Abstract
The current study describes a simple, rapid and eco-friendly method for the synthesis of silver nanoparticles (AgNPs) using Excoecaria agallocha (E. agallocha) leaf extract as stabilizer, bioreductant and capping agent. Synthesized AgNPs were characterized by UV–Visible spectroscopy (UV–Vis), X-ray diffraction (XRD), fourier transform infrared (FT-IR) spectroscopy, field emission scanning electron microscopy (FESEM) and energy dispersive X-ray spectroscopy (EDX). Generation of AgNPs was initially confirmed with the color change from yellow to dark brown which produces intense absorbance spectra at 440 nm in UV–Vis spectroscopy without any shifting of peaks. Further, XRD pattern confirms that the synthesized AgNPs was face centered cubic (fcc) crystalline in structure with an average size of 20 nm. On the other hand, FTIR spectrum reveals that the active metabolites like water soluble phenolic compounds, flavonoids, methylene groups, amides and carboxylate groups. These active biocompounds plays a vital role in the reduction of Ag+ into their nanoscale values, it also acts as a stabilizing and surface functionalization agent. FESEM micrographs of synthesized AgNPs shows spherical and hexagonal shaped well dispersed particles in the dimension ranging between 23 and 42 nm. EDAX confirms the presence of silver (Ag) as the major constituent element without any impurities; also substantiate the stability of generated AgNPs. The biomedical insights of nanoparticles (NPs) were assessed through radical scavenging and antibacterial properties. Additionally, synthesized AgNPs was also exhibits an excellent cytotoxic effect against human breast carcinoma cell lines (MCF-7). This study proves that synthesized AgNPs can be developed as a potential nano-drug formulation to combat pathogenic disease and also for the expansion of breast cancer therapy.
Keywords: Green synthesis, Silver nanoparticles, Excoecaria agallocha, Antimicrobial activity, Breast cancer
Introduction
Nanobiotechnology is an emerging and energetic field of research on particles at nano scale level. Development of environmentally benign bioprocess for the synthesis of NPs is one of the most important areas of research in material science and system engineering (Laura Castro et al. 2014). There are diverse physical and chemical methodologies have been followed for the synthesis of NPs, but they remain toxic, expensive and required hazardous chemicals as reducing agents (Chen et al. 2007). In other hand, there is an urgency to develop a high yield, cost effective, non-toxic and an eco-friendly procedure for the synthesis of NPs. As a result, green nanotechnology fulfills the above stated requirements. The main concept of green nanotechnology is to be exploited the locally available biodiversity with potential, novel and an economic concept of bioprospecting. Boisselier and Astruc (2009) reported the bio-molecules like proteins, phenols, saponins, flavonoids are not only playing a role in reducing the ions into particles but also play an important role in the capping of NPs.
Excoecaria agallocha (Euphorbiaceae, eye blinding tree) is an important medicinal mangrove tree which is used for the treatment of rheumatism, paralysis, cutaneous infection, leprosy, lauric, palmitic, linolenic, linoleic, oleic, stearic and also having potential antimicrobial and anti-tumor activity (Jayaweera 1980; Padmanabhan and Ayyakkannu 1985; Karalai et al. 1994). Green synthesis of AgNPs using plant extracts have been studied previously using several medicinal plants which includes Rhizophora mucronatas (Gnanadesigan et al. 2011), Acalypha indica (Krishnaraj et al. 2010), Phyllanthus emblica (Rosarin et al. 2010), Panicum virgatum (Mason et al. 2012), Piper nigrum (kumar et al. 2014), Glycine max (Vivekanandhan et al. 2009), Murraya Koengii (Christensen et al. 2011), Rosa rugosa (Dubey et al. 2010) and Camellia sinensis (Vilchis-Nestor et al. 2008).
