Abstract
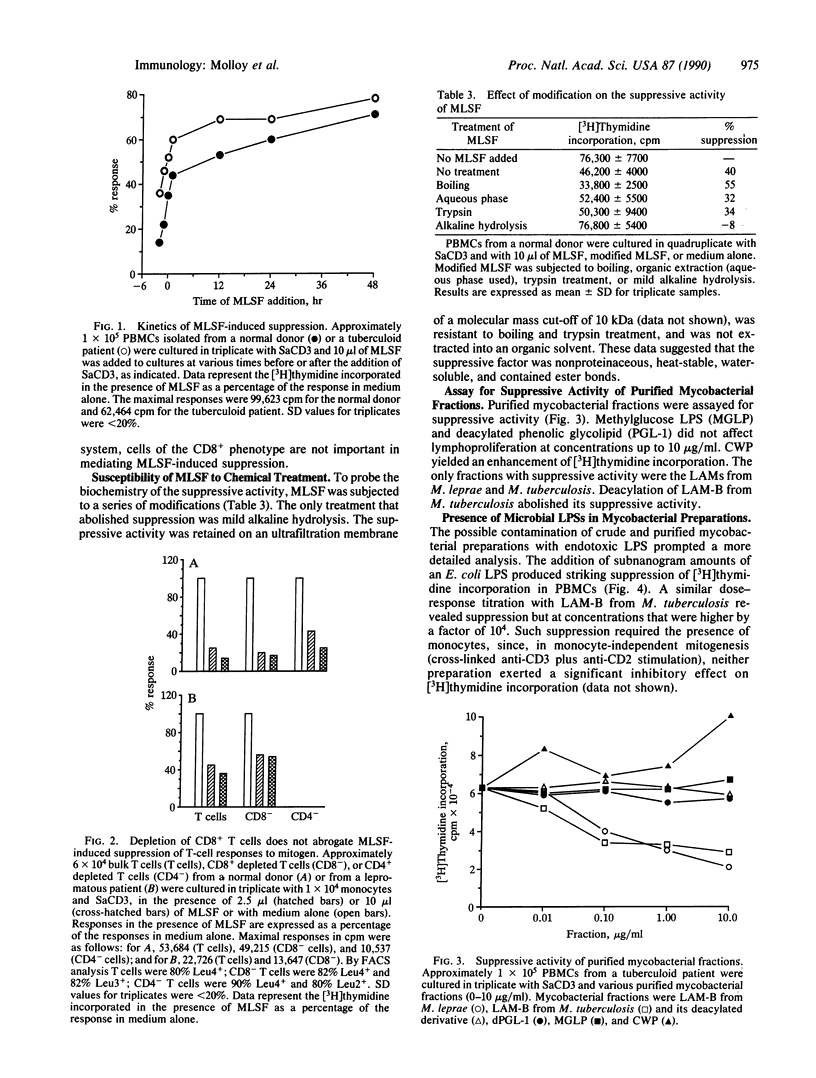
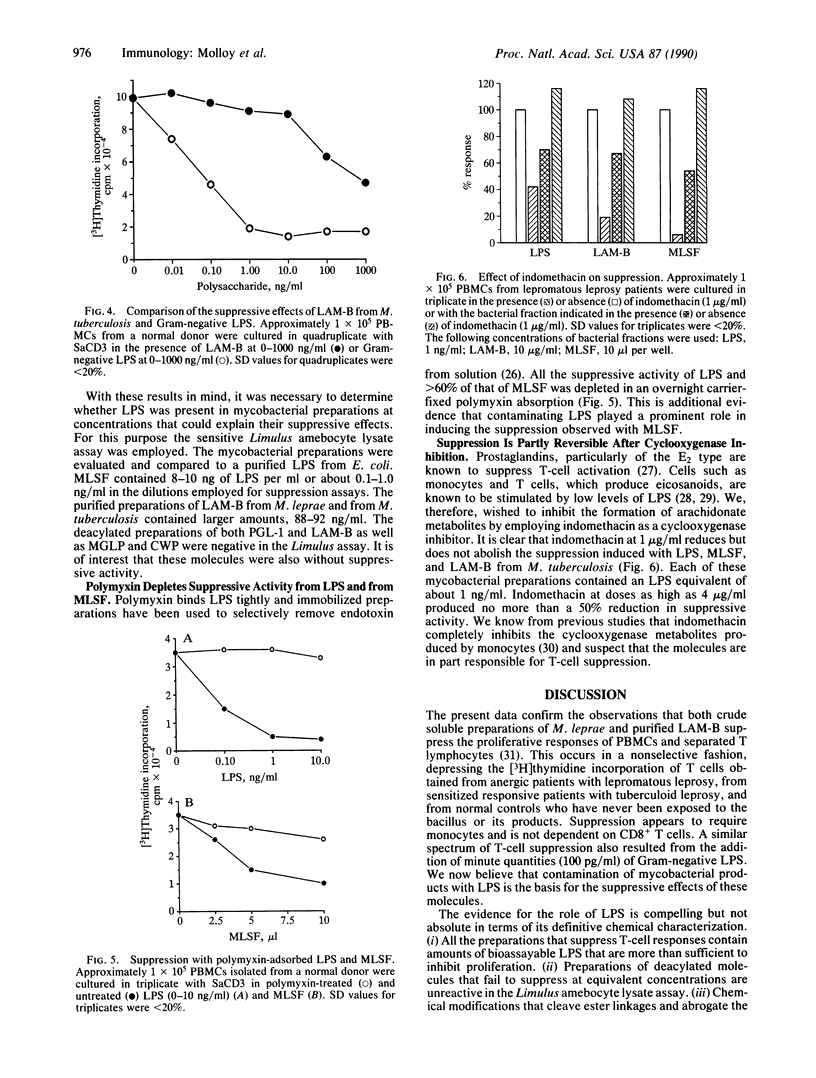
Addition of soluble molecules obtained from sonicated Mycobacterium leprae markedly suppressed the proliferative response to the mitogen anti-CD3 of peripheral blood mononuclear cells and isolated T cells. Suppression was nonspecific and occurred with cells from lepromatous and tuberculoid leprosy patients as well as control donors. The purified lipoarabinomannans from M. leprae and Mycobacterium tuberculosis had a similar spectrum of inhibition whereas their deacylated derivatives were without effect. All mycobacterial preparations of either a crude or purified state, which suppressed cellular responses, contained appreciable quantities of bacterial lipopolysaccharide by the Limulus amebocyte assay. Contamination with lipopolysaccharide could account for the extent and nonselectivity of the T-cell suppression. Suppression was also monocyte-dependent and in part due to the release of arachidonate metabolites of the cyclooxygenase pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Cohen D. S., Wright S. D., Cohn Z. A. Bacterial lipopolysaccharides prime macrophages for enhanced release of arachidonic acid metabolites. J Exp Med. 1986 Jul 1;164(1):165–179. doi: 10.1084/jem.164.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A. A. Protein myristoylation as an intermediate step during signal transduction in macrophages: its role in arachidonic acid metabolism and in responses to interferon gamma. J Cell Sci Suppl. 1988;9:151–167. doi: 10.1242/jcs.1988.supplement_9.8. [DOI] [PubMed] [Google Scholar]
- Clement L. T., Tilden A. B., Dunlap N. E. Analysis of the monocyte Fc receptors and antibody-mediated cellular interactions required for the induction of T cell proliferation by anti-T3 antibodies. J Immunol. 1985 Jul;135(1):165–171. [PubMed] [Google Scholar]
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Spagnuolo P. J. Suppression of antigen and mitogen induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979 Dec;123(6):2689–2695. [PubMed] [Google Scholar]
- Ellner J. J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978 Dec;121(6):2573–2579. [PubMed] [Google Scholar]
