Abstract
Hepatic affection by granulomatous inflammation in schistosomiasis suggested that a potential anti-pathology vaccine could be generated based on limiting the presence of hazardous hepatocytes induced apoptosis and caused reduction of granulomas number and size . So, this work is concerned with experimental assessment of the efficacy of different Schistosoma mansoni antigens (SEA, SWAP and combined SEA and SWAP) on murine liver after challenge by Schistosoma infection, histopathological, histochemical and molecular investigations were performed on sixty male laboratory bred Swiss Albino mice. A schedule of vaccination and challenge infection was followed and performed on 6 mice groups (each of ten); control normal (G1), control infected (G2), adjuvant received then infected (G3), SEA + adj. received then infected (G4), SWAP + adj. received then infected (G5) and SEA + SWAP + adj. received then infected (G6).
Animals were euthanized 10 weeks post infection.
Vaccination efficacy was assessed by histopathological, histochemical and molecular studies on murine hepatic tissues.
Results showed that:
The combined (SEA + SWAP) antigens were better in reducing the number and diameter of the hepatic granulomas, with more protection of the hepatocytes DNA, in addition to more decrease of hepatocytes induced apoptosis and fragmentation as demonstrated by molecular assay.
Keywords: Schistosoma mansoni, Hepatocytes DNA, Induced apoptosis, Fragmentation, Molecular study
Introduction
Schistosomiasis is a parasitic disease caused by blood flukes of the genus Schistosoma (WHO 2010). More than 240 million people are infected with schistosomiasis, and an estimated 700 million people are at risk of infection in 76 countries where the disease is considered endemic, as their agricultural work, domestic chores, and recreational activities expose them to infested water. Globally, 200,000 deaths are attributed to schistosomiasis annually (WHO 2013).
Schistosoma has a typical trematode vertebrate–invertebrate life cycle with the human being the definitive host. Adult Schistosoma parasites live as pairs within the capillary blood vessels where the female is carried in the gynecophoric canal of the male, they copulate and the female then lays spined eggs intravascular, some eggs pass through the vessel wall where they reach the exterior with urine or stools (Cribb et al. 2001). Eggs that are not shed successfully may remain in the tissues or be swept back to the portal circulation (Houston et al. 2004), where they embolise to the liver initiating granulomatous inflammatory response, Soluble egg antigen (SEA) coming from seeded eggs in liver tissue activates Th1-polarized response (Pearce 2005), while S. mansoni adult worm antigens(SWAP) modulate the effects of Th1 and Th2 responses through immunosuppressive cytokines as interleukin-10 (IL-10) and transforming growth factor beta (TGF-β), the balance between Th1, Th2 determines the outcome of Schistosoma infections (Milner et al. 2010).
Hepatomegaly, secondary to granulomatous inflammation then collagen deposits lead to the progressive portal hypertension, oesophageal or gastric varices, splenomegaly, and hypersplenism (Ross et al. 2002).
The generation of granulomatous inflammation in schistosomiasis suggested that a potential anti-pathology vaccine could be generated based on limiting the presence of hazardous cytokines and caused reduction in granuloma no. and size (Kerishina 1991) which is the main pathology in schistosomiasis especially on ending by fibrosing reactions against the parasite eggs in the liver (Taylor et al. 2006).
SEA is a glycoprotein secreted by the miracidium, passed through microscopic pores within the egg shell. It can induce granuloma formation in the form of aggregation of eosinophils, neutrophils and macrophages (Hams et al. 2013) also basophils (Anyan et al. 2013). This reaction acts as a barrier by sequestering the toxic and antigenic egg substances, it simultaneously destroys the parenchyma of the involved organs (liver; Taylor et al. 2006), the granuloma is thus both friend and foe during infection (Hams et al. 2013).
During hepatic schistosomiasis, there was shrinkage and some necrosis of hepatocytes around granulomas (Soomro et al. 2005). SEA also induce apoptosis (programmed cell death) in hepatic cells through the TNF-related apoptosis inducing ligand/death receptor 5 and caspase-dependent pathways (Duan et al. 2014). In addition caspases disregulate hepatocytes DNA repair and replication through the cleavage of DNA topoisomerase and terminal deoxy nucleotidyl transferase enzymes. They also cleave DNAase which has a high specific activity that results in fragmentation of hepatocytes nuclear DNA (Shams El Din 2011). In liver injuries, the resulted pathologic apoptosis is unregulated and can be massive leading to cell lysis (Bechmann et al. 2008), may promote fibrogenesis, resulting in cirrhosis (Canbay et al. 2003).
In the context of schistosomiasis, where parasites do not multiply in the mammalian host (Gryseels et al. 2006), an effective vaccine would contribute greatly to a decrease in morbidity associated with schistosomiasis via protective immune responses leading to reduced worm burdens, egg production and the induced hepatocytes pathologic changes (McManus and Loukas 2008).
Antigens tested in vaccination include adult worm antigens e.g. (SWAP), egg antigens e.g. (SEA) and cercarial antigens e.g. (CAP) (Montesano et al. 1999). The combination of two vaccines provides augmentation of the protective immunity and reduction of hepatic immunopathology (Khalifa et al. 2011), while Fang et al. (2012) postulated that the development of a multivalent (cocktail) antigens vaccine may be the way forward.
Aim of the work
To use different preparations of antischistosomal crude antigens as potential vaccines and assess the degree of the protection presented by these antigens preparations to murine hepatocytes after challenge by Schistosoma infection by histopathological, histochemical and molecular studies (DNA changes and fragmentation).
Materials and methods
Type of the study: Experimental study.
Time of the study: This study was started on February 2013 and completed on May 2014.
Location of the study: The current work was conducted at the laboratories of the medical Parasitology and Biochemistry Departments, Faculty of Medicine, Zagazig University and Theodor Bilharz Research Institute, Imbaba, Giza, Egypt.
Infective cercariae: According to Liang et al. (1987), S. mansoni Egyptian strain cercariae were obtained from infected laboratory bred B. alexandrina snails which were purchased from Schistosome Biological Supply Center at Theodor Bilharz Research Institute, Imbaba, Giza, Egypt. After exposure to light for at least 4 h, S. mansoni cercariae shed from the snails were used to infect the experimental animals of the study by subcutaneous injection of ±80 cercariae.
Animals: The current work was carried out on male laboratory bred and parasites free Swiss albino mice, about 8 weeks’ old age and 18–20 g in weight for each mouse at the beginning of the experiment. Mice were obtained from Schistosome Biological Supply Center at Theodor Bilharz Research Institute (TBRI). The mice were maintained on a standard commercial pelleted diet with free accessible water and in an air condition animal house at 20–22 °C all over the time of study.
Ethical aspects
All procedures related to animal experimentation in the present study met the International Guiding Principles for Biomedical Research Involving Animals as issued by the International Organizations of Medical Sciences and approved by the ethics committee of the Faculty of Medicine, Zagazig University.
