Abstract
To detection and genotype of Toxoplasma gondii isolated from soil in Ahvaz, southwest of Iran. Between August 2011 and May 2012 at different sites located in the area of the Ahvaz city south west Iran. A total of 200 soil samples were taken from different points of the region. Oocysts were recovered using the flotation method. Then, PCR reactions targeting the GRA6 gene were performed for specific T. gondii detection. The positive samples were studied by RFLP (random amplified fragment length polymorphism) using MseI enzymes to confirm the parasite linage. Toxoplasma DNA was found in 18 samples. Among them, 12 samples were successfully genotyped as GRA6 type III and 6 as GRA6 Type II. This is the first investigation detecting and genotyping T. gondii oocyst in environmental soil samples of Ahvaz, South west of Iran. The results of this study indicated that soil contaminated with T. gondii oocysts especially in public park may play a role in the epidemiology of human toxoplasmosis in southwest of Iran.
Keywords: Toxoplasma gondii, Soil, PCR, Iran
Introduction
Toxoplasma gondii is an obligate intracellular coccidian. It causes toxoplasmosis, the most prevalent parasitic infections in humans. The definitive host for the parasite is cat and the intermediate hosts are the wide range of warm-blooded vertebrates including humans, birds, livestock, and marine mammals. It is estimated that 20–90 % of people in the world are contaminated with Toxoplasma (Robert-Gangneux and Darde 2012). Toxoplasmosis continues to be public health problem and the seroprevalence of this infection has been reported 39.3 % in the Iranian general papulation (Daryani et al. 2014).
Toxoplasma gondii is capable of infecting a wide variety of nucleated cell types and is a facultative heteroxenous, polyxenous protozoon that has developed several potential acquired and congenital routes of transmission within and between different host species. In immunocompetent individuals, many cases of toxoplasmosis are asymptomatic. However, infections can be problematic and severe in immunocompromised groups such as patients with HIV (Robert-Gangneux and Darde 2012). Also in non-immune pregnant women, toxoplasmosis can result in congenital transmission and fetal toxoplasmosis. This can be dangerous for fetus and infants including abortion, neonatal death, or fetal and newborn abnormalities such as encephalomyelitis, retinochoroiditis, intracranial calcifications, and hydrocephalous (Saki et al. 2015a, b).
The definitive hosts, domestic and wild felids in which T. gondii undergoes sexual replication, shed environmentally resistant oocysts, the infective stage for other hosts, including humans. An infected cat may shed as many as one billion oocysts during a primary infection. Therefore, cats have a main role in the epidemiology of toxoplasmosis. Oocysts are extremely durable and have been reported to survive for several months and remain infectious to mice inoculated with the soil samples (Dabritz and Conrad 2010). One of the most common transmission routes of toxoplasmosis is ingestion or inhalation of sporulated oocysts that may be present in water, soil, fruits, and vegetables contaminated with the faeces of infected cats (Khademvatan et al. 2014; Tenter et al. 2000).
In recent years, techniques with high specific and sensitive for recover and identify of T. gondii oocysts in environmental samples were developed (Dumetre and Darde 2003). Although there are several studies conducted on different aspects of Toxoplasma in Iran but there is poor information about oocyst distribution in soil. Therefore, the aim of current study was to estimate the occurrence of T. gondii oocysts in soil in Ahvaz, southwest of Iran as well as determine the genotype of the detected parasites.
Materials and methods
Study area
Ahvaz is a city located in the southwest of Iran, built on the banks of the Karun River and capital for Khūzestān Province. The city has an average elevation of 20 m above sea level. Ahvaz has a desert climate with very hot summers and mild, short winters. The humidity in sometimes exceeding 90 %. However, in winters, the minimum temperature can fall to around +5 °C.
Sampling
Two hundred soil samples were collected between August 2011 and May 2012 at different sites located in the area of the Ahvaz city southwest of Iran. The samples were collected from sand pits, playgrounds, public parks and areas around rubbish dumps where cats that may excrete T. gondii oocysts often appear. Samples were prepared using the following procedure: 400 g of the soil were taken from the surface layer of the ground (the depth of 2–5 cm), dried at room temperature for 24 h, and then sieved. Finally, 40 g of the soil prepared in this way were used to further examinations (Mizgajska-Wiktor 2005).