Emerging multiple drug resistance (MDR) bacteria created a demand for an immediate need to identify novel antibacterial agents with large surface area (Konoshima et al. 2001; Anjaneyulu and Rao 2003; Sadhasivam et al. 2010). By the advancement of nanotechnology, AgNPs were identified for their appreciable antimicrobial potential. However, the mode of action is not clearly understood, but it suppresses the microorganisms by interacting with their membrane, enzymes, proteins or nucleic acids to inhibit cell proliferation (Singh et al. 2011; Kumar et al. 2014). Green synthesized NPs have exhibited excellent antioxidant activity (Nagaraj and Yong 2013; Swamy et al. 2015; Kumar et al. 2014). Bio-synthesized AgNPs was performed to analyze the cytotoxic effect against MCF-7 breast cancer cell lines (Saxena et al. 2010; Akim et al. 2013). Hence, the present study was focused on the green synthesis of multifunctional AgNPs using E.agallocha leaf extract as novel bioreductant towards various applications in nanomedicine.
Materials and methods
Biosynthesis of AgNPs
The fresh leaves of Excoecaria agallocha were collected from Pichavaram Mangrove forest and confirmed by the Department of Botany, Annamalai University, Annamalai Nagar, Tamilnadu, India. The collected leaves were washed in running water and shade-dried at room temperature for 10 days. The fully dried leaves were powdered with a sterile electric blender. The powdered samples were preserved in an airtight container and away from the sunlight for further use. Phytochemical screening of E. agallocha aqueous leaf extracts were performed to identify the phytochemical constituents (Abou El-Nour et al. 2010). Two grams of powdered leaf were mixed with 100 ml of de-ionized sterile water and heat at 100 °C for 30 min. Filter was made using Whatman No. 1 filter paper and the residue was re-extracted using vacuum pumps. The AgNPs was prepared according to the procedure suggested by Song and Kim (2009).
Characterization of AgNPs
For the synthesis of AgNPs, the reaction mixture was prepared by blending 20 ml of 1 mM silver nitrate (AgNO3) solution with 2 ml of aqueous leaf extract. The formation of AgNPs was observed by scanning the absorbance spectra of reaction mixture at regular interval with UV–Vis spectrophotometer (Hitachi-U-2001) from 300 to 800 nm wavelength range. The AgNPs solution was dropped on glass on XPERT-PRO, D-8, with 30 kv, 40 mA with Cu kα radians at 2θ for XRD measurement. XRD is a rapid analytical technique used for phase identification of a crystalline material and provides information on unit-cell dimensions. The crystallite size was calculated using the Scherrer’s formula, D = Kλ/βcosθ, where K is the Scherrer’s constant (shape factor), λ is the X-ray wavelength and β is the Full width Half Maximum (FWHM) and θ is the angle of diffraction. The dry powder of the NPs was prepared deionised water and centrifuge at 10,000 rpm for 15 min for FT-IR study. The residue was re-suspended in sterile de-ionized water to remove the biological impurities. Pure residue was dried in oven at 70 °C overnight, the dried NPs was mixed with KBr powder and pelletized after drying the transmittance were recorded using Perkin-Elmer spectrum instrument resolution of 4 cm−1 in the transmission mode of 4000–400 cm−1. The surface study of the AgNPs was examined by FESEM (BRUKER-INDIA, FESEM). Thin film of the sample was prepared with aluminum foil by dropping a small amount of the sample onto the copper grid. The reduced Ag+ ions were dried on an aluminum-foil-coated copper grid and EDX analysis of the sample was performed using FESEM equipped with an EDX attachment.
Antibacterial and antioxidant activities of AgNPs
The antibacterial property of bio-synthesized AgNPs and E. agallocha aqueous leaf extract was performed against gram-positive [Staphylococcus aureus (MTCC-9760), Bacillus cereus (MTCC-6840)] and gram-negative [Salmonella typhi (MTCC-8767), Pseudomonas aeruginosa (MTCC-10462)] bacteria by Kirby-Bauer disk diffusion method (Bauer et al. 1966). Briefly, the bacteria cultures swabbed uniformly on Mulller-Hinton Agar (MHA) plates using sterile cotton swabs. To evaluate the antibacterial, the different concentrations of (25, 50 and 100 mg/L) of AgNPs were prepared. The activity was compared with aqueous leaf extract and sparfloxacin (5 µg). The inoculated Pertiplates were incubated at 37 °C for 24 h and after the incubation period, the inhibition zone was measured. Different concentrations of AgNPs (10, 20, 30, 40 and 50 µl/ml) were prepared, from the each concentration 3 ml of AgNPs solution was mixed with 1 ml of DPPH solution for the antioxidant study and the ascorbic acid (5 µg/ml) used as control. The tubes were incubated in dark place for 30 min and absorbance was read at 517 nm using UV–Visible spectrophotometer (Braca et al. 2001).