Materials
Schistosomal antigens preparation
Schistosomal crude antigens (SWAP and SEA) were obtained from Schistosome Biological Supply Center at Theodor Bilharz Research Institute (TBRI), Imbaba, Giza, Egypt.
Adjuvants
Freund’s complete adjuvant (FCA) was obtained from Sigma Chemical Co., St Louis, MO, USA and emulsified in phosphate-buffered saline (PBS) at a ratio of 2:1.
Experimental design
Sample size
Sixty albino mice. Mice were divided into six groups (ten mice in each group).
Preparation of antigens
SWAP was prepared according to the method of Salih et al. (1978) and SEA was prepared according to the method of Boros and Warren (1970), the protein content was estimated using Bio-Rad kit (Bio-Rad Laboratories, Hercules, CA, USA) (Bradford 1976) and the final concentration was adjusted with PBS to a concentration 50 μg/ml and stored at −70 °C until use.
Antigens administration regimens
The immunization schedule was performed according to the method of Nabih and Soliman (1986). Each mouse was sensitized with an initial S.C. injection of 200 μl of the extracted antigen with a total antigen concentration of 30 μg protein. After 2 weeks, a second S.C. injection of 200 μl of the same antigen was taken and diluted to contain 20 μg protein. The antigen was combined with complete Freund’s adjuvant at a 1:1 ratio and injected S.C. (Smithers et al. 1989).
Infection of mice
Infection of mice was done by subcutaneous injection with about ±80 S. mansoni cercariae suspended in 0.2 ml solution (cercariae in distelled water) 3 weeks from the initial S.C. antigen injection. The suspension was injected into the loose skin of the back of the mouse using an insulin syringe (1 cm length; Peters and Warren 1969).
Animal groups
Group 1: Control non infected group.
Group 2: Infected control group (infected by ±80 S. mansoni cercariae by subcutaneous injection).
Group 3: Mice were subcutaneously injected by complete Freund’s adjuvant (CFA) and then infected by ±80 S. mansoni cercariae (adj. + infected group).
Group 4: Mice were subcutaneously injected by soluble egg antigen (SEA) + complete Freund’s adjuvant, and then infected (SEA + adj. + infected group).
Group 5: Mice were subcutaneously injected by soluble worm antigen preparation (SWAP) + complete Freund’s adjuvant, and then infected (SWAP + adj. + infected group).
Group 6: Mice were subcutaneously injected by SWAP and SEA + complete Freund’s adjuvant, and then infected (SEA + SWAP + adj. + infected group).
Vaccination efficacy assessment
Animals were sacrificed by cervical dislocation 10 weeks post infection. Efficacy of the vaccination was assessed by histopathological, histochemical and molecular studies via DNA changes and fragmentation.
Histopathological and histochemical studies of the hepatic tissues
By using H&E and Feulgen stains to highlight the number, size (diameter) and type of liver granulomas, the hepatocytes nucleic DNA changes as a reflection of hepatocytes induced apoptosis were also detected via Feulgen stain.
Haematoxylin and Eosin (H&E) staining (Von Lichtenberg 1962)
10 % formalin fixed paraffin embedded liver tissues were cut into sections (4 μm thick) and stained by (H&E) stain and examined microscopically on different magnifications (100× and 400×) to show liver granulomas count, diameter and type either cellular, fibrocellular or fibrous. The number of hepatic granulomas was determined by semiquantitative method in five randomly selected microscopic fields/liver section at high power magnification. The diameter of hepatic granuloma was measured using graduated eyepiece lenses, considering only lobular granulomas containing central ova. Two perpendicular maximal diameters were measured to get the mean diameter for each granuloma. In five randomly selected microscopic fields/liver sections were examined at high power magnification (Ali and Hamed 2006).
Feulgen-methylene blue (Gravin et al. 1976)
The Feulgen reaction depends on DNA acid hydrolysis. Fixating agents using strong acids were avoided to prevent over de-staining. DNA stained bright red, and the background counterstained green in the normal hepatocytes. Grades of apoptosis according to color changes of hepatocytes nucleic DNA were evaluated as: faint staining of liver cell DNA (marked apoptosis); pale red staining of liver cell DNA (moderate apoptosis); less than bright red staining of liver cell DNA (mild apoptosis); bright red staining of liver cell DNA (normal). On examination of the stained slides we use oil immersion lense (1000×) to detect hepatocytes nucleic DNA changes (Etewa et al. 2013).
Molecular study
For detection of hepatocytes DNA changes (fragmentation) used for assessment of the efficacy of the tested antigens as potential vaccine and their role in hepatocytes protection against experimental schistosomiasis in the murine models (Kasibhatla et al. 2006):
Liver tissue specimens (25 mg) were transferred to 1.5-ml sterile microcentrifuge tubes. Then centrifuged at 2000 rpm in an Eppendorf table top centrifuge for 5 min at 4 °C and the supernatant was removed.
20 μl of TES (Tris–EDTA–sodium dodecyl sulfate) lysis buffer were added. Cell pellet was mixed with TES lysis buffer by stirring with a wide-bore pipette tip.
10 μl of RNase Cocktail were added and mixed well by flipping the tip of the tube then incubated for 30–120 min at 37 °C.
10 μl of proteinase K were added, mixed by flipping the tip of the tube, and incubated at 50 °C for at least 90 min.
5 μl of 6X DNA loading buffer were added and DNA samples were loaded into dry wells of a 1–1.5 % agarose gel in TAE containing 0.5 μg/ml ethidium bromide.
The gel was run at a low voltage, which improves resolution of DNA fragments (i.e., 35 V for ~4 h or until loading dye has run two thirds of the way down the gel).
DNA ladders are finally visualized by a UV transillumination and documented by photography. Apoptotic cells showed DNA shearing or laddering, while DNA from viable cells will stay on the top of the gel as a high-molecular-weight band.
By using DNA extraction and gel electrophoresis the degrees of hepatocyte DNA fragmentation were classified according to the shearing of DNA into multiple bands into mild, moderate and severe (Hassab-El Nabi and El Hassaneen 2008).
Statistical analysis
Data were entered, checked and analyzed using statistical computer program Statistical package for Social Sciences (SPSS version 16 windows). Data were expressed as the mean ± standard deviation (SD). Comparison between the mean values of different parameters in the studied groups was performed using one way analysis of variance (ANOVA) test, with paired (t) test for comparison between means of two groups. Chi square test was used for comparing between the qualitative data.
Results
The results of the histopathological and histochemical studies (Figs. 1, 2):
Fig. 1.