Oocyst recovery
In order to concentrate and recover the oocysts from the soil samples, the flotation method using sucrose solution (specific gravity 1.200) was performed as described by Matsuo with some modification (Matsuo et al. 2004). Briefly, 40 g soil sample was suspended in 100 ml tap water, filtered through 2 layer gaze, centrifuged at 3000 rpm for 10 min and then the supernatant was discarded. The sediment was then re-suspended in 10 ml of sucrose solution (specific gravity 1.200), centrifuged as described above, and the supernatant was transferred to new tube. The supernatant, after dilution with distilled water (1:10), was centrifuged as described above and obtained sediment was considered to DNA extraction.
DNA extraction and GRA6 amplification
The DNA for PCR was extracted by the commercial Genomic Mini Kit (Bioneer, South Korea), according to the manufacturer’s instructions, and then DNA was stored at −20 °C. To remove of the PCR inhibitors present in the soil, heating and cooling (at 90 °C for 10 min followed by −80 °C for 10 min repeatedly for 5 times( of the samples was performed.
The conventional PCR was performed on all DNA samples to amplify a fragment from the GRA6 gene, in the T. gondii genome, as described by Saki et al. (2013). For specific T. gondii detection, PCR reaction was performed with the use of a pair of primers forward primer, 5′-GTAGCGTGCTTGTTGG CGAC-3′, and reverse primer, 5′-ACAAGACATAGAGTGCCCC-3, targeting the Approximate 800 bp fragment of the GRA6 gene (Saki et al. 2013).
Amplification was performed according to the conditions described by Saki et al. (2013). Ten microliters of the PCR product were subjected to electrophoresis on a 1.5 % agarose gel stained with ethidium bromide. One hundred bp molecular weight marker (Fermentase life science, Germany) was included on each gel for base-pair comparisons. The analysis was done by visualizing on a UV illuminator. To confirm PCR results, control T. gondii DNA (GenBank: AB703306.1) was used.
PCR based restriction fragment length polymorphism analysis (PCR–RFLP)
The GRA6 amplification products from collected isolates were digested using restriction enzyme MseI in 5 h at 37 °C, and the digested products were analyzed on 2 % agarose gel followed by staining with ethidium bromide and visualization under UV.
Results
Two hundred samples were examined with the PCR and then restriction fragment length polymorphism detection methods based on the T. gondii GRA6 gene. T. gondii DNA was found in 18 samples (9 %). This study revealed that samples collected from public park were more contaminated than others (Table 1). Amplification of the GRA6 gene resulted in a single product (approx. 800 bp) for all positive samples. According to the restriction patterns obtained after separation by electrophoresis (Fig. 1), two linage II (3) and linage III (8) were found.
Table 1.
The prevalence of T. gondii lineages in different soil samples of Ahvaz, southwest of Iran
| Soil samples | No | Pos | Linage II | Linage III |
|---|---|---|---|---|
| sand pits | 40 | 2 | 0 | 2 |
| playgrounds | 50 | 4 | 2 | 0 |
| public parks | 60 | 8 | 2 | 6 |
| Areas around rubbish dumps | 50 | 4 | 2 | 4 |
| Total | 200 | 18 | 6 | 12 |
Fig. 1.
