Abstract
Cutaneous leishmaniasis (CL) is vector borne parasitic disease, considered as public health problem especially in border of Iran and Iraq, Dehloran County (Musian district). The aim of this study was molecular identification of Leishmania parasites in sandfly as vectors of Leishmaniasis. Totally 280 female sandflies were trapped by sticky traps from 7 rural areas of Musiyan in September–November 2012. All sandflies were identified using morphological characters of the head and abdominal terminalia. DNA was extracted from female sandflies and Leishmania was identified using PCR and sequencing. All 280 trapped sandflies were identified as Phelobotumus Papatasi and Leishmania infections were detected in 3.2 % out of 280 female sandflies. All leishmania were identified as L. major and submitted in Gene bank as: LC014642.1, LC014641.1, LC014640.1 and LC014639.1. Frequency of Phlebotomus Papatasi and infection with L. major in studied regions showed that this vector is dominant in these areas.
Keywords: Sandfly, L. major, Characterization, Iran
Introduction
Leishmaniasis is a major public health problem caused by Leishmania protozoa from class of Kinetoplastida. Based on clinical manifestation, Leishmaniasis are seen in different forms: Cutaneous (CL), Mucocutaneous (MCL), Diffius (DCL) and Visceral Leishmaniasis (VL). Epidemiological studies show that 12 million people around the world are involved with the disease; 350 million people are at risk and annually 2 million new cases are added. More than 90 % of cases of cutaneous Leishmaniasis occur in 10 countries: Afghanistan, Algeria, Saudi Arabia, Iran, Syria, Bolivia, Brazil, Colombia, Nicaragua and Peru (WHO 2010).
Cutaneous Leishmaniasis has long existed in Iran and region is one of the endemic areas of cutaneous Leishmaniasis and known since ancient times. Currently, around 20,000 cases of the disease reported annually that the real number is probably several times more than this (Islamic Republic of Iran Ministry of Heath and Medical Education 2007).
The disease occurs as endemic areas in Central, Northeastern, West and Southern parts of Iran (Hajjaran et al. 2013) and exists in rural (Zoonotic) and urban (Anthroponotic) types. Causative agent of urban leishmaniasis or Anthroponotic Cutaneous leishmaniasis (ACL) is Leishmania tropica and its vector is Phlebotomus sergenti sandfly, the main reservoir of the disease is human. But Leishmania major causes Zoonotic type or Zoonotic Cutaneous Leishmaniasis (ZCL); Phlebotomus papatasi is the main vector and sandflies as P. anderjevi, P. mongolensis, P. ansarii and P. alexandri have been identified as vectors in rodents. Tatera indica rodents are the main reservoirs of ZCL in Iran (4.5). The only confirmed sandfly as the main vector of ZCL in Iran is the haematophagous females of P. papatasi (Diptera: Psychodidae) (6.7). A large number of sandflies from endemic areas of Iran have been described and L. major Promastigote infection have been detected from P. papatasi, Para phlebotomus subgenus including P. caucasicus, P. mongoliensis, P. ansari, Sergentomyia sintoni etc. (Nadim and Seyedi-Rashti 1971; Yaghoobi-Ershadi et al. 1996; Yaghoobi-Ershadi et al. 1995; Nadim et al. 1968a, b; Javadian and Mesghali 1974; Yaghoobi-Ershadi and Javadian 1996; Tashakori et al. 2006).
Previously several traditional laboratory methods including culturing or inoculating to sensitive lab animals carried out for detection of Leishmani sp. from infected Phlebotomus.
Also, given the clinical and geographical data as well as laboratory methods, Leishmania species in human, animal reservoir and vectors have been identified and declared as Leishmania major, L. tropica and L. infantum.
However, the above method definitely lacks the efficiency of new methods as determining the nucleotide sequence of the gene under study and phylogenetic analysis (Davami et al. 2010; Muller and Schlein 2011; Strelkova et al. 2001; Mirzaei et al. 2011; Hajjaran et al. 2009).
One of Iran endemic region is located in Southwest (Ilam province).
Most of the cases reported in this area are from Musiyan district. The course of the disease over recent years shows that especially Musian district is one of the endemic foci of cutaneous Leishmaniasis. This is very important in terms of public health in this border region (Yaghoobi-Ershadi 2012; Kavarizadeh et al. 2013).
Finding Leishmania naturally, infected female sandflies is essential to determine a sandfly as vector of Leishmaniosis, however finding the parasite in the sandflies is not a definite reason because most of the Leishmania infections in vectors are temporary and life cycle of parasites in the infected sandflies may be not completed and removed from the vectors (Nadim et al. 1968a, b).
In this study, hunting of sandflies done at the end of the active seasons of adult sandflies, when the rate of vectors infection are increasing. However, limited studies have been done in the past only to determine species of sandflies in Musiyan district, no studies have been done on the amount and type of infection by Leishmania parasite. (RanjbarKermani 1989; Javadian et al. 1997).