MTT assay
MCF-7 cell lines were collected from National Centre for Cell Science (NCCS), Pune, India. The cell viability was estimated using the MTT calorimetric technique. The cell lines were cultured in 96 well plate for 6 h at 37 °C and kept in CO2 incubator. 100 µL of MTT solution [3-(4,5-dimethlthiazoyl-2)-2,5-diphenyltetrazolium bromide, a yellow tetrazole] without phenol red with series of 20–100 µg/L was added to each well and incubated for 6 h at 37 °C, for the reduction of MTT by metabolically active cells. Purple color formazone crystals were formed which dissolved in 100 µL of dimethyl sulphoxide (DMSO) and read at 540 nm absorbance in an ELISA plate reaader (Thermo, Multiskan). The percentage of viability was calculated by using the following formula (Sukirtha et al. 2012),
Results and discussion
Synthesis of AgNPs
Preliminary phytochemical investigation of E.agallocha aqueous extract shows the presence alkaloids, saponin, phenol, tannin and flavanoid (Table 1) (Jayanta kumar et al. 2009; Deepa and Padmaja 2014). Synthesis of NPs was initially observed by color change from colorless to yellow when the extract mixed with AgNO3, after the incubation time yellow color was changed in dark brown in color (Fig. 1a, b), which designates the formation of AgNPs.
Table 1.
Phyto-chemical screening of crude aqueous leaf extract of E. agallocha
| Si. no | Phytochemicals | Result |
|---|---|---|
| 1. | Alkaloids | + |
| 2. | Glycosides | − |
| 3. | Tannins | + |
| 4. | Saponins | + |
| 5. | Flavanoids | + |
| 6. | Phenolic compounds | + |
| 7. | Cardiac glycosides | − |
Fig. 1.

a Synthesis of NPs using E. agallocha leaf extract with Ag+ ions at initial point and b reaction mixture after 5 h
Characterization of AgNPs
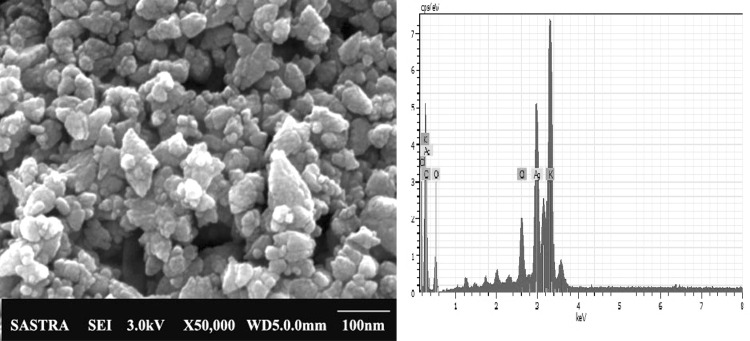
The color change is mainly due to excitation of surface plasmonic resonance which generates maximum absorbance spectra at 440 nm without any shifting (Fig. 2), which further confirms the generation of AgNPs with unique optical properties (Mohamed et al. 2014). XRD pattern of AgNPs showed the peak values 32.03°, 38.06°, 43.62°, 46.29° and 64.42° at 2θ and corresponding to (111), (111), (200), (120) and (110) planes reflect the face centered cubic (FCC) crystalline structure (Fig. 3). The size of the AgNPs ranged between 17.85 and 23.98 nm with the average size of 20 nm. The plane values (111) and (200) coincident with Mostafa et al. (2014), Ponarulselvam et al. (2012), Murali Krishna et al. (2015). In addition, (110) plane value coincident with Geethalakshmi and Sarada (2010) and (120) value coincident with Jayaseelan et al. (2012). FTIR transmittance of AgNPs and E.agallocha leaf extract imitating a complex nature of functional groups (Fig. 4). The bands at 3373.07, 2930.07, 1636.25, 1381.96 and 1039.71 cm−1 are corresponding to O–H stretching in alhocols, flavonoids and phenolic (Mittal et al. 2014), large asymmetric –CH stretches of methylene groups (Gregory et al. 2012), C=O stretching of amides (Rajasekar et al. 2013), symmetrical stretching of carboxylate groups and C–O stretches