- Garcia-Peñarrubia P., Bankhurst A. D., Koster F. T. Prostaglandins from human T suppressor/cytotoxic cells modulate natural killer antibacterial activity. J Exp Med. 1989 Aug 1;170(2):601–606. doi: 10.1084/jem.170.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen R., Gaudernack G., Leivestad T., Vartdal F., Thorsby E. Activation of resting, pure CD4+, and CD8+ cells via CD3. Requirements for second signals. Scand J Immunol. 1987 Aug;26(2):197–205. doi: 10.1111/j.1365-3083.1987.tb02252.x. [DOI] [PubMed] [Google Scholar]
- Halvorsen R., Leivestad T., Gaudernack G., Thorsby E. Accessory cell-dependent T-cell activation via Ti-CD3. Involvement of CD2-LFA-3 interactions. Scand J Immunol. 1988 Sep;28(3):277–284. doi: 10.1111/j.1365-3083.1988.tb01449.x. [DOI] [PubMed] [Google Scholar]
- Hunter S. W., Fujiwara T., Brennan P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J Biol Chem. 1982 Dec 25;257(24):15072–15078. [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Hunter S. W., McNeil M., Modlin R. L., Mehra V., Bloom B. R., Brennan P. J. Isolation and characterization of the highly immunogenic cell wall-associated protein of Mycobacterium leprae. J Immunol. 1989 Apr 15;142(8):2864–2872. [PubMed] [Google Scholar]
- Jacobs D. M., Morrison D. C. Inhibition of the mitogenic response to lipopolysaccharide (LPS) in mouse spleen cells by polymyxin B. J Immunol. 1977 Jan;118(1):21–27. [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Kaplan G., Sampaio E. P., Walsh G. P., Burkhardt R. A., Fajardo T. T., Guido L. S., de Miranda Machado A., Cellona R. V., Abalos R. M., Sarno E. N. Influence of Mycobacterium leprae and its soluble products on the cutaneous responsiveness of leprosy patients to antigen and recombinant interleukin 2. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6269–6273. doi: 10.1073/pnas.86.16.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Convit J., Rubinstein A., Bloom B. R. Activated suppressor T cells in leprosy. J Immunol. 1982 Nov;129(5):1946–1951. [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Fields J. P., Bloom B. R. Lepromin-induced suppressor cells in patients with leprosy. J Immunol. 1979 Oct;123(4):1813–1817. [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Rothman W., Reinherz E., Schlossman S. F., Bloom B. R. Delineation of a human T cell subset responsible for lepromin-induced suppression in leprosy patients. J Immunol. 1980 Sep;125(3):1183–1188. [PubMed] [Google Scholar]
