Abstract
The role of immunomodulation in the therapeutic treatment of visceral leishmaniasis has gained eminence in view of moderate to severe drawbacks of the currently available drugs like toxicity, drug resistance and prohibitive costs. The potential for modulation of the immune system of many herbal plants can be tapped to address these problems. We conducted the present research study to investigate the antileishmanial and immunomodulatory effects of Ocimum sanctum Linn. and Cocos nucifera Linn. during the progression of visceral leishmaniasis in BALB/c mouse model. The IC50 values of the ethanolic leaf extract of O. sanctum and that of the aqueous husk-fibre extract of C. nucifera against the sodium stibogluconate (SSG) susceptible strain (MHOM/IN/80/Dd8) were found to be 73.3 and 62 µg/ml respectively. On treatment of infected BALB/c mice with the extracts, we observed a reduction in hepatic parasite load by 43.63 % (O. sanctum), 65.42 % (C. nucifera) and 75.61 % (O. sanctum + C. nucifera) at 1st post treatment day (p.t.d.), while at 15th p.t.d., the reduction was 73.61 % (O. sanctum), 76.59 % (C. nucifera) and 94.12 % (O. sanctum + C. nucifera). This was accompanied by an up-scaling of the DTH response, skewing of the humoral response towards Th1 type and hepatoprotection in the form of normalization of liver function tests. Overall, administration of the extracts of these two plants in combination as compared to their administration alone rescued the affected mice from the disease greatly, which can be attributed to their antileishmanial and immunomodulatory activities.
Keywords: Visceral leishmaniasis, Leishmania donovani, Ocimum sanctum, Cocos nucifera, Medicinal plants
Introduction
Leishmaniases are a group of diseases caused by Leishmania parasites and are recognized as a major health problem. Leishmania spp. persist as intracellular parasites in the vertebrate hosts, while they exist as free-living forms in the intestine of the invertebrate host i.e., sand fly (El-On et al. 2009). Four types of clinical manifestations of this disease spectrum are mainly widespread around the world - visceral leishmaniasis (VL), cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL) and post kala azar dermal leishmaniasis (PKDL) (Sen and Chatterjee 2011).
Visceral leishmaniasis is responsible for an estimated 20,000–40,000 deaths and 200,000–400,000 cases globally per year, with more than 9 out of 10 cases occurring in India, Bangladesh, Sudan, South Sudan, Ethiopia and Brazil (Alvar et al. 2012). It also causes a loss of over 2 million DALYs (disability-adjusted life years) (Mathers et al. 2007).
Leishmania parasites are known to disable the anti-pathogen activity of the macrophages for their survival inside the host by preventing the formation of phagolysosomes, antigen presentation to T-cells, and suppression of the cell-mediated immunity. To outwit these moves adopted by the parasites, therapeutic approaches should be able to confer restoration of the signaling between the macrophages and T-cells, in addition to the activation of the Th1 arm of cell-mediated immunity so that the macrophages can eliminate intracellular amastigotes (Chouhan et al. 2014). This parasite-driven down regulation of the immune system can be reversed by means of immunomodulation, which means stimulation or modulation of any component of the immune system by some substance—biological or synthetic in nature (Sharma and Singh 2009), besides treatment with the antileishmanial drugs.
The conventional drugs used for the treatment for leishmaniasis have a long list of drawbacks. So, an imperative need for the development of novel, effective, and safer drugs for the treatment of VL on a priority basis has been stressed upon (Mondal et al. 2014). There exists a rich local ethno-botanical bibliography describing the use of Indian medicinal plants in various disease conditions like bronchial asthma, chronic fever, cold, diabetes, diarrhea, arthritis, cough, malaria, dysentery, convulsions, skin diseases, insect bites, gastric, hepatic, cardiovascular and immunological disorders etc. (Chopra et al. 1956). Over one hundred plants reportedly exhibit leishmanicidal activity, and this number is increasing steadily (Atta-ur-Rahman et al. 2008).
About 80 % of the patients in India, 85 % in Burma and 90 % in Bangladesh are treated by the health practitioners using traditional system of medicine (Prakash and Gupta 2005). The immense chemical diversity and range of the bioactivity of the medicinal plant parts have led to the development of hundreds of pharmaceuticals. Extracts derived from such plants offer novel possibilities to obtain new compounds that might show activity against Leishmania parasites in vitro and in vivo. The current study, therefore, was focused on searching for new alternatives to treat leishmaniasis using components from the medicinal plants and their derivatives, employing BALB/c mouse as the animal model.
Materials and methods
Parasite strain and its culture
Leishmania donovani promastigotes of strain MHOM/IN/80/Dd8 (originally obtained from the London School of Hygiene and Tropical Medicine, London, were maintained in vitro at 22 ± 1 °C by serial cultures after every 48–72 h in Novy, McNeal & Nicolle (NNN) medium, supplemented with Eagle’s Minimum Essential Medium (MEM; pH 7.2) (Rao et al. 1984) and in RPMI-1640 medium (pH 7.2) containing 7–10 % Fetal Calf Serum (FCS; complete medium) (Raina and Kaur 2012). Both the culture media contained antibiotics streptomycin (200 units/ml), benzyl penicillin (40 μg/ml) and gentamycin (40 μg/ml).
Plant materials and preparation of their extracts
Plants
O. sanctum Linn. (Tulsi; Holy Basil) whole plant was collected from different areas of Chandigarh and from the Botanical Garden of Panjab University, Chandigarh. It was identified and authenticated by the Department of Botany, Panjab University, Chandigarh vide voucher no. 5044. Ripe fruit of C. nucifera Linn. (coconut) was procured locally from Chandigarh and was identified and authenticated by the Department of Botany, Punjabi University, Patiala vide voucher no. 187.
Preparation of the extracts
Leaves of O. sanctum were used for the extraction employing ethanol as the extraction solvent (Soxhlet 1879). Briefly, leaves were plucked from the fresh plants, washed, weighed, dried in the shade and ground in a mixer to make a fine powder. Extraction was carried out by consistent heating of the powder in ethanol at 70 °C. The extract, obtained thus, was evaporated to dryness using a Rota-Evaporator and stored at −4 °C till further use.