vibration of alhocolic groups (Zahir and Rahuman 2012). The slight shift in peak positions 771.03 and 616.74 cm−1 of the synthesized AgNPs indicates that the various phytochemical present in the leaf extract. Existences of the functional groups represented the capping of different molecules, which worked together with the metals, contributes the reduction of ions, and followed by stabilization of NPs. So, it states the secondary metabolites available in the form of phytochemicals are understood to be bio-reducing agents (Mohana Roopan et al. 2013). FESEM micrographs clearly displays that the green synthesized AgNPs were spherical, hexagonal in shape some particles were irregular and mostly aggregated with size ranged from 23 to 42 nm (Fig. 5b). In EDX, a sharp peak indicates the reduction of silver metal with strong and weak peaks indicated the other groups of K, Ca, Na, Mg, S and Cl also bound to the surface of the AgNPs (Fig. 5a) (Mallikarjuna et al. 2014).
Fig. 2.
UV–visible spectrum of biosynthesized AgNPs and its Plasmon excitation upon the interaction with E. agallocha extract
Fig. 3.
XRD pattern of synthesized AgNPs using E. agallocha leaf extract
Fig. 4.
FTIR spectrum of green synthesized AgNPs and E. agallocha leaf extract
Fig. 5.
a EDX and b FESEM analysis of synthesized AgNPs using E. agallocha leaf extract
Antibacterial and antioxidant activities of AgNPs
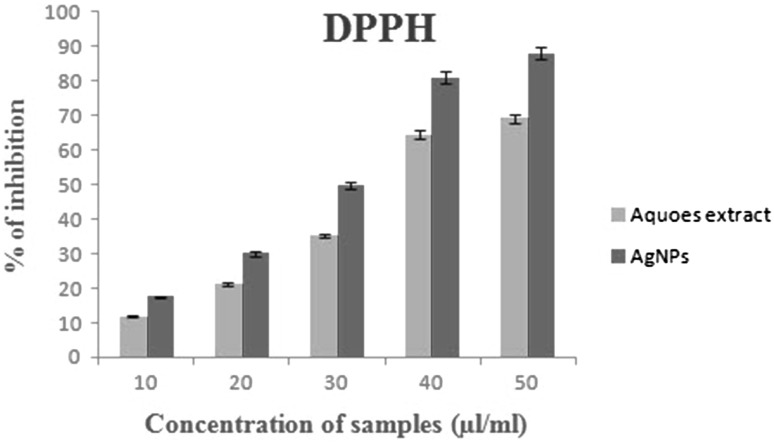
Antibacterial activity of AgNPs was found to have a higher inhibitory effect when compared to aqueous leaf extract (Fig. 6). The synthesized AgNPs shows higher inhibition zone against gram-negative bacteria (Salmonella typhi and Pseudomonas aeruginosa) compared to the gram-positive bacteria (Staphylococcus aureus and Bacillus cereus). Here, the phenomenon is well understood that the particle surface area and size of green synthesized NPs played a vital role in their interaction with bacterial cells. Due to their large surface area and size of AgNPs produces an electric effect. This effect increased attaching strength of the NPs to the cell membrane and penetrated into the bacteria. Inhibitory effect differed cell wall structures between gram negative and positive of bacteria. According to Kaviya et al. (2011) and Muthuvel et al. (2014) the gram-negative bacteria exhibited higher antibacterial activity than a gram-positive bacteria because of their the cell wall texture. This contains a polypeptide thick layer and polysaccharide chain, form more complex structure lead to difficult to penetration of the AgNPs compared to gram-negative bacteria where the cell wall possessed thin layer of peptioglycon. DPPH is a stable chemical and it will be reduced by the addition of hydrogen or electrons. The radical scavenging activity of AgNPs and E. agallocha leaf aqueous extract were quantified by UV–Vis spectroscopy with changing the DPPH color from purple to yellow. The inhibition percentage of the DPPH activity of AgNPs was higher with the increased concentrations (Fig. 7). This observation is coincident with Piper longum fruits (Reddy et al. 2014).