- Moreno C., Mehlert A., Lamb J. The inhibitory effects of mycobacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin Exp Immunol. 1988 Nov;74(2):206–210. [PMC free article] [PubMed] [Google Scholar]
- Myrvang B., Godal T., Ridley D. S., Fröland S. S., Song Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin Exp Immunol. 1973 Aug;14(4):541–553. [PMC free article] [PubMed] [Google Scholar]
- Nath I., Singh R. The suppressive effect of M. leprae on the in vitro proliferative responses of lymphocytes from patients with leprosy. Clin Exp Immunol. 1980 Sep;41(3):406–414. [PMC free article] [PubMed] [Google Scholar]
- Salgame P. R., Mahadevan P. R., Antia N. H. Mechanism of immunosuppression in leprosy: presence of suppressor factor(s) from macrophages of lepromatous patients. Infect Immun. 1983 Jun;40(3):1119–1126. doi: 10.1128/iai.40.3.1119-1126.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner G. L., Atlaw T., Touw J., Belehu A. Antigen-specific suppressor cells in subclinical leprosy infection. Lancet. 1981 Dec 19;2(8260-61):1372–1377. doi: 10.1016/s0140-6736(81)92798-7. [DOI] [PubMed] [Google Scholar]
- Touw J., Stoner G. L., Belehu A. Effect of Mycobacterium leprae on lymphocyte proliferation: suppression of mitogen and antigen responses of human peripheral blood mononuclear cells. Clin Exp Immunol. 1980 Sep;41(3):397–405. [PMC free article] [PubMed] [Google Scholar]
- Wadee A. A., Sher R., Rabson A. R. Production of a suppressor factor by human adherent cells treated with mycobacteria. J Immunol. 1980 Sep;125(3):1380–1386. [PubMed] [Google Scholar]
- Young J. W., Steinman R. M. Accessory cell requirements for the mixed-leukocyte reaction and polyclonal mitogens, as studied with a new technique for enriching blood dendritic cells. Cell Immunol. 1988 Jan;111(1):167–182. doi: 10.1016/0008-8749(88)90061-5. [DOI] [PubMed] [Google Scholar]



