E6 is unable to induce p53 degradation.
DISCUSSION
In this study, we show that E6 from both nononcogenic HPV1 and oncogenic HPV16 has the ability to alter regulation of the cell cycle in primary human cells. Both viral proteins stimulate proliferation when expressed in POFs. Interestingly, the endogenous levels of p16INK4a were higher in cells expressing HPV E6s than in the control cells. Several studies have demonstrated that the accumulation of p16INK4a in proliferating cells corresponds to a functional loss of pRb. This phenomenon can be explained by the fact that the p16INK4a gene is transcriptionally repressed by pRb (7, 8). Cells harboring pRb mutations or overexpressing the HPV16 E7 oncoprotein, which promotes pRb degradation, have high levels of p16INK4a in order to counteract the loss of cell cycle control (7). Thus, the accumulation of p16INK4a in E6-expressing cells indicates a loss of functionality of the pRb pathway. We have observed that pRb is hyperphosphorylated in cells containing HPV1 or HPV16 E6, even in the presence of ectopic levels of p16INK4a. Accordingly, the activities of the kinases involved in pRb phosphorylation (cyclin D/CDK4, cyclin D/CDK6, and cyclin A/CDK2 complexes) are elevated in E6-expressing cells. Taking into consideration all these data, it is highly likely that E6 proteins induce proliferation by promoting activation of the CDK complexes and consequently of pRb hyperphosphorylation. In agreement with this model, it has previously been shown that E6 is not able to overcome a G1 arrest imposed by the overexpression of a pRb mutant in which all CDK phosphorylation sites were mutated (10). The inactivation of pRb functions in E6 cells is also supported by the elevated levels of cellular proteins that are encoded by E2F-regulated genes, such as E2F-1, cyclin A, and CDC2.
Expression of oncogenic ras in primary cells induces cell cycle arrest and premature senescence as a consequence of the accumulation of cell cycle inhibitors such as p53, p16INK4a, and p21WAF1/CIP1 (17). We have shown here that both HPV1 E6 and HPV16 E6 can counteract the negative effect of ras on cell proliferation. Since HPV1 E6 is not able to promote p53 degradation, we conclude that the reversal of ras-induced G1 arrest is independent of p53 inactivation. To further confirm the activity of E6 in cell cycle deregulation, we used immunofluorescence staining of cells coexpressing ras and HPV16 E6 or HPV1 E6 to show that antiproliferative (p16INK4a) and proliferative (Ki67) markers were coexpressed in the same cells. In contrast, the presence of Ki67 and p16INK4a was mutually exclusive in normal cells.
Together, these data demonstrate that E6 is able to promote cell cycle progression in the presence of high levels of p16INK4a. The E6-induced neutralization of p16INK4a functions may be explained by two different mechanisms. It is possible that E6 protein directly inactivates p16INK4a, by, for instance, changing its cellular localization. Alternatively, E6 may increase the levels of CDK complexes responsible for pRb phosphorylation. Our data support the second hypothesis. First, we observed that p16INK4a localization did not differ in cells with or without E6. Second, we observed that CDK2 activity is strongly stimulated in E6-expressing cells. This event correlates with increased levels of the positive regulatory subunit cyclin A and down-regulation of the CDK2 inhibitor p21WAF1/CIP1. Overexpression of p21WAF1/CIP1 by use of a heterologous promoter decreases the E6-induced proliferation, showing that down-regulation of this cell cycle inhibitor is a key mechanism for the G1/S transition promoted by the viral protein.
In conclusion, our data show that, like the E7 protein, E6 is able to alter the regulation of the restriction point in the G1 phase of the cell cycle. However, the two viral proteins act by different mechanisms.
Acknowledgments
We are grateful to Marian Mansour and the other members of our laboratory for their cooperation, Konstantinos Alevizopoulos and Bruno Amati for the pBabe-puro/p16INK4a construct, Michele Pagano for the CDK4 and CDK6 antibodies, Giorgia Randi for statistical analysis, and John Cheney and Leonie Ringrose for critical readings of the manuscript.
