Abstract
Cryptosporidiosis is an emerging waterborne protozoan disease and one of the major causes of neonatal diarrhea in humans and animals. But the disease remains under diagnosed due to lack of availability of special stains in majority of laboratories at primary health centers. Therefore, it requires a rapid screening test for routine diagnosis in conventional laboratory set up. In this pursuit, the present study was planned. During this study, fecal samples from 100 representative animals randomly selected from 17 out breaks of bovine calf diarrhea, were stained with modified Ziehl Neelsen staining (mZN) and Leishman’s stain to demonstrate cryptosporidial oocysts and for routine fecal examination, respectively. By mZN staining, 25 cases confirmed the presence of cryptosporidial oocysts. However, examination of Leishman’s stained fecal smears revealed round hollow unstained bodies resembling cryptosporidia in 20 cases. Therefore, a comparative morphometric analysis was made between the two techniques to determine their relative efficacy in demonstrating cryptosporidia in the feces of affected animals. The analyses showed that the Leishman’s stain can be effective in making a presumptive diagnosis of cryptosporidiosis with a little experience. Confirmation of cryptosporidiosis was done by histopathological examination of intestinal sections of calves died during these out breaks. The findings appear to have great clinical value for routine laboratory screening of fecal samples for cryptosporidiosis as conventional Romanowsky stains are readily available and used for multipurpose examination in most of the laboratories at grass root level. Perusal of literature proved this to be the first attempt at easy diagnostics for cryptosporidiosis.
Keywords: Cryptosporidium, Diagnosis, mZN staining, Leishman’s staining, Morphometry
Introduction
Cryptosporidiosis is a widely reported diarrheal disease caused by an apicomplexan intracellular-extracytoplasmic protozoan parasite of Cryptosporidium spp., which is an important zoonotic entero-pathogen of neonatal and young bovine calves (Gay 2005). The disease is mainly water borne (Karanis et al. 2007), affects a wide range of species and its oocysts are highly resistant, therefore, it keeps on circulating in the environment. The humans are always at risk of infection through contaminated water and milk (Bhat et al. 2012). Of lately, disease is becoming increasingly important, thus, laboratory testing of Cryptosporidium has become mandatory in gastrointestinal disease outbreaks in the developed world. However, in India, the disease has attracted subdued attention and most laboratories fail to diagnose it routinely. A number of methods have been developed to detect Cryptosporidium spp. infection in faeces, such as flotation method of oocysts and modified Ziehl Neelsen’s staining method (Cole et al. 1999), immunofluorescence assay and flow cytometry (Cole et al. 1999), ELISA (Werner et al. 2004), chromatographic lateral flow immunoassay (Singla et al. 2013), PCR and loop-mediated isothermal amplification of DNA (Bakheit et al. 2008). The organism is usually identified on the basis of laborious, relatively expensive and time consuming staining, including modified Ziehl Neelsen’s and FAT staining of the faecal samples to identify the oocysts of the parasite in people and animals (Casemore et al. 1985). However, these are not routinely used protocols in the developing world, including India. On the other hand in majority of the grass root laboratories, Romanowsky staining is available for routine haematology and faecal examination. Therefore, if with little effort and experience the cryptosporidium infection could be identified in the faecal samples of human and animal origin, it would be worthwhile to incorporate it as a routine screening procedure. The aim of the present study was to evaluate and validate the routinely used Leishman’s staining technique with specific mZN staining in routine diagnosis of cryptosporidial diarrhoea for multiple species application.
Materials and methods
Faecal samples from 100 cases of diarrheic bovine calves were collected directly from the rectum during 17 outbreaks of diarrhoea in the peri-urban belt of Ludhiana, Punjab. Multiple faecal smears were prepared with the help of sterile ear bud. The air dried smears were fixed in methanol for 3 min and stained by modified Ziehl–Neelsen (OIE 2008). The sister smears made from similar cases were air dried and immersed in the working solution of Leishman stain as per the protocol of Jain (1986). Briefly, the Leishman Stain was prepared by dissolving 150 mg of Leishman powder in 100 ml of pure methanol. Air dried faecal smears were stained with undiluted stain for 1–2 min. Then diluted the stain on the smear with the double amount of buffer and mixed gently. Allowed the diluted stain to react for 5–15 min and washed the excessive stain with distilled water. The smears were air dried and then examined at 40×and 100× objectives. The morphology and staining characteristics of cryptosporidial oocysts as well as morphometric analysis (with software DPZ BSW, Olympus, Japan) of the oocysts was done to compare the efficacy of both the staining methods in identifying cryptosporidial oocysts by employing Student’s t test. The representative tissue samples from the intestine of all these dead calves were processed for routine histopathology and special staining by mZN and the organisms were demonstrated in tissue sections.
Results and discussion
Modified ZN staining of multiple faecal smears confirmed the presence of cryptosporidial oocysts in 25 cases of bovine calf diarrhoea. The cryptosporidian oocysts appeared as bright red, oval to spherical (3–5 μm) bodies (Fig. 1). On the other hand, examination of Leishman’s stained faecal smears revealed round to oval, hollow unstained bodies resembling cryptosporidia in 20 cases (Fig. 2). A comparison of morpho-meteric analysis of cryptosporidial oocysts by two staining techniques revealed no significant differences in their size and morphology. The average size of the Leishman’s stained bodies and modified Zeihl Neelsen’s stained oocysts of cryptosporidium was found to be 4.26 ± 0.29 µm and 4.24 ± 0.28 µm, respectively. Forty-three animals succumbed due to severe diarrhoea during the process of investigation of these outbreaks. Intestinal sections revealed Cryptosporidium oocysts predominantly in the areas that showed necrotic enteritis (Figs. 3, 4).
Fig. 1.
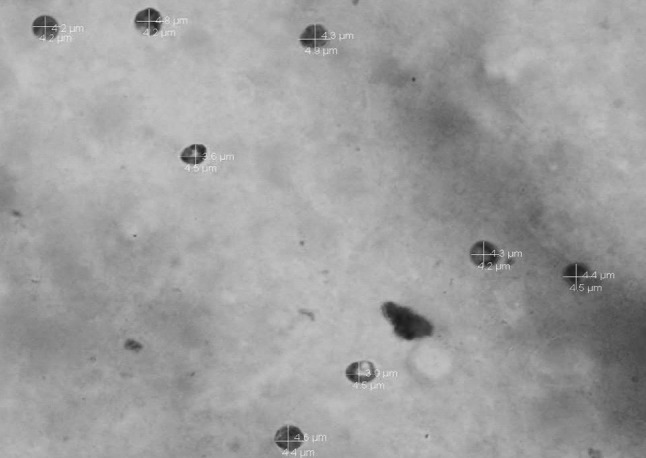
Morphometery of cryptosporidial bodies stained with mZN stain, original magnification 1000×
Fig. 2.
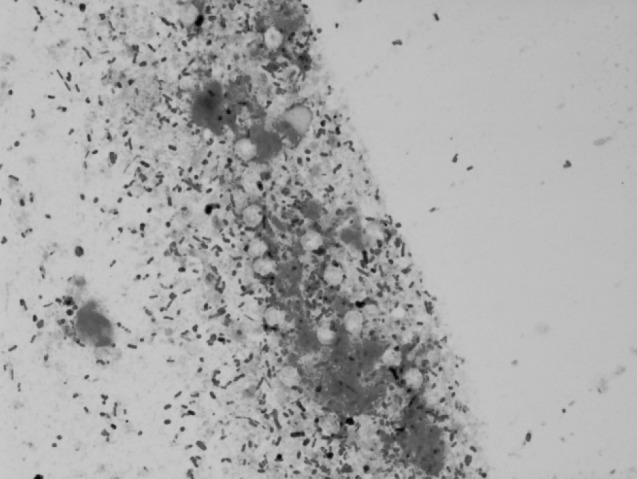
Leishman stained faecal smear showing hollow, round but unstained bodies resembling cryptospridia1000×
Fig. 3.
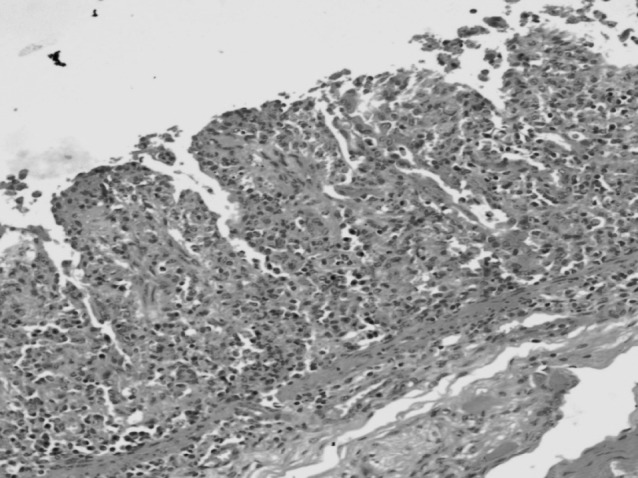
Section of intestine showing marked necrotic enteritis HE 200×
Fig. 4.
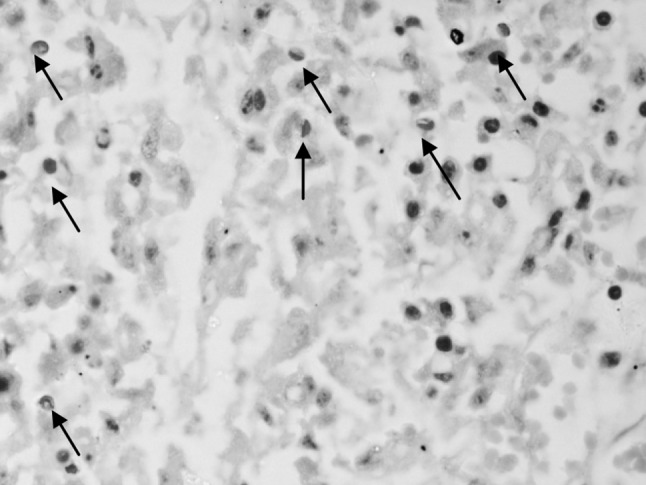
Higher magnification of another section of intestine showing clear cut cryptosporidian oocysts deep in the mucosa 1000×
The usage of special mZN staining as a diagnostic method for the cryptosporidiosis in people and animals is well known (Bhat et al. 2012; Ruest et al. 1998; Singh et al. 2006). However, there seems to be no report of demonstration of cryptosporidial oocysts by conventional Romanowsky staining. Therefore, from the present investigation, it is concluded that Leishman’s stain could be effective in making a presumptive diagnosis of cryptosporidiosis with a little experience and effort. It is also suggested that the latter technique might also find a foot hold as a routine screening test for the diagnosis of cryptosporidial infection in various species of animals as well as people.
References
- Bakheit MA, Torra D, Palomino LA, Thekisoe OM, Mbati PA, Ongerth J, Karanis P. Sensitive and specific detection of Cryptosporidium species in PCR-negative samples by loop-mediated isothermal DNA amplification and confirmation of generated LAMP products by sequencing. Vet Parasitol. 2008;158:11–22. doi: 10.1016/j.vetpar.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Bhat SA, Juyal PD, Singla LD. Prevalence of cryptosporidiosis in neonatal buffalo calves in Ludhiana District of Punjab, India. Asian J Anim Vet Adv. 2012;7:512–520. doi: 10.3923/ajava.2012.512.520. [DOI] [Google Scholar]
- Casemore DP, Armstrong M, Sands RL. Laboratory diagnosis of cryptosporidiosis. J Clin Pathol. 1985;38:1337–1341. doi: 10.1136/jcp.38.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DJ, Snowden K, Cohen ND, Smith R. Detection of Cryptosporidium parvum in horses: thresholds of acid-fast stain, immunofluorescence assay, and flow cytometry. J Clin Microbiol. 1999;37:23–26. doi: 10.1128/jcm.37.2.457-460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay CC. Intestinal diseases in ruminants. In: Kahn CM, editor. Merck veterinary manual. 9. Whitehouse station: Merck and Co; 2005. pp. 220–233. [Google Scholar]
- Jain NC. Schalm’s veterinary hematology. 4. Philadelphia: Lea and Febiger; 1986. [Google Scholar]
- Karanis P, Kourenti C, Smith H. Waterborne transmission of protozoan parasites; a worldwide review of outbreaks and lessons learnt. J Water Health. 2007;5:1–38. doi: 10.2166/wh.2006.002. [DOI] [PubMed] [Google Scholar]
- OIE (2008) Cryptosporidiosis dkjhiobjoid dfkjgoidsb. In: OIE manual of diagnostic tests and vaccines for terrestrial animals, 6th edn. Office International des Epizooties press, Paris, ISBN-13: 9789290447184. 1192-1215
- Ruest N, Faubert GM, Couture Y. Prevalence and geographical distribution of Giardia spp. and Cryptosporidium spp. in dairy farms in Quebec. Can Vet J. 1998;39:697–700. [PMC free article] [PubMed] [Google Scholar]
- Singh BB, Sharma R, Kumar H, Banga HS, Aulakh RS, Gill JPS, Sharma JK. Prevalence of Cryptosporidium parvum infection in Punjab (India) and its association with diarrhea in neonatal dairy calves. Vet Parasitol. 2006;140:162–165. doi: 10.1016/j.vetpar.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Singla LD, Gupta MP, Singh H, Singh ST, Kaur P, Juyal PD. Antigen based diagnosis of Cryptosporidium parvum infection in faeces of cattle and buffalo calves. Indian J Anim Sci. 2013;83:37–39. [Google Scholar]
- Werner A, Sulima P, Majewska AC. Evaluation and usefulness of different methods for detection of Cryptosporidium in human and animal stool samples. Wiad Parazytol. 2004;50:209–220. [PubMed] [Google Scholar]


