Abstract
Senna alexandrina Mill. has been used for antimicrobial activity. In the present study, the crude ethanolic extract of the plant and a synthetic compound Sennoside were tested in vitro on Hymenolepis diminuta to evaluate its potential anthelmintic efficacy through ultrastructural changes. Worms were maintained between rat model and beetle and the test parasites were exposed to different concentrations of crude ethanolic leaf extracts of S. alexandrina. Praziquantel was used as a reference drug. Dose dependent efficacy was observed in terms of motility and time of mortality in all treated parasites. Ultrastructural micrography revealed irrevocable destruction all over the body tegument accompanied with sloughing of microtriches and swellings of the basal lamina. Vacuolization of the syncytium along with sparsely cytoplasmic cytons and depletion of parenchymatous layer were observed accompanied by deformities in the cell organelles. Extensive deformities in the tegument indicates that the plant extract alter membrane permeability of the parasite leading to paralysis and subsequent death. Thus, S. alexandrina can be regarded as a potential anthelmintic agent.
Keywords: Anthelmintic, Senna, Cestode, Hymenolepis, Tegument
Introduction
Intestinal worm infections in human are still amongst the most common till today. The impact of cestode (tapeworms) is considerable and can be endemic (and under-reported) in developing regions of the world (Scholz et al. 2009). Parasites have shown resistance to existing drugs and their high costs warrants the search for alternative sources of anthelmintic drugs (Abdel-Ghaffar et al. 2011). Screening and proper evaluation of the claimed medicinal plants could offer possible alternatives that may both be sustainable and environmentally acceptable.
Senna alexandrina Vahl. (family: Fabaceae), commonly known as Senna, is a perennial shrub found abundant throughout South India and West Bengal (Souza et al. 2011). Medicinal properties of Senna were first explored by Arabians since 900 AD (Abulafatih 1987). Due to its great reputation in the Unani system of medicine, it has been accepted by various traditional medical systems of the world, including Ayurveda, Homeopathy and Allopathy in Indian, British, US and many other pharmacopoeias of the world (Shahina 1994). The laxative principle sennoside isolated from Senna leaves and pods, forms the important ingredients in purgative medicines and are dispensed in tablets (Verloop et al. 2004).
Extracts of this plant are used in folk medicine to treat certain gastrointestinal disorders (Souza et al. 2011). Scientific credence was given to the plant for its anticestodal activity on Railietina tetragona integument disruption by Kundu and Lyndem (2012). Cestodes depend on its tegument for nutrient absorption and protection as it lacks digestive system (Halton 2004). Being so vital to the parasite, any change in its tegument caused by a drug becomes important. In view of this, the present study was taken up to further validate the anthelmintic efficacy of the plant by ascertaining if the plant derived components and its crude extract caused any alteration in the tegumental architecture at the sub-cellular level of another cestode parasite Hymenolepis diminuta as this parasite is easily maintained in laboratory rodent model.
Materials and methods
Preparation of plant extract
Fresh plant voucher specimen VBSL-3 was deposited in the Central National Herbarium (CNB), Kolkata, India. Semi dried leaves of S. alexandrina were collected from herbal shop at West Bengal and identified by a scientist from CNB Kolkata, washed with distilled water, allowed to dry in an oven at 50 °C, and crushed to powder. About 250 g of the powder was extracted with 1 l of ethanol (90 %) in Soxhlet apparatus for 7–8 h, and the final crude extract (11.85 g) was recovered by using rotary evaporator and stored at 4 °C until further use.
Drugs and chemicals
Ethanol was supplied by Bengal Chemicals, Kolkata, India and the reference drug Praziquantel (PZQ) with trade name Distocide (composed of 600 mg PZQ) is a product of Chandrabhaghat Pharma Pvt Ltd., Mumbai, India. Sennoside A and B were purchased from Sigma Aldrich, USA.
Parasites
Hymenolepis diminuta was maintained by routine passage through Sprague-Dawley rats and beetle Tribolium confusum (intermediate host). Each host was infected with 8 cysticercoids. At 20–22 days post-infection, worms were recovered from rat intestine. Experiments with animals were performed following the approval of the protocol by the Institutional Animal Ethnics Committee (IAEC) of Visva-Bharati University.