REFERENCES
- 1.Caldeira, S., I. Zehbe, R. Accardi, I. Malanchi, W. Dong, M. Giarrè, E.-M. de Villiers, R. Filotico, P. Boukamp, and M. Tommasino. 2003. The E6 and E7 proteins of the cutaneous human papillomavirus type 38 display transforming properties. J. Virol. 77:2195-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clifford, G. M., J. S. Smith, M. Plummer, N. Munoz, and S. Franceschi. 2003. Hum. papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br. J. Cancer 88:63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diehl, F., B. Beckmann, N. Kellner, N. C. Hauser, S. Diehl, and J. D. Hoheisel. 2002. Manufacturing DNA microarrays from unpurified PCR products. Nucleic Acids Res. 30:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elbel, M., S. Carl, S. Spaderna, and T. Iftner. 1997. A comparative analysis of the interactions of the E6 proteins from cutaneous and genital papillomaviruses with p53 and E6AP in correlation to their transforming potential. Virology 239:132-149. [DOI] [PubMed] [Google Scholar]
- 5.Giarrè, M., S. Caldeira, I. Malanchi, F. Ciccolini, M. J. Leão, and M. Tommasino. 2001. Induction of pRb degradation by the human papillomavirus type 16 E7 protein is essential to efficiently overcome p16INK4a-imposed G1 cell cycle arrest. J. Virol. 75:4705-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kastner, A., E. Moyse, S. Bauer, F. Jourdan, and G. Brun. 2000. Unusual regulation of cyclin D1 and cyclin-dependent kinases cdk2 and cdk4 during in vivo mitotic stimulation of olfactory neuron progenitors in adult mouse. J. Neurochem. 74:2343-2349. [DOI] [PubMed] [Google Scholar]
- 7.Khleif, S. N., J. DeGregori, C. L. Yee, G. A. Otterson, F. J. Kaye, J. R. Nevins, and P. M. Howley. 1996. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc. Natl. Acad. Sci. USA 93:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li, Y., M. A. Nichols, J. W. Shay, and Y. Xiong. 1994. Transcriptional repression of the D-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Cancer Res. 54:6078-6082. [PubMed] [Google Scholar]
- 9.Liu, Y., J. J. Chen, Q. Gao, S. Dalal, Y. Hong, C. P. Mansur, V. Band, and E. J. Androphy. 1999. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. J. Virol. 73:7297-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malanchi, I., S. Caldeira, M. Krützfeldt, M. Giarre, M. Alunni-Fabbroni, and M. Tommasino. 2002. Identification of a novel activity of human papillomavirus type 16 E6 protein in deregulating the G1/S transition. Oncogene 21:5665-5672. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani, F., and L. Banks. 2001. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene 20:7874-7887. [DOI] [PubMed] [Google Scholar]
- 12.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munger, K., J. R. Basile, S. Duensing, A. Eichten, S. L. Gonzalez, M. Grace, and V. L. Zacny. 2001. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene 20:7888-7898. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen, M., S. Song, A. Liem, E. Androphy, Y. Liu, and P. F. Lambert. 2002. A mutant of human papillomavirus type 16 E6 deficient in binding α-helix partners displays reduced oncogenic potential in vivo. J. Virol. 76:13039-13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel, D., S. M. Huang, L. A. Baglia, and D. J. McCance. 1999. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 18:5061-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrano, M., E. Gomez-Lahoz, R. DePinho, D. Beach, and D. Bar-Sagi. 1995. Inhibition of ras-induced proliferation and cellular transformation by p16INK4. Science 267:249-252. [DOI] [PubMed] [Google Scholar]
- 18.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 19.Song, S., H. C. Pitot, and P. F. Lambert. 1999. The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals. J. Virol. 73:5887-5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas, M., D. Pim, and L. Banks. 1999. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene 18:7690-7700. [DOI] [PubMed] [Google Scholar]
- 21.Tomakidi, P., D. Breitkreutz, N. E. Fusenig, J. Zoller, A. Kohl, and G. Komposch. 1998. Establishment of oral mucosa phenotype in vitro in correlation to epithelial anchorage. Cell Tissue Res. 292:355-366. [DOI] [PubMed] [Google Scholar]
- 22.Tomakidi, P., N. E. Fusenig, A. Kohl, and G. Komposch. 1997. Histomorphological and biochemical differentiation capacity in organotypic co-cultures of primary gingival cells. J. Periodontal Res. 32:388-400. [DOI] [PubMed] [Google Scholar]
- 23.Tommasino, M. 2001. Early genes of human papillomaviruses, p. 266-272. In M. Schwab (ed.), Encyclopedic reference of cancer. Springer-Verlag, Heidelberg, Germany.
- 24.Zehbe, I., A. Ratsch, M. Alunni-Fabbroni, A. Burzlaff, E. Bakos, M. Durst, E. Wilander, and M. Tommasino. 1999. Overriding of cyclin-dependent kinase inhibitors by high and low risk human papillomavirus types: evidence for an in vivo role in cervical lesions. Oncogene 18:2201-2211. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann, H., R. Degenkolbe, H.-U. Bernard, and M. J. O'Connor. 1999. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J. Virol. 73:6209-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.zur Hausen, H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2:342-350. [DOI] [PubMed] [Google Scholar]