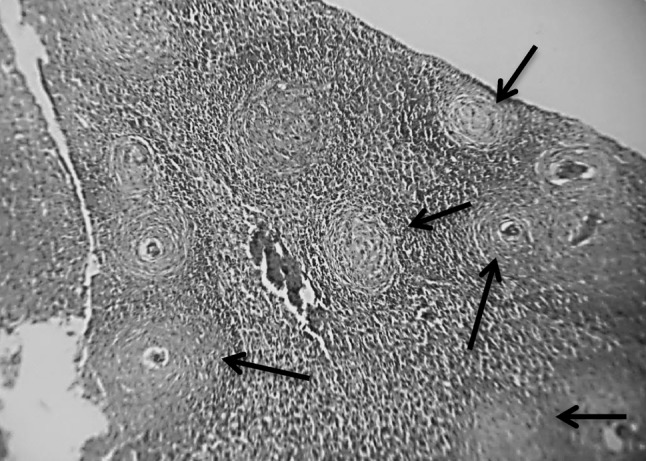
Section in murine liver of Schistosoma mansoni infected control group (G2) showing numerous subcapsular and deeper granulomas (arrows; cellular, fibrocellular, fibrous), areas of degenerated hepatic tissue (degenerated hepatocytes), some granulomas revealed central ova surrounded by a space with an association of multiple inflammatory cells, macrophages, eosinophils and lymphocytes (H&E ×100)
Fig. 2.
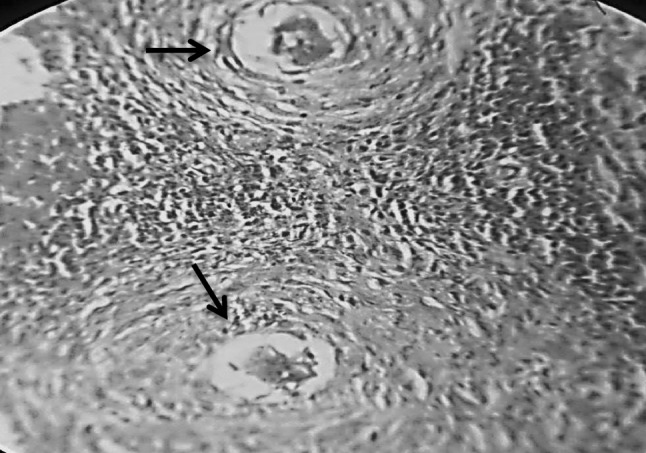
Section in murine liver from (G3) (adjuvant then infected by Schistosoma mansoni) showing two close bilharzial fibrocellular granulomas formed of Schistosoma mansoni ova surrounded by lymphocytes, plasma cells, eosinophils, histocytes and little fibrosis (H&E ×400)
The results of the histopathological study (Figs. 3, 4, 5):
Fig. 3.
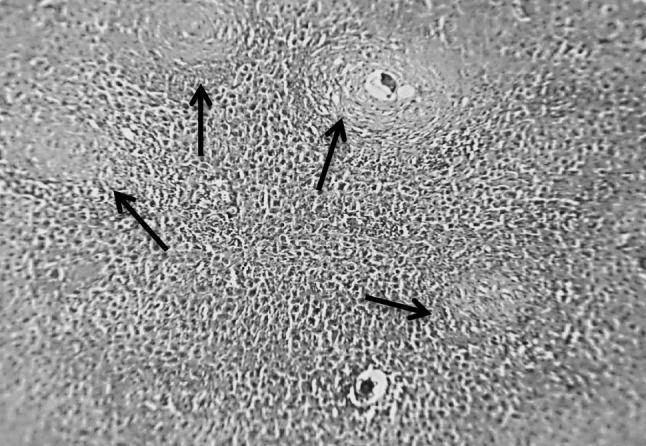
Section in murine liver from (G4) (SEA + adjuvant then infected by S. mansoni) showing small sized granulomas (arrows; fibrocellular and fibrous) some of the granulomas revealed central degenerated ova. The granulomas formed mainly of fibroblasts, macrophages, lymphocytes and collagen fibers. There was an improvement of the hepatic tissue configuration (H&E ×100)
Fig. 4.
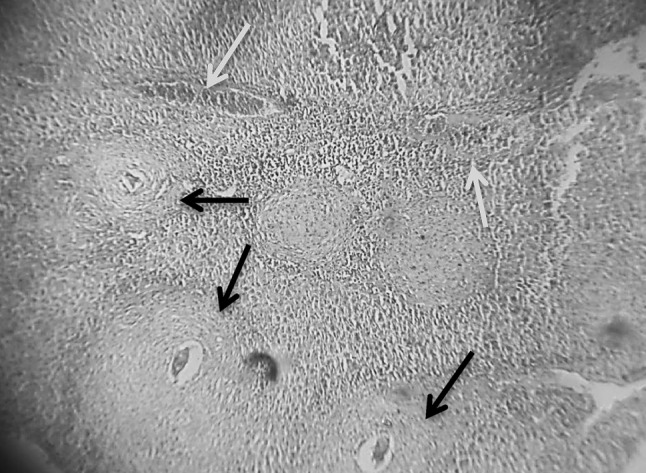
Section in murine liver from (G5) (SWAP + adjuvant then infected by S. mansoni) showing moderate sized granulomas (arrows; fibrocellular and fibrous) with central degenerated ova. There was a little bit improvement in the hepatic tissue configuration. Areas of hemorrhages were seen (blue arrow; H&E ×100) (color figure online)
Fig. 5.
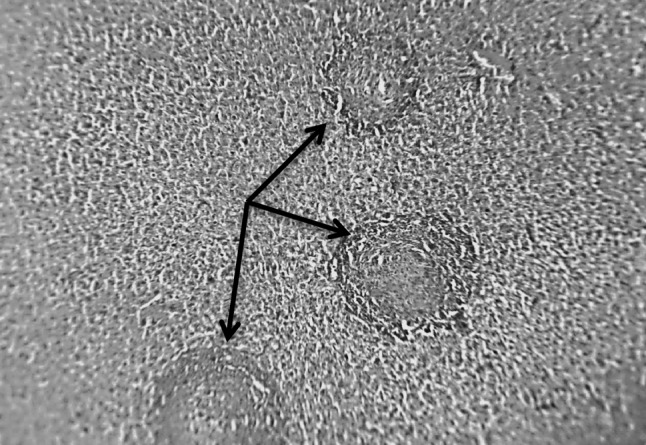
Section in murine liver from (G6) (SEA + SWAP + adjuvant then infected by S. mansoni) showing small sized, fibrocellular and fibrous granulomas (arrows) with marked reduction in the size and number of granulomas. Eggs were not seen in the center of the granuloma. Healthy hepatic tissue was seen in between the few scattered granulomas (H&E ×100)
The evaluation of the different vaccines by the histopathological study showed that the usage of combined antigens (SWAP + SEA) led to changes in hepatic granuloma cell content towards increase in fibroblasts, fibrocytes and collagen fibers with more fibrocellular and fibrous granulomas, while the hepatic tissue configuration was nearly kept. These positive changes was also recorded on using SEA antigen but less than that with combined antigen, while SWAP antigen was inferior to SEA antigen.