In vitro treatment
Worms were washed several times in 0.9 % phosphate buffer saline (PBS) and placed in four groups. Group 1 containing crude extract of S. alexandrina (10, 20 and 40 mg/ml concentration), Group 2, PZQ (0.0010, 0.0025, and 0.0050 mg/ml); Group 3, Sennoside A and Group 4, Sennoside B (both having concentrations 0.025, 0.1 and 0.5 mg/ml), all prepared with 0.9 % PBS at pH 7.4 and 1 % dimethylsulfoxide (DMSO). One set with only PBS and 1 % DMSO was used as a control. All experiments were maintained at 37 ± 1 °C in the incubator. Time of paralysis (PT) was noted at different time intervals when no movement could be observed except when shaken vigorously or kept in slightly warmer PBS whereas time of mortality (TM) was recorded when worms neither moved when shaken vigorously, nor when dipped in warm PBS.
Assessment of plant treatment of electron microscope studies
Paralysis of worms at 40 mg/ml with plant extract was observed within a time span comparable with that of PZQ, at 005 mg/ml but at different concentrations. Immediately after paralysis, treated worms and control were fixed for scanning electron microscope (SEM) studies following standard method Roy and Tandon (1991) and viewed in a Jeol JSM 6360 at an electron accelerating voltage of 20 kV.
Other sets of worms after paralysis were washed gently with warm PBS and fixed for transmission electron microscope (TEM) studies according to the standard protocol of Sophisticated Analytical Instrumentation Facility (SAIF), North Eastern Hill University (NEHU). JEOL JEM-100 CX-II electron microscope was used for observation in ultrathin sections at a magnification range of 2000–27,000 X.
Statistical analysis
Data are presented as mean values ± standard deviations for each group (n = 6). For determining statistical significance, standard deviation and analysis of variance (ANOVA) at 5 % level of significance were employed; P < 0.001 was considered significant.
Results
Dose response studies
Dose dependent efficacy was observed with different concentrations of S. alexandrina crude extracts. The post-paralytic time was comparatively shorter in all concentrations of S. alexandrina compared to PZQ. Treatment with a different concentration of Sennoside A and B does not show any dose response in terms of motility and mortality, but after 36 h of incubation slow movements were observed in a concentration of 0.5 mg/ml. However control parasite survived up to 69.22 ± 0.23 h (Table 1).