Fig. 6.
Antibacterial activity of synthesized AgNPs using E. agallocha leaf extract by disk diffusion method (A commercial antibiotic, C Control (AgNO3); 1–25, 2–50 and 3–100 µl of AgNPs solution)
Fig. 7.
Estimation of DPPH in phytosynthesized AgNPs and plant leaf extact
In vitro anticancer activity of AgNPs
In vitro cytotoxic effect of AgNPs was screened against the MCF-7 cell lines. The cell viability of MCF-7 cell lines was observed for 24, 48 and 72 h, after treatment with biosynthesized AgNPs. From Table 2, the increasing concentration of AgNPs by decreasing cell viability of MCF-7 cell lines. The dose dependent cell viability was measured in AgNPs treated MCF-7 cells. The fifty percent of cell death indicated the inhibitory concentration (IC50) value of AgNPs against the MCF-7 cell lines. After 72 h of incubation of cell lines showed higher 92 % of dead cells than 24 and 48 h. However, the toxicity of synthesized AgNPs showed a higher effect than E. agallocha leaf extract and AgNO3 at the same incubation period (Vivek et al. 2012; Castiglioni et al. 2015; Mollick et al. 2015).
Table 2.
Percentage of cells Viability on MCF-7 cells was measured after treatment with biosynthesized AgNPs in 24, 48 and 72 h by MTT assay
| Treatment | 24 h | 48 h | 72 h | |||
|---|---|---|---|---|---|---|
| Dead (%) | Viable (%) | Dead (%) | Viable (%) | Dead (%) | Viable (%) | |
| MCF-7 cell + leaf extract | 22.00 ± 0.10 | 78.00 | 37.00 ± 0.03 | 63.00 | 46.12 ± 1.02 | 54.00 |
| MCF-7 cell + AgNO3 | 16.01 ± 0.91 | 84.00 | 21.02 ± 0.12 | 79.00 | 23.21 ± 1.57 | 77.32 |
| MCF-7 cell + 50 µg/mL AgNPs | 35.10 ± 1.02 | 65.21 | 46.21 ± 0.59 | 54.20 | 64.21 ± 0.3 | 36.02 |
| MCF-7 cell + 100 µg/mL AgNPs | 50.01 ± 1.21 | 50.00 | 75.20 ± 1.15 | 25.00 | 92.00 ± 1.06 | 8.00 |
Conclusion
In the present study, AgNPs were effectively synthesized using a Mangrove plant E.agallocha as stabilizing and capping agents. The green synthesized AgNPs were characterized by XRD, FESEM, FT-IR and EDX for structural morphology, functional groups and elemental studies respectively. The average size of the AgNPs was 20 nm with face centered cubic structure. Further, biomedical prospective of AgNPs were characterized in in vitro antioxidant and antibacterial activities. In addition, cytotoxic potential of AgNPs expressed the cell death against MCF-7 breast cancer’s cell lines. The productivity of this current investigation demonstrates a wide range of applications of bioactive AgNPs synthesized by green method.