The results of the histochemical study (Figs. 6, 7, 8, 9, 10, 11):
Fig. 6.
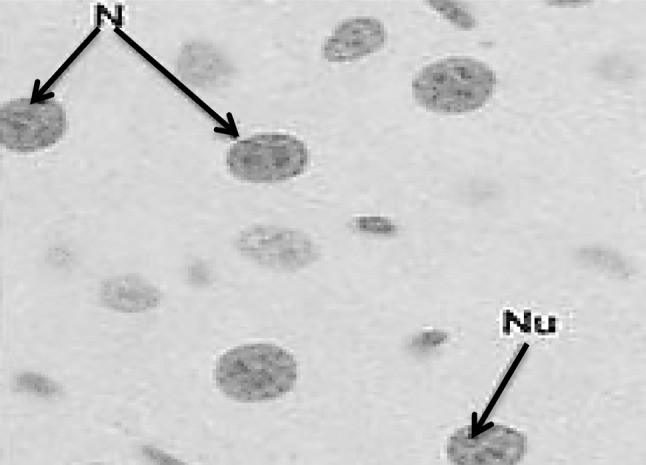
Section in murine liver from control normal group (G1) showing normal hepatocyte nuclei with circular contour, intact nuclear membrane, regularly distributed chromatin, one or two nucleoli and bright red staining of the DNA. N nucleus, Nu nucleolus (Feulgen ×1000)
Fig. 7.
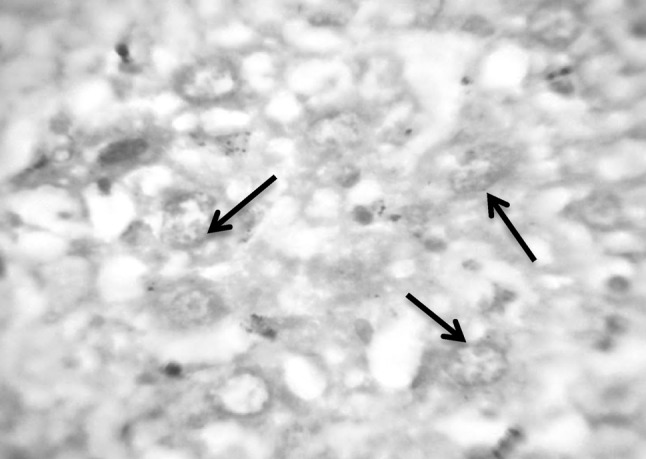
Section in murine liver from (G2) control infected mice by S. mansoni: showing hepatocytes in different stages of degeneration with vacuolated cytoplasm. The nuclei showed fragmentation (arrows), chromatolysis, partially destroyed nuclear membrane and very pale red staining of the DNA (Feulgen ×1000)
Fig. 8.
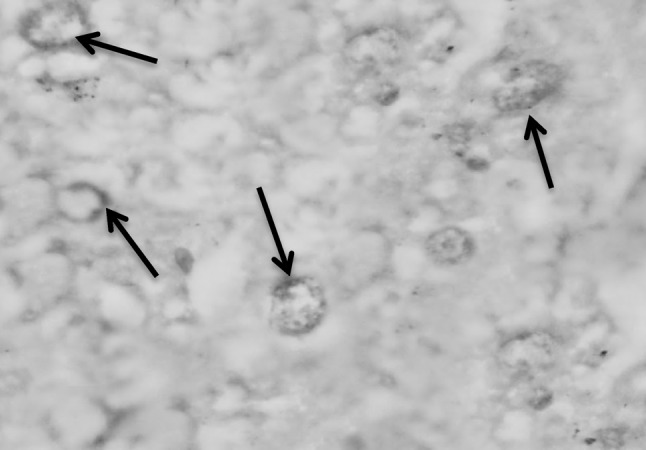
Section in murine liver from (G3; adjuvant then infected by S. mansoni) showing degenerated hepatocytes where the nuclei showing fragmentation (arrows), chromatolysis, partially destroyed nuclear membrane with faintly red staining of the DNA (Feulgen ×1000)
Fig. 9.
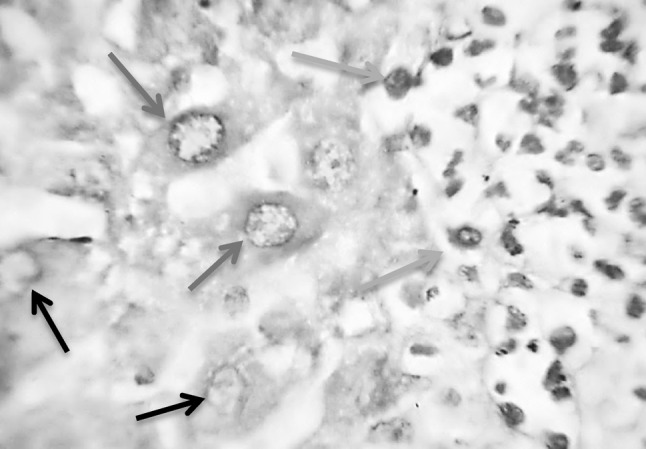
Section in murine liver from (G4; SEA + adjuvant then infected by S. mansoni) showing hepatocytes with improved configuration, where the hepatic cell nuclei restored its vesicular healthy condition with intact healthy distributed chromatin and light red staining of the DNA (red arrow), although still present few other degenerated hepatocytes with vacuolated cytoplasm and partially destroyed nuclear membrane (black arrows). On the right side of the picture some nuclei of the granulation tissue cells are seen (green arrows; Feulgen ×1000) (color figure online)
Fig. 10.

Section in murine liver from (G5; SWAP + adjuvant then infected by S. mansoni) showing hepatocytes with improved configuration, where the hepatic cell nuclei restored its vesicular healthy condition with intact healthy distributed chromatin (black arrow), few other degenerated hepatocytes with vacuolated cytoplasm, partially destroyed nuclear membrane with light red staining of the DNA (red arrows; Feulgen ×1000)
Fig. 11.
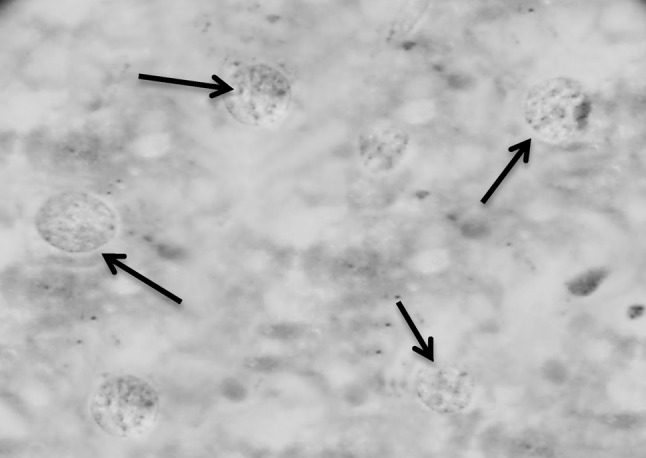
Section in murine liver from (G6; SWAP + SEA + adjuvant then infected by S. mansoni) showing hepatocytes with good configuration regarding the state of the nuclei, where the hepatic cell nuclei are vesicular, healthy with intact healthy distributed chromatin, intact nuclear membrane, normal shape and average size, one or two nucleoli and nearly bright red staining of the DNA (black arrows; Feulgen ×1000)
So, the histochemical study evaluation showed that best protection presented to hepatocytes DNA was that of the combined antigens (SWAP + SEA), followed by SEA then SWAP antigen (Table 1).