Table 1.
Effects of crude leaf extract of S. alexandrina, Sennoside A, Sennoside B and PZQ on H. diminuta
| Concentration (mg/ml) | PT (h) | TM (h) | |
|---|---|---|---|
| S. alexandrina | 10 | 10.64 ± 0.2 | 25.24 ± 0.29 |
| 20 | 5.38 ± 0.2 | 20.01 ± 0.11 | |
| 40 | 2.32 ± 0.1 | 16.65 ± 0.3 | |
| PZQ | 0.0010 | 1.18 ± 0.04 | 22.21 ± 0.12 |
| 0.0025 | 0.67 ± 0.03 | 18.80 ± 0.05 | |
| 0.0050 | 0.31 ± 0.01 | 17.31 ± 0.13 | |
| Sennoside A | 0.025 | – | – |
| 0.1 | – | – | |
| 0.5 | After 36 h slow movement | – | |
| Sennoside B | 0.025 | – | – |
| 0.1 | – | – | |
| 0.5 | After 36 h slow movement | – |
Survivability of control parasite is 69.22 ± 0.23 h. Each value represented as mean ± SD (n = 6)
PT time of paralysis, TM time of mortality
Ultrastructural alterations
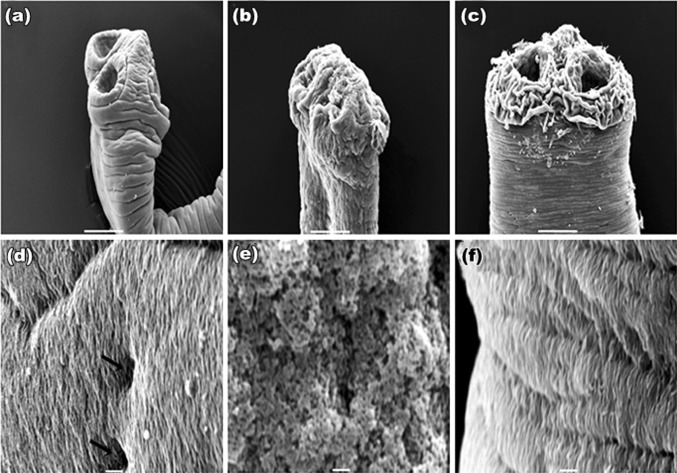
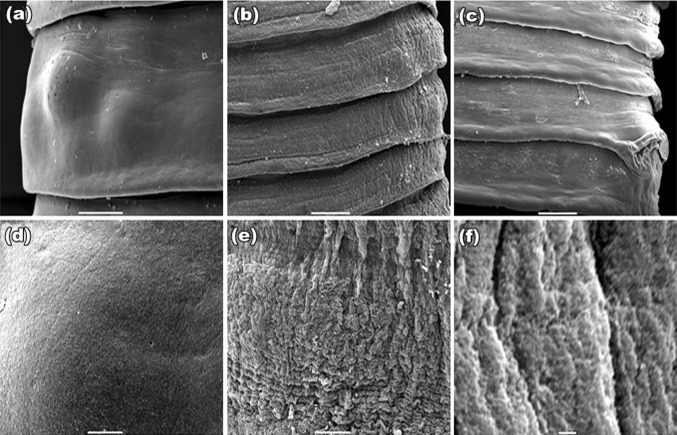
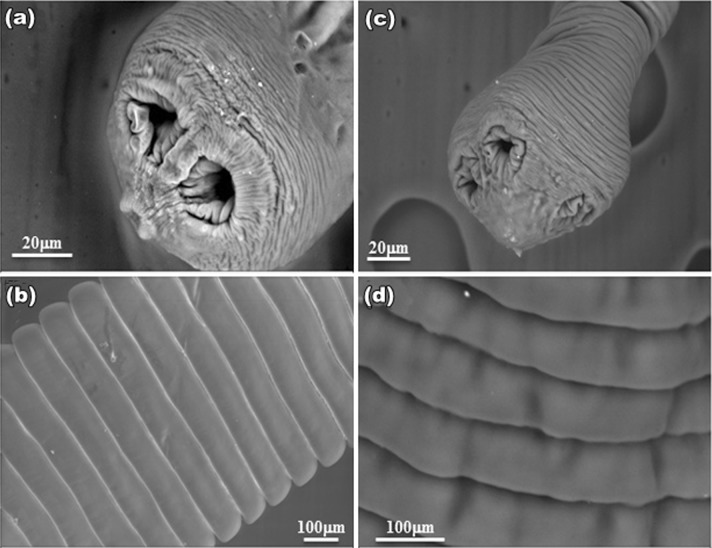
SEM studies of control worm revealed a normal body contour with scolex at its anterior end (Fig. 1a). The tegument of proglottids revealed a uniform, trapezoid shape with densely covered microtriches giving worm surface a velvety appearance (Fig. 1d). Treated worms possessed irrevocable destruction all over the general topography of the body; tegument of the scolex was extensively damaged, and the suckers get constricted with plant extracts (Fig. 1b) as that observed in PZQ (Fig. 1c). Microtriches completely loses their velvety appearance and intense sloughing and papillae become indistinguishable (Fig. 1e) which is of more damage than drug treated parasite (Fig. 1f). The overall trapezoid shape of the proglottid found in control (Fig. 2a), became disorganized. Shrinkage in the proglottids and constrictions at their rims occurred in both plant and PZQ-treated worms (Fig. 2b, c respectively). At higher magnifications, the velvety appearance found at gravid proglottids (Fig. 2d) is lost and exfoliation in the tegument occurred in both plant- and PZQ-treated parasites (Fig. 2e, f respectively). On the other hand, worms exposed to Sennoside A and B (Fig. 3a–d) showed less wrinkles, and insignificant damage as compared to PZQ and plant crude extracts.