References
- Abdel-Aziza MS, Shaheen MS, El-Nekeety AA, Abdel-Wahhab MA. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J Saudi Chem Soc. 2014;18(4):356–363. doi: 10.1016/j.jscs.2013.09.011. [DOI] [Google Scholar]
- Abou El-Nour KM, Eftaiha A, Al-Warthan A, Ammar RA. Synthesis and applications of silver nanoparticles. Arab J Chem. 2010;3:135–140. doi: 10.1016/j.arabjc.2010.04.008. [DOI] [Google Scholar]
- Akim AM, Tung EE, Chong PP, Hamzah MY, Dahlan KZM (2013) Nanoparticle-encapsulated tamoxifen inducing cytotoxic effect on Mcf-7 breast cancer cell lines. In: Proceedings of 4th International conference on biomedical engineering in vietnam IFMBE, vol 49, pp 226–228. (doi:10.1007/978-3-642-32183-2)
- Anjaneyulu AS, Rao VL. Seco diterpenoids from Excoecaria agallocha L. Phytochemistry. 2003;62(4):585–589. doi: 10.1016/S0031-9422(02)00269-8. [DOI] [PubMed] [Google Scholar]
- Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493–496. [PubMed] [Google Scholar]
- Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38(6):1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- Braca A, De Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia tarapotensis. J Nat Prod. 2001;64(7):892–896. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- Castiglioni S, Cazzaniga A, Perrotta C, Maier JA. Silver nanoparticles-induced cytotoxicity requires ERK activation in human bladder carcinoma cells. Toxicol Lett. 2015;237(3):237–243. doi: 10.1016/j.toxlet.2015.06.1707. [DOI] [PubMed] [Google Scholar]
- Castro L, Blázquez ML, Muñoz JÁ, González FG, Ballester A. Mechanism and applications of metal nanoparticles prepared by bio-mediated process. Rev Adv Scie Eng. 2014;3(3):199–216. doi: 10.1166/rase.2014.1064. [DOI] [Google Scholar]
- Chen M, Feng YG, Wang X, Li TC, Zhang JY, Qian DJ. Silver nanoparticles capped by oleylamine: formation, growth and self-organization. Langmuir. 2007;23:5296–5304. doi: 10.1021/la700553d. [DOI] [PubMed] [Google Scholar]
- Christensen L, Vivekanandhan S, Misra M, Mohanty AK. Biosynthesis of silver nanoparticles using Murraya koenigii leaf: an investigation on the effect of broth concentration in reduction mechanism and particle size. Adv Mater Lett. 2011;2(6):429–434. doi: 10.5185/amlett.2011.4256. [DOI] [Google Scholar]
- Deepa M, Padmaja CK. Preliminary phytochemical analysis and thin layer chromatography of the extracts of Excoecaria agallocha L. Int J Pharmal Sci Res. 2014;5(10):4535–4542. [Google Scholar]
- Dubey SP, Lahtinen M, Sillanpää M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf A Physicochem Eng Asp. 2010;364(1–3):34–41. doi: 10.1016/j.colsurfa.2010.04.023. [DOI] [Google Scholar]
- Geethalakshmi R, Sarada DVL (2010) Synthesis of plant-mediated silver nanoparticles using Trianthema decandra extract and evaluation of their anti microbial activities. Int J Eng SciTech 2(5):970–975. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.165.5420&rep=rep1&type=pdf
- Gnanadesigan M, Anand M, Ravikumar S, Maruthupandy M, Vijayakumar V, Selvam S, Dhineshkumar M, Kumaraguru AK. Biosynthesis of silver nanoparticles by using mangrove plant extract and their potential mosquito larvicidal property. Asian Pac J Trop Med. 2011;4(10):799–803. doi: 10.1016/S1995-7645(11)60197-1. [DOI] [PubMed] [Google Scholar]
- Jayanta Kumar P, Tapan Kumar P, Sakti KR, Nabinkumar D, Hrudyanth T. Phytochemical screening and Antimicrobial assessment of leaf extract of Excoecaria agallocha L. a Mangal species of Bhitarkanika, Orissa, India. Adv Nat Appl Sci. 2009;3(2):241–246. [Google Scholar]
- Jayaseelan C, Rahuman AA, Rajakumar G, Santhoshkumar T, Kirthi AV, Marimuthu S, Bagavan A, Kamaraj C, Zahir AA, Elango G, Velayutham K, Rao KV, Karthik L, Raveendran S. Efficacy of plant-mediated synthesized silver nanoparticles against hematophagous parasites. Parasitol Res. 2012;111(2):921–933. doi: 10.1007/s00436-011-2473-6. [DOI] [PubMed] [Google Scholar]
