Abstract
Echinorhynchus veli (George and Nadakal, 1978), an acanthocephalid worm infesting the estuarine flat fish, Synaptura orientalis, was collected from the Veli lake, Kerala. The parasite was recovered from the intestine of the host fish. The detailed surface morphology was studied with the help of scanning electron microscope. The study revealed a cylindrical, medially swollen proboscis with a flat apex, backward directed hooks, each with smooth surface, broad base, pointed tip and an epidermal elevation at the point of insertion. A pair of sensory pits was seen at the base of the proboscis. The neck was well developed with densely packed epidermal micropores. Paired sensory pits were seen at the base of the neck and a collar between it and the trunk. The epidermis of the trunk has microtriches and micropores. The female genital pore was circular, and terminal in an elevated orifice. In male, the copulatory bursa was directed ventrally, with well-defined rim and several sensory papillae.
Keywords: Acanthocephala, Echinorhynchus veli, Synaptura orientalis, Microtriches
Introduction
Cosmopolitan in distribution, acanthocephalid worms are intestinal parasites of many terrestrial and aquatic vertebrates. They have a complex life cycle involving vertebrates as definitive hosts and arthropods as intermediate hosts (Crompton and Nickol 1985). Adult worms cause nutrient depletion, tissue damage, cellular mobilization and tumour formation in hosts. In heavy infections they cause intestinal occlusion and emigrate to even unusual locations (Nickol 2006). The present acanthocephalan, Echinorhynchus veli (E. veli) infecting the flat fish, Synaptura orientalis was first reported by George and Nadakal (1978). Their light microscopic studies revealed sexual dimorphism, pseudosegmentation and division of the body into proboscis, neck and trunk (George and Nadakal 1978).
The preliminary studies revealed that proboscis was armed with 12–15 longitudinal rows of hooks, each row having 10–14 hooks. The posterior end of male had a copulatory bursa. Some of the worms had orange colour due to carotenoid pigments (George and Nadakal 1985). In the original description, not much has been focused on the surface details of this worm and hence the present work was undertaken.
Materials and methods
The worms were collected from the fish, Synaptura orientalis caught from Veli lake, Thiruvananthapuram, Kerala, India were transferred to physiological saline and fixed overnight in 3 % glutaraldehyde in 0.1 M phosphate buffer at room temperature. The samples were dehydrated in ascending series of ethanol. Drying was done with liquid CO2 in a critical point drier (HCP-02 Hitachi) and sputter coated with a gold–palladium complex in a gold ion-sputtering unit (E101, Hitachi). The coated specimens placed on a grid were examined in a scanning electron microscope (Hitachi-s-2400) at an accelerating voltage of 15 kV and appropriate photomicrograph were taken.
Results
The proboscis was cylindrical, medially swollen and apically flat (Fig. 1). The hooks, were arranged quinquintially and posteriorly directed. The hooks had a smooth surface. Each hook has a broad base with a prominent epidermal elevation at the point of insertion and pointed tip (Fig. 2). The flat apex was devoid of hooks. Paired sensory pits (SP), neck (N), and collar (C) were observed at the base of proboscis (Figs. 3, 4, 5). The neck was well developed and shorter than proboscis, with densely packed epidermal micropores. Paired sensory pits were seen on the posterior side of the proboscis (Fig. 3). The epidermis of the trunk had microtriches (MT) and epidermal micropores (EM) of different dimensions (Fig. 6). Female genital pore was terminal and circular in an elevated orifice (Fig. 7). Copulatory bursa in male had a well-defined rim and many papillae (Figs. 8, 9).
Fig. 1.
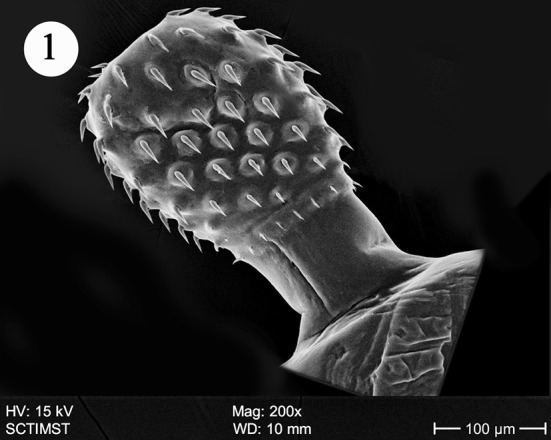
Scanning electron micrograph of Echinorhynchus veli showing proboscis
Fig. 2.