Fig. 1.
SEM of scolex of H. diminuta: a control: scolex with suckers; b S. alexandrina: showing shrunken scolex; c PZQ: sloughed off tegument near suckers. All bars, 50 μm. d control: velvety appearance with distinguished papillae (rightwards arrow); e S. alexandrina: microtriches totally sloughed off, deep pits occurred in the tegument; f PZQ: papillae covered with loose microtriches. All bars, 1 μm
Fig. 2.
SEM of matured proglottid: a control: typical trapezoid with defined rim; b S. alexandrina: shrinkage; c PZQ: shrinkage and blebbings at the rims of the proglottid. All bars, 20 μm; d control: smooth velvety appearance; e S. alexandrina tearing and vacuolization occur; f PZQ vacuolization
Fig. 3.
SEM of H. diminuta treated with Sennoside A: a Scolex showed very little shrinkage (bar 20 μm); b mature proglottids remain unaltered and revealed control like architecture (bar 100 μm). Sennoside B; c Scolex showed very little shrinkage (bar 20 μm); d matured proglottid showed little infoldings (bar 100 μm)
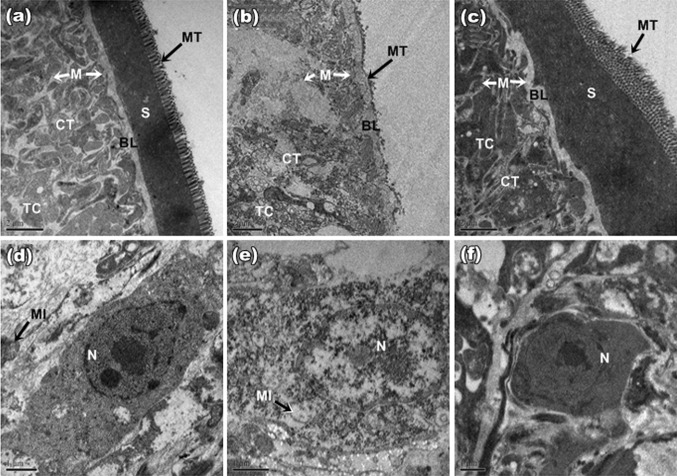
TEM studies of control worm revealed typical cestode structure with outer tegument having abundant microtriches (M) serially localized followed by a compact syncytial layer (S). Distal cytoplasm is an electron dense and has an abundance of tegumentary cytons (TC). Basal lamina (BL) and subtegumental muscles (M) are well organized (Fig. 4a). Nucleus bearing well defined nucleolus with condensed chromatin region and electron dense nucleoplasm (Fig. 4d). With S. alexandrina treatment (Fig. 4b) degenerative changes were noticeable in the tegument. It appeared as stripped right down to the BL and the remnants of basal infolds left behind were highly swollen. Serially arranged row of microtriches were totally damaged and clumped. Tegument appeared to be completely sloughed off, leaving an exposed BL that was only present in remnants and there was a release of underlying structures to the outside at the basal lamina. Parenchymatous muscle layer showed intense degradation and was loosely stacked up with a loss in continuity in the plant extract. Worms treated with PZQ also showed major damage to the tegument, with microthrix layer damaged in many places and the muscle stacks showed degeneration (Fig. 4c). The parenchymal cytons of treated parasite showed complete loss of connections with surrounding parenchyma and the chromatin in nucleus appeared clumped into large areas of electron-dense heterochromatin in both plant and drug treated parasite (Fig. 4e, f).
Fig. 4.