Husk fiber of the fruit of C. nucifera was used for the extraction employing distilled water as extraction solvent (Mendonça-Filho et al. 2004). Briefly, husk fiber of fruit was peeled-off and dried in sunlight to evaporate any moisture and then finely ground. The powder was boiled in distilled water and the resulting crude aqueous extract was first filtered by a coarse filter paper and then by Whatman Filter paper No. 1. The crude extract was concentrated and dried using a lyophillizer. Thereafter, the dried powder was scrapped from the vials, weighed and stored at −4 °C till further use.
Sodium stibogluconate (SSG)
SSG was purchased from M/s. Wellcome Research Laboratories, United Kingdom. Pellets of SSG were crushed and ground to fine powder followed by its dissolution in PBS in a water bath at 72 °C to make a concentration of 1 mg/ml. SSG was injected at a dose of 40 mg/kg b wt intraperitoneally into the infected mice for 5 days following Sodhi et al. (1992).
Dose response curve of the extracts
A total of 2 × 106 parasites per ml of early stationary phase L. donovani promastigotes in RPMI-1640 complete medium supplemented with 10 % FCS were dispensed into 24-well culture plates. Stock solution of the extracts was prepared in DMSO solution (one part DMSO in seven parts ethanol) and PBS in the ratio of 1:9, which was further diluted with RPMI-1640, to make various concentrations (5, 10, 20, 40, 60, 80 and 100 µg/ml) of extracts. The culture medium was supplemented with the extracts of the selected plant parts in triplicate series. Negative control cultures were supplemented with an equal volume of the normal saline. Parasite cultures were further incubated at 22 ± 1 °C and then their number was counted after 48 h by trypan blue dye exclusion method using Neubauer’s Chamber. The percentage growth inhibition was calculated as follows:
IC50 was obtained by plotting a linear dose–response curve of various concentrations (5–100 μg/ml) of the extract used.
Experimental animals and their infection
Inbred BALB/c mice of either sex, 5–6 weeks old, weighing 20–25 g were procured from the Institute of Microbial Technology, Chandigarh. They were kept in the experimental wing of the Central Animal House, Panjab University and fed with water and mouse feed, ad libitum. Mice were infected intravenously with 1 × 107 stationary phase promastigotes of L. donovani.
Treatment of infected mice
Treatment of infected BALB/c mice with the plant extracts prepared from O. sanctum and C. nucifera was begun 30 days after infection. The standard drug sodium stibogluconate (SSG) was also used for the treatment of one group of infected mice as a reference drug. The plant extracts were dissolved in the Standard Suspension Vehicle (SSV; 500 mg carboxy methyl cellulose, 500 μl benzyl alcohol, 400 μl Tween-80 in 100 ml of 0.9 % aqueous sodium chloride).
Preparation of the required doses of extracts and their administration
Ethanolic extract of O. sanctum and aqueous extract of C. nucifera was dissolved in the Standard Suspension Vehicle to get a required dose of 500 mg/kg b wt, each. Both the extracts were administered through the oral route daily for a period of 7 days.
Groups of experimental animals
Mice were divided into nine groups, namely (1) uninfected mice, (2) infected mice (3) infected mice treated with SSG (4) infected mice treated with the extract of O. sanctum (5) infected mice treated with the extract of C. nucifera (6) infected mice treated with a combination of extracts of O. sanctum and C. nucifera (7) uninfected mice treated with the extract of O. sanctum (8) uninfected mice treated with the extract of C. nucifera ix) uninfected mice treated with the extracts of both O. sanctum and C. nucifera. All groups comprised of six mice each.
Assessment of delayed type hypersensitivity (DTH) response in mice
Assessment of DTH responses was done following the method of Kaur et al. (2008). Briefly, all the groups of mice were challenged in their right and left foot pads with a subcutaneous injection of leishmanin (prepared in PBS) and PBS alone, respectively, 48 h before the actual sacrifice. Prior to sacrificing mice, the thickness of foot pads was measured with a Vernier calliper. Results were expressed as mean ± SD of percentage increase in the thickness of the right foot pad as compared to the left footpad.
Assessment of infection in the liver of mice
Liver of mice sacrificed on 1st and 15th post treatment days (p.t.d.) was removed and weighed immediately. A section of the liver was cut and rinsed with MEM to wash-off the blood; impression smears were made on clean grease-free slides from the surface of the cut ends, and subsequently stained with Giemsa after air-drying. A total of 100 cells were observed in each slide under 100X objective using immersion oil and amastigotes in them were counted. Parasite load was assessed in terms of Leishman–Donovan Units (LDU) (Bradley and Kirkley 1977) and calculated as follows:
Collection of serum
Blood was collected from all the mice after anaesthetization (conforming to the approved Institutional Animal Ethics Committee guidelines) on different post infection or post treatment days and serum was isolated from it for performing ELISA and various biochemical tests.
ELISA for parasite-specific IgG1 and IgG2a isotypes
Levels of the parasite-specific antibodies IgG1 (Th2-specific) and IgG2a (Th1-specific) were assessed by Enzyme Linked Immunosorbent Assay (ELISA) as per Kaur et al. (2008). Briefly, crude antigen was prepared by subjecting the log phase promastigotes to six cycles of freeze–thawing, followed by centrifugation at 10,000 r.p.m. at 4 °C. The supernatant from this centrifugate was collected, and its protein concentration was estimated by the method of Lowry et al. (1951). It was subsequently used for coating 96-well ELISA microplates. Thereafter, serum samples from various animal groups were added at two-fold serial dilution, followed by the addition of isotype specific HRP-conjugated secondary antibodies (rabbit anti-mouse IgG1 or IgG2a), H2O2 (substrate) and diaminobenzidene (chromogen) in succession. The absorbance was read on an ELISA plate reader at 450 nm.
Preparation of the soluble Leishmania antigen (SLA)
Log phase promastigotes from NNN medium were subjected to 6 cycles of freeze-thaw after washing thrice with PBS (pH 7.2). The solid debris was removed by centrifugation at 10,000 rpm for 15 min at 4 °C, and the protein content of the antigen was estimated by the method of Lowry et al. (1951). The supernatant was stored at −20 °C till further use.
