Abstract
The bopyrid isopods are common in wild Macrobrachium spp. but not common in aquaculture condition. This is the first study that reports the parasitizing of bopyrid isopods on the cultured M. malcolmsonii. Bopyrid isopod (Probopyrus buitendijki) was identified in the branchial cavities of the fresh water prawn, M. malcolmsonii from grow-out culture pond at Kuriyamangalam, India. Macrobrachium malcolmsonii is a new host for P. buitendijki. A total of 1323 M. malcolmsonii were checked for this study. The overall prevalence of the parasitic infestation was reached 46.2 %. The parasitic infection was higher in female (83 %) than in male (3.4 %). Highest prevalence of infestation was found in the median size group (7–8 cm) (58.7 %). Infected females were not berried unlike uninfected prawns. The parasites cause infertility and does not found any organ deformities due to the infestation. The parasite was inversely attached in the gill chamber with no lesion on the gill but the infected branchial chamber became bulged.
Keywords: Bopyrid isopod, Probopyrus buitendijki, Fresh water prawn, Macrobrachium malcolmsonii, Prevalence
Introduction
Macrobrachium malcolmsonii (H. Milne-Edwards, 1844), commonly known as the monsoon river prawn is the second largest prawn after the Giant river prawn M. rosenbergii (de Man, 1879). It is a fast growing prawn which is widely distributed throughout the Indian subcontinent (Hossain et al. 2012). Macrobrachium malcolmsonii is an omnivorous bottom dwelling prawn, feeding on decomposing plants and animals, small worms, insects and their larvae, and freshly molted conspecific specimens.
The parasitic isopods infecting fishes and crustaceans can induce serious problems in the wild (Koesharyani et al. 1999; Papapanagiotou et al. 1999; Kent 2000; Papapanagiotou and Trilles 2001; Thatcher and Blumenfeldt 2001). The bopyrid isopods are common in wild Macrobrachium spp. but not common in aquaculture condition (Oliveira 2000). They are holoparasites on decapod crustaceans (Markham 1986) and affect the reproductive potential of host (Van Wyk 1982). However, published information regarding parasitizing of bopyrid isopods on grow out cultured M. malcolmsonii is lacking. Hence, this paper reports the parasitizing of bopyrid isopods on the cultured M. malcolmsonii for the first time.
Materials and methods
Macrobrachium malcolmsonii was cultured in fresh water pond from the middle of December 2010 to May 2011. The prawn juveniles were collected from the wild, transported in oxygenated bags and stocked into the pond at a density of 40,000/ha. Both bore well and canal waters were used for the pond. All prawn juveniles were parasite free at the time of stocking into the ponds. The prawns were fed with commercial pellet feed. The stocked prawns were sampled every 10 days once since 30th days of culture. During the second sampling (January last week of 2011) period, from the 40th day of culture (DOC), the prawns were sampled to check the growth and the incidence of parasitic infections. On the 80th day of culture, the prawns were investigated for the isopod parasitic infestation. The parasites were identified according to the description given by Markham (1985). Probopyrus buitendijki was identified according to the following morphological characters: the black pigment present in the posterior lateral region of the pereomeres, head uncleft, unequally pentagonal in outline, about one fifth length of the body. The incidence, sex and distribution of parasites in the left and right branchial cavities of the host were noted and the length and weight were measured. Prevalence and mean intensity were calculated according to Bush et al. (1997).