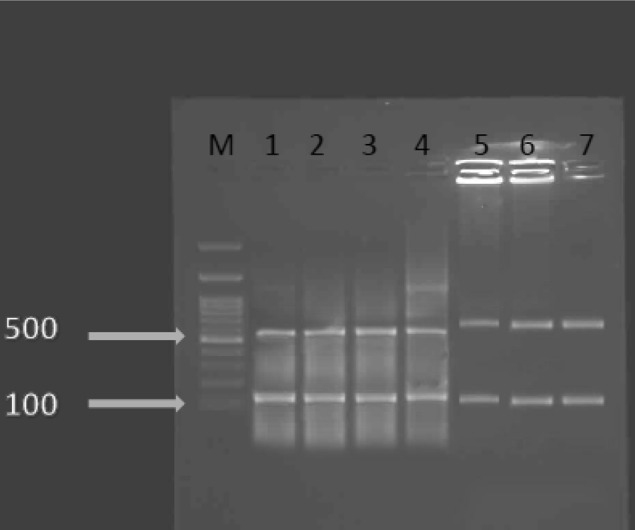
PCR–RFLP assay of the GRA6 locus. Lane I corresponds to the PCR product of control T. gondii DNA (GenBank: AB703306.1) as a positive control. Lanes 2–4 represent type III lineage. Lane 5–7 represent type II lineage. Lane M corresponds to the DNA molecular weight marker (100 bp DNA ladder, Gibco BRL)
Discussion
Because Toxoplasma oocysts are resistant to environmental conditions, soil can be as a large and important source of T. gondii infection (Dabritz and Conrad 2010). The level of soil contamination and the dynamics of this contamination are mostly unknown due to little or absent studies in the area, Khuzestan, southwest of Iran. This is the first investigation detecting and genotyping T. gondii oocyst in environmental soil samples in this point.
In this study, we used PCR–RFLP assay at GRA6 locus, previously had been exploited as a target for molecular genotyping of T. gondii strains (Saki et al. 2013). PCR–RFLP assay at GRA6 locus demonstrated a three clonal lineage in T. gondii population with RFLP variations being identified by MseI restriction endonucleases.
Current study indicated 9 % of the soil samples were positive for two types III and II of T. gondii with type III dominancy. The results were consistent with those of a previous report (Tavalla et al. 2013). They showed 13 of 150 soil samples (8.7 %) were positive. Of them, nine were type III (69 %), three were mixed of type I and III (23 %) and 1 of the samples was type I (8 %) (Tavalla et al. 2013). Studies on different samples in the worlds showed different results of contaminations and type dominancy. Studies of Howe et al. (1997) and Mondragon et al. (1998) revealed type II to be the most prevalent lineage accounting for 80 and 83.3 % respectively of T. gondii strains isolated from patients and pigs. In the study of Howe and Sibley, 1995, type III was more common in animals than in human toxoplasmosis (Howe and Sibley 1995).
Study of Behzadi et al. (2003) on 21 T. gondii isolates from humane and mice showed type II was the predominant lineage, accounting for 85.7 % of the isolates. Type I was found in 14.3 % of cases with more presentation in animal samples, whereas type III lineage was not seen (Behzadi et al. 2003). Study in Iran on genotyping of T. gondii isolates from chickens and ducks indicated all the isolates were type III whereas type I and II isolates and mixed genotypes were not found (Zia-Ali et al. 2007).
Current study revealed that public park was more contaminated with T. gondii oocysts than other places. Humid shady areas have an important role in surviving and retaining of T. gondii oocysts ability to become infectious in the environment. The contact with the soil of this area can be associated with an increased risk of maternal toxoplasmosis (Decavalas et al. 1990). Also humans can become infected indirectly when they ingest food and other consumable products that have been infected with oocysts contaminated soil. Because of the public health importance, studies on more public park soil samples and other potential environmental sources for detection of T. gondii oocysts contamination rate are needed.
The prevalence of T. gondii in human, animals and birds is thought as a good indicator of distribution of T. gondii ooccyts in the environment (Tenter et al. 2000). In our previous study on bird’s hosts in southwest of Iran (Khuzestan province), PCR results showed, 16.46 % of the samples were infected to T. gondii (Khademvatan et al. 2013). In mentioned study, type II and III lineage of T. gondii were dominant in bird’s hosts in southwest of Iran. In conclusion, current study indicated that soil contaminated with T. gondii oocysts especially in public park may play a role in the epidemiology of human toxoplasmosis in southwest of Iran.
Acknowledgments
This study is Granted (No. OG-91105) by the Ahvaz Jundishapur University of Medical Science. The authors would like to thank medical parasitology department for their precious valuable collaboration.