- Jayaweera DMA. Medicinal plants (Indigenous and exotic) used in Ceylon—Part III. Colombo: The National Science Council of Sri Lanka; 1980. [Google Scholar]
- Karalai C, Wiriyachitra P, Opferkuch HJ, Hecker E. Cryptic and free skin irritants of the daphnane and tigliane types in latex of Excoecaria agallocha. Planta Med. 1994;60:351–355. doi: 10.1055/s-2006-959499. [DOI] [PubMed] [Google Scholar]
- Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasan K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochem Acta Part A Mol Biomol Spectrosc. 2011;79(3):594–598. doi: 10.1016/j.saa.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Konoshima T, Konishi T, Takasaki M, Yamazoe K, Tokuda H. Anti-tumor-promoting activity of the diterpene from Excoecaria agallocha. II. Biol Pharm Bull. 2001;24(12):1440–1442. doi: 10.1248/bpb.24.1440. [DOI] [PubMed] [Google Scholar]
- Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B Biointerfaces. 2010;76(1):50–56. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Kumar B, Smita K, Cumbal L, Debut A. Synthesis of silver nanoparticles using Sacha inchi (Plukenetia volubilis L.) leaf extracts. Saudi J Biolo Sci. 2014;21(6):605–609. doi: 10.1016/j.sjbs.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallikarjuna K, John Sushma N, Narasimha G, Manoj L, Raju BDP. Phytochemical fabrication and characterization of silver nanoparticles by using Pepper leaf broth. Arab J Chem. 2014;7(6):1099–1103. doi: 10.1016/j.arabjc.2012.04.001. [DOI] [Google Scholar]
- Mason C, Vivekanandhan S, Misra M, Mohanty AK. Switchgrass (Panicum virgatum) extract mediated green synthesis of silver nanoparticles. World J Nano Sci Eng. 2012;2(2):47–52. doi: 10.4236/wjnse.2012.22008. [DOI] [Google Scholar]
- Mittal AK, Bhaumika J, Kumar S, Banerjee UC. Biosynthesis of silver nanoparticles: elucidation of prospective mechanism and therapeutic potential. J Coll Interface Sci. 2014;415:39–47. doi: 10.1016/j.jcis.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Mohana Roopan S, Rohit Madhumitha G, Abdul Rahuman A, Kamaraj C, Bharathi A, Surendra TV. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. J Ind Crop Prod. 2013;43:631–696. doi: 10.1016/j.indcrop.2012.08.013. [DOI] [Google Scholar]
- Mostafa MHK, Eman HI, El-Baghdadyc KZ, Mohameda D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab J Chem. 2014;7(6):1131–1139. doi: 10.1016/j.arabjc.2013.04.007. [DOI] [Google Scholar]
- Mollick MR, Dipak R, Sandeep Kumar D, Chattopadhyay S, Bhowmick B, Maity D, Mondal D, Pattanayak S, Roy S, Chakraborty M, Chattopadhyay D. Studies on green synthesized silver nanoparticles using Abelmoschus esculentus (L.) pulp extract having anticancer (in vitro) and antimicrobial applications. Arabian J Chem. 2015 [Google Scholar]
- Murali Krishna I, Bhagavanth Reddy G, Veerabhadram G, Madhusudhan A. Eco-friendly green synthesis of silver nanoparticles using Salmalia malabarica: synthesis, characterization, antimicrobial, and catalytic activity studies. App Nanosci. 2015 [Google Scholar]
- Muthuvel A, Adavallan K, Balamurugan K, Krishnakumar N. Biosynthesis of gold nanoparticles using Solanum nigrum leaf extract and screening their free radical scavenging and antibacterial properties. Biomed Prev Nutr. 2014;4(2):325–332. doi: 10.1016/j.bionut.2014.03.004. [DOI] [Google Scholar]
- Nagaraj B, Yong RL. Synthesis of silver nanoparticles using Satsuma mandarin (Citrus unshiu) peel extract: a novel approach towards waste utilization. Mat Lett. 2013;109(15):31–33. [Google Scholar]