Results
Macrobrachium malcolmsonii was parasitized by the bopyrid, Probopyrus buitendijki (Horts, 1910) (Fig. 1). Higher infestation in the different size group of M. malcolmsonii was reported (Fig. 2). The length and width of the parasite varied from 7 to 17 mm (13 ± 3 mm) and 5 to 12 mm (8.4 ± 2.3 mm) respectively. The infected female prawns were not berried but the uninfected female prawns were berried. The parasites were attached on the inner side of the carapace of the host and the infected carapace became bulge in the outside. On the 80th day of culture, of 1323 prawns, 611 were parasitized in the gill chambers. The prevalence was reached 46.2 %. The infection rate was almost the similar in the both left and the right side of the gill chamber. The parasite was inversely attached in the gill chamber with no lesion on the gill but the infected branchial chamber became bulged. There was only a slightly variation, the left branchial chamber being higher infected (51.4 %) than the right one (48.6 %). The highest prevalence (83 %) was observed in the female prawns, rather than in the male (3.4 %) (Table 1). However, the percentage of infestation was highest (58.74 %) in the medium group prawns (7–8 cm) than the lowest size prawns (<6) (28.9 %) (Table 2).
Fig. 1.
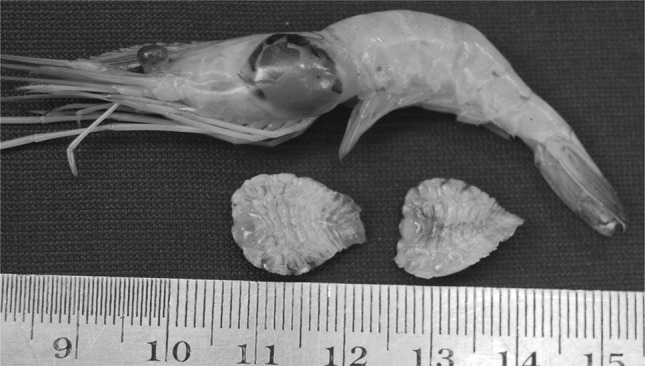
Probopyrus buitendijki in the gill chamber of Macrobrachium malcolmsonii and two isolated specimens
Fig. 2.

Probopyrus buitendijki in the gill chamber of several specimens belonging to the different size group of Macrobrachium malcolmsonii
Table 1.
Infestation of Macrobrachium malcolmsonii by Probopyrus buitendijki
| Total No. of prawns examined | No. of prawns parasitized (%) | No. of male examined | No. of female examined | No. of male parasitized (%) | No. of female parasitized (%) | No. of prawns parasitized in left branchial chamber (%) | No. of prawns parasitized in right branchial chamber (%) |
|---|---|---|---|---|---|---|---|
| 1323 | 611 (46.2) | 612 | 711 | 21 (3.4) | 590 (83) | 313 (51.2) | 298 (48.8) |
Table 2.
Variation of the isopod infestation (Probopyrus buitendijki) on different size group of the fresh water prawn Macrobrachium malcolmsonii
| Size group (cm) | No. of prawns examined | No. of prawns parasitized | % of infestation | Length of the parasite (mm) | Width of the parasite (mm) |
|---|---|---|---|---|---|
| <6 | 128 | 37 | 28.9 | 7–9 | 5–6 |
| 6–7 | 156 | 77 | 49.36 | 10–12 | 7–9 |
| 7–8 | 295 | 173 | 58.74 | 12–14 | 8–9 |
| 8–9 | 337 | 169 | 50.1 | 14–16 | 9–11 |
| 9–10 | 317 | 112 | 35.3 | 15–17 | 10–11 |
| 10< | 90 | 43 | 47.8 | 17 | 12 |
The length and width of the parasite varied from 7 to 17 mm (13 ± 3 mm) and 5 to 12 mm (8.4 ± 2.3 mm) respectively
Discussion
In this study, we report that this bopyrid species parasitize cultured Macrobrachium malcolmsonii from India with high infection rate reaching 46.2 %. In this study, canal water might be the source of parasites as pond was supplied with canal water. The highest infections were observed in the female prawns and median size group prawns. Similar result was also observed by Gopalakrishnan et al. (2009) in Parapenaeopsis stylifera. The higher infection in female than male prawns might be due to female had more fatty acids reserve for reproductive purpose. The high prevalence of infestation in the medium size groups might be due to most of the females are in the medium size groups. Published information regarding these issues are still lacking. Therefore, more research is needed regarding these issues.
