Abstract
The objective of the study was to reveal physiological link between sex specific engorgement pattern and evading mechanism of ticks against oxidative stress as well as acaricides. Quantitative determination of nitric oxide radical scavenging, superoxide dismutase activity and reduced glutathione (GSH) concentrations in salivary gland and gut extracts of male and female Hyalomma anatolicum anatolicum (Acari: Ixodidae) ticks established significant variation in antioxidant responses between two sexes of the ticks. Higher activity of these antioxidants and GSH depletion in females clearly indicate stronger antioxidant defense in female ticks which is to combat host mediated oxidative assault during feeding for greater engorgement and reproductive stress. The females are also better equipped with the mechanism of acaricide resistance as evidenced by higher expression of esterases than males in unfed whole tick extracts in current study.
Keywords: Hyalomma anatolicum anatolicum, Oxidative stress, Antioxidants, Esterases, Acaricide resistance
Introduction
Ticks are of vast importance due to their ability to transmit an impressive variety of infectious agents during their blood feeding from respective vertebrate hosts. Hyalomma anatolicum anatolicum (H. a. anatolicum) tick is one of the most important ectoparasite of cattle and buffaloes with a wide host range. It acts as the vector of tropical theileriosis causing annual economic loss of around US $800 million in India (Devendra 1995). Attachment and feeding takes several days to complete for ixodid species, the majority of the blood meal is not taken up until the last day of engorgement (Kemp et al. 1982). Successful engorgement of ticks relies on a pharmacy of chemicals located in their complex salivary glands and secreted in tick saliva that helps them to protect against host mediated oxidative assault (Francischetti et al. 2009). Ticks have evolved antioxidant defenses to circumvent the oxidative assault as evidenced by presence of considerable amount of nitric oxide radical scavenging, superoxide dismutase (SOD) activities and reduced glutathione (GSH) concentrations in Hyalomma ticks (Wu et al. 2010; Ghosh et al. 2014). The tick salivary cocktail also contain esterases which helps in metabolic resistance against acaricides by over expression through gene amplification and point mutations that has been well studied in aphid Myzus persicae (Devonshire et al. 1993) and Drosophila melanogaster (Mutero et al. 1994) respectively. A more detailed knowledge about the expression of these salivary active components is required to understand their importance and association with tick feeding. In this study, we describe the heterogeneity in expression of esterases and antioxidant response between the two sexes of H. a. anatolicum ticks which differ considerably in engorgement pattern where females engorge several hundred times during a single blood meal with maximum increase in size, especially at late feeding stage unlike males which rarely engorge more than two fold from their unfed weight (Chen et al. 2012; Ma et al. 2013) .
Materials and methods
Collection of ticks
Partially fed adult male and female H. a. anatolicum ticks were randomly collected by detaching them from buffaloes from the villages around Hisar, Haryana, India for antioxidant assay where as the unfed male and female ticks were collected from the moulted nymphs at the walls and ground of the animal sheds for esterase profiling.
Dissection of ticks
The partially fed ticks were glued to the bottom of a Petri dish with their dorsal surface upward and placed on ice for 20 min. Using fine scalpel blade and fine tip forceps, ticks were incised along the dorsal-lateral margin, and the dorsal integuments were removed under a stereoscopic dissection microscope (Magnus MSZ-TR Trinocular Version). Salivary glands and gut were removed by the method of Wu et al. (2010) and transferred into preservation buffer (pH 6.0) containing 0.1 M phosphate buffer saline (PBS), 5 % glycerol, protease inhibitor cocktail (Sigma, P2714), and stored at −40 °C until extract preparation.
Extract preparation
The isolated organs from partially fed ticks and the whole unfed ticks were homogenized under ice using tissue homogenizer (IKA T10 basic Ultra-Turrox) in 0.1 M PBS, pH 6.0, containing protease inhibitor cocktail. The homogenate was centrifuged at 10,000 rpm for 15 min at 4 °C. Then the supernatant was filtered through 0.22 µ syringe filter (MILLEX-GV) and stored at −40 °C until further analysis.
Estimation of anti-oxidants
Anti-oxidant activities were estimated in salivary gland and gut extracts of male and female semi-fed H. a. anatolicum ticks.