TEMs of tegument of H. diminuta: a Control: intact microthrix (MT) cover the free surface of the syncytium (S). Connective tissue (CT) and superficial musculature (M) with non-disrupted basal lamina (BL). b S. alexandrina: MT are totally damaged and clumped, exposing BL and CT, sparse syncytium with few tegumentary cytons (TC) and intense vacuolization; degradation of musculature (M); c PZQ: MT disorganized and stripped off. All bars, 2 μm. d Control: distinct nucleus (N) with well defined nucleolus, condensed chromatin, electron dense nucleoplasm and mitochondria (MI); e S. alexandrina: vacuolized N with electron lucency inside, mitochondrial damage observed; f PZQ: nuclear membrane irregular, no vacuolization in N. All bars, 1 μm
As no noticeable effects observed on the tegument of treated worms with Sennosides A and B, no TEM studies were undertaken on this aspect.
Overall damage caused by the plant is more pronounced than that of the drug in terms of structural alteration.
Discussion
The present investigation illustrates that Senna extract showed high potential anthelmintic efficacy in H. diminuta. TM of worms in control medium had an insignificant with PT, which validates that there was no precession of paralysis to death. Similar studies were also observed with many ethnomedicinal plants (Ferreira et al. 2011; Kundu and Lyndem 2012; Kundu et al. 2012; Vijaya and Yadav 2014). A dose-dependent efficacy was observed in treated worms as an increase in concentration shortens the paralysis period. These observations were also recorded by some authors (Schmahl et al. 2007; Xiao et al. 2010; Yadav and Tangpu 2012). Further, many chemically derived marketed drugs showed dose-dependent efficacy as well as tegumental alterations (Markoski et al. 2006). Although significant mortality was not observed in lowest concentration (10 mg/ml), still a considerable significant paralysis occurred. It may therefore be suggested that plant extracts possibly exert a reversible action on the neuromuscular system of worms and though it did not cause mortality for some time to follow, but once paralyzed, it took very short time for mortality to commence. Thus, it may be suggested that it possesses a vermifugal activity in nature and the inactiveness caused would last long enough for the parasites to be swept out of the host’s body (Martin et al. 1997).
The first and most obvious pathological effects of plant treatment observed under SEM were blebs on the worm’s surface. Many of these blebs were partially covered with perforated membrane. Surface blebbing is a common feature observed in helminthes after treatment with either drug or plant extracts (Meaney et al. 2004; Bashter et al. 2011; Hossain et al. 2012; Giri and Roy 2014). This can be regarded as a stress response resulting from emergency repair to a damaged tegument and may be induced by many harmful events (Perez-Serrano et al. 1994; Stitt and Fairweather 1994).
The contour of a scolex, its suckers and organization of the proglottids and microtriches were severely deformed and clumped. Such distortions may be attributed to tegumental enzymes since they are the primary target of such actions (Das et al. 2004; Dasgupta et al. 2010). Further, the present study also revealed infoldings on the edge of proglottid which may be due to an increase in chloride ion conductance of worm muscle membrane producing hyperpolarization and reducing excitability that could lead to muscle relaxation and flaccid paralysis (Martin 1985).
Progressive erosion of BL as a consequence of exposure to the test materials were observed in the present study, are indicative of the destructive effects on the parasite. Depletion of the parenchyma cells in treated worms was evidenced by TEM. This corresponds to the decrease in total glycogen content (Wastling et al. 1994). Glycogen depletion and mitochondrial dysfunction would, in combination, impair the tegument through reduced glycolysis. In the PZQ treated parasites, most of the damage induced were tegument, subtegument degeneration, but on a much lesser scale than the plant extract-treated ones. Some of the responses seen, like stripping of tegument, increase in electron lucency of the nucleus and surrounding parenchyma, are typical of a generalized stress response and have been described in other helminthes as well (Buchanan et al. 2003; O’Neill et al. 2009; Dasgupta et al. 2010). The ultrastructural changes such as, clumping of chromatin into heterochromatin are indicative of protein synthesis inhibition (Stitt and Fairweather 1996). Botanicals like artemisinins are known to cause a collapse of the membrane potential of mitochondria, leading to their swelling and inhibition of electron transfer and oxidative phosphorylation (Wakabayashi and Karbowski 2001; Li et al. 2005). However there are compounds or phytochemicals that possess less activity than crude extract (Bizimenyera et al. 2006). This is also depicted in our findings with Sennosides. The results suggest that the sennoside may have only purgative effect but not anthelmintic effect and therefore it may be speculated that other compounds present in the plant may be the active ingredients responsible for the anthelmintic activity as S. alexandrina revealed the presence of alkaloids, flavonoids, glycosides, saponins and sterols (Ahmed et al. 2012).
