Abstract
Microscopic examination of thin blood smear of a domestic pigeon brought to Teaching Veterinary Clinical Complex, Junagadh, Gujarat revealed mature and immature halter shaped gametocytes of Haemoproteus columbae encircling the nucleated erythrocytes. The disease was diagnosed as acute infection of pigeon malaria more notably known as pseudomalaria. The parasitic infection was further confirmed by polymerase chain reaction assay targeting the Cytochrome b (cyt b) gene fragment amplifying 207 base pair (bp) fragment. Pseudolynchia canariensis, the vector for H. columbae was also recovered from underneath the feathers of the affected pigeon.
Keywords: cyt b, Haemoproteus columbae, PCR, Pigeon, Pseudomalaria, Pseudolynchia canariensis
Introduction
Haemoproteus is the largest genus of avian haemosporidian parasites distributed all over the world (Krizanauskiene et al. 2013). It is the most common intracellular haemo parasite of birds, reptiles and amphibians being reported from various bird species (Burry-Caines and Bennett 1992). Among the birds, pigeons are considered to be attractive and raised as pets for thousands of years as a means of religious and/or cultural symbols. Pigeons suffer from variety of metazoan and protozoan infections. In this regard, Haemoproteus columbae, transmitted by Pseudolynchia canariensis, a hippoboscid blood sucking fly affects domestic as well as wild pigeons. The disease caused by H. columbae in pigeon is called as pigeon malaria or pseudomalaria and is lethal chiefly in young pigeons. The parasite widely occurs in pigeons of tropical and subtropical regions. The disease can be diagnosed by observation of halter or crescent shaped gamonts in the erythrocytes partially encircling the nucleus of the host cell (Soulsby 1982). From Indian perspectives, a higher prevalence of H. columbae in pigeons has been documented form Uttar Pradesh and Mumbai (Shinde et al. 2008; Jahan et al. 2011). However, informations regarding occurrence of pseudomalaria particularly, in pigeons is scanty from this geographical part of the country. This paper highlights the diagnosis of a case of severe H. columbae infection in an adult domestic pigeon by conventional as well as polymerase chain reaction (PCR) based assay targeting the cyt b gene fragment.
Materials and methods
Case history and sample collection
An adult domestic pigeon was brought to the Teaching Veterinary Clinical Complex (TVCC), College of Veterinary Science & A.H., Junagadh Agricultural University, Junagadh, Gujarat with a history of reduced feed intake and symptoms of dullness, dyspnea, torticollis and frequent diarrhea. Blood sample was collected aseptically from wing vein by vein-puncture in vials containing EDTA and used for conventional and molecular based assay to detect the infection. The body hair coat was also carefully searched for the presence of ectoparasites, if any.
Conventional parasitological method
Thin blood smears were prepared and subjected to Giemsa staining method following the standard protocol (Soulsby 1982). Briefly, the blood smears were prepared, air dried, fixed in methanol, stained with Giemsa, and examined under oil immersion lens of compound binocular microscope (100×) for the detection of haemoprotozoan parasite.
Molecular diagnostic method
Preparation of genomic DNA
Genomic DNA was extracted from whole blood collected in EDTA coated vacutainer using GENEJET whole blood genomic DNA purification mini kit (Thermo Scientific, Lithuania) as per the given protocol. Briefly, 10 µL of nucleated pigeon blood was taken and the volume was adjusted to 200 µL with 1× PBS. Then, 20 µL of Proteinase K solution was added, mixed by vortexing followed by addition of 400 µL of lysis solution. The above sample was incubated at 56 °C for 10 min. Then 200 µL of absolute ethanol was added. The prepared mixture in spin column was centrifuged at 6000g for 1 min. 500 µL of wash buffer I (WB I) was then added, centrifuged for 1 min at 8000g followed by addition of 500 µL of wash buffer II (WB II) with centrifugation at 20,000g for 3 min. After each centrifugation, the flow through was discarded. Finally in order to elute genomic DNA, 100 µL of elution buffer was added to the centre of the spin column, incubated for 2 min at room temperature and centrifuged for 1 min at 8000g. The purified DNA was collected immediately in downstream applications and stored at −20 °C.