Scanning electron micrograph of Echinorhynchus veli showing the hook
Fig. 3.
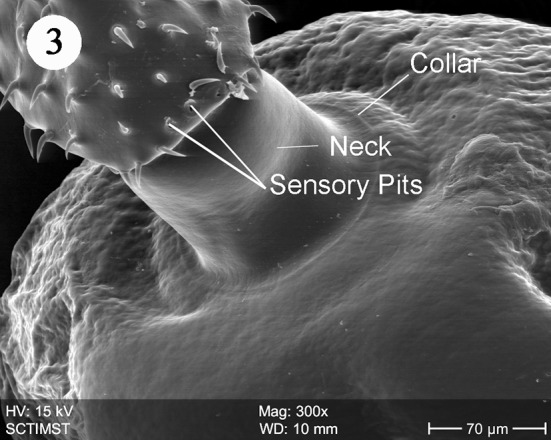
Scanning electron micrograph of Echinorhynchus veli showing sensory pits and collar
Fig. 4.
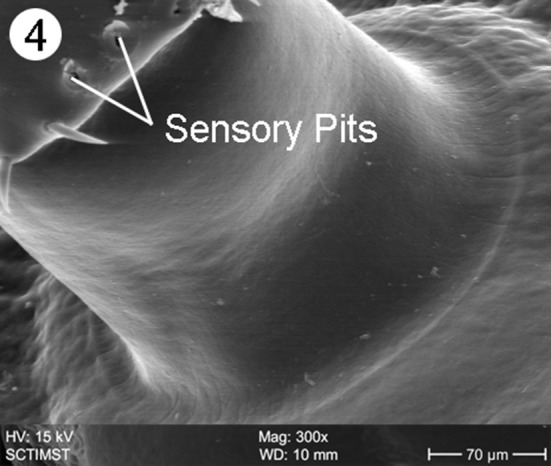
Closer view of sensory pits of Echinorhynchus veli
Fig. 5.
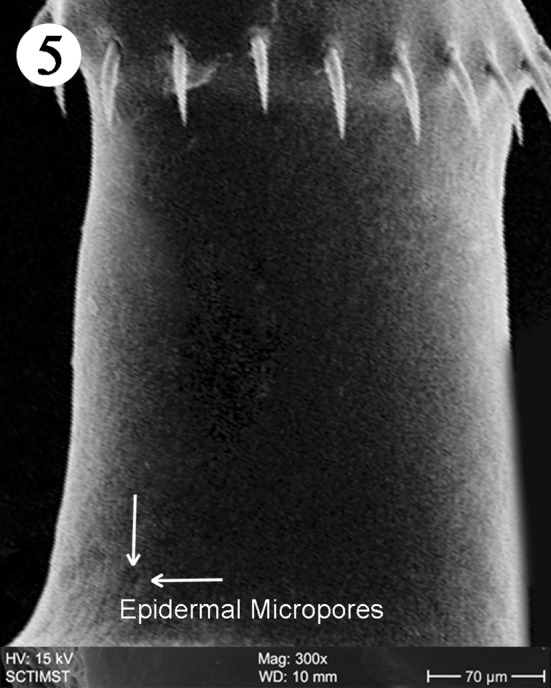
Scanning electron micrograph of Echinorhynchus veli showing neck region
Fig. 6.
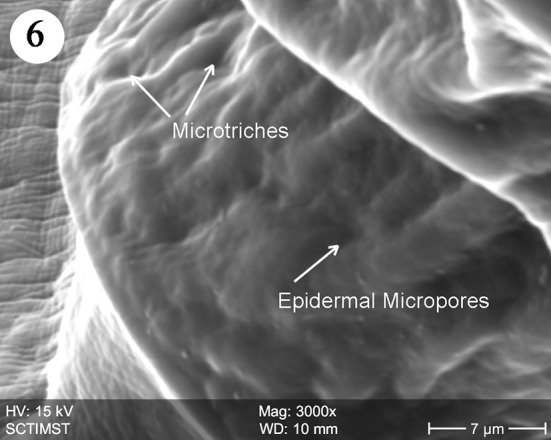
Scanning electron micrograph of trunk of Echinorhynchus veli showing microtriches
Fig. 7.