The highest infestation rate was observed in the median group size prawns and there is not much variation of parasite infestation in the left and right branchial chambers of the prawns. Gopalakrishnan et al. (2009) reported that of 68 P. stylifera parasitized by Epipenaeon ingens, 38 bopyrids were attached in the right gill chamber and 30 in the left side. The length and width of the parasite were directly proportioned to the length of the prawn, which revealed that the parasite infestation start from the small size group of the host and grown according to the host growth. Bopyrids are large ectoparasites inducing usually a prominent bulge on the side of cephalothorax of infected prawns (Cash and Bauer 1993). In the parasitized mud-prawns (U. stellata), it was observed an enlarged branchial chamber, or branchial gall, as a result of the branchiostegite deformation. The branchial chamber, gills appeared splayed and flattened but showed no other signs of damage (Astall et al. 1996). However, Truesdale and Mermilliod (1977) reported that the presence of the parasite in the gill chamber usually induces a visible swelling and discoloration on the branchiostegite.
Of major importance, our study showed that the female M. malcolmsonii prasitized by Probopyrus buitendijki did not carry eggs whereas uninfected females were berried. The prawns became infertile (could not produce eggs). It may be due to the stress of the host due to parasite. The effects of bopyrid parasites include infertility (interruption of vitellogenesis) and morphological alteration of secondary sexual characters in male prawns (Beck 1980; Schuldt and Rodrigues 1985; Ordinetz-Collart 1990). The bopyrid, P. pandalicola prevents also the reproduction of Palaemonetes pugio (Chaplin-Ebanks and Curran 2007). The said parasitic isopod is one of the prime threat to the emerging prawn industry of India and also severely affect the reproductive potential of the host both in captive and also in the wild.
Acknowledgments
We are grateful to the Director and Dean, CAS in Marine Biology, Faculty of Marine Sciences, Annamalai University, Parangipettai, India for the facility provided throughout the study period. We acknowledge the financial support by University Grants Commission (UGC) Major Project F. No–39–569/2010 (SR). We also would like to thank the fish venders and fish farmers in and around Bhuvanagiri, for the prawn supply.
References
- Astall CM, Taylor AC, Atkinson RJA. Notes on some branchial isopods parasitic on upogebiid mud shrimps. J Mar Biol Assoc UK. 1996;76:821–824. doi: 10.1017/S0025315400031489. [DOI] [Google Scholar]
- Beck JT. Effect of an isopod castrator, Probopyrus pandalicola, on the sex characters of one of its caridean shrimp hosts, Palaemonetes paludosus. Biol Bull. 1980;158:1–15. doi: 10.2307/1540753. [DOI] [Google Scholar]
- Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol. 1997;83:575–583. doi: 10.2307/3284227. [DOI] [PubMed] [Google Scholar]
- Cash CE, Bauer RT. Adaptations of the branchial ectoparasite Probopyrus pandalicola (Isopoda: Bopyridae) for survival and reproduction related to ecdysis of the host, Palaemonetes pugio (Caridea: Palaemonidae) J Crustac Biol. 1993;13:111–124. doi: 10.2307/1549126. [DOI] [Google Scholar]