Table 1.
The results of the used methods for histochemical and histopathological studies to detect the induced apoptosis by Feulgen stain and granuloma size (diameter) and number in the different groups
| Group | Induced apoptosis | Granuloma (diameter and number) | |
|---|---|---|---|
| By Feulgen stain | Diameter (µm) Mean ± SD |
Number (mean ± SD) | |
| G2 | Severe | 357.33 ± 53.08 | 69.17 ± 12.5 |
| G3 | Severe | 341 ± 31.95 | 64.5 ± 12.1 |
| G4 | Moderate | 223.17 ± 14.12*** | 25.3 ± 5.96*** |
| G5 | Moderate | 241.5 ± 18.29*** | 28.66 ± 6.5*** |
| G6 | Mild | 164.67 ± 17.95*** | 7.67 ± 2.16*** |
*** Very high significant difference from infected control at p < 0.001
There were high significant difference among the results of G4, G5 and G6 regarding granuloma diameter and number, the induced apoptosis was directly proportional with the granuloma no. and size i.e. increased with the increase in granuloma no. and size as in G2 and G3 and decreased with the decrease in granuloma no. and size as in G4, G5 and G6.
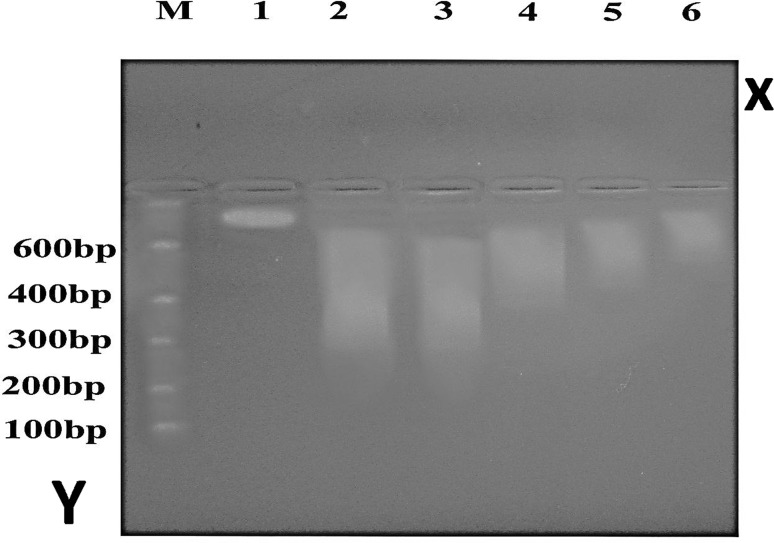
The molecular study of hepatocyte DNA fragmentation (induced apoptosis) by gel electrophoresis
This molecular study was performed to assess the effects of different Schistosoma mansoni antigens used as potential vaccines and challenged by Schistosoma infection in murine models by detection of hepatocyte DNA changes and fragmentation by gel electrophoresis in the control and tested group.
By using DNA extraction and gel electrophoresis, the degrees of hepatocyte DNA fragmentation (induced apoptosis) were classified according to the density of apoptotic bands into mild, moderate, dense and severe i.e. induced apoptosis was the other face of hepatocyte DNA fragmentation. The results were shown in (Fig. 12):
Fig. 12.
Results of DNA fragmentation (induced apoptosis) using gel electrophoresis. M: molecular weight marker. Lane (1): control non infected group (G1). Lane (2): control infected group (G2). Lane (3): Adjuvant + infected group (G3). Lane (4): SEA + adjuvant + infected group (G4). Lane (5): SWAP + adjuvant + infected group (G5). Lane (6): SEA + SWAP + adjuvant + infected group (G6)
Axis Y: represent number of DNA base pair in the DNA marker (ladder).
Axis X: represent the control and vaccinated groups.
Lane (1): showing the normal DNA with high molecular weight which stopped at the top of the gel (no fragmentation).
Lane (2) and (3): showing high density of the apoptotic bands denoting severe DNA fragmentation.
Lane (4) and (5): showing slight decrease in the density of the apoptotic bands denoting moderate DNA fragmentation.
Lane (6): showing the least density of the apoptotic bands denoting very mild DNA fragmentation (Table 2).
Table 2.
Percentages of hepatocyte DNA fragmentation in both control and vaccinated groups by gel electrophoresis
| Groups | −ve (%) | Mild (%) | Moderate (%) | Severe (%) | Total (%) | Chi square χ2 | p value |
|---|---|---|---|---|---|---|---|
| G1 | 100 | 0 | 0 | 0 | 100 | 58.76*** | <0.001 |
| G2 | 0 | 0 | 0 | 100 | 100 | ||
| G3 | 0 | 0 | 33.3 | 66.7 | 100 | ||
| G4 | 16.7 | 33.3 | 50 | 0 | 100 | ||
| G5 | 33.3 | 33.3 | 16.7 | 16.7 | 100 | ||
| G6 | 33.3 | 66.7 | 0 | 0 | 100 |
*** There is very high significant difference between all groups at p < 0.001
The DNA fragmentation method of evaluation showed that there was an inverse relationship between the degree of induced apoptosis (degree of hepatocyte DNA fragmentation) and the degree of protection presented by the tested antigens to hepatocyte DNA.
The evaluation by the molecular study using hepatocytes DNA fragmentation revealed that the combined vaccine (G6) showed the lowest degree of induced apoptosis followed by SEA vaccine group (G4) then SWAP vaccine group (G5), while the control infected group (G2)and the control infected + adj. group (G3) showed the highest degree of induced apoptosis i.e. the highest degree of the protection against induced apoptosis was presented by the combined tested antigens in G6 followed by SEA in G4 then SWAP in G5 (Table 3).
Table 3.