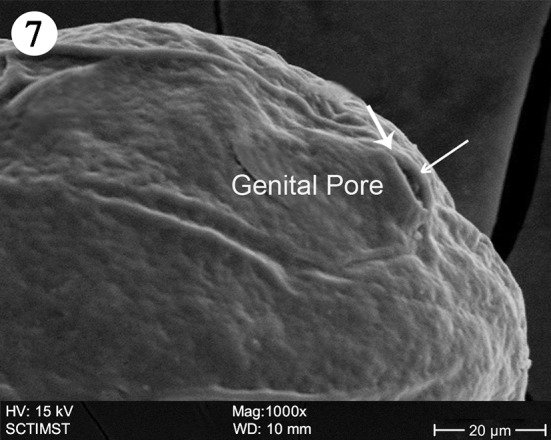
Scanning electron micrograph of Echinorhynchus veli showing female genital pore
Fig. 8.
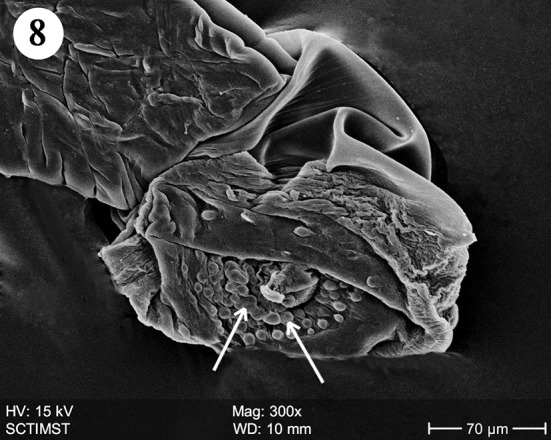
Scanning electron micrograph of male Echinorhynchus veli showing copulatory bursa
Fig. 9.
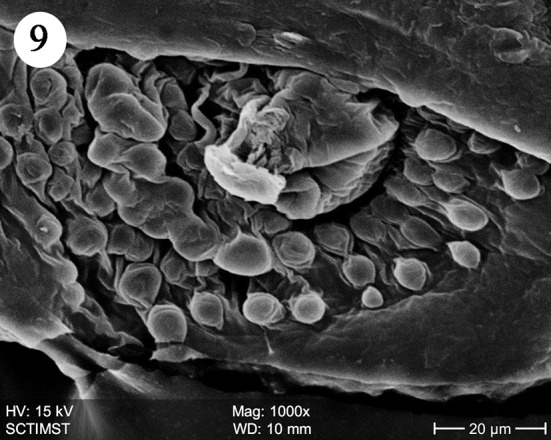
Scanning electron micrograph of male Echinorhynchus veli showing copulatory bursa (ventral bordering view)
Discussion
The present scanning electron microscope (SEM) study on E. veli revealed surface details of the proboscis, neck, epidermal micropores and microtriches, female genital opening and special features of male copulatory bursa. The surface features of proboscis in E. veli are almost similar in certain aspects, to those in Acanthocephalus lucii, except the hooks (Amin et al. 2011) in contrast to a short, subcylindrical and almost globular proboscis in Neoacanthocephalus (Alava and Aguirre, 2005). In A. lucii hooks are uniformly slender throughout the length of proboscis (Amin et al. 2011). But in E. veli, the hooks are progressively decreased in size posteriorly. Again, the epidermal elevations at the base of hooks are absent in A. lucii. In Tenuiproboscis sp (Sanil et al. 2011) observed a progressively decreasing size of hooks from anterior to posterior region, but the posterior most row of spines was larger. As in most acanthocephalan species, hook surface is smooth in E. veli in contrast to surface striations in Porrorchis indicus (Abd-El-Moaty and Taeleb, 2011), Dentitruneus truttae (Dezfuli et al. 2008) and Rhadinorhynchus ornatus (Amin et al. 2009). Dezfuli et al. (2008) speculated that the striations may help effective attachment to the host intestinal wall. The paired pits at the base of the proboscis similar to those in E. veli were reported in Acanthogyrus tilapiae by Amin and Heckmann (2012) and suggested a sensory function. Hence the pits observed in E. veli, may be considered as sensory in function.