Senna extract caused swelling of the basal infolds and intense vacuolization of the tegument as observed herein. Swelling of the basal infolds could have an osmotic basis, due to impairment of energy-dependent ion pumps (Anderson and Fairweather 1995; Meaney et al. 2004). Further, vacuolization of the tegument has been known to be induced by a triggering of calcium ion flux with PZQ (Jiraungkoorskul et al. 2006).
Conclusion
From the present study, it can be concluded that the plant extracts have significant structural effects on the parasites and thus support the plausibility of this plant as an anthelmintic agent. Further investigation is, however required to isolate and reveal the active compound (s), mode of action before clinical investigation in target species.
Acknowledgments
The authors gratefully acknowledge the University Grants Commission (UGC), New Delhi (No. UGC/SR/40-385/2011), for providing financial assistance through a major research project sanctioned to Larisha M. Lyndem and UGC Research Fellowship in Science for Meritorious Students to the first author. We also wish to thank the Department of Zoology, VisvaBharati and SAIF, NEHU for providing infrastructural support.
Author contribution
The major part of the experimental work was performed by the first author. To carry out this work the second and fourth author maintained the parasitic model between Sprague-Dawley rats and beetle Tribolium confusum while the third author is helping in the crude extract preparation, the last and corresponding author is finalizing the manuscript.
Compliance with ethical standards
Conflict of interest
None.
References
- Abdel-Ghaffar F, Margit S, Al-Rasheid KAS, Strassen B, Fischer K, Aksu G, Klimpel S, Mehlhorn H. The effects of different plant extracts on intestinal cestodes and on trematodes. Parasitol Res. 2011;108:979–984. doi: 10.1007/s00436-010-2167-5. [DOI] [PubMed] [Google Scholar]
- Abulafatih HA. Medicinal plants of Southwestern Saudi Arabia. Econ Bot. 1987;41(3):354–360. doi: 10.1007/BF02859051. [DOI] [Google Scholar]
- Ahmed S, Zahid A, Abidi S, Meer S. Ant-emetic activity of four species of Genus Cassia in chicks. IOSR J Pharm. 2012;2(3):380–384. [Google Scholar]
- Anderson HR, Fairweather I. Fasciola hepatica: ultrastrucural changes to the tegument of juvenile flukes following incubation in vitro with the deacetylated (amine) metabolite of diamphenathide. Int J Parasitol. 1995;25(3):319–333. doi: 10.1016/0020-7519(94)00105-W. [DOI] [PubMed] [Google Scholar]
- Bashter AR, Hassanein M, Abdel-Ghaffar F, Al-Rasheid K, Hassan S, Mehlhorn H, Al-Mahdi M, Morsy K, Al-Ghamdi A. Studies on monieziasis of sheep I. Prevalence and anthelminthic effects of some plant extracts a light and electron microscopic study. Parasitol Res. 2011;108:177–186. doi: 10.1007/s00436-010-2060-2. [DOI] [PubMed] [Google Scholar]
- Bizimenyera ES, Githiori JB, Eloff JN, Swan GE. In vitro activity of Peltophorum africanum Sond (Fabaceae) extracts on the egg hatching and larval development of the parasitic nematode Trichostrongylus colubriformis. Vet Parasitol. 2006;142:336–343. doi: 10.1016/j.vetpar.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Buchanan JF, Fairweather I, Brennan GP, Trudgett A, Hoey EM. Fasciola hepatica: surface and internal tegumental changes induced by treatment in vitro with the sulphoxide metabolite of albendazole (‘Valbazen’) Parasitology. 2003;126(Pt 2):141–153. doi: 10.1017/S0031182002002664. [DOI] [PubMed] [Google Scholar]