Comparison among the results of histopathological, histochemical and molecular studies in the different groups
| Item | Group | ||||
|---|---|---|---|---|---|
| G2 | G3 | G4 | G5 | G6 | |
| Granuloma no. | 69.17 ± 12.5 | 64.5 ± 12.1 | 25.33 ± 5.96*** | 28.66 ± 6.5*** | 7.67 ± 2.16*** |
| Granuloma size | 357.33 ± 53.08 | 341 ± 31.95 | 223.17 ± 14.12 *** | 241.5 ± 18.29 *** | 164.67 ± 17.95 *** |
| Apoptosis by Feulgen | Severe | Severe | Moderate | Moderate | Mild |
| −ve fragmentation | 0 % | 0 % | 16.7 % | 33.3 % | 33.3 % |
| Mild fragmentation | 0 % | 0 % | 33.3 % | 33.3 % | 66.7 % |
| Moderate fragmentation | 0 % | 33.3 % | 50 % | 16.7 % | 0 % |
| Severe fragmentation | 100 % | 66.7 % | 0 % | 16.7 % | 0 % |
*** Very high significant difference from infected control at p < 0.001
In this table, the results were in consequence showing that the combined antigens presented the best protection to the hepatocytes, while the results of all tests used for evaluating the impact of the tested potential antischistosomal vaccines cleared that molecular method of assessment by (DNA fragmentation) was clearer and so, more accurate than that of histopathological and histochemical semiquantitative and descriptive methods.
Discussion
The main pathology in schistosomiasis is granulomatous and fibrosing reactions against the parasite eggs, which in the case of Schistosoma mansoni takes place in the liver and intestines; this reaction is an immunopathological process mediated by CD4 + Th lymphocytes specific for schistosome egg antigens (Taylor et al. 2006). Granuloma formation is initiated by antigens secreted by the miracidium through microscopic pores within the egg shell, these antigens are glycoproteins, known as soluble egg antigens (SEA) which can induce granuloma by inducing the host cellular immune response (Schmitt 2006). This reaction commences by the accumulation of CD4+ Th2-cells and followed by the aggregation of eosinophils and neutrophils around the alternatively activated macrophages (M2; Hams et al. 2013). Also, similar findings were detected by Affify (1999) who noted that the antigenicity of SEA was more powerful than SWAP because it moves through the intestinal wall and become closely encircled by host cells. Also, in the liver it becomes closely surrounded at first by parenchymal cells and later by granuloma cells.
Zahran (2002) and Khalifa et al. (2011) reported that combination of two vaccines provides augmentation of the protective immunity and reduction of hepatic immunopathology.
The previous findings highlighted the histopathological evaluation results of the current study, it was noticed that the most effective vaccine with very high significant reduction in the number and size of hepatic granulomas was the SEA + SWAP combined antigens with fibrocellular to fibrous granulomatous composition, followed by the SEA then SWAP antigens. On the other hand, insignificant reduction was detected in granuloma number and size in the Freund’s group, with cellular to fibrocellular composition when compared to the control infected group. So, these results indicated the increase in Th1 response by its specific cytokines which was reflected positively on the number and size of hepatic Schistosoma granulomas leading to decrease in number and size of them and change of the cellular content.
These results were in agreement with that of Ismail (2005) who reported that the most effective vaccine with very high significant reduction in granuloma number and size was the combined (SEA + SWAP + CAP) vaccine followed by SEA then SWAP vaccine candidates. The current results were also, agreed with that of Etewa et al. (2014) who reported that the most effective vaccine with very high significant reduction in the number and size of hepatic granulomas was the cocktail (CAP + SWAP + SEA + BCG) vaccine followed by SEA then SWAP vaccine. Meanwhile, insignificant reduction was detected in the adjuvant-injected group.
Regarding granuloma cellular composition, The present results were in accordance with that of Boros (1989) who stated that granuloma formation is mediated by a T-cell response to egg secreted SEA which together with TNF-α induce recruitment of many inflammatory cells including macrophages, lymphocytes, plasma cells, eosinophils, neutrophils and fibroblasts.
In schistosomiasis, it was reported that schistosome worms may use apoptosis as a survival strategy to establish infection in their host, and can influence the development or maintenance of different clinical manifestations (Etewa et al. 2013). Apoptosis is a tightly regulated process in which cells establish an inducible non-necrotic cellular death process. It has a major role in balancing cell proliferation and remodeling tissue activity in many organisms (Fuchs and Steller 2011). Apoptosis has been defined morphologically by the presence of cytoplasmic shrinkage (pyknosis), chromatin condensation, nuclear fragmentation (karyorhexis), the presence of nuclear membrane blebbing while the maintenance of an intact plasma membrane which may retain its integrity as the nucleus fragments into apoptotic bodies (Kroemer et al. 2005).
Hepatic schistosomiasis could suppress the activation of hepatic cells by emitting soluble antigens which promote liver cells apoptosis, induce morphological changes in the hepatocytes, inhibit cell proliferation and enhance cell-cycle arrest at the G1 phase. Schistosoma soluble antigens induce apoptosis in hepatic cells through the TNF-related apoptosis-inducing ligand/death receptor 5 and caspase-dependent pathways (Duan et al. 2014).
One of the classic features of apoptosis is the cleavage of the genomic DNA into oligonucleosomal fragments represented by multiples of 180–200 bp. Visualizing these DNA fragments using agarose gel electrophoresis can aid in characterizing an apoptotic event (Kasibhatla et al. 2006).
In schistosomiasis, deposition of eggs in liver induces a severe inflammatory reaction, which participates in elimination of eggs but may at the same time cause damage of DNA and liver cell apoptosis (Sayed et al. 2006). The inflammatory cellular reaction in response to different parasite antigens with local areas of degeneration, pyknotic nuclei and massive congestion leads to blood stasis with subsequent hypoxia. The hypoxia raises the hydrostatic pressure which in turn results in entry of fluids into the cells. These events affect the mitochondria which are very sensitive organelles to changes in osmotic pressure and oxygen tension. These changes may also lead to functional damage of liver DNA and its metabolism (Wang and Lin 2013).
So the study of DNA fragmentation and apoptosis in liver tissues of S. mansoni infected mice after vaccination showed that the antischistosomal vaccines effectually decreased DNA fragmentation. The combined (SWAP + SEA) followed by SEA then SWAP antigens together with Freund’s adjuvant apparently caused a significant reduction in hepatic parasite load which in turn decreased soluble antigens production that induce liver apoptosis by different mechanisms as explained by El-Sharkawi et al. (2002).
In the work in our hands; hepatocyte apoptosis evaluated by detection of DNA hydrolysis (using Feulgen histochemical technique) revealed that the best hepatocyte DNA protection with the least apoptotic changes was obtained with the combined (SWAP + SEA) antigens, followed by SEA then SWAP antigens, when compared to control infected group that showed marked hepatocyte apoptosis. On the other hand, Freund’s adjuvant alone (G3) was almost ineffective and associated with marked liver cell apoptotic changes, these findings are not in accordance with Etewa et al. (2014) who reported that giving Freund’s adjuvant alone may produce moderate apoptosis due to nonspecific stimulation of immune responses.