The neck in E. veli is well developed, shorter than proboscis, devoid of hooks and with paired pits towards the basal part. In Tenuiproboscis sp. neck is elongated (Sanil et al. 2011). Rhadinorhynchus dorsoventrospinosus has a prominent neck with a posterior ring of hooks (Al Ghamdi 2013). In Acanthocephalus ranae, neck is very short and conical (Heckmann et al. 2011). Paired sensory pits were reported in A. lucii (Amin et al. 2011). The collar reported in the present study is of a unique nature. Nadakal et al. (1990) reported a collar-like swelling at the anterior part of the trunk in Metarhadinorhynchus valiathurae. We speculate a muscular origin of the collar, supporting the function of retraction or extrusion of the proboscis. The trunk epidermis in E. veli has micropores and microtriches increasing the surface area of nutrient absorption. There are both micropores and microtriches in some acanthocephalans (Amin et al. 2009). Microtrichus are highly specialised microvilli covering the entire surface of the tegument of cestodes and some acanthocephalans. Micropores are part of the lacunar system of acanthocephalans (Hammond 1968) and serving as pinocytic invaginations leading to the underlying canalicular system as shown in the SEM and TEM studies in Corynosoma strumosum (Heckman et al. (2013). The trunk surface of Sclerocollum sp. has minute scale like spines arranged in oblique lines whereas that of S. rubrimaris has small pores and sclerotised plates on its anterior portion (Abdou and Mahfouz 2006).
The female genital aperture in E. veli is circular and terminal in an elevated orifice. It is slit-like and lateral in A. lucii but in an elevated orifice (Amin et al. 2011). In Pomphorhynchus spindletruncatus, it is slit-like but not elevated (Heckmann et al. 2010). It is slit-like and terminal with two muscular lips in A. ranae (Heckmann et al. 2011).
Copulatory bursa in E. veli is directed ventrally with well defined rim and many papillae. The papillae may be sensory in function as suggested by Heckmann et al. (2011) in A. ranae. In A. lucii also it is directed ventrally and heavily muscular with a prominent rim but devoid of sensory papillae (Amin et al. 2011).
Conclusion
The study revealed the details of the proboscis, hooks, neck, epidermal micropores, microtriches, sensory pits copulatory bursa, and female genital pore, which were not observed under light microscope. The proboscis is cylindrical, paired with conspicuous pits. At the base of the proboscis hooks were arranged quinquintially. Neck is well developed with epidermal microspores. The trunk epidermis has microtriches and epidermal microspores Female genital pore is terminal and the copulatory bursa in male is provided with many papillae.
Acknowledgments
The authors thank the authorities of Mar Ivanios College for providing facilities for the present work and the University of Kerala for the fellowship awarded to the senior author. We are also thankful to Sree Chitra Thirunal Institute for Medical Science and Technology for the technical assistance rendered.