- Chaplin-Ebanks SA, Curran MC. Prevalence of the bopyrid isopod Probopyrus pandalicola in the grass shrimp, Palaemonetes pugio, in four tidal creeks on the South Carolina–Georgia coast. J Parasitol. 2007;93:73–77. doi: 10.1645/GE-3537.1. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan A, Rajkumar M, Ravichandran S, Trilles JP, Vasanthan TM. Identification of Parapenaeopsis stylifera, a new host for Epipenaeon ingens. J Environ Biol. 2009;30:1063–1064. [PubMed] [Google Scholar]
- Hossain MY, Ohtom J, Jaman A, Saleha J, Robert LVJ. Life history traits of the Monsoon River prawn Macrobrachium malcolmsonii (Milne-Edwards, 1844) (Palaemonidae) in the Ganges (Padma) River, northwestern Bangladesh. J Freshw Ecol. 2012;27:131–142. [Google Scholar]
- Kent ML. Marine netpen farming leads to infections with some unusual parasites. Int J Parasitol. 2000;30:321–326. doi: 10.1016/S0020-7519(00)00018-7. [DOI] [PubMed] [Google Scholar]
- Koesharyani I, Zafran Yuasa K, Hatai K. Two species of capsalid monogeneans infecting cultured humpback grouper Cromileptes altivelis in Indonesia. Fish Pathol. 1999;34:165–166. doi: 10.3147/jsfp.34.165. [DOI] [Google Scholar]
- Markham JC. A review of the bopyrid isopods infesting caridean shrimps in the Northwestern Atlantic Ocean, with special reference to those collected during the Hourglass cruises in the Gulf of Mexico. Mem Hourglass Cruises. 1985;7:1–156. [Google Scholar]
- Markham JC (1986) Evolution and zoogeography of the Isopoda Bopyridae, parasites of Crustacea Decapoda. In: Gore RH, Heck KL (eds) Crustacean Biography, Crustacean Issues 4, Balkema, Rotterdam, pp 143–164
- Oliveira E. The population structure of Probopyrus floridensis (Isopoda, Bopyridae). A parasite of Macrobrachium potiuna (Decapoda, Palaemonidae) from the Pereque River Paranagua Basin, South Brazil. Crustaceana. 2000;73:1095–1108. doi: 10.1163/156854000505100. [DOI] [Google Scholar]
- Ordinetz-Collart O. Interactions centre le ectoparasite Probopyrus bithynis (Isopoda, Bopyridae), et l’ un de ses hotes, la crevette Macrobrachium amazonicum (Decapoda, Palaemonidae) Crutaceana. 1990;58:258–269. doi: 10.1163/156854090X00174. [DOI] [Google Scholar]
- Papapanagiotou EP, Trilles JP. Cymothoid parasite Ceratothoa parallela inflicts great losses on cultured gilthead sea bream Sparus aurata in Greece. Dis Aquat Organ. 2001;45:237–239. doi: 10.3354/dao045237. [DOI] [PubMed] [Google Scholar]
- Papapanagiotou EP, Trilles JP, Photis G. First record of Emetha audouini, a cymothoid isopod parasite, from cultured sea bass Dicentrarchus labrax in Greece. Dis Aquat Organ. 1999;38:235–237. doi: 10.3354/dao038235. [DOI] [PubMed] [Google Scholar]
- Schuldt M, Rodrigues AC. Biological and pathological aspect of parasitism in the branchial chamber of Palaemonetes argentinus (Crustacea: Bopyridae) by infestation with Probopyrus cf. oviformis (Crustacea: Isopoda) J Invertebr Pathol. 1985;45:139–146. doi: 10.1016/0022-2011(85)90002-3. [DOI] [Google Scholar]
- Thatcher VE, Blumenfeldt CL. Anilocra montti sp. n. (Isopoda, Cymothoidae) a parasite of caged salmon and trout in Chile. Rev Bras Zool. 2001;18:269–275. doi: 10.1590/S0101-81752001000500023. [DOI] [Google Scholar]
- Truesdale FM, Mermilliod WJ. Some observation on the host–parasite relationship of Macrobrachiun ohione (Smith) (Decapoda, Palaemonidae) and Probopyrus bithynis Richardson (Isopoda, Bopyridae) Crutaceana. 1977;32:216–220. doi: 10.1163/156854077X00665. [DOI] [Google Scholar]
- Van Wyk PM. Inhibition of the growth and reproduction of the porcellanid crab Pachycheles rudis by the bopyrid isopods, Aporobopyrus muguensis. Parasitology. 1982;85:459–473. doi: 10.1017/S0031182000056249. [DOI] [Google Scholar]