Nitric oxide radical scavenging activity
Nitric oxide scavenging activity of the extracts was measured by the method of Sreejayan and Rao (1997). Different concentrations of fresh extracts in standard phosphate buffer were incubated with 5.0 mM sodium nitroprusside at 25 °C for 3 h. After incubation 0.5 ml of incubated solution was removed and mixed with equal volume of Griess reagent (equal amount of 0.1 % naphthylethylenediamine dihydrochloride in distilled water and 1 % sulfanilamide in 5 % concentrate phosphoric acid). The absorbance of the chromophore was taken at 546 nm against blank without sodium nitroprusside, using UV–VIS spectrophotometer (Thermo Scientific Multiskan Spectrum—UV/Vis microplate and cuvette spectrophotometer). All data were taken in triplicate.
Superoxide dismutase (SOD) activity
The activity of SOD in tissue extracts was measured by the method of Marklund and Marklund (1974), modified by Madesh and Balasubramanian (1998). Estimation of SOD activity was performed by adding 0.65 ml of PBS and 30 µl of 1.25 mM MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to each of the tubes marked as sample, control and blank. Then 10 µl of tissue homogenate was added to the sample tube followed by addition of 100 µM pyrogallol (75 µl) to all the tubes. After 5 min of incubation at room temperature, 0.75 ml of DMSO (Dimethyl sulfoxide) was added into all the tubes to terminate the reaction followed by addition of 10 µl tissue homogenate to the control tube. The absorbance was measured at 570 nm. The activity of the SOD was calculated as follows
y, % inhibition of MTT reduction by SOD protein; DF, dilution factor.
Reduced glutathione (GSH) assay
The concentration of GSH was estimated by the method of Ellman (1959) modified by Beutler (1971). Tissue homogenate (500 µl) in phosphate buffer (pH-7.4) containing 0.02 M ethylenediaminetetraacetate (EDTA) was mixed with 400 µl of distilled water and 100 µl of 50 % Trichloroacetic acid (TCA) solution. The contents were incubated at room temperature for 20 min and centrifuged at 3000 rpm for 15 min. 800 µl of supernatant was taken and 1 ml of 1 M Tris–HCl (pH-8.0) buffer was added to it. Then 80 µl of DTNB reagent [0.14 M sodium chloride, 0.009 M disodium hydrogen phosphate and 40 mg/100 ml 5,5′-dithiobis (2-nitrobenzoic acid)] was added and kept in reducing condition at room temperature for 15 min. The absorbance was measured at 412 nm. A standard curve of GSH was prepared using concentrations ranging from 0.02 to 0.1 mM of GSH in 0.02 M EDTA and the values of the samples were extrapolated from that curve.
Native Polyacrylamide gel electrophoresis (PAGE) for esterase profiling
The extracts from whole unfed male and female ticks were used for esterase profiling Non-denaturing PAGE was performed using the Laemmli (1970) buffer system without sodium dodecyl sulfate (SDS) and a gel system consisting of a 4 % (w/v) stacking gel and a 12 % (w/v) separating gel. Equal amount of protein from both male and female tick extracts were loaded and electrophoresis was conducted at a constant voltage of 90 volt for 4 h at 4 °C in pre-chilled buffer containing 87 mM Tris and 13 mM glycine (pH 8.3). The gel was stained by pre-incubation in 100 mM sodium phosphate buffer (pH 6.5) for 30 min at 37 °C, followed by incubation in 100 mM phosphate buffer containing 3.2 mM α-naphthyl acetate and 2.4 mM Fast Blue R/R Salt (SIGMA) for 60 min in the dark at 37 °C (Stauffer et al. 1992).
Estimation of protein concentrations
Extracted protein from tick organs as well as whole tick homogenates were quantified by Bradford (1976) method using BSA as the standard.
Statistical analysis
The data were subjected to standard error of means (SE) and analysis of Variance (ANOVA) for statistical significance (Snedecor and Cochran 1967).
Results
Anti-oxidant response
Antioxidant activity in terms of nitric oxide radical scavenging, SOD activity and GSH concentration were estimated in different organs of H. a. anatolicum ticks.