Our results were in agreement with Soomro et al. (2005) who reported that during hepatic Schistosomiasis, there was shrinkage and some necrosis of hepatocytes around granulomas with massive infiltration with leucocytes and many clumps of Schistosoma eggs.
Additionally, similar results were obtained by Etewa et al. (2013) who reported mild apoptosis occurred in mice vaccinated by S. mansoni tegumental antigen (TA55) plus IL-12, as compared to the marked hepatocyte apoptotic changes in the control infected group using Feulgen histochemical technique for the assessment of apoptosis. The results were also in agreement with Etewa et al. (2014) who reported mild apoptosis occurred in mice vaccinated by S. mansoni combined antigens (CAP + SEA + SWAP), as compared to the severe hepatocyte apoptotic changes in the control infected group.
On the other hand, results of the present work were against that reported by Botelho et al. (2009) who found that S. haematobium antigens led to increased proliferation and decreased apoptosis of the bladder epithelial cells. This controversy was explained by down-regulation of tumor suppressor factor (p27) and up-regulation of anti-apoptotic molecule (Bcl-2) and these factors may be the causes of bladder cancer.
By using DNA extraction and gel electrophoresis, the degrees of hepatocyte DNA fragmentation (induced apoptosis) were classified according to the density of apoptotic bands into mild, moderate, dense and severe i.e. apoptosis was the reported face of hepatocyte DNA fragmentation(El-Bendarya et al. 2014). So, the current results revealed that the best hepatocyte DNA protection with the least DNA fragmentation was obtained with the combined (SWAP + SEA) antigens, followed by SEA then SWAP antigens i.e. the highest degree of the protection against induced apoptosis was presented by the combined tested vaccine followed by SEA vaccine then SWAP vaccine, when compared to control infected group that showed severe DNA fragmentation. On the other hand, Freund’s adjuvant alone (G3) was almost ineffective and associated with marked DNA fragmentation.
In addition the present findings are similar to that of Eid et al. (2014) who explained that high DNA fragmentation level quantified in hepatic tissues of infected mice potentially concurrent with the elevation in the level of oxidative/nitrosative stress parameters associated with inflammatory granulomatous reactions, while Ma et al. (2011) reported that oxidative/nitrosative DNA damage in S. haematobium-associated bladder cancer.
Conclusion
On comparing all tests used for assessment, the results were in consequence and showing that the combined antigens (SEA + SWAP) as a potential vaccine were the best regarding hepatocytes protection. The results of all tests used for evaluating the efficacy of the tested potential anti-schistosomal vaccines cleared that the molecular method of assessment by (DNA fragmentation) was clearer and more accurate, so better than semiquantitative descriptive histopathological and histochemical methods of assessment.
References
- Affify HA (1999) Detection of circulating schistosomal antigens in serum and urine samples in patients with schistosomiasis. M.D. thesis, Faculty of Medicine, Zagazig University
- Ali SA, Hamed MA. Effect of Ailanthus ltissima and Zizyphus spina-christi on bilharzial infestation in mice: histological and histopathological studies. J Appl Sci. 2006;6:1437–1446. doi: 10.3923/jas.2006.1437.1446. [DOI] [Google Scholar]
- Anyan WK, Seki T, Kumagai T, Obata-Ninomiya K, Furushima-Shimogawara R, Kwansa-Bentum B, Akao N, Bosompem KM, Boakye DA, Michael D, Wilson MD, Karasuyama H, Ohta N. Basophil depletion downregulates Schistosoma mansoni egg-induced granuloma formation. Parasitol Int. 2013;62:508–513. doi: 10.1016/j.parint.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Bechmann LP, Marquitan G, Jochum C, Saner F, Gerken G, Canbay A. Apoptosis versus necrosis rate as a predictor in acute liver failure following acetaminophen intoxication compared with acute-on-chronic liver failure. Liver Int. 2008;28(5):713–716. doi: 10.1111/j.1478-3231.2007.01566.x. [DOI] [PubMed] [Google Scholar]
- Boros DL. Immunopathology of Schistosoma mansoni infection. Clin Microbiol Rev. 1989;2:250–269. doi: 10.1128/CMR.2.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros DL, Warren KS. Delayed hypersensitivity type III: granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970;132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho M, Ferreira AC, Oliveira MJ, Domingues A, Machado JC, José Manuel J, da Costa C. Schistosoma haematobium total antigen induces increased proliferation, migration and invasion, and decreases apoptosis of normal epithelial cells. Int J Parasitol. 2009;39(10):1083–1091. doi: 10.1016/j.ijpara.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Canbay A, Guicciardi ME, Higuchi H, Feldstein A, Bronk SF, Rydzewski R, Taniai M. Cathepsin-B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Investig. 2003;112:152–159. doi: 10.1172/JCI200317740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribb TH, Bray RA, Littlewood DTJ, Pichelin SP, Herniou EA. The digenea. In: Littlewood DTJ, Bray RA, editors. Interrelationships of the platyhelminthes. London: Taylor & Francis; 2001. pp. 168–185. [Google Scholar]
- Duan Y, Gu X, Zhu D, Sun W, Chen J, Feng J, Song K, Xu F, He X. Schistosoma japonicum soluble egg antigens induce apoptosis and inhibit activation of hepatic stellate cells: a possible molecular mechanism. Int J Parasitol. 2014;44:217–224. doi: 10.1016/j.ijpara.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Eid JI, Mohammed AR, Hussien NA, El-Shennawy AM, Magda M, Noshy MM, Mohamed A. In vivo antioxidant and antigenotoxic evaluation of an enaminone derivative BDHQ combined with praziquantel in uninfected and Schistosoma mansoni infected mice. J Appl Pharm Sci. 2014;4(5):25–33. [Google Scholar]
- El-Bendarya M, Hawas S, Elhammady D, Al-Hadidy AM, Rizk H. Expression of Fas (CD95) and Bcl-2 in peripheral blood mononuclear cells in patients with chronic HCV and schistosomiasis. Egypt J Basic Appl Sci. 2014;1:136–143. doi: 10.1016/j.ejbas.2014.10.002. [DOI] [Google Scholar]
- El-Sharkawi IM, Ibrahim AA, El Bassuoni MA. Neutrophil apoptosis in acute and chronic Schistosoma mansoni infection. J Egypt Soc Parasitol. 2002;32(2):507–516. [PubMed] [Google Scholar]
- Etewa SE, El settawy MA, Sadek GS. Potential role of Interleukin 4 and 12 as adjuvants to tegumental antigen in a vaccination model for murine Schistosomiasis mansoni. PUJ. 2013;6(1):65–76. [Google Scholar]
- Etewa SE, AbdEl-Aal NF, Metwally AS, Abd El Bary EH, Radwan H. Histopathological assessment of different vaccines in experimental mansoniasis. AARJMD. 2014;1(17):2319–2801. [Google Scholar]
- Fang R, Feng H, Hu M, Khan MK, Wang L, Zhou Y, Zhao J. Evaluation of immune responses induced by SAG1 and MIC3 vaccine cocktails against Toxoplasma gondii. Vet Parasitol. 2012;187(1–2):140–146. doi: 10.1016/j.vetpar.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147(4):742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravin AJ, Hall BJ, Brissie RA, Spicer SS. Cytochemical differentiation of nucleic acid with schiff methylene blue sequence. J Histochem Cytochem. 1976;24:587–590. doi: 10.1177/24.4.58023. [DOI] [PubMed] [Google Scholar]
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- Hams E, Aviello G, Fallon PG. The Schistosoma granuloma: friend or foe? Front Immunol. 2013;4(89):1–8. doi: 10.3389/fimmu.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassab-El Nabi SE, El Hassaneen YA. Detection of DNA damage, molecular apoptosis and production of homemade ladder by using simple techniques. Biotechnology. 2008;7(3):514–522. doi: 10.3923/biotech.2008.514.522. [DOI] [Google Scholar]
- Houston S, Kowalewska-Grochowska K, Naik S, McKean J, Johnson ES, Warren K. First report of Schistosoma mekongi infection with brain involvement. Clin Inf Dis. 2004;38:1–6. doi: 10.1086/379826. [DOI] [PubMed] [Google Scholar]
- Ismail OA (2005) Study of the efficacy of adult worm, cercarial and egg antigens in protection against experimental intestinal schistosomiasis. M.D. thesis, Faculty of Medicine, Suez Canal University
- Kasibhatla S, Amarante-Mendes GP, Finucane D, Brunner T, Wetzeland EB, Douglas R, Green DR. Analysis of DNA fragmentation using agarose gel electrophoresis. Cold Spring Harb Protoc. 2006 doi: 10.1101/pdb.prot4429. [DOI] [PubMed] [Google Scholar]
- Kerishina TF. In vivo regulation of S. japonicum granuloma formation by an IL-2 antagonist. Parasitology. 1991;102:243. doi: 10.1017/S0031182000062557. [DOI] [PubMed] [Google Scholar]
- Khalifa RMA, Elnadi NA, Omran EK, Abdel-Tawab RA. Immunological response and the probability of production of vaccine for schistosome parasites. Egypt J Med Sci. 2011;32(2):547–570. [Google Scholar]
- Kroemer G, El-Deiry WS, Golstein P. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12(2):1463–1467. doi: 10.1038/sj.cdd.4401724. [DOI] [PubMed] [Google Scholar]
- Liang YS, John I, Bruce JI, David AB (1987) Laboratory cultivation of schistosome vector snails and maintenance of schistosome life cycle. In: Proceedings of first Sine-American symposium, vol 1, p 34
- Ma N, Thanan R, Kobayashi H, Hammam O, Wishahi M, El Leithy T, Hiraku Y, Amroel K, Oikawa S, Ohnishi S, Murata M, Kawanishi S. Nitrative DNA damage and Oct3/4 expression in urinary bladder cancer with Schistosoma haematobium infection. Biochem Biophys Res Commun. 2011;414:344–349. doi: 10.1016/j.bbrc.2011.09.073. [DOI] [PubMed] [Google Scholar]
- McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21:225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner T, Reilly L, Nausch N, Midzi N, Mduluza T, Maizels R, Mutapi F. Circulating cytokine levels and antibody responses to human Schistosoma haematobium: IL-5 and IL-10 levels depend upon age and infection status. J Parasite Immunol. 2010;32:710–721. doi: 10.1111/j.1365-3024.2010.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano M, Daniel A, Colley G, George L, Freeman JR, Evan SW. Neonatal exposure to idiotype induces S. mansoni egg antigen-specific cellular and humoral immune responses. J Immunol. 1999;163:898–905. [PubMed] [Google Scholar]
- Nabih I, Soliman AM. Studies on fresh water snails, specific intermediate host for schistosomiasis. II. Isolation of total protein from native and irradiated snails. Cell Mol Biol. 1986;32:315–317. [PubMed] [Google Scholar]
- Pearce EJ. Priming of the immune response by schistosome eggs. J Parasite Immunol. 2005;27(7–8):265–270. doi: 10.1111/j.1365-3024.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- Peters AP, Warren KS. A rapid method of infecting mice and other laboratory animals with Schistosoma mansoni subcutaneous injection. J Parasitol. 1969;55:558–563. doi: 10.2307/3277297. [DOI] [Google Scholar]
- Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, McManus DP. Schistosomiasis. N Engl J Med. 2002;346:1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- Salih SY, Bartlett A, Voller A. Detection of antibodies by enzyme immunoassay in human Schistosoma mansoni infection a clinical chemotherapeutic study. J Trop Parasitol. 1978;29:409–412. [PubMed] [Google Scholar]
- Sayed AA, Cook SK, William DL. Redox balance mechanisms in Schistosoma mansoni rely on peroxiredoxins and albumin and impilicate peroxiredoxins as novel drug targets. J Biol Chem. 2006;281(25):17001–17010. doi: 10.1074/jbc.M512601200. [DOI] [PubMed] [Google Scholar]
- Schmitt I (2006) Schistosomiasis––The burden of disease and trends of intervention. International Health Practice
- Shams El Din SA. Histopathological, histochemical and immunehisto chemical studies of experimental Heterophyiasis in dogs and the protecting role of Praziquantel. PUJ. 2011;4(2):185–192. [Google Scholar]
- Smithers SR, Hackett F, Ali OP, Simpson AJG. Protective immunization of mice against schistosoma mansoni with purified adult worm surface membranes. Parasite Immunol. 1989;11:301–318. doi: 10.1111/j.1365-3024.1989.tb00669.x. [DOI] [PubMed] [Google Scholar]
- Soomro NM, Arijo AG, Qureshi TA, Runham NW, Doenhoff MJ (2005) Effect of Schistosome infection on liver of mice. J Agric Soc Sci 1813–2235/2005/01– 3–219–222. http://www.ijabjass.org
- Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J Immunol. 2006;176:5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- Von Lichtenberg FC. Host response to eggs of Schistosoma mansoni. I Granuloma formation in the sensitized laboratory mouse. Am J Pathol. 1962;41:711–731. [PMC free article] [PubMed] [Google Scholar]
- Wang K, Lin B. Pathophysoilogical significance of hepatic apoptosis. ISRN Hepatol. 2013;2013(2013):740149. doi: 10.1155/2013/740149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2010) Schistosomiasis. http://www.who.int/mediacentre/factsheets/fs115/en/. Accessed 5 Oct 2010
- World health Organization (2013) Schistosomiasis. A major public health problem. http://www.who.int/schistosomiasis/epidemiology/en Accessed 22 Aug 2013
- Zahran SGH (2002) Immunological studies on the synergistic action of different vaccines against schistosomiasis in mice. Ph.D. thesis, National Research Center, Ain Shams University, Cairo, Egypt