Therefore, identification of Leishmania sp. and its molecular type in the infected vector of ZCL with Nested-PCR molecular technique was considered for the first time in the region.
Materials and methods
Study area
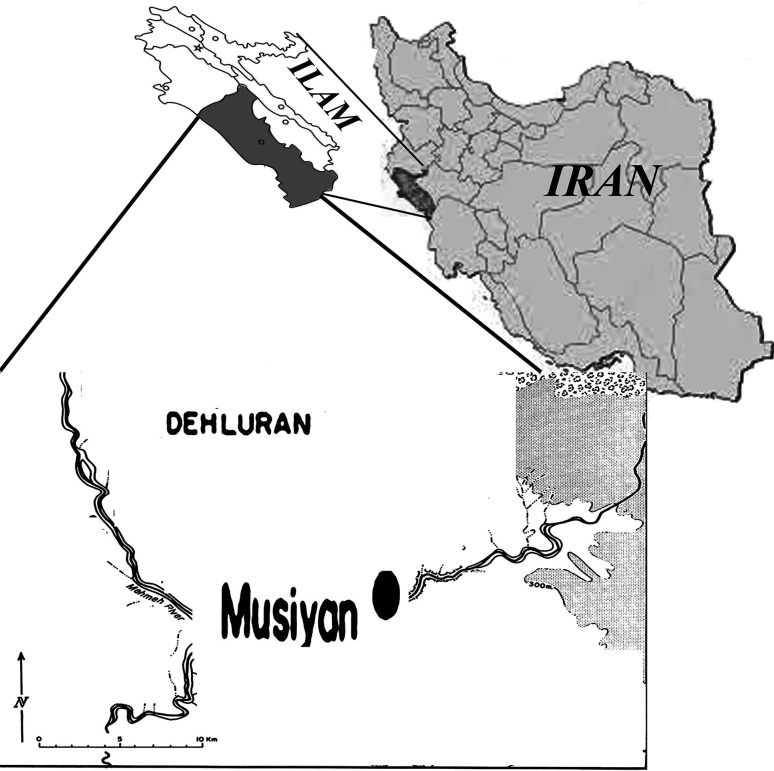
Ilam province is located in the west of Iran with an area of about 20,133 km2 and located at 32°31′20′′N, 47°22′3′′E Dehloran county, Musiyan city, located in bordering Iraq (Fig. 1) and it neighbors Khuzestan province in the south, and has a warm climate with hot summers and mild, short winters (Mansoori et al. 2009).
Fig. 1.
Locations of Musiyan, districts in Dehloran, Ilam province of Iran, where sandflies screened for Leishmania infections
Sample collection
Two hundred eighty Sandflies were sampled from 7 different villages of Dehloran county, Musiyan city; Patak-e Arab, Cham Hendi, Nahr Anbar, EynKhvosh, Shahrak-e Nasr, Shahrak-e Fath and Borom.
Samples were collected from, Rodent burrows, and indoor outdoor the places using sticky traps in September to November 2012.
Briefly, in every trapping 90 traps were established in sunset and gathered before sunrise, all trapped sandflies washed by ethanol 96 and were identified by using morphological characters of the head and Spermatheca (Nadim and Javadian 1976; Seydei-Rashti and Nadim 1992; Lewis 1982).
DNA extraction and Nested-PCR-based diagnosis
DNA was extracted from the thorax and was attached to the anterior abdomen of each sandflies using a standard extraction procedure with the QIA DNeasy blood and tissue kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions (Parvizi et al. 2005).
DNA concentration was measured at 260 nm using a spectrophotometer. For molecular diagnosis, primers were chosen from variable segments on minicircles of kinetoplast DNA(ref) as CSB1XR (ATT TTT CGC GAT TTT CGC AGA ACG) and CSB2XF (CGAGTA GCA GAA ACT CCC GTT CA) for the first round, and LiR (TCG CAG AACGCC CCT) and 13Z (ACT GGG GGTTGG TGT AAA ATAG) for the second (Noyes et al. 1998).
Amplifications were carried out using an AccuPower PCR PreMixkit (Bioneer. Co, Daejeon, Korea).The thermo cycler used (MyCycler; Bio-Rad, Hercules, CA) was set to give 5 min at 94 °C, followed by 30 cycles, each of 30 s at 94 °C, 90 s at 55 °C and 90 s at 72 °C, and then a final extension for 5 min at 72 °C. The PCR product of first step was diluted 1:9 with ultrapure water and then 1 ml of this dilution used for the second round of PCR using the same conditions and reaction mixture as the first round.
Electrophoresis performed after adding a 5 µl sample of the PCR products to a 1.5 % agarose and the gel was stained with ethidium bromide. Bands were observed by ultraviolet transillumination. (BioSystematica, Devon, UK).
Nucleotide sequences
DNA derived from PCR amplification was subjected to sequencing by MWG (Ebersberg, Germany). The data were analyzed using Chromas software (http://www.technelysium.com.au/Chromas.htm) and compared with the sