Abstract
Ticks and tick-borne diseases are the main problems affecting the livestock production in Egypt. Bovine babesiosis has adverse effects on the animal health and production. A comparison of Giemsa stained blood smears, polymerase chain reaction (PCR) and nested PCR (nPCR) assays for detection of Babesia bovis infection in Egyptian Baladi cattle (Bos taurus) in reference to reverse line blot was carried out. The sensitivity of PCR and nested PCR (nPCR) assays were 65 and 100 % respectively. Giemsa stained blood smears showed the lowest sensitivity (30 %). According to these results using of PCR and nPCR target for B. bovis, [BBOV-IV005650 (BV5650)] gene are suitable for diagnosis of B. bovis infection. The 18Ss rRNA partial sequence confirmed that all the positive samples were Babesia bovis and all of them were deposited in the GenBank databases (Accession No: KM455548, KM455549 and KM455550).
Keywords: Babesia bovis, Giemsa, 18Ss rRNA, PCR, nPCR
Introduction
Babesia bovis infection is a tick-borne parasite of cattle transmitted by Rhipicephalus annulatus ticks. The first description of this disease in Egypt was in 1947 by Nagati (1947). B. bovis infection is a worldwide distributed and considered as one of the most important destructive diseases of cattle (McCosker, 1981; Ibrahim et al. 2013 and Elsify et al. 2015). The clinical signs include fever, hemoglobinuria, acute anemia, and nervous signs (Mosqueda et al. 2012; Radwan et al. 2013). Low parasitemia carrier state is usually developed after survival of the animals; they serve as a reservoir of the parasite (Mahoney, 1969). The diagnosis of bovine babesiosis depends mainly on clinical and microscopic examination of Giemsa stained blood smear which is the conventional laboratory technique. Presence of piroplasms is the gold standard for the diagnosis, especially during the acute stage of the disease. Indeed, during this stage the number of parasites inside the erythrocytes is high enough to be detected easier by microscopical examination (Bose et al. 1995). In carrier animals this method is less sensitive since the parasitaemia is low. In this case, molecular tools using PCR and nested PCR increase the sensitivity. They are more sensitive and specific, providing rapid and accurate results (Oliveira-Sequeira et al. 2005; Costa-Junior et al. 2006; Martins et al. 2008). This study aim to compare four diagnostic techniques, namely Giemsa stained blood smears, PCR and nested PCR for detection of Babesia bovis infection in Egyptian Baladi cattle (Bos taurus). The Reverse line blot (RLB) was the reference technique.
Materials and methods
Animals
Baladi breed cattle belong to different localities in Assuit governorate in Upper Egypt (Egypt) were admitted to the Veterinary Teaching Hospital. They were subjected to clinical examination according to Radostits et al. (2000).
Parasitological diagnosis
Blood samples were collected directly from the ear vein and used for preparation of blood smears (Coles, 1986).
Molecular diagnosis
Whole blood samples were collected directly from the jugular vein on vacutainer tube containing EDTA and stored at −20 °C until used. DNA extraction was performed with QIAamp DNA blood Mini kit, (Qiagen, Ltd, UK) according to manufacturer’s instruction. PCR and nested PCR (nPCR) amplifications were carried out by using specific primers to B. bovis, [BBOV-IV005650 (BV5650)] F, 5′-CCGGAATTCCAAATGGCAACAAAGGTTGA-3′R, 5′-CCGCTCGAGGGAGCAGCGTATTACTTCCTCACGT-3′, F1, 5′-CGAGGATTTGGTAGACCTCATC-3′, R2 5′-CGTAAAATGTGTACAACTATTT-3′, respectively (Figueroa et al. 1993; Aboulaila, et al. 2010a, b). RLB was performed with a set of 18Ss rRNA Babesia spp.-catchall primers (RLB-F2 5′-GAC ACA GGG AGG TAG TGA CAA G-3′, Nested RLB-F 5′-GAC AAG AAA TAA CAA TAC RGG GC-3′, RLB-R2 5′CTA AGA ATT TCA CCT CTG ACA GT-3′) (Gubbels et al. 1999). The products were subjected to electrophoresis in 2 % agarose gel and then visualized under ultraviolet (UV) light after staining with ethidium bromide (Sigma-Aldrich). Positive DNA products from 18Ss rRNA primer were purified and then cloned into pDrive Cloning vector using QIAGEN Kit (Qiagen, Ltd, UK) according to manufacturer’s instructions. The amplicons were sequenced in both directions (Molecular Biology Research Unite, Assiut University, Egypt), and subjected to BLAST similarity searches.
Statistical analysis
Giemsa stained blood smears, PCR and nested PCR were compared with RLB as a reference test. Evaluation parameters included sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and combined predictive value (CPV) (Thrusfield, 2005).
Results
Giemsa stained blood smears confirmed the infection in 7.9 % (6/76) of the examined animals. The positive animals showed specific clinical signs of babesiosis, including fever (>40 °C) and hemoglobin urea with different intensities of Boophilus (Rhipicephalus) annulatus ticks infestations. PCR and nested PCR detected the DNA target of Babesia bovis in 17.1 % (13/76) and 26.3 % (20/76) of examined cattle respectively. RLB assay confirmed the infection in 26.3 % (20/76) of the animals (Table 1, Figs. 1, 2, 3, 4, 5). The PCR showed 100 % specificity for detection of Babesia bovis infection but their sensitivity varied between 30 and 100 % (Table 2). Partial sequences were deposited in the GenBank databases under accession numbers (KM455548, KM455549 and KM455550).
Table 1.
Infection rates according to Giemsa stained blood smears, PCR, nPCR and RLB for detection of Babesia bovis in tick infested cattle
| Diagnostic assays | Positive | ±SD (%) |
|---|---|---|
| Blood smear | 6/76 | 7.89 ± 25.32 |
| BV5650 PCR | 13/76 | 17.11 ± 25.23 |
| BV5650 nPCR | 20/76 | 26.31 ± 26.41 |
| RLB | 20/76 | 26.31 ± 26.41 |
Fig. 1.

Giemsa stained blood smear showing Babesia bovis piroplasms
Fig. 2.
Babesia bovis PCR products. Lane M: DNA ladder 100 bp, Lanes 1, 3, 5, 7 and 8: positive PCR with bands of 720 bp
Fig. 3.
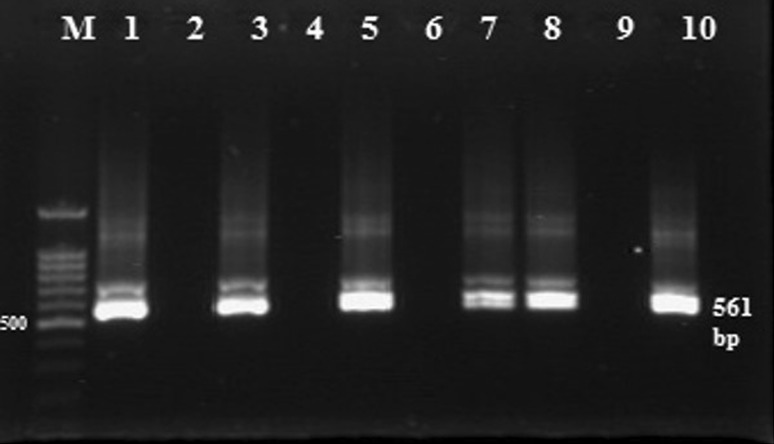
Babesia bovis nested PCR products. Lane M: DNA ladder 100 bp, Lanes 1, 3, 5, 7, 8 and 10: positive PCR with bands of 561 bp
Fig. 4.
Babesia bovis RLB-PCR products. Lane M: DNA ladder 100 bp, Lanes 1, 4, 6, 7 and 10: positive PCR
Fig. 5.
Babesia bovis nested RLB-PCR products. Lane M: DNA ladder 100 bp, Lanes 1, 2, 4, 6, 7 and 10: positive PCRs both of them gave bands of 460 and 520 bp
Table 2.
Evaluation of Giemsa stained blood smears, PCR and nPCR against RLB as a reference test for Babesia bovis detection in tick infested cattle
| Diagnostic assays | TP | TN | FP | FN | Sensitivity | Specificity | PPV | NPV | CPV |
|---|---|---|---|---|---|---|---|---|---|
| Blood film | 6 | 56 | 0 | 14 | 30 | 100 | 100 | 80 | 81.58 |
| BV5650 PCR | 13 | 56 | 0 | 7 | 65 | 100 | 100 | 88.89 | 90.79 |
| BV5650 nPCR | 20 | 56 | 0 | 0 | 100 | 100 | 100 | 100 | 100 |
TP true positive, TN true negative, FP false positive, FN false negative
Discussion
Bovine babesiosis is one of the most important diseases in Egypt. It has an adverse effect on both production and reproduction of cattle. B. bovis and B. bigemina are the most common parasites in cattle (Ibrahim et al. 2013; Elsify et al. 2015). The prevalence of them usually becomes different according to the investigated locality. The prevalence of B. bovis is higher than B. bigemina in Dakahlia Governorate, Egypt it was 7.3 and 1.2 %, respectively according to El-Ashker a et al. (2015) while Ibrahimet et al. (2013) reported that the prevalence of B. bigemina is higher than B. bovis it was 5.30 and 3.97 %, respectively in Beheira and Faiyum, Governorates Egypt. In Upper Egypt PCR and nPCR of BV5650 gene are recently used in detection of B. bovis infection in randomly collected field samples from baladi breed cattle. The molecular assay, specially nested PCR of BV5650 gene was the most sensitive test (100 %) followed by BV5650 gene PCR (65 %) and blood smears examination (30 %) this came in agreement with El-Ashker et al. (2015) and Aboulaila, et al. (2010a, b) the first study reported that molecular assay was more sensitive than conventional method and both of them should be in combination to detect both clinically infected animals and carrier state; the second study reported that using of PCR and nPCR of gene BV5650 had the highest sensitivity rate when applied on field samples collected from cattle in Ghana, Mongolia and Brazil, respectively. This could be attributed to the presence of several gene copies in the genome; also the sequence conservation among strains may be playing an important role in these findings according to Aboulaila, et al. (2010a, b). Giemsa stained blood smears, PCR and nPCR of BV5650 gene revealed high PPV (100 %). These findings indicate that during further investigations, these tests will have the ability to detect the infected animals correctly. The NPVs of Giemsa stained blood smears and PCR were relatively low as 80 and 88.9 %, respectively; if compared with nPCR BV5650 which revealed 100 % NPV. These results indicate the ability of nPCR BV5650 to detect true negative if compared with Giemsa stained blood smears and BV5650 PCR assay. The combined predictive values were 81.6, 90.8 and 100 % for Giemsa stained blood film and BV5650 PCR and nPCR assays.
In conclusion, PCR and nPCR methods based on BV5650 gene detection should be recommended for the detection of B. bovis infection in cattle under field conditions and for epidemiological studies.
Future study
A recent type of molecular assay like Loop-Mediated isothermal amplification (LAMP) test is more applicable, not expensive and not need sophisticated instruments. This molecular assay is more sensitive than conventional method, especially in the carrier state. We will start to use it for confirmation of infection in randomly collected field samples and evaluate its validity to use in epidemiological studies.
Acknowledgements
This work was supported by DFG project “Molecular epidemiology network for promotion and support of delivery of life vaccines against Theileria parva and Theileria annulata infection in Eastern and Northern Africa” (DFG project SE862/2-1).
Compliance with ethical standards
Conflict of interest
Author confirmed that there is no conflict of interest.
References
- AbouLaila M, Yokoyama N, Igarashi I. Development and evaluation of a nested PCR based on spherical body protein 2 genes for the diagnosis of Babesia bovis infection. Vet Parasitol. 2010;139:45–50. doi: 10.1016/j.vetpar.2009.12.013. [DOI] [PubMed] [Google Scholar]
- AbouLaila M, Yokoyama N, Igarashi I. Development and evaluation of two nested PCR assays for the detection of Babesia bovis from cattle blood. Vet Parasitol. 2010;172:65–70. doi: 10.1016/j.vetpar.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Bose R, Jorgensen WK, Dalgliesh RJ, Friedhoff KT, De vos AJ. Current state and future trends in the diagnosis of babesiosis. Vet Parasitol. 1995;57:61–67. doi: 10.1016/0304-4017(94)03111-9. [DOI] [PubMed] [Google Scholar]
- Coles EH. Veterinary clinical pathology. 4. London and Toronto Philadelphia: W B Saunders Company; 1986. pp. 46–56. [Google Scholar]
- Costa-Junior LM, Rabelo EML, Filho OAM, Ribeiro MFB. Comparison of different direct diagnostic methods to identify Babesia bovis and Babesia bigemina in animals vaccinated with live attenuated parasites. Vet Parasitol. 2006;139:231–236. doi: 10.1016/j.vetpar.2006.02.034. [DOI] [PubMed] [Google Scholar]
- El-Ashker M, Hotzel H, Gwida M, El-Beskawy M, Silaghi C, Tomaso H. Molecular biological identification of Babesia, Theileria, and Anaplasma species in cattle in Egypt using PCR assays, gene sequence analysis and a novel DNA microarray. Vet Parasitol. 2015;207:329–334. doi: 10.1016/j.vetpar.2014.12.025. [DOI] [PubMed] [Google Scholar]
- Elsify A, Sivakumar T, Nayel M, Salama A, Elkhtam A, Rizk M, Mosaab O, Sultan K, Elsayed S, Igarashi I, Yokoyama N. An epidemiological survey of bovine Babesia and Theileria parasites in cattle, buffaloes, and sheep in Egypt. Parasitol Int. 2015;64:79–85. doi: 10.1016/j.parint.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Figueroa JV, Chieves LP, Johnson GS, Buening GM. Multiplex polymerase chain reaction based assay for the detection of Babesia bigeminaBabesia bovis and Anaplasma marginale DNA in bovine blood. Vet Parasitol. 1993;50:69–81. doi: 10.1016/0304-4017(93)90008-B. [DOI] [PubMed] [Google Scholar]
- Gubbels JM, de Vos AP, van der Weide M, Viseras J, Schouls LM, de Vries E, Jongejan F. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J Clin Microbiol. 1999;37:1782–1789. doi: 10.1128/jcm.37.6.1782-1789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim HM, Adjou Moumouni PF, Mohammed-Geba K, Sheir SK, Hashem IS, Cao S. Molecular and serological prevalence of Babesia bigemina and Babesia bovis in cattle and water buffalos under small-scale dairy farming in Beheira and Faiyum Provinces, Egypt. Vet Parasitol. 2013;198:187–192. doi: 10.1016/j.vetpar.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Mahoney DF. Bovine babesiosis: a study of factors concerned in transmission. Ann Trop Parasitol. 1969;63:1–14. doi: 10.1080/00034983.1969.11686595. [DOI] [PubMed] [Google Scholar]
- Martins TM, Pedro OC, Caldeira RA, do Rosario VE, Neves L, Domingos A. Detection of bovine babesiosis in Mozambique by a novel seminested hot-start PCR method. Vet Parasitol. 2008;153:225–230. doi: 10.1016/j.vetpar.2008.01.037. [DOI] [PubMed] [Google Scholar]
- McCosker PJ. The global importance of babesiosis. In: Ristic M, Krier JP, editors. babesiosis. New York: Academic Press; 1981. pp. 1–24. [Google Scholar]
- Mosqueda J, Olvera-Ramírez A, Aguilar-Tipacamú G, Cantó GL. Current Advances in Detection and Treatment of Babesiosis. Curr Med Chem. 2012;19:1504–1518. doi: 10.2174/092986712799828355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagati HE. Some new and rare records of piroplasmosis with a list of the species of Babesia and Theileria so far recorded from Egypt. Vet Rec. 1947;59:145–147. [PubMed] [Google Scholar]
- Oliveira-Sequeira TC, Oliveira MC, Araujo JP, Amarante AF. PCR-based detection of Babesia bovis and Babesia bigemina in their natural host Boophilus microplus and cattle. Int J Parasitol. 2005;35:105–111. doi: 10.1016/j.ijpara.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Radostits OM, Gay CC, Blood DC, Hinchcliff KW. A text book of veterinary medicine. 9. Tindal and Cox: Baieller; 2000. pp. 1328–1329. [Google Scholar]
- Radwan AM, Ibrahim MA, Abdalla AA, AL-Hosary AA, Ahmed LS (2013) Conventional detection of Babesia bovis in tick infested cattle and its effect on the hematological profile. In: 16th ISAH congress. Nanjing, China, 5–9 May 2013, pp 170–172
- Thrusfield M. Veterinary epidemiology. 3. New Jersey: Blackwell science publishing; 2005. pp. 313–317. [Google Scholar]