Liver function tests
Liver function tests (LFTs) were performed by estimating the activity of serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT) alkaline phosphatase (ALP) and bilirubin using commercial kits (M/s. Reckon Diagnostics Pvt. Ltd, Vadodara) as per manufacturer’s instructions.
Renal function tests
Renal function tests (RFTs) were performed by estimating the concentrations of blood urea and serum creatinine using commercial kits (M/s. Reckon Diagnostics Pvt. Ltd, Vadodara) as per manufacturer’s instructions.
Ethical clearance
The ethical clearance for conducting the animal experiments was obtained from the Institutional Animal Ethics Committee, Panjab University, Chandigarh.
Statistical analysis
All the results were statistically tested by unpaired Student t-test.
Results
IC50 value
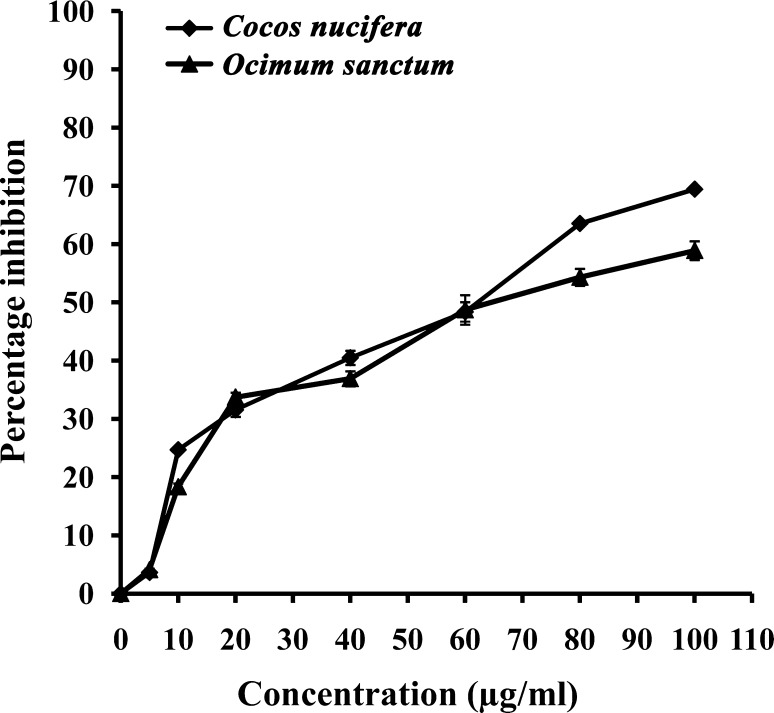
IC50 value of ethanolic extract from O. sanctum against promastigotes of Leishmania donovani was found to be 73.3 µg/ml and that of aqueous extract from C. nucifera was found to be 62 µg/ml (Fig. 1).
Fig. 1.
Dose response curve of the ethanolic leaf extract of Ocimum sanctum and aqueous husk fiber extract of Cocos nucifera against in vitro culture of the promastigotes of L. donovani
Hepatic parasite load
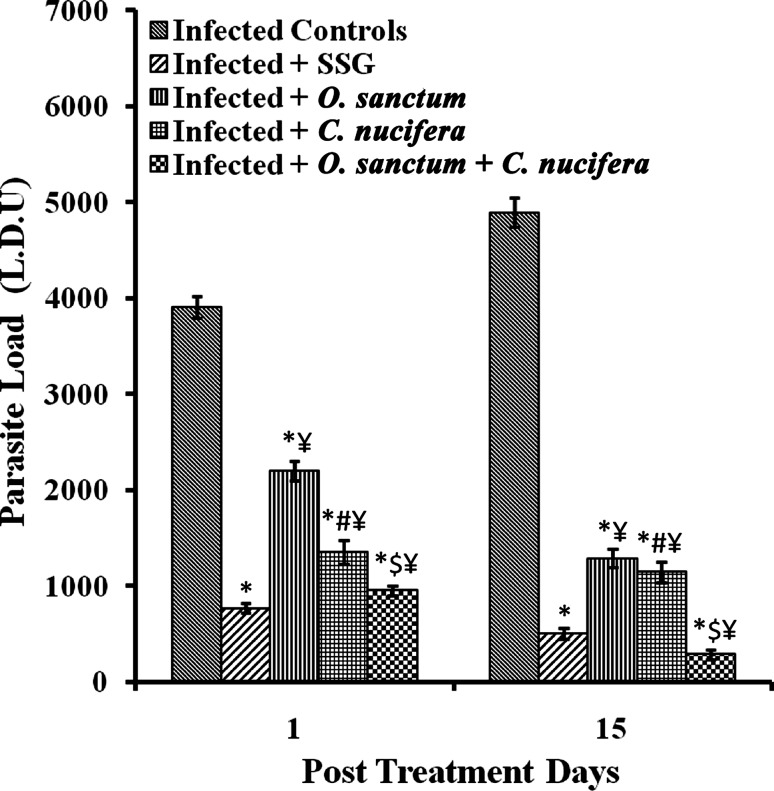
Parasite load in terms of Leishman Donovan Units (LDU) in the liver of L. donovani infected BALB/c mice after 1 and 15 p.t.d. significantly decreased (p < 0.0001) in all the groups of treated mice having infection in comparison to the untreated infected mice. The reduction in parasite load followed the trend O. sanctum > C. nucifera > O. sanctum + C. nucifera. The combination of the extracts of O. sanctum and C. nucifera was more effective than even SSG in significantly (p < 0.0001) reducing hepatic parasite load in infected mice (Fig. 2).
Fig. 2.
Parasite load in various groups of BALB/c mice. Results are expressed as Mean ± SD of 6 mice. p value: Infected v/s Infected + SSG/Infected + O. sanctum/Infected + C. nucifera/Infected + O. sanctum + C. nucifera. *p < 0.0001. p value: Infected + O. sanctum v/s Infected + C. nucifera # p < 0.0001. p value:Infected + O. sanctum/Infected + C. nucifera v/s Infected + O. sanctum + C. nucifera. $ p < 0.0001. p value: Infected + SSG v/s Infected + O. sanctum/Infected + C. nucifera/Infected + O. sanctum + C. nucifera ¥ p < 0.0001
IgG1 and IgG2a antibody responses
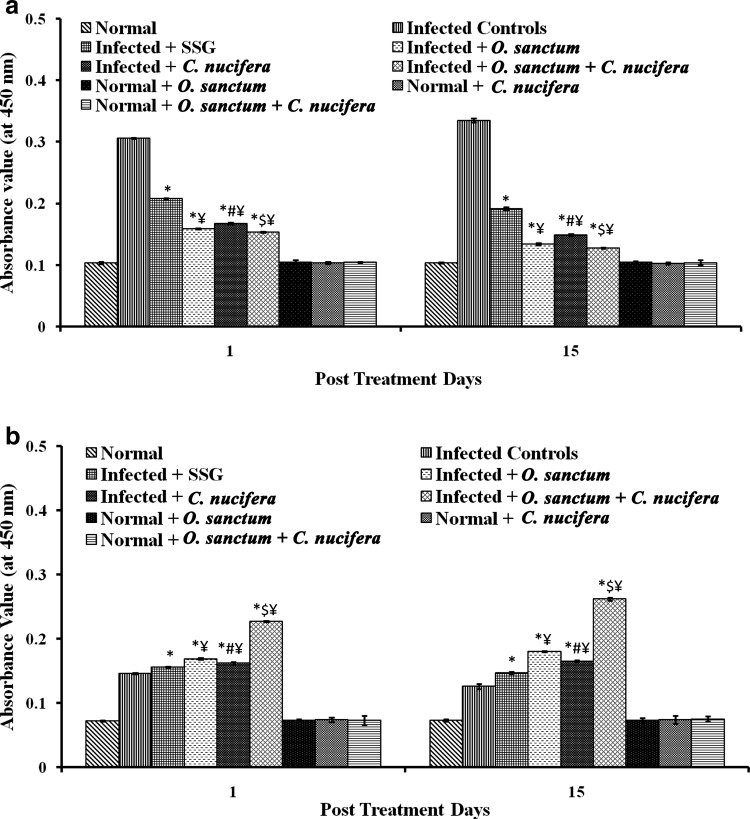
The levels of IgG1 and IgG2a antibody isotypes indicate a bias towards the non-protective Th2 and the healing Th1 responses, respectively. In the present study, the IgG1 levels were significantly lowered (p < 0.0001) in all the groups of the treated infected mice in comparison to untreated infected mice at 1 and 15 p.t.d., for which the effect followed the trend C. nucifera < O. sanctum < C. nucifera + O. sanctum. The combination of extracts was the most effective in lowering IgG1 response even when compared to SSG-treated mice. The IgG2a levels significantly increased (p < 0.0001) in all groups of treated infected mice in comparison to the untreated infected mice at 1 and 15 p.t.d. Rise in the IgG levels followed the trend C. nucifera + O. sanctum > O. sanctum > C. nucifera. The administration of combination of both extracts increased the IgG2a levels by the highest margin in the infected mice than in the mice that were treated by O. sanctum or C. nucifera, alone even when compared with SSG for the same effect (Fig. 3).
Fig. 3.
Antibody levels in various groups of BALB/c mice. a IgG1, b IgG2a. Results are expressed as Mean ± SD of 6 mice. p value: Infected v/s Infected + SSG/Infected + O. sanctum/Infected + C. nucifera/Infected + O. sanctum + C. nucifera. *p < 0.0001. p value: Infected + O. sanctum v/s Infected + C. nucifera. # p < 0.0001. p value: Infected + O. sanctum/Infected + C. nucifera v/s Infected + O. sanctum + C. nucifera. $ p < 0.0001. p value: Infected + SSG v/s Infected + O. sanctum/Infected + C. nucifera/Infected + O. sanctum + C. nucifera ¥ p < 0.0001
Delayed type hypersensitivity (DTH) responses
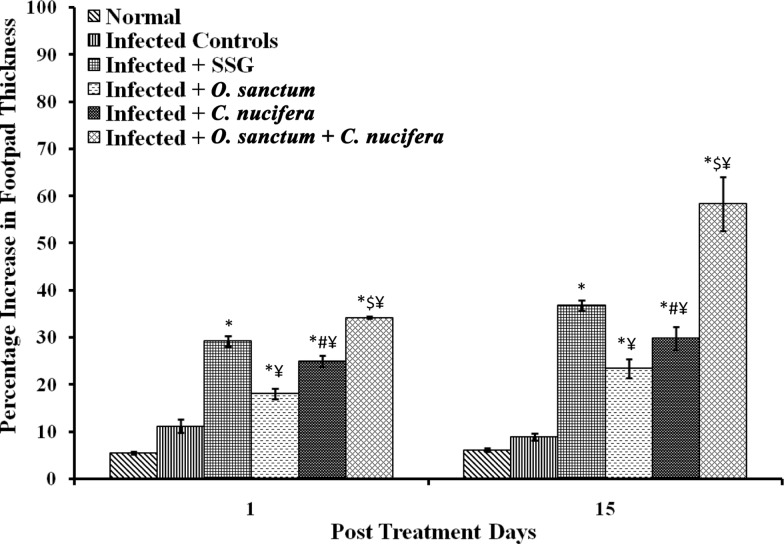
DTH responses were heightened significantly (p < 0.0001) in all groups of treated infected mice in comparison to untreated infected mice at 1 and 15 p.t.d. It followed the trend C. nucifera + O. sanctum > C. nucifera > O. sanctum. The combination of both the extracts was more effective in significantly (p < 0.0001) increasing DTH responses in infected mice even more than that in mice treated with SSG on 1 and 15 p.t.d respectively (Fig. 4).
Fig. 4.
DTH responses in various groups of BALB/c mice. Results are expressed as Mean ± SD of 6 mice. p value: Infected v/s Infected + SSG/Infected + O. sanctum/Infected + C. nucifera/Infected + O. sanctum + C. nucifera. *p < 0.0001. p value: Infected + O. sanctum v/s Infected + C. nucifera. # p < 0.0001.. p value: Infected + O. sanctum/Infected + C. nucifera v/s Infected + O. sanctum + C. nucifera. $ p < 0.0001. p value: Infected + SSG v/s Infected + O. sanctum/Infected + C. nucifera/Infected + O. sanctum + C. nucifera ¥ p < 0.0001
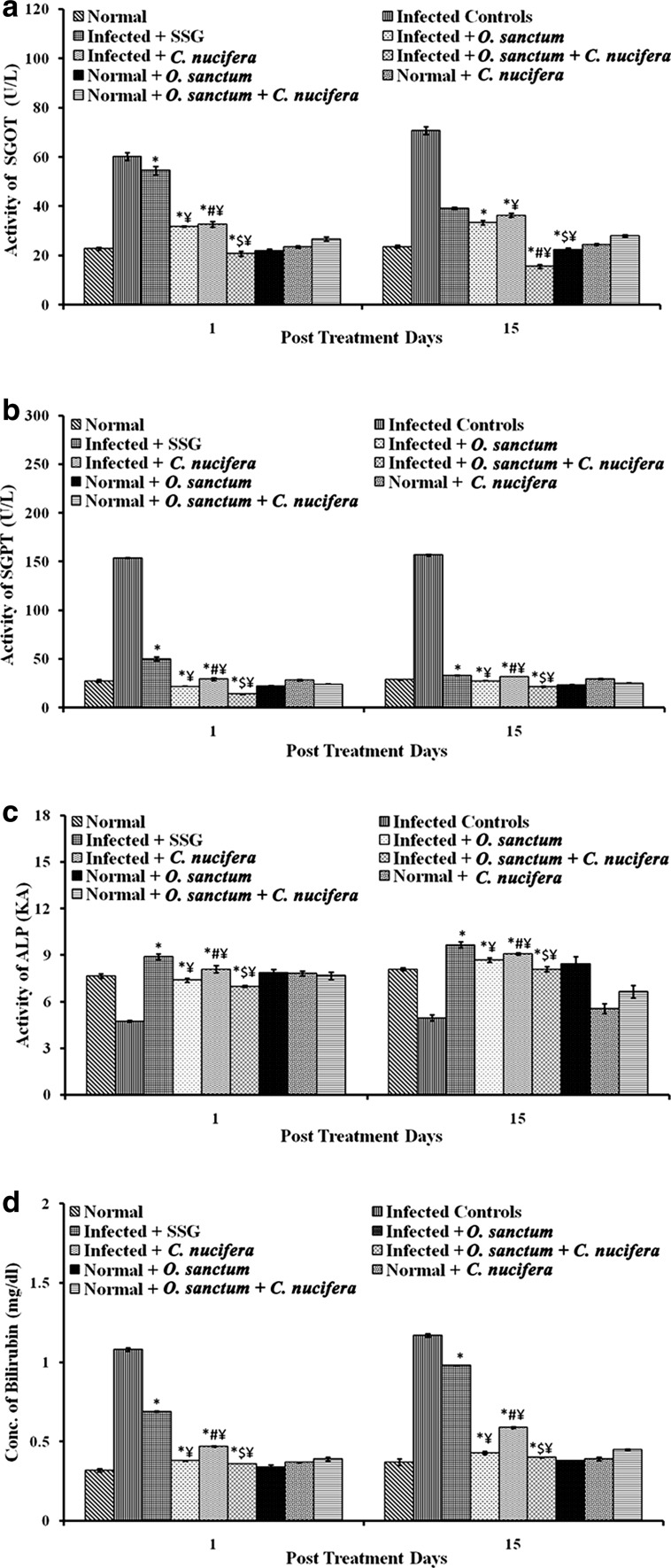
Liver function tests
In comparison to the infected mice, in which SGOT, SGPT and ALP activity was above normal range, all the groups of mice treated with plant extracts exhibited markedly significant (p < 0.0001) restoration of the activity of these enzymes to within the normal range (in the unininfected mice) at 1 and 15 p.t.d. It was seen in all the cases that treatment with the combination of extracts of O. sanctum and C. nucifera was more efficient at restoring the normal activity of these enzymes than either of the two plant extracts alone. The bilirubin concentration that shot up to the above normal values in the infected mice was also restored to within the normal range both on 1 and 15 p.t.d. as a result of the treatment with the plant extracts. The effects followed the same trend as in case of the liver enzymes tested i.e., the combination of the two extracts showed the best effect (Fig. 5).
Fig. 5.
Liver functions tests in various groups of BALB/c mice. a SGOT activity, b SGPT activity, c ALP activity, d bilirubin concentration. Results are expressed as Mean ± SD of 6 mice. p value: Infected v/s Infected + SSG/Infected + O. sanctum/Infected + C. nucifera/Infected + O. sanctum + C. nucifera. *p < 0.0001. p value: Infected + O. sanctum v/s Infected + C. nucifera. # p < 0.0005. p value: Infected + O. sanctum/Infected + C. nucifera v/s Infected + O. sanctum + C. nucifera. $ p < 0.0001. p value: Infected + SSG v/s Infected + O. sanctum/Infected + C. nucifera/Infected + O. sanctum + C. nucifera ¥ p < 0.0001
Renal function tests
In the uninfected mice, infected-only mice and infected mice treated with the plant extracts, concentration of blood urea and serum creatinine was found to be within the normal range i.e., within 10–45 mg/dl and 0.85–1.35 mg/dl respectively, both on 1 and 15 p.t.d.
Discussion
The current therapeutic options for leishmaniasis have majorly become outdated because of many downsides like intrinsic variation in drug sensitivity of the seventeen Leishmania species known to infect humans, acquired resistance to the pentavalent drugs in active endemic regions, decreased efficacy in immunosuppressed patients; especially in patients with HIV co-infection, besides other serious problems like cardiotoxicity, pancreatitis, hepatotoxicity, nephrotoxicity, requirement of long periods of hospitilization etc. (Croft et al. 2006).
Ocimum sanctum (Tulsi) has endless biological applications namely, its documented use as an anti-bacterial, anti-viral, anti-allergic, cardioprotective, hepatoprotective, nephroptotective, anti-diabetic and immunomodulator (Pattanayak et al. 2010). The therapeutic applications of C. nucifera include its usage as anti-viral (Esquenazi et al. 2002), hepatoprotective (Loki and Rajamohan 2003), anti-oxidant, anti-bacterial (Oyi et al. 2010), cardioprotective, anti-protozoal and as an immunomodulator (DebMandal and Mandal 2011).
Therefore in the present study, the antileishmanial and the immunomodulatory potential of O. sanctum and C. nucifera were assessed. After the determination of IC50 concentration of the prepared extracts in vitro, their antileishmanial and immunomodulator effects were probed in terms of percentage reduction in the parasite load in liver, increased DTH response, increased levels of Th1 immune response specific IgG2a antibody, and normalization of hepatic parameters.
The extracts of O. sanctum and C. nucifera alone and in combination caused a significant decline in the parasite load in all infected but untreated mice after treatment as compared to the infected-only mice. A remarkably good reduction was seen in the infected mice treated with the combination of both O. sanctum and C. nucifera extracts. The reduction was even more than the infected mice treated with SSG.
In the humoral immune responses, diminished levels of IgG1 and elevated levels of IgG2a point towards the establishment of a protective immune response, also called as Th1 type response (Sharma and Singh 2009). We observed a significant decrease in the levels of IgG1 antibody and an increase in the levels of IgG2a antibody in all the groups of treated infected mice in comparison to the infected controls. A significant fall in the IgG1 levels and a rise in the IgG2a levels in the group of infected mice treated with the extract of O. sanctum alone were in line with the earlier studies on immunomodulatory potential of O. sanctum on the Swiss albino mice immunized with the sheep red blood cells (SRBCs) and subsequently treated with the various doses of the alcoholic extract for 2 weeks. This treatment brought about a significant rise in the antibody titre as compared to the aqueous extract at a same dose (Vaghasiya et al. 2010). A significant fall in the IgG1 levels and a concomitant rise in the IgG2a levels in the group of infected mice treated with the extracts of C. nucifera alone in comparison to the infected controls were in corroboration with an earlier study on C. nucifera by Costa et al. (2010), wherein the authors found the butanolic extract from the husk fibre raising the circulating antibody levels on 14 and 21 days after the SRBC boost. Many other studies also support the immunomodulatory activity of O. sanctum, like immunostimulation in cattle suffering from the subclinical mastitis (Mukherjee et al. 2005) as well as increased IL-2 gene expression and heightened IL-2 production in male wistar rats (Goel et al. 2010).
DTH responses are evoked after intradermal injection of an antigen in an animal which has had a previous encounter with the same antigen. DTH comprises of two phases, an initial sensitization phase after the primary contact with antigen (when sensitized Th1 cells get activated and undergo multiplication) and a later effector phase (which includes induration, swelling and monocytic infiltration into the site of the lesion within 24–72 h). This response is due to CD4+ and CD8+ cells, though CD4+ Th1 cells are more actively involved than CD4+ Th2cells. An increase in swelling at the site of injection corresponds to a heightened cellular immune response (Black 1999; Dashputre and Naikwade 2010). For Leishmania-infected mice, the percentage increase in foot-pad thickness in response to leishmanin corresponds to an increased DTH response. In the present study, we observed a significant increase in DTH response in all the groups of infected mice after treatment with the plant extracts, as compared to the infected-only mice. Further, there was a progressive increase in the DTH responses in all treated groups of mice on 15th p.t.d. than on 1st p.t.d. These results were in agreement with a report about the immunomodulatory aspects of O. sanctum by Goel et al. (2010) who observed a boost in the number of T and B lymphocytes in male wistar rats due to the administration of aqueous extract of the plant, and by Costa et al. (2010) who found the butanolic extract of C. nucifera triggering DTH responses in swiss albino mice immunized with SRBCs. The DTH response in infected mice treated with a combination of both the plant extracts was found to be even higher than in the infected mice treated with SSG. Hence, treatment with the combination of extracts from both the plants appears to be the most effective in eliciting cellular responses.
Liver is the central organ of metabolism with numerous independent vital functions that are complex to measure and quantify. It is the seat of detoxification and is responsible for the secretion of bile and the production of blood clotting factors (Sharma and Sharma 2009). The liver enzymes are very much of diagnostic value as their levels are altered due to an infection and these abnormal levels are analysed as a tool to assess disease progression or recovery (Limdi and Hyde 2003). VL patients show a number of marked changes in the liver ranging from histological alterations (inflammatory changes, fibrosis, necrosis, etc.) to an increase in the level of liver enzymes. Commonly monitored liver enzymes in VL patients are SGOT, SGPT, ALP and bilirubin. These tests are performed to check the health of liver and effect of drug induced toxicity on enzyme levels (el Hag et al. 1994). SGOT and SGPT levels are elevated in case of VL patients as the L. donovani amastigotes inhabiting liver cells damage them, causing these enzymes to move out of the cells. This leads to their increased blood levels (Nemati et al. 2012). It was found in the present study that the activity of enzymes SGOT, SGPT, ALP and the concentration of bilirubin was significantly above the normal range in infected but untreated mice that was restored to the normal levels in infected mice treated with O. sanctum or C. nucifera, alone or in combination. Nemati et al. (2012) have also reported an increase in the activity of SGOT and SGPT in L. major infected BALB/c mice. The hepatoprotective nature of O. sanctum has been widely reported in earlier studies. Jeba et al. (2011) reported that the aqueous extract of O. sanctum leaves was able to restore the normal levels of SGOT and SGPT in Wistar rats. Similarly, the ethanolic extract of the leaves of O. sanctum was found to ameliorate the hepatotoxicity induced by anti-tubercular drugs in albino rats (Ubaid et al. 2001). Cocos nucifera has also been documented to possess hepatoprotective property, since the liquid endosperm of C. nucifera was reported to be hepatoprotective in alloxan-induced diabetes in Sprague–Dawley rats by restoring SGOT, SGPT and ALP activity to within normal range as compared to normal controls (Preetha et al. 2013). The SSG-treated mice in our study exhibited intense hepatotoxicity as indicated by the abnormal range of activity of the enzymes of liver function tests. The SSG induced hepatotoxicity has been reported by Roychoudhury and Ali (2008) also.
Various changes in the activity of renal enzymes have been reported during severe toxicity with drugs. Hence, renal function tests are an important tool in assessing the suitability of a therapeutic agent. In the present study, the concenteration of blood urea and serum creatinine was found to be within the normal range in the infected but untreated mice. The levels in the infected mice treated with O. sanctum or C. nucifera alone, or in combination were also were found to be within the normal range. Treatment with the standard drug SSG caused slightly raised concentration of blood urea and serum creatinine, though it still remained within the normal range. Thus, we found using O. sanctum and C. nucifera alone and in combination to be safer than using SSG.
New and alternative drug sources of anti-leishmanial compounds which are less toxic, easily available and cost-effective is the need of the present times. These requirements can best be fulfilled by the plant products and their derivatives that act as a source for more than 50 % of all the drugs in clinical use in the world (Gurib-Fakim 2006). The World Health Organization has estimated that approximately 80 % of the world population relies on traditional medicines for their health care (Newman and Cragg 2007). Plant-based drugs play a very important role in the fight against numerous diseases, since a large section of world population has no access to the conventional pharmacological treatment (owing to cost and availability) and there is an increasing inefficiency of conventional medicines in terms of the serious side-effects and toxicity. Thus, easy access and ethnobotanical knowledge has propelled the use of plant-based drugs (Rates 2001). Leishmaniasis is associated with the immunological dysfunction of T cells, natural killer cells and in particular, incapacitation of macrophages which ultimately leads to the establishment of the parasite. Hence, immunomodulation that involves recovery of the Th1 responses and enhanced nitric oxide production is an important aspect of this disease (Kaye et al. 2004). Immunomodulatory properties of plants have been known since ancient times. Many plants are known to enhance macrophage activation as well as cell mediated and humoral immune responses (Agarwal and Singh 1999). The concept of using natural immunomodulators to treat parasitic infections including leishmaniasis holds a great potential in achieving the control of this disease. These natural immunomodulatory molecules can serve as scaffolds for the synthesis and discovery of new drugs. Also, their use in synergy with the existing drugs may involve the functional manipulation of multiple molecular targets leading to an improved therapeutic efficacy and reduced toxicity.
In the light of above discussion, the results of the present study obtained with the extracts of O. sanctum and C. nucifera are promising as they have anti-leishmanial and immunomodulatory properties. The plant extracts used showed a better healing potential than SSG and in terms of drug-induced toxicity in treated mice. Thus, the result of this study provides an insight into plant-based drugs which could be used for effective treatment of visceral leishmaniasis without causing harmful effects. These plants could further be investigated in other animal models such as hamsters for establishing their anti-leishmanial and immunomodulatory activity.
Acknowledgments
The authors wish to acknowledge the contribution of DST-PURSE grant received by Panjab University, Chandigarh towards the funding of this work.
Authors contribution
SK & PR conceived the study and corrected the manuscript; GB, JK & RK conducted the experiments and prepared the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Agarwal S, Singh VK. Immunomodulators: a review of studies on Indian medicinal plants and synthetic peptides. PINSA. 1999;B65:179–204. [Google Scholar]
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta-ur-Rahman, Samreen, Atia-tul-Wahab, Choudhary MI. Discovery of leishmanicidal agents from medicinal plants. Pure Appl Chem. 2008;80(8):1783–1790. doi: 10.1351/pac200880081783. [DOI] [Google Scholar]
- Black CA. Delayed type hypersensitivity: current theories with an historic perspective. Dermatol Online J. 1999;5(1):7. [PubMed] [Google Scholar]
- Bradley DJ, Kirkley J. Regulation of Leishmania populations within the host. I. The variable course of Leishmaniadonovani infections in mice. Clin Exp Immunol. 1977;30:119–129. [PMC free article] [PubMed] [Google Scholar]
- Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants. Publications and Informations Directorate. New Delhi: CSIR; 1956. [Google Scholar]
- Chouhan G, Islamuddin M, Sahal D, Afrin F. Exploring the role of medicinal plant-based immunomodulators for effective therapy of leishmaniasis. Front Immunol. 2014;5:193. doi: 10.3389/fimmu.2014.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C, Bevilaqua C, Nunes-Pinheiro D, Morais S, Farias V, Oliveira L, Braga R. Immunomodulatory activity of Cocos nucifera L. in mice. Ciência Anim. 2010;20(1):57–64. [Google Scholar]
- Croft S, Seifert K, Yardley V. Current scenario of drug development for leishmaniasis. Ind J Med Res. 2006;123:399–410. [PubMed] [Google Scholar]
- Dashputre N, Naikwade N. Preliminary immunomodulatory activity of aqueous and ethanolic leaves extracts of Ocimum basilicum Linn. in mice. Int J Pharm Tech Res. 2010;2(2):1342–1349. [Google Scholar]
- DebMandal M, Mandal S. Coconut (Cocos nucifera L.: Arecaceae): in health promotion and disease. Asian Pac. J Trop Med. 2011;4(3):241–247. doi: 10.1016/S1995-7645(11)60078-3. [DOI] [PubMed] [Google Scholar]
- el Hag IA, Hashim FA, el Toum IA, Homeida M, el Kalifa M, el Hassan AM. Liver morphology and function in visceral leishmaniasis (Kala-azar) J Clin Pathol. 1994;47(6):547–551. doi: 10.1136/jcp.47.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-On J, Ozer L, Gopas J, Sneir R, Golan-Goldhirsh A. Nuphar lutea: in vitro anti-leishmanial activity against Leishmania major promastigotes and amastigotes. Phytomedicine. 2009;16(8):788–792. doi: 10.1016/j.phymed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Esquenazi D, Wigg M, Miranda M, Rodrigues H, Tostes J, Rozental S, da Silva A, Alvianod C. Antimicrobial and antiviral activities of polyphenolics from Cocos nucifera Linn. (Palmae) husk fiber extract. Res Microbiol. 2002;153:647–652. doi: 10.1016/S0923-2508(02)01377-3. [DOI] [PubMed] [Google Scholar]
- Goel A, Singh D, Kumar S, Bhatia A. Immunomodulating property of Ocimum sanctum by regulating the IL-2 production and its mRNA expression in rat spenocytes. Asian Pac J Trop Med. 2010;3(1):8–12. doi: 10.1016/S1995-7645(10)60021-1. [DOI] [Google Scholar]
- Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Asp Med. 2006;27(1):1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Jeba R, Vaidyanathan R, Rameshkumar G. Immunomodulatory activity of aqueous extract of Ocimum sanctum in rat. Int J Pharma Biomed Res. 2011;2(1):33–38. [Google Scholar]
- Kaur S, Kaur T, Garg N, Mukherjee S, Raina P, Athokpam V. Effect of dose and route of inoculation on the generation of CD4+ Th1/Th2 type of immune response in murine visceral leishmaniasis. Parasitol Res. 2008;103(6):1413–1419. doi: 10.1007/s00436-008-1150-x. [DOI] [PubMed] [Google Scholar]
- Kaye PM, Svensson M, Ato M, Maroof A, Polley R, Stager S, Zubairi S, Engwerda CR. The immunopathology of experimental visceral leishmaniasis. Immunol Rev. 2004;201:239–253. doi: 10.1111/j.0105-2896.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- Limdi JK, Hyde GM. Evaluation of abnormal liver function tests. Postgrad Med J. 2003;79:307–312. doi: 10.1136/pmj.79.932.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loki AL, Rajamohan T. Hepatoprotective and antioxidant effect of tender coconut water on CCl4 induced liver injury in rats. Ind J Biochem Biophys. 2003;40:354–357. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mathers CD, Ezzati M, Lopez AD. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl Trop Dis. 2007;1:e114. doi: 10.1371/journal.pntd.0000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonça-Filho R, Rodrigues I, Alviano D, Santosa A, Soares R, Alviano C, Lopes A, Rosa M. Leishmanicidal activity of polyphenolic-rich extract from husk fiber of Cocos nucifera Linn. (Palmae) Res Microbiol. 2004;155:136–143. doi: 10.1016/j.resmic.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Mondal D, Alvar J, Hasnain MG, Hossain MS, Ghosh D, Huda MM, Nabi SG, Sundar S, Matlashewski G, Arana B. Efficacy and safety of single-dose liposomal amphotericin B for visceral leishmaniasis in a rural public hospital in Bangladesh: a feasibility study. Lancet Glob Health. 2014;2(1):e51–e57. doi: 10.1016/S2214-109X(13)70118-9. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Dash PK, Ram GC. Immunotherapeutic potential of Ocimum sanctum (L) in bovine subclinical mastitis. Res Vet Sci. 2005;79:37–43. doi: 10.1016/j.rvsc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Nemati S, Jafary S, Nahrevanian H, Farahmand M, Nahrevanian S. Immuno-biochemical alterations in Leishmania major infected BALB/c mice after immunization with killed leishmania vaccine and BCG as adjuvant. Curr Res J Biol Sci. 2012;4(6):706–712. [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70(3):461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- Oyi AR, Onaolapo JA, Obi RC. Formulation and antimicrobial studies of coconut (Cocos nucifera Linne) oil. Res J Appl S Eng Tech. 2010;2(2):133–137. [Google Scholar]
- Pattanayak P, Behera P, Das D, Panda S. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: an overview. Pharmacogn Rev. 2010;4(7):95–105. doi: 10.4103/0973-7847.65323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash P, Gupta N. Therapeutic uses of Ocimum sanctum Linn. (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J Physiol Pharmacol. 2005;49(2):125–131. [PubMed] [Google Scholar]
- Preetha PP, Devi VG, Rajamohan T. Comparative effects of mature coconut water (Cocos nucifera) and glibenclamide on some biochemical parameters in alloxan induced diabetic rats. Rev Bras Farmacog. 2013;23(3):481–487. doi: 10.1590/S0102-695X2013005000027. [DOI] [Google Scholar]
- Raina P, Kaur S. Knockdown of LdMC1 and Hsp70 by antisense oligonucleotides causes cell-cycle defects and programmed cell death in Leishmaniadonovani. Mol Cell Biochem. 2012;359(1–2):135–149. doi: 10.1007/s11010-011-1007-y. [DOI] [PubMed] [Google Scholar]
- Rao RR, Mahajan RC, Ganguly NK. Modified media for in vitro cultivation of Leishmania promastigotes. A comparative study. Bull PGI. 1984;18:125–128. [Google Scholar]
- Rates SM. Plants as source of drugs. Toxicon. 2001;39(5):603–613. doi: 10.1016/S0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Roychoudhury J, Ali N. Sodium stibogluconate: therapeutic use in the management of visceral leishmaniasis. Indian J Biochem Biophys. 2008;45:16–22. [Google Scholar]
- Sen R, Chatterjee M. Plant derived therapeutics for the treatment of Leishmaniasis. Phytomedicine. 2011;18(12):1056–1069. doi: 10.1016/j.phymed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Sharma B, Sharma U. Hepatoprotective activity of some indigenous plants. Int J Pharm Tech Res. 2009;1(4):1330–1334. [Google Scholar]
- Sharma U, Singh S. Immunobiology of leishmaniasis. Ind J Exp Biol. 2009;47:412–423. [PubMed] [Google Scholar]
- Sodhi S, Kaur S, Mahajan RC, Ganguly NK, Malla N. Effect of sodium stibogluconate and pentamidine on in vitro multiplication of Leishmania donovani in peritoneal macrophages from infected and drug-treated BALB/c mice. Immunol Cell Biol. 1992;70:25–31. doi: 10.1038/icb.1992.4. [DOI] [PubMed] [Google Scholar]
- Soxhlet F. Die gewichtsanalytische Bestimmung des Milchfettes. Dingler’s Polytechnisches J. 1879;232:461–465. [Google Scholar]
- Ubaid R, Anantrao K, Jaju JB, Mateenudin MD. Effect of Ocimum sanctum leaf extract on hepatotoxicity induced by antitubercular drugs in rats. Indian J Physiol Pharmacol. 2001;47(4):465–470. [PubMed] [Google Scholar]
- Vaghasiya J, Datani M, Nandkumar K, Malaviya S, Jivani N. Comparative evaluation of alcoholic and aqueous extracts of O. sanctum for immunomodulatory activity. Int J Pharm. Biol Res. 2010;1(1):25–29. [Google Scholar]