Nitric oxide radical scavenging activity
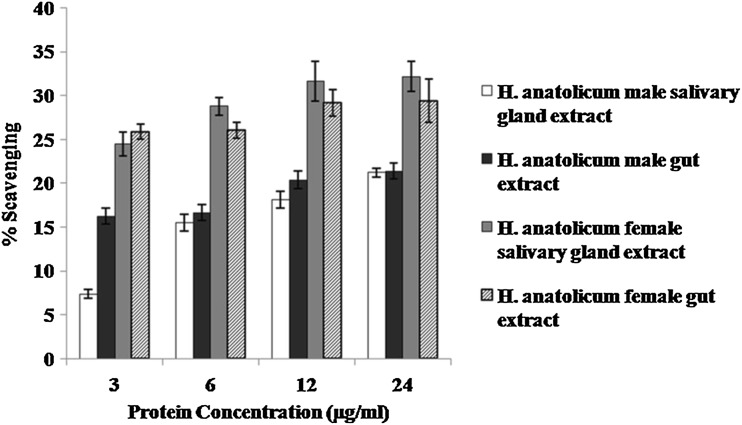
Nitric oxide radical scavenging activity is increased with increase in protein concentrations (3–24 µg/50 µl) in all the extracts (Fig. 1). A significantly (p < 0.05) higher activity was observed in female than male salivary gland and gut extract at all the different protein concentrations.
Fig. 1.
Nitric oxide scavenging activity (%) in salivary gland and gut extracts of semi-fed adult male and female Hyalomma anatolicum anatolicum ticks. Data presented as Mean ± SE
Superoxide dismutase (SOD) activity
Significantly higher (p < 0.05) SOD activity (Fig. 2) was only found in female salivary gland extract than its male counterpart but no such activity was detected in the gut extract of the ticks.
Fig. 2.
Superoxide dismutase activity (%) in salivary gland extracts of semi-fed adult male and female Hyalomma anatolicum anatolicum ticks. Data presented as Mean ± SE
Reduced glutathione (GSH) concentration
Significantly lower (p < 0.05) GSH concentrations (Fig. 3) were found in female salivary gland extract than male in most of the protein concentrations taken where as in case of gut extracts, the males have shown significantly higher (p < 0.05) values than females in most of the protein concentrations except the lowest one.
Fig. 3.
Reduced glutathione (GSH) concentration in salivary gland and gut extracts of semi-fed adult male and female Hyalomma anatolicum anatolicum ticks. Data presented as Mean ± SE
Esterase profiling
Native-PAGE resolves unfed female whole tick extract esterases into three major bands of variable mobility; one band of relatively lower mobility was retained in stacking and separating gel interface. The unfed male whole tick extract showed marked variations from the female (Fig. 4) in esterase expression in terms of absence of two upper bands as well as the lesser band intensity in case of the third band as the amount of protein loaded was same in both the lanes.
Fig. 4.

Esterase profile of whole tick extract from unfed adult female and male Hyalomma anatolicum anatolicum ticks in 12 % Native-PAGE. A unfed female; B unfed male
Discussions
Ticks are constantly challenged with reactive oxygen and nitrogen species (ROS and RNS) generated from endogenous and exogenous sources during their prolonged attachment with the vertebrate host for successful completion of blood meal (Kemp et al. 1982; Francischetti et al. 2009; Saeaue et al. 2011). Host mediated exogenous oxidative assault is more in case of females as it proportionate with their greater engorgement and feeding behavior than males. The females are further prone to endogenous reproductive stress. So, they require a complex mode of antioxidant defense during host-parasite interactions. In the present study, higher nitric oxide radical scavenging activity in SGE and gut extract of partially fed females than males may help them to combat higher feeding stress, especially after mating as well as the reproductive stress. The females are also supported by their significantly higher status of SOD activity in SGE than the males to circumvent the extra burden. Lower SOD activity in male SGE correlates with minimum feeding stress on males due to their lesser feeding and engorgement pattern. In case of GSH status, females have shown relatively lower concentrations than male in both the organ extracts. GSH depletion in females may be due to greater consumption of GSH by glutathione peroxidase mediated disposal of H2O2 which will be more in females as it is exposed to maximum oxidative stress. Because the superoxide radical (O2 •−) produced in stress response is dismuted by SOD to oxygen (O2) and hydrogen peroxide (H2O2) (Fridovich 1995). This H2O2 is also strong oxidative agent which is finally disposed off by hydrogen peroxidase and GSH is used as hydrogen supplier in this reaction (Halliwell and Gutteridge 1989). Thus GSH depletion is more during higher stress response in females. This is in accordance with Saeaue et al. (2011) and Ghosh et al. (2014) where higher stress is associated with more GSH depletion in the vectors. So the above discussions about the differential anti-oxidant responses particularly in term of nitric oxide radical scavenging, SOD activity and GSH status clearly reflect about the stronger antioxidant defense machinery in females to encounter greater feeding as well as reproduction stress which is in accordance to sex specific engorgement pattern in H. a. anatolicum ticks.