- Das B, Tandon V, Saha N. Effect of phytochemicals of Flemingia vestita (Fabaceae) on glucose 6-phosphate dehydrogenase and enzymes of gluconeogenesis in a cestode (Raillietina echinobothrida) Comp Biochem Physiol C Toxicol Pharmacol. 2004;139(123):141–146. doi: 10.1016/j.cca.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Roy B, Tandon V. Ultrastructural alterations of the tegument of Raillietina echinobothrida treated with the stem bark of Acacia oxyphylla (Leguminosae) J Ethnopharmacol. 2010;127(2):568–571. doi: 10.1016/j.jep.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Ferreira JFS, Peaden P, Keiser J. In vitro trematocidal effects of crude alcoholic extracts of Artemisia annua, A. absinthium,Asimina triloba and Furmura officinalis. Parasitol Res. 2011;109:1585–1592. doi: 10.1007/s00436-011-2418-0. [DOI] [PubMed] [Google Scholar]
- Giri BR, Roy B. Resvaratrol induced structural and biochemical structure alterations in the tegument of Raillietina echinobothrida. Parasitol Int. 2014;63:432–437. doi: 10.1016/j.parint.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Halton DW. Microscopy and the helminth parasite. Micron. 2004;35:361–390. doi: 10.1016/j.micron.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Hossain E, Chandra G, Nandy AP, Mandal SC, Gupta JK. Anthelmintic effect of a methanol extract of leaves of Dregea volubilis on Paramphistomum explanatum. Parasitol Res. 2012;110:809–814. doi: 10.1007/s00436-011-2558-2. [DOI] [PubMed] [Google Scholar]
- Jiraungkoorskul W, Sahaphong S, Sobhon P, Riengrojpitak S, Kangwanrangsan N. Schistosoma mekongi: the in vitro effect of praziquantel and artesunate on the adult fluke. Exp Parasitol. 2006;113:16–23. doi: 10.1016/j.exppara.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Kundu S, Lyndem LM. In vitro screening for cestocidal activity of three species of Cassia plants against the tapeworm Raillietina tetragona. J Helminthol. 2012;87:154–159. doi: 10.1017/S0022149X12000156. [DOI] [PubMed] [Google Scholar]
- Kundu S, Roy S, Lyndem LM. Cassia alata L: potential role as anthelmintic agent against Hymenolepis diminuta. Parasitol Res. 2012;111:1187–1192. doi: 10.1007/s00436-012-2950-6. [DOI] [PubMed] [Google Scholar]
- Li W, Mo W, Shen D, Sun L, Wang J, Lu S, Gitschier JM, Zhou B. Yeast model uncovers dual roles of mitochondria in the action of artemisinin. PLoS Genet. 2005;1(3):0329–0334. doi: 10.1371/journal.pgen.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoski MM, Trindale ES, Cabrera G, Laschuk A, Galanti N, Zaha A, Nader HB, Ferreira HB. Praziquantel and albendazole damaging action on in vitro developing Mesocestoides corti (Platyhelminths: Cestoda) Prasitol Int. 2006;55:51–61. doi: 10.1016/j.parint.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Martin RJ. γ-Aminobutyric acid- and piperazin-activated single-channel currents from Ascaris summ body muscle. Br J Pharmacol. 1985;84:445–461. doi: 10.1111/j.1476-5381.1985.tb12929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RJ, Robertson AP, Bjorn H. Target sites of anthelmintics. Parasitology. 1997;11:S111–S124. [PubMed] [Google Scholar]
- Meaney M, Fairweather I, Brennan GP, Forbes AB. Transmission electron microscope study of the ultrastructural changes induced in the tegument and gut of Fasciola hepatica following in vivo drug treatment with clorsulon. Parasitol Res. 2004;92:232–241. doi: 10.1007/s00436-003-1036-x. [DOI] [PubMed] [Google Scholar]