References
- Abd-El-Moaty SM, Taeleb AA. Ultrastructural study on the body surface of Porrorchis indicus (Acanthocephala: Plagiorhynchidae) from the Egyptian cuculus, Centropus senegalensis aegyptius (Aves : Cuculidae) J Am Sci. 2011;7:571–577. [Google Scholar]
- Abdou NE, Mahfouz ME. Ultrastructural and genetic diversity studies of two Sclerocollum (Acanthocephala) species infecting Siganid and Lutianid fishes from the Red Sea, Egypt. J Egypt Soc Parasitol. 2006;36:1035–1056. [PubMed] [Google Scholar]
- Al Ghamdi AO. Description of Rhadinorhynchus dorsoventrospinosus (Acanthocephala: Rhadinorhynchidae) from the red spot emperor Lethrinus lentjan with new host and locality records in Saudi Arabia. J Egypt Soc Parasitol. 2013;43:209–214. doi: 10.12816/0006378. [DOI] [PubMed] [Google Scholar]
- Alava JJ, Aguirre WE. Scanning electron microscopy of Neoechinorhynchus sp. (Acanthocephala: Neoechinorhynchidae), a possible new species of intestinal parasite of the Tallfin Croaker Micropogonias altipinnis (Guenther, 1864) Parasitol Latinoam. 2005;60:48–53. doi: 10.4067/S0717-77122005000100007. [DOI] [Google Scholar]
- Amin OM, Heckmann RA. An SEM study of Acanthogyrus (Acanthosentis) tilapiae (Acanthocephala: Quadrigyridae) from Africa documenting previously unreported features and host parasite interface. Sci Parasitol. 2012;13:57–63. [Google Scholar]
- Amin OM, Heckmann RA, Radwan NA, Anchundia JS, Alcivar MA. Redescription of Rhadinorhynchus ornatus (Acanthocephala: Rhadinorhynchidae) from skipjack tuna, Katsuwonus pelamis, collected in the Pacific Ocean off South America, with special reference to new morphological features. J Parasitol. 2009;95:656–664. doi: 10.1645/GE-1804.1. [DOI] [PubMed] [Google Scholar]
- Amin OM, Heckmann RA, El-Naggar AM. Revisiting the morphology of Acanthocephalus lucii (Acanthocephala: Echinorhynchidae) in Europe, using SEM. Sci Parasitol. 2011;12:185–189. [Google Scholar]
- Crompton DWT, Nickol BB. Biology of the Acanthocephala. Cambridge: Cambridge University Press; 1985. [Google Scholar]
- Dezfuli BS, Giovinazzo G, Lui A, Giari L. Inflammatory response to Dentitruncus truttae (Acanthocephala) in the intestine of brown trout. Fish Shellfish Immunol. 2008;24:726–733. doi: 10.1016/j.fsi.2007.11.013. [DOI] [PubMed] [Google Scholar]
- George PV, Nadakal AM. Four new species of Acanthocephala from brackish and freshwater fishes of Kerala. Aquat Biol. 1978;3:79–90. [Google Scholar]
- George PV, Nadakal AM. Biochromy of Acanthocephalid worm Echinorhynchus veli (George & Nadakal, 1978) parasitic in fish Synaptura orientalis (Bl & Sch) Jpn J Parasitol. 1985;34:68. [Google Scholar]
- Hammond RA. Observations of the body surface of some acanthocephalans. Nature. 1968;218:872–873. doi: 10.1038/218872a0. [DOI] [Google Scholar]
- Heckmann RA, Oguz MC, Amin OM, Dusen S, Tepe Y, Aslan B. Host and geographical distribution of Pomphorhynchus spindletruncatus (Acanthocephala: Pomphorhynchidae) in turkey with an enhanced description from new fish and amphibian hosts, using SEM and histopathological notes. Sci Parasitol. 2010;11:129–139. [Google Scholar]
- Heckmann RA, Amin OM, Tepe Y. Acanthocephalus ranae (Acanthocephala: Echinorhynchidae) from amphibians in Turkey, with special reference to new morphological features revealed by SEM, and histopathology. Sci Parasitol. 2011;12:23–32. [Google Scholar]
- Heckmann RA, Amin OM, EI-Naggar AM. Micropores of Acanthocephala, a Scaning Electron Microscopy study. Sci Parasitol. 2013;14:105–113. [Google Scholar]
- Nadakal AM, John KO, Jacob A. Metarhadinorhynchus valiathurae Sp.nov., an acanthocephalid worm parasitic in the marine fish, Caranx melampygus (Cuv. & Val.) Zoo Anzei. 1990;225:377–382. [Google Scholar]
- Nickol BB. Phylum Acanthocephala. In: Woo PTK, editor. Fish diseases and disorders, second edition: protozoan and metazoan infections. UK: Wallingford; 2006. pp. 444–465. [Google Scholar]
- Sanil NK, Asokan PK, John Lijo, Vijayan KK. Pathological manifestations of the acanthocephalan parasite, Tenuiproboscis sp. in the mangrove red snapper (Lutjanus argentimaculatus) (Forsskal, 1775), a candidate species for aquaculture from Southern India. Aquaculture. 2011;310:259–266. doi: 10.1016/j.aquaculture.2010.10.027. [DOI] [Google Scholar]