- Padmanabhan K, Ayyakkannu K (1985) Bioactive compounds from marine plants. II. Antibiotic properties of Excoecaria agallocha L. from Pichavaram mangroves. In: Bosale LJ (ed) Proceedings of national symposium of biology, utilization and conservation Mangroves. Shivaji University, Kolhapur, pp 319–321
- Ponarulselvam S, Panneerselvam C, Murugan K, Aarthi N, Kalimuthu K, Thangamani S. Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pac J Trop Biomed. 2012;2(7):574–580. doi: 10.1016/S2221-1691(12)60100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekar P, Priyadharshini S, Rajarajeshwari T, Shivashri C. Bio-inspired synthesis of silver nanoparticles using Andrographis paniculatawhole plant extract and their antimicrobial activity over pathogenic microbes. Int J Res Biomed Biotech. 2013;3(3):47–52. [Google Scholar]
- Reddy NJ, Nagoor Vali D, Rani M, Rani SS. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. J Mater Sci Eng. 2014;34:115–122. doi: 10.1016/j.msec.2013.08.039. [DOI] [PubMed] [Google Scholar]
- Rosarin FS, Arulmozhi V, Nagarajan S, Mirunalini S. Antiproliferative effect of silver nanoparticles synthesized using Amla on Hep2 cell line. Asian Pac J Trop Med. 2010;6(1):1–10. doi: 10.1016/S1995-7645(12)60193-X. [DOI] [PubMed] [Google Scholar]
- Sadhasivam S, Shanmugam P, Yun K. Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf B: Biointerfaces. 2010;81(1):358–362. doi: 10.1016/j.colsurfb.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Saxena A, Tripathi RM, Singh RP. Biological synthesis of silver nanoparticles by using onion (Allium cepa) extract and their antibacterial activity. Dig J Nanometer Biostruct. 2010;5:427–432. [Google Scholar]
- Singh C, Sharma V, Naik PK, Khandelwal V, Singh H. Green biogenic approach for synthesis of gold and silver nanoparticles using Zingiber officinale. J Nanomater Bios. 2011;6:535–542. [Google Scholar]
- Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009;32(1):79–84. doi: 10.1007/s00449-008-0224-6. [DOI] [PubMed] [Google Scholar]
- Sukirtha R, Priyanka K, Antony JJ, Kamalakkannan S, Thangam R, Gunasekaran P, Krishnana M, Achiraman S. Cytotoxic effect of green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and Lymphoma mice model. Proc Biochem. 2012;47(2):273–279. doi: 10.1016/j.procbio.2011.11.003. [DOI] [Google Scholar]
- Swamy MK, Sudipta KM, Jayanta K, Balasubramanya S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl Nanosci. 2015;5(1):73–81. doi: 10.1007/s13204-014-0293-6. [DOI] [Google Scholar]
- Vilchis-Nestor AR, Sanchez-Mendieta V, Camacho-Lopez MA, Gomez-Espinosa RM, Camacho-Lopez MA, ArenasAlatorre J. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater Lett. 2008;62(17–18):3103–3105. doi: 10.1016/j.matlet.2008.01.138. [DOI] [Google Scholar]
- Vivek R, Thangam R, Muthuchelian K, Gunasekaran P, Kaveri K, Kannan S. Green synthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Proc Biochem. 2012;47:2405–2410. doi: 10.1016/j.procbio.2012.09.025. [DOI] [Google Scholar]
- Vivekanandhan S, Misra M, Mohanty AK. Biological synthesis of silver nanoparticles using Glycine max (soybean) leaf extract: an investigation on different soybean varieties. J Nanosci Nanotech. 2009;9(12):6828–6833. doi: 10.1166/jnn.2009.2201. [DOI] [PubMed] [Google Scholar]
- Von White G, II, Kerscher P, Brown RM, Morella JD, McAllister W, Dean D, Kitchens CL. Green synthesis of robust, biocompatible silver nanoparticles using garlic extract. J Nanomater. 2012 doi: 10.1155/2012/730746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahir AA, Rahuman AA. Evaluation of different extracts and synthesised silver nanoparticles from leaves of Euphorbia prostrata against Haemaphysalis bispinosa and Hippobosca maculata. Vet Parasitol. 2012;187(3–4):511–520. doi: 10.1016/j.vetpar.2012.02.001. [DOI] [PubMed] [Google Scholar]