Funding
Research Deputy, Ahvaz Jundishapur University of Medical Sciences.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Behzadi R, Roohvand F, Razavi MR, Hovanessian A, Assmar M. Genotyping of Toxoplasma gondii strains isolated from patients and mice by PCR–RFLP assay. Iran J Biotechnol. 2003;1(2):83. [Google Scholar]
- Dabritz HA, Conrad PA. Cats and Toxoplasma: implications for public health. Zoonoses Public Health. 2010;57(1):34–52. doi: 10.1111/j.1863-2378.2009.01273.x. [DOI] [PubMed] [Google Scholar]
- Daryani A, et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: a systematic review and meta-analysis. Acta Trop. 2014;137:185–194. doi: 10.1016/j.actatropica.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Decavalas G, Papapetropoulou M, Giannoulaki E, Tzigounis V, Kondakis XG. Prevalence of Toxoplasma gondii antibodies in gravidas and recently aborted women and study of risk factors. Eur J Epidemiol. 1990;6(2):223–226. doi: 10.1007/BF00145798. [DOI] [PubMed] [Google Scholar]
- Dumetre A, Darde ML. How to detect Toxoplasma gondii oocysts in environmental samples? FEMS Microbiol Rev. 2003;27(5):651–661. doi: 10.1016/S0168-6445(03)00071-8. [DOI] [PubMed] [Google Scholar]
- Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172(6):1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- Howe DK, Honore S, Derouin F, Sibley LD. Determination of genotypes of Toxoplasma gondii strains isolated from patients with toxoplasmosis. J Clin Microbiol. 1997;35(6):1411–1414. doi: 10.1128/jcm.35.6.1411-1414.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khademvatan S, Saki J, Yousefi E, Abdizadeh R. Detection and genotyping of Toxoplasma gondii strains isolated from birds in the southwest of Iran. Br Poult Sci. 2013;54(1):76–80. doi: 10.1080/00071668.2013.763899. [DOI] [PubMed] [Google Scholar]
- Khademvatan S, Abdizadeh R, Rahim F, Hashemitabar M, Ghasemi M, Tavalla M. Stray cats gastrointestinal parasites and its association with public health in ahvaz city, South Western of Iran. Jundishapur J Microbiol. 2014;7(8):e11079. doi: 10.5812/jjm.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo J, Kimura D, Rai SK, Uga S. Detection of Toxoplasma oocysts from soil by modified sucrose flotation and PCR methods. Southeast Asian J Trop Med Public Health. 2004;35(2):270–274. [PubMed] [Google Scholar]
- Mizgajska-Wiktor H. Recommended method for recovery of Toxocara and other geohelminth eggs from soil. Wiad Parazytol. 2005;51(1):21–22. [PubMed] [Google Scholar]
- Mondragon R, Howe DK, Dubey JP, Sibley LD. Genotypic analysis of Toxoplasma gondii isolates from pigs. J Parasitol. 1998;84(3):639–641. doi: 10.2307/3284743. [DOI] [PubMed] [Google Scholar]
- Robert-Gangneux F, Darde ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saki J, Khademvatan S, Soltani S, Shahbazian H. Detection of toxoplasmosis in patients with end-stage renal disease by enzyme-linked immunosorbent assay and polymerase chain reaction methods. Parasitol Res. 2013;112(1):163–168. doi: 10.1007/s00436-012-3120-6. [DOI] [PubMed] [Google Scholar]
- Saki J, Mohammadpour N, Moramezi F, Khademvatan S. Seroprevalence of Toxoplasma gondii in women who have aborted in comparison with the women with normal delivery in Ahvaz, southwest of Iran. Sci World J. 2015;2015:764369. doi: 10.1155/2015/764369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saki J, Shafieenia S, Foroutan-Rad M. Seroprevalence of Toxoplasmosis in diabetic pregnant women in Southwestern of Iran. J Parasit Dis. 2015 doi: 10.1007/s12639-015-0735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavalla M, et al. Genotyping of Toxoplasma gondii isolates from soil samples in Tehran, Iran. Iran J Parasitol. 2013;8(2):227–233. [PMC free article] [PubMed] [Google Scholar]
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12–13):1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zia-Ali N, Fazaeli A, Khoramizadeh M, Ajzenberg D, Darde M, Keshavarz-Valian H. Isolation and molecular characterization of Toxoplasma gondii strains from different hosts in Iran. Parasitol Res. 2007;101(1):111–115. doi: 10.1007/s00436-007-0461-7. [DOI] [PubMed] [Google Scholar]


