Abstract
Pneumocystis pneumonia due to Pneumocystis jirovecii infection is an emerging health problem not only for HIV-infected patients but also for other immunocompromised patients in many countries. We compared Gomori methenamine silver (GMS), Toluidine Blue O (TBO) and Giemsa staining methods using standard procedures. The sensitivity and specificity of GMS were 100 %. The sensitivity and specificity of TBO were 96 and 100 %, respectively. The sensitivity and specificity of Giemsa stain were 84 and 90 %, respectively. Only GMS had positive and negative predictive values of 100 % while PPV and NPV for TBO were 100 and 90.9 %, and for Giemsa stain were 95.4 and 69.2 %, respectively. Therefore, our results suggest that if TBO or Geimsa stains are used as the primary staining methods in a clinical laboratory, then confirmation with a GMS staining method should be performed to increase the sensitivity and specificity of the final test result.
Keywords: Pneumocystis jirovecii, Staining method, Sensitivity, Specificity
Introduction
Pneumocystis jirovecii is the causative agent of Pneumocystis pneumonia (PCP) in patients with acquired immunodeficiency syndrome (AIDS) and other immunosuppressived patients especially in transplant recipient patients (Calderón-Sandubete et al. 2002). It is demonstrated that PCP can developed in 5–15 % of patients who underwent kidney, lung and heart–lung transplantation (Goto and Oka 2011). PCP has non-specific clinical symptoms such as dyspnea, fever and nonproductive cough that are similar to many infectious agents. Although a variety of polymerase chain reaction (PCR) methods, such as nested-PCR and real-time PCR, have been developed for the diagnosis of PCP, these assays are not conventional in most clinical microbiology laboratories and are not easily available, especially in developing countries (Bandt and Monecke 2007; Contini et al. 1998; Larsen et al. 2002). Therefore, the standard laboratory method for diagnosis of PCP is microscopic examination of the clinical specimen such as induced sputum, bronchoalveolar lavage (BAL) and lung biopsy after some types of staining methods(Takahashi et al. 2002).
Immunosuppressived patients due to AIDS and use of immunosuppressive therapy are increasing worldwide especially in developing countries. Following this condition, laboratories are receiving increasing numbers of requests for the diagnosis of P. jirovecii. The purpose of this study was to compare three commonly used staining methods for the direct detection of P. carinii (as a model for P. jirovecii) in the BAL of experimentally infected rats.
Materials and methods
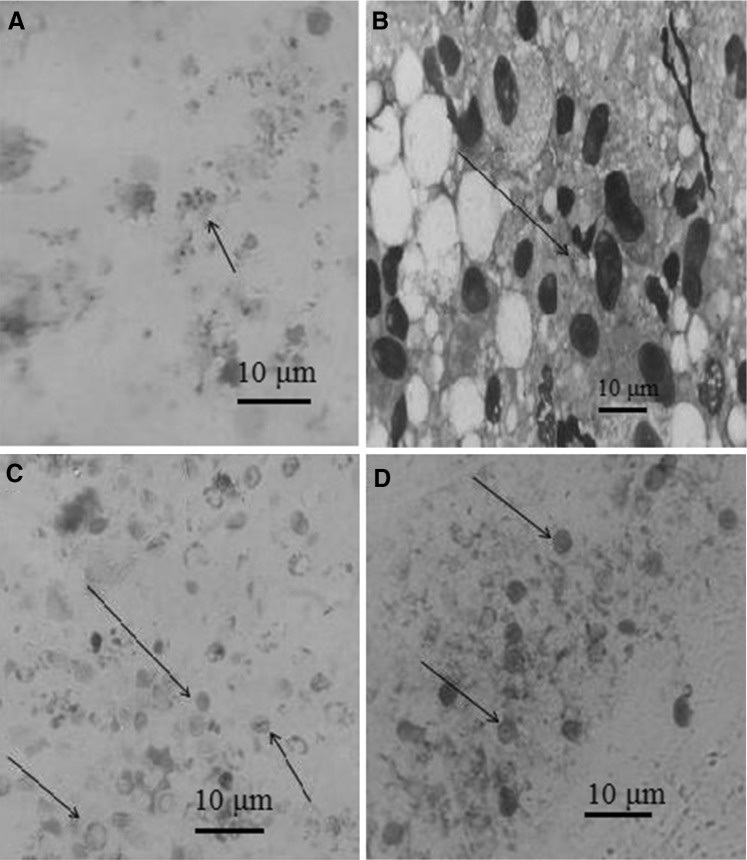