- O’Neill JF, Johnston RC, Halferty L, Brennan GP, Keiser J, Fairweather I. Adult triclabendazole-resistant Fasciola hepatica: morphological changes in the tegument and gut following in vivo treatment with artemether in the rat model. J Helminthol. 2009;83:151–163. doi: 10.1017/S0022149X09344934. [DOI] [PubMed] [Google Scholar]
- Perez-Serrano J, Casado N, Denegri G, Rodriguez-Caabeiro F. The effects of albendazole and albendazole sulphoxide combination-therapy on Echinococcus granulosus in vitro. Int J Parasitol. 1994;24(2):219–224. doi: 10.1016/0020-7519(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Roy B, Tandon V. Usefulness of tetramethylsilane in the preparation of helminth parasites for scanning electron microscopy. Riv Parassitol. 1991;8:207–215. [Google Scholar]
- Schmahl G, Mehlhorn H, Harder A, Klimpel S, Krieger KJ. Effect of a combination of emodepside plus praziquantel against larval and adult stages of nematodes (Trichuris muris,Angistrongylus cantonensis) in rodents. Parasitol Res. 2007;101:77–84. doi: 10.1007/s00436-007-0614-8. [DOI] [Google Scholar]
- Scholz T, García HH, Kuchta R, Wicht B. Update on the human broad tapeworm (genus Diphyllobothrium) including clinical relevance. Clin Microbiol Rev. 2009;22(1):146–160. doi: 10.1128/CMR.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahina AG. A hand book of Arabian medicinal plants. Boca Raton: CRC Press; 1994. [Google Scholar]
- Souza DE, Pereira MO, Bernardo LC, Carmo FS, Fonseca AS, Bernardo-Filho M. An experimental model to study the effects of a senna extract on the blood constituent labelling and biodistribution of a radiopharmaceutical in rats. Clinics (Sao Paulo) 2011;66(3):483–486. doi: 10.1590/S1807-59322011000300021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt AW, Fairweather I. The effect of the sulphoxide metabolite of triclabendazole (Fasinex) on the tegument of mature and immature stages of the liver fluke Fasciola hepatica. Parasitology. 1994;108(Pt5):555–567. doi: 10.1017/S0031182000077428. [DOI] [PubMed] [Google Scholar]
- Stitt AW, Fairweather I. Fasciola hepatica: disruption of the vitelline cells in vitro by the sulphoxide metabolite of triclabendazole. Parasitol Res. 1996;82(4):333–339. doi: 10.1007/s004360050122. [DOI] [PubMed] [Google Scholar]
- Verloop Q, Liebenberg W, Marais AF, Lötter AP, de Villiers MM. Compound laxative formulations for substituting phenolphthalein with sennosides A & B in solid dosage forms. Trop J Pharm Res. 2004;3(1):265–277. [Google Scholar]
- Vijaya, Yadav AK. In vitro anthelmintic assessment of the phytochemicals against Hymenolepis diminuta, a zoonotic tapeworm. J Parasit Dis. 2014 doi: 10.1007/s12639-014-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi T, Karbowski M. Structural changes of mitochondria related to apoptosis. Biol Signals Recept. 2001;10:26–56. doi: 10.1159/000046874. [DOI] [PubMed] [Google Scholar]
- Wastling JM, Mackenzie K, Chappell LH. Cyclosporin A: drug treatment in vivo affects the kinetics of [14C] glucose transport in Hymenolepis microstoma in vitro. Parasitology. 1994;108(Pt2):223–228. doi: 10.1017/S0031182000068323. [DOI] [PubMed] [Google Scholar]
- Xiao SH, Xue J, Li-li X, Zhang Y. Effectiveness of mefloquine against Clonorchis sinensis in rats and Paragonimus westermani in dogs. Parasitol Res. 2010;107:1391–1397. doi: 10.1007/s00436-010-2007-7. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Tangpu V. Anthelmintic activity of ripe fruit extract of Solanum myriacanthum Dunal (Solanaceae) against experimentally induced Hymenolepis diminuta (Cestoda) infections in rats. Parasitol Res. 2012;110(2):1047–1053. doi: 10.1007/s00436-011-2596-9. [DOI] [PubMed] [Google Scholar]