PCR protocol
The PCR assay was optimized targeting a portion of cyt b of H. columbae (Doosti et al. 2014). The sequences of the primers used were as follows:
H. columbae-F: 5′-TTA GAT ACA TGC ATG CAA CTG GTG-3′
H. columbae-R: 5′-TAG TAA TAA CAG TTG CAC CCC AG-3′.
PCR assay in a final volume of 25 µL was carried out in a PCR thermal cycler (Applied Biosystems, USA). The master mix consisted of 2.5 µL of Dream Taq buffer (Thermo Scientific, USA), 0.5 µL of 10 mM dNTP mix (Thermo Scientific, USA), 1 µL each (20 pmol) of the primers, 0.2 µL of recombinant Taq DNA Polymerase (Thermo Scientific, USA) and 2 µL of template DNA isolated from infected pigeon blood. The volume was made up to 25 µL with nuclease-free water. The PCR cycling conditions were set in automated thermal cycler with the following programme: initial denaturation at 94 °C for 4 min, 35 cycles of denaturation at 94 °C for 60 s, annealing at 60 °C for 45 s and extension at 72 °C for 30 s with the final extension at 72 °C for 7 min. The amplified PCR products were resolved by electrophoresis on a 1.2 % agarose gel and visualized using gel documentation system (Vilverlourmat, Bioprint ST4, Germany).
Results and discussion
Infections with Haemoproteus spp. are generally asymptomatic owing to low pathogenicity of the parasite. Clinical examination of the pigeon revealed dullness, depression, dyspnoea, torticollis and frequent diarrhea as reported by other workers (Nematollahi et al. 2012; Varshney et al. 2014). Careful examination of body hair coat revealed the presence of louse fly Pseudolynchia canariensis underneath the wing which acts as the vector for H. columbae (Fig. 1). Microscopic examination of Giemsa stained thin blood smear revealed intra-erythrocytic halter shaped gametocytes encircling the host cell nuclei (Fig. 2). The present observations are similar with that of earlier workers (Weisman 2007; Nematollahi et al. 2012; Borkataki et al. 2013). The parasitaemia in the current study was recorded around 5 %. High parasitaemia can lead to clinical illness in stressed or immunocompromised birds (Greiner and Ritchie 1994) and heavy mortality has been reported due to acute form of infection in nestling pigeons (Dey et al. 2008). The infection was further confirmed by PCR assay targeting a 207 bp fragment of cyt b gene specific for H. columbae (Fig. 3). From India, a higher prevalence of H. columbae in pigeons has been documented form Uttar Pradesh (U.P.) and Mumbai (Shinde et al. 2008; Jahan et al. 2011). Jahan et al. (2011) reported 55.63 % infectivity of H. columbae in rock pigeons of Bareilly regions, U.P. The mean parasitic load of the infected pigeons was 1.68 gametocytes/100 RBC’s. Nandi and Bennett (1997) also documented a prevalence of 54.4 % H. columbae infection in pigeons of U.P. Similar result (58.33 %) has been stated by Shinde et al. (2008) from urban localities of Mumbai, India. Surveys indicate that ecological factors, vector abundance and susceptibility of the bird might play a role in determining the incidence and levels of parasitaemia in different regions.
Fig. 1.

Dorsal view of Pseudolynchia canariensis
Fig. 2.
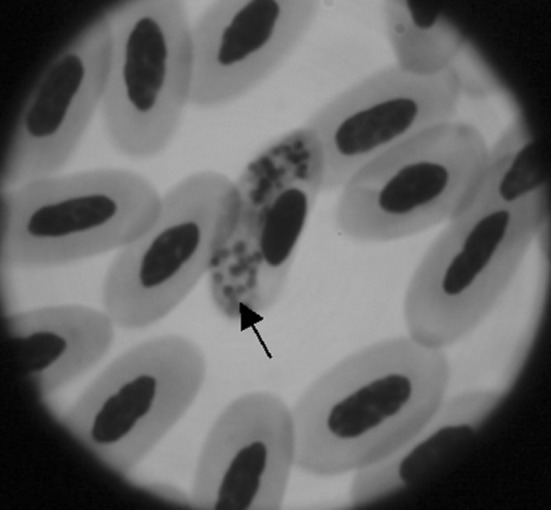
Giemsa stained thin blood smear showing gamont stage of Haemoproteus columbae (marked with arrow) inside the RBC (×100)
Fig. 3.
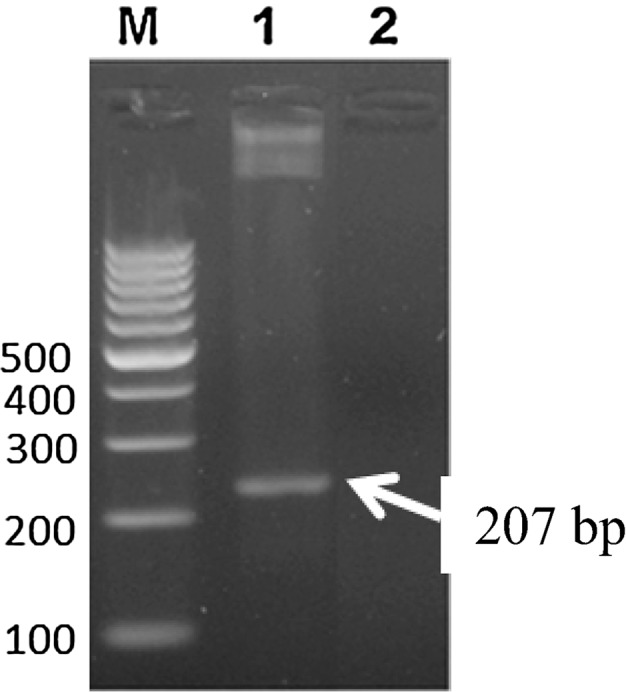
Agarose gel (1.2 %) electrophoresis showing PCR product of cyt b gene fragment specific to H. columbae. Lane M 100 bp DNA ladder. Lane 1 positive amplification of 207 bp specific for H. columbae from the blood of infected pigeon. Lane 2 no amplification from blood of healthy pigeon (negative control)
There is no valid treatment protocol for pigeons infected with H. columbae on account of its nonpathogenic nature. In the present case, the domestic pigeon was provided with Vitamins in the drinking water for 3 weeks to boost up immunity.
In summary, the present study reveals a case of an acute pigeon malaria in a domestic pigeon, its diagnosis by conventional microscopy completed with molecular technique like PCR assay. Presently there appears to no published report on occurrence of pseudomalaria in pigeons from this geographical part of the country. Considering the lack of information about the prevalence of pigeon blood parasites, more conventional along with precise molecular surveys seem to be necessary in this part of the country.
Acknowledgments
The authors would like to acknowledge the officer in Incharge, TVCC, Veterinary College, Junagadh, Gujarat and Principal and Dean, College of Veterinary Science & A.H., Junagadh for providing the necessary facilities.
References
- Borkataki S, Katoch R, Goswami P, Godara R, Khajuria JK, A Yadav, Kour R, Mir I. Incidence of Haemoproteus columbae in pigeons of Jammu district. J Parasit Dis. 2013;39:426–428. doi: 10.1007/s12639-013-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burry-Caines JR, Bennett GF. The haemoproteidae (Apicomplexa: Haemosporina) of the avian families Fringillidae and Emberizidae sensulato. Can J Zool. 1992;70:1149–1160. doi: 10.1139/z92-161. [DOI] [Google Scholar]
- Dey AR, Begum N, Anisuzzaman Khan MAHNA, Mondal MMH. Haemoprotozoan infection in ducks: prevalence and pathology. Bangladesh J Vet Med. 2008;6:53–58. doi: 10.3329/bjvm.v6i1.1339. [DOI] [Google Scholar]
- Doosti A, Ahmadi R, Mohammadalipour Z, Zohoor A (2014) Detection of Haemoproteus columbae in Iranian pigeons using PCR. In: International conference on Biological, Civil and Environmental Engineering, pp 36–38
- Greiner EC, Ritchie BW. Parasites. In: Ritchie BW, Harrison GJ, Harrison LR, editors. Avian medicine: principles and application. Lake Worth, FL: Wingers; 1994. pp. 1007–1029. [Google Scholar]
- Jahan N, Chandra R, Mohammad S. Parasitimic load of haematozoan parasite in rock pigeon. Recent Res Sci Technol. 2011;3:9–11. [Google Scholar]
- Krizanauskiene A, Iezhova TA, Sehgal RNM, Carlson JS, Palinauskas V, Bensch S, Valkiunas G. Molecular characterization of Haemoproteus sacharovi (Haemosporida, Haemoproteidae), a common parasite of columbiform birds, with remarks on classification of haemoproteids of doves and pigeons. Zootaxa. 2013;3613(1):85–94. doi: 10.11646/zootaxa.3616.1.7. [DOI] [PubMed] [Google Scholar]
- Nandi NC, Bennett GF. The prevalence, distribution and check list of avian haematozoa in the Indian subcontinent. Rec Zool Surv Ind. 1997;96(1–4):83–150. [Google Scholar]
- Nematollahi A, Ebrahimi M, Ahmadi A, Himan M. Prevalence of Haemoproteus columbae and Trichomonas gallinae in pigeons (Columba domestica) in Isfahan, Iran. J Parasit Dis. 2012;36(1):141–142. doi: 10.1007/s12639-011-0082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde GN, Gantne ML, Singh A. Prevalence of parasites in pigeons (Columbia liviadomestica) of Mumbai. J Vet Parasitol. 2008;22:65–66. [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. London: Bailliere Tindal; 1982. [Google Scholar]
- Varshney JP, Deshmukh VV, Chaudhary PS. Pseudomalaria (Haemoproteus columbae) in pigeon shelter. Intas Polivet. 2014;15(I):176–177. [Google Scholar]
- Weisman J (2007) Haemoproteus infection in avian species. In: Veterinary clinical pathology clerkship program, University of Georgia College of Veterinary Medicine, Athens, GA