We used adult male Sprague–Dawley rats weighing about 280 g, obtained from Pasture Institute of Iran. The animal care and procedure of the experiment in this study were approved by Institutional Animal Care and Research Advisory Committee of Aja University of Medical Science. Techniques for rat’s infection and preparation of bronchial lavage were performed, as described by Walzer et al. (1980) with some modification. Briefly, the rats were divided into two groups. Group A consisted of 50 rats on the treatment of increasing dose (from 0.4 to 1.2 mg) Dexamethasone (Daropakhsh drug’s company, Iran) injected subcutaneously weekly, low (8 %) protein diet, and tetracycline (1 mg/ml) in the drinking water for 8 to 9 weeks to produce P. carinii infection. Group B (controls) composed of 20 rats, which received no Dexamethasone, ate a regular diet, and drank tap water with or without tetracycline. The rats were sacrificed by exposure to halothane in a closed container. Bronchial lavage was performed by the method of Masur and Jones (1987) and lavage fluids were processed for staining (Milder et al. 1980). Giemsa staining was performed (with 2 ml of Giemsa stain in 40 ml of 6.7 mM phosphate buffer at pH 7.2) on methanol fixed BAL smears, as described previously (Wolfson et al. 1990). The modified Toluidine Blue O staining was performed by the method of Gosey et al. (1985). For Grocott-Gomori methenamine silver (GMS) staining procedure was performed by the method of Procop et al. (2004). All experiments were examined twice in parasitology and mycology laboratories, and positive or negative findings were confirmed by a professional parasitologist and mycologist. The samples were considered as a true positive if Pneumocystis cysts or trophozoites were found by at least two out of three staining methods (Fig. 1a–d). Contrarily, samples were considered as negative if all stains were negative or only one stain was positive after 30 min of visual examination under light microscopy. To calculating of sensitivity, specificity, PPV and NPV we used of standard statistic procedures, as described previously (Aghamolaie et al. 2014).
Fig. 1.
Pneumocystis in rat BAL specimen under light microscopy. a Trophozoites of Pneumocystis staining with Geimsa stain (×10), b Cyst walls of Pneumocystis staining with Geimsa stain (×40), c Cyst walls of Pneumocystis staining with Gomori’s methenamine silver stain (×10), d Cyst walls of Pneumocystis staining with Toluidine Blue O stain (×10)
Results
Of the specimens provided from rats of group A (treated with Dexamethasone), all were detected by at least two staining methods. Thus, these specimens were considered as true positive. Of the true positive specimens, all (100 %) were detected by the GMS, 42 of 50 (84 %) were detected by Giemsa stain, and 48 of 50 (96 %) were detected by TBO. Also, according to the results of staining methods, all specimens provided from rats of group B (control group) considered as true negative. GMS and TBO stains not have false-positive results. But, false-positive result was 2 of 20 (10 %) for Giemsa stain. False-negative results for TBO and Giemsa stains were 2 of 50 (4 %) and 7 of 50 (14 %), respectively. The results for the sensitivity of staining methods, the specificity, positive and negative predictive values, and cost and time spent for each staining method were calculated, and presented in Table 1.
Table 1.