The esterases are known to be associated with different physiological processes in insects such as reproduction (Richmond et al. 1980), juvenile hormonal level regulation (Kort and Granger 1981), nervous system functioning and development of insecticide resistance (Guillemaud et al. 1997). The tick esterases also play important roles in host immunomodulation against tick antigens, tick mediated transmission and establishment of pathogens to the host (Temeyer and Tuckow 2016). Theileria transmission is influenced by sexual difference in ticks where female ticks have higher infection rates and levels for Theileria parva and Theileria taurotragi than male ticks. Whereas Amblyomma variegatum males have higher infection rates and levels for Theileria mutans than females (Shaw and Young 1994). In current study, greater expression of different esterases in H. a. anatolicum females (Fig. 4) indicate towards the major role of females in tick life cycle as well as tick mediated pathogen transmission in the current tick species. Whole tick extracts were used to get the maximum number of esterases. Though only three major bands were detected in whole tick extracts in this study rather many other bands detected in different stages of life cycle in different species of ticks by many other studies (Jamroz et al. 2000; Villarino et al. 2003; Baffi et al. 2005, 2007; Al-Shammery and Fetoh 2011) but it clearly indicates about the sexual polymorphism in esterase expression in H. a. anatolicum ticks. Moreover, esterase activity is directly linked with acaricide resistance (Abbas et al. 2014). So, less number of esterase bands observed in current study may be related with more succeptibility of Indian H. a. anatolicum tick to acaricides. Variations in esterase expression may occur during the feeding. So, unfed ticks were used to identify the polymorphic variations at the basal level.
This is the preliminary study describing the putative role and expression of different anti-oxidants and esterases in H. a. anatolicum ticks. Further studies are going on for identification of their temporal expression and characterization of associated mechanisms to develop improved tick control strategies.
Acknowledgments
This research was supported partly with grants from University Grant Commission (UGC), Govt. of India.
Authors’ contributions
MG and NS designed the experiment, data analyses and final corrections of the manuscript. MG and AKS collected the sample. AKS identified the tick species and contributed in dissection of ticks. MG, RK and RSG performed the dissection of ticks, wet lab analyses, and data analyses. All authors participated in manuscript preparation. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- Abbas RZ, Zamanb MR, Colwell DD, Gillearde J, Iqbal Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet Parasitol. 2014;203:6–20. doi: 10.1016/j.vetpar.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Al-Shammery KA, Fetoh BA. Protein and esterase profile patterns of the camel tick Hyalomma (Hyalomma) dromedarii (Koch) (Acari: lxodidae) in Hail and Qassim Regions, Saudi Arabia. IJESE. 2011;2:53–60. [Google Scholar]
- Baffi MA, Pereira CD, Souza GRL, Bonetti AM, de Ceron CR, Goulart Filho LR. Esterase profile in a pyrethroid-resistant Brazilian strain of Boophilus microplus cattle ticks (Acari, Ixodidae) Genet Mol Biol. 2005;28:749–753. doi: 10.1590/S1415-47572005000500016. [DOI] [Google Scholar]
- Baffi MA, Pereira CD, Souza GRL, de Ceron CR, Bonetti AM. Esterase profile in the postembryonic development of Rhipicephalus microplus. Pesqui Agropecu Bras. 2007;42:1183–1188. doi: 10.1590/S0100-204X2007000800016. [DOI] [Google Scholar]
- Beutler E (1971) Red cell metabolism manual of biochemical methods. In: Beutler E (ed). Academic Press, London, pp. 68–70
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen X, Yu Z, Guo L, Li L, Meng H, Wang D, Liu R, Liu J. Life cycle of Haemaphysalis doenitzi (Acari: Ixodidae) under laboratory conditions and its phylogeny based on mitochondrial 16S rDNA. Exp Appl Acarol. 2012;56:143–150. doi: 10.1007/s10493-011-9507-8. [DOI] [PubMed] [Google Scholar]
- Devendra C (1995) Animal production system in South-East and East Asia: potential and challenges for research. In: Gardiner PR, Devendra C (eds) Global agenda for livestock research. Proceedings of a consultation, 18–20 January 1995. ILRI (International Livestock Research Institute), Nairobi, Kenya, pp 41–48
- Devonshire AL, Williamson MS, Moores GD, Field LM. Analysis of the esterase genes conferring insecticide resistance in peach-potato aphid, Myzus perspicae. Biochem J. 1993;294:569–574. doi: 10.1042/bj2940569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Francischetti IM, Sá-Nunes A, Mans BJ, Santos IM, Ribeiro JMC. The role of saliva in tick feeding. Front Biosci. 2009;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Sangwan N, Sangwan AK. Variations in free radical scavenging activities and antioxidant responses in salivary glands of Hyalomma anatolicum anatolicum and Hyalomma dromedarii (Acari: Ixodidae) ticks. Vet World. 2014;7(10):876–881. doi: 10.14202/vetworld.2014.876-881. [DOI] [Google Scholar]
- Guillemaud T, Makate N, Raymond M, Hirst B, Callghan A. Esterase gene amplification in Culex pipiensis. Insect Mol Biol. 1997;6:319–327. [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC (1989) In: Free Radical in Biology and Medicine, 2nd edn. Clarendon Press, Oxford
- Jamroz RC, Guerrero FD, Pruett JH, Oehler DD, Miller RJ. Molecular and biochemical survey of acaricide resistance mechanisms in larvae from Mexican strains of southern cattle tick. J Insect Physiol. 2000;46:685–695. doi: 10.1016/S0022-1910(99)00157-2. [DOI] [PubMed] [Google Scholar]
- Kemp DH, Stone BF, Binnington KC. Tick attachment and feeding: role of the mouthparts, feeding apparatus, salivary gland secretions and host response. In: Obenchain FD, Galun R, editors. Physiology of ticks. Oxford: Pergamon Press; 1982. pp. 119–168. [Google Scholar]
- Kort CA, Granger NA. Regulation of the juvenile hormone titer. Annu Rev Entomol. 1981;26:1–28. doi: 10.1146/annurev.en.26.010181.000245. [DOI] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ma M, Guan G, Chen Z, Liu Z, Liu A, Gou H, Ren Q, Li Y, Niu Q, Yang J, Yin H, Luo J. The life cycle of Haemaphysalis qinghaiensis (Acari: Ixodidae) ticks under laboratory conditions. Exp Appl Acarol. 2013;59:493–500. doi: 10.1007/s10493-012-9617-y. [DOI] [PubMed] [Google Scholar]
- Madesh M, Balasubramanian KA. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys. 1998;35:184–188. [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxyde anion radical in the auto oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Mutero A, Pralavorio M, Bride JM, Fournier D. Resistance associated point mutations in insecticide-insensitive acetylcholinesterase. PNAS. 1994;91:5922–5926. doi: 10.1073/pnas.91.13.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond RC, Gilbert DG, Sheehan KB, Gromko MH, Butterworth FM. Esterase 6 and reproduction in Drosophila melanogaster. Science. 1980;207:1483–1485. doi: 10.1126/science.6767273. [DOI] [PubMed] [Google Scholar]
- Saeaue L, Morales NP, Komalamisra N, Vargas REM. Antioxidative systems defense against oxidative stress induced by blood meal in Aedes aegypti. Southeast Asian J Trop Med Public Health. 2011;42(3):542–549. [PubMed] [Google Scholar]
- Shaw MK, Young AS (1994) The biology of Theileria species in ixodid ticks in relation to parasite transmission. In: Advances in Disease Vector Research, vol 10. Springer-Verlag, New York, pp 23–63 (1254)
- Snedecor GW, Cochran WJ. Statistical methods. 7. New Delhi: Oxford and IBG Publishing Co.; 1967. [Google Scholar]
- Sreejayan N, Rao MNA. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- Stauffer C, Leitinger R, Simsek Z, Schreiber JD, Führer E. Allozyme variation among nine Austrian Ips typographus (L.) (Coleoptera, Scolytidae) populations. J Appl Entomol. 1992;114:17–25. doi: 10.1111/j.1439-0418.1992.tb01091.x. [DOI] [Google Scholar]
- Temeyer KB, Tuckow AP. Tick Salivary Cholinesterase: A Probable Immunomodulator of Host–parasite Interactions. J Med Entomol. 2016;2016:1–5. doi: 10.1093/jme/tjv252. [DOI] [PubMed] [Google Scholar]
- Villarino MA, Waghela SD, Wagner GG. Biochemical detection of esterases in the adult female integument of organophosphate-resistant Boophilus microplus (Acari, Ixodidae) J Med Entomol. 2003;40:52–57. doi: 10.1603/0022-2585-40.1.52. [DOI] [PubMed] [Google Scholar]
- Wu J, Wang Y, Liu H, Yang H, Ma D, Li J, Li D, Lai R, Yu H. Two immunoregulatory peptides with antioxidant activity from tick salivary glands. J Biol Chem. 2010;285:1–19. doi: 10.1074/jbc.X109.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]