Statistical parameters of different stains used for detecting of Pneumocystis jirovecii
| Method | No. of PSa/TPSb | No. of NSc/TNSd | SEN (%)e | SPEC (%)f | PPV (%)j | NPV (%)h | Time consumed (min) | Cost consumed ($) |
|---|---|---|---|---|---|---|---|---|
| GMS | 50/50 | 20/20 | 100 | 100 | 100 | 100 | 90 | 3.5 |
| G | 42/50 | 18/20 | 84 | 90 | 95.4 | 69.2 | 25 | 2 |
| TB0 | 48/50 | 20/20 | 96 | 100 | 100 | 90.9 | 20 | 2.5 |
aPositive slides detected by methods
bTrue positive samples
cNegative slides detected by methods
dTrue negative samples
eSensitivity (measures the proportion of positives that are correctly identified)
fSpecificity (measures the proportion of negatives that are correctly identified)
jPositive predictive value (is the probability that subjects with a positive screening test truly are positive)
hNegative predictive value (is the probability that subjects with a positive screening test truly are positive)
Although all true-positive specimens were found by the GMS, this number was not significantly different from those detected by the TBO (P = 0.495) stain. But, the number of true-positives samples detected by Giemsa stain was significantly fewer than those detected by the GMS (P = 0.006) and TBO (P = 0.045) stains. GMS and TBO stains did not have false-positive results. Although the number of false-positives observed in Giemsa stain (two samples) was more than those observed in staining with GMS and TBO (not have false-positive), but this difference was not statistically significant (P = 0.487).
Discussion
In developing countries, P. jirovecii infection is an emerging health problem not only for HIV-infected patients, but also for other patients who receive immunosuppressive drugs during their treatments (Goto and Oka 2011). Therefore, there is an important necessity for laboratories that use of the best possible method to detection the Pneumocystis infection, because there are major differences in the treatment of pneumonia caused by Pneumocystis versus pneumonia caused by other microorganisms (Procop et al. 2004). Pneumocystis is mostly detected by examining induced sputum or BAL specimens of suspicious patients, although evaluating BAL specimens is more costly and its collection is slightly risky for the patients. For a definitive diagnosis of PCP, a variety of staining methods, such as Giemsa stains, Methenamine silver, Calcofluor white, and Toluidine blue, can be used (Lautenschlager et al. 1996).
Calcofluor white staining is a rapid, easy and inexpensive method for detecting of Pneumocystis, but its disadvantage is that it requires a fluorescence microscope that may be not available in many laboratories in developing countries.
Here we found that the Geimsa staining was not an effective staining method for detection of Pneumocystis due to its low sensitivity (84 %), weak specificity (90 %) and negative predictive value (69.2 %). Of interest, significant number of specimens contaminated with Pneumocystis was not detected by the Geimsa staining while being detected by GMS and TBO. Consistantly, a weak sensitivity and a low negative predictive value was reported for Geimsa method when it was used for the detection of Pneumocystis in rat BAL specimens (Wolfson et al. 1990). Moreover, the number of false-positive results with the Geimsa stain was greater than that reported for each of the other stains (Mohebali et al. 2002).
In our study, the GMS stain was the most sensitive staining method and may be useful for screening of Pneumocystis as a routine laboratory trial. However, in comparison with two other utilized methods it was more time-consuming and costly. Moreover, in accordance with a previous study (Mohebali et al. 2002) here we also found that the GMS stain had a high specificity, PPV, and NPV. These findings, however, are in contrast with Procop et al. (2004) study that reported low sensitivity for GMS.
Although, sensitivity, NPV, and cost of TBO stain were located somewhere between those of the GMS and Geimsa stains, this staining method showed high specificity and PPV similar to GMS. The sensitivity, specificity, NPV and PPV of TBO satin in our study is in agreement with Mohebali et al. (2002) study.
There were some limitations to our study. Firstly, the staining methods used in this study are not specifically staining Pneumocystis species, although they are often used to diagnosis of Pneumocystis in medical laboratories. Secondly, the Pneumocystis sp. that infects rats is P. carinii and differs from those infecting humans (P. jirovecii), although they have same pathway to cause of the PCP infection.
In conclusion results of this study revealed that GMS and TBO stains have the best parameters for routine use in clinical laboratories with limited facilities. TBO was cost effective and less time-consuming method but GMS had highest sensitivity and specificity. Therefore, our results suggest that it is better the TBO staining is being used as the primary staining methods in a clinical laboratory for fast and cost benefit evaluation method. Then, confirmation with more costly and time consuming GMS staining should be performed especially for the suspicious samples to increase the sensitivity and specificity of the final test results.
Acknowledgments
The authors are very grateful to the protozoology unit staffs in AJA University of Medical Science and Shahid Beheshti University of Medical Science, for their technical assistance.
Author contributions
Conceived and designed the experiments: MMH and AR. Performed the experiments: MMH, HG and ME. Analyzed the data: AR and SM. Critical revision of article: ASAM.
Compliance with ethical standards
Conflict of interest
None of the above authors have any conflict of interest.
Refrences
- Aghamolaie S, Rostami A, Fallahi S, et al. Evaluation of modified Ziehl–Neelsen, direct fluorescent-antibody and PCR assay for detection of Cryptosporidium spp. in children faecal specimens. J Parasit Dis. 2014 doi: 10.1007/s12639-014-0614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandt D, Monecke S. Development and evaluation of a real-time PCR assay for detection of Pneumocystis jiroveci. Transpl Infect Dis. 2007;9:196–202. doi: 10.1111/j.1399-3062.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- Calderón-Sandubete EJ, Varela-Aguilar JM, Medrano-Ortega FJ, et al. Historical perspective on Pneumocystis carinii infection. Protist. 2002;153:303–310. doi: 10.1078/1434-4610-00107. [DOI] [PubMed] [Google Scholar]
- Contini C, Villa MP, Romani R, et al. Detection of Pneumocystis carinii among children with chronic respiratory disorders in the absence of HIV infection and immunodeficiency. J Med Microbiol. 1998;47:329–333. doi: 10.1099/00222615-47-4-329. [DOI] [PubMed] [Google Scholar]
- Gosey LL, Howard RM, Witebsky FG, et al. Advantages of a modified toluidine blue O stain and bronchoalveolar lavage for the diagnosis of Pneumocystis carinii pneumonia. J Clin Microbiol. 1985;22:803–807. doi: 10.1128/jcm.22.5.803-807.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto N, Oka S. Pneumocystis jirovecii pneumonia in kidney transplantation. Transpl Infect Dis. 2011;13:551–558. doi: 10.1111/j.1399-3062.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- Larsen HH, Masur H, Kovacs JA, et al. Development and evaluation of a quantitative, touch-down, real-time PCR assay for diagnosing Pneumocystiscarinii pneumonia. J Clin Microbiol. 2002;40:490–494. doi: 10.1128/JCM.40.2.490-494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager I, Lyytikainen O, Jokipii L, et al. Immunodetection of Pneumocystis carinii in bronchoalveolar lavage specimens compared with methenamine silver stain. J Clin Microbiol. 1996;34:728–730. doi: 10.1128/jcm.34.3.728-730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur H, Jones TC. The interaction in vitro of Pneumocystis carinii with macrophages and L-cells. J Exp Med. 1987;147:157–170. doi: 10.1084/jem.147.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milder JE, Walzer PD, Coonrod JD, et al. Comparison of histological and immunological techniques for detection of Pneumocystis carinii in rat bronchial lavage fluid. J Clin Microbiol. 1980;11:409–417. doi: 10.1128/jcm.11.4.409-417.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebali M, Mirbakhsh M, Keshavarz H. Rapid detection of Pneumocystis carini in spiratory specimens of rats by calcofluor white staining. Iran J Public Health. 2002;31:108–110. [Google Scholar]
- Procop G, Haddad S, Quinn J, et al. Detection of Pneumocystis jiroveci in respiratory specimens by four staining methods. J Clin Microbiol. 2004;42:3333–3335. doi: 10.1128/JCM.42.7.3333-3335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Goto M, Endo T, et al. Pneumocystis carinii carriage in immunocompromised patients with and without human immunodeficiency virus infection. J Med Microbiol. 2002;51:611–614. doi: 10.1099/0022-1317-51-7-611. [DOI] [PubMed] [Google Scholar]
- Walzer P, Powell R, Yoneda K, et al. Growth characteristics and pathogenesis of experimental Pneumocystis carinii pneumonia. Infect Immun. 1980;27:928–937. doi: 10.1128/iai.27.3.928-937.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson JS, Waldron M, Sierra L. Blinded comparison of a direct immunofluorescent monoclonal antibody staining method and a Giemsa staining method for identification of Pneumocystis carinii in induced sputum and bronchoalveolar lavage specimens of patients infected with human immunodeficiency virus. J Clin Microbiol. 1990;28:2136–2138. doi: 10.1128/jcm.28.9.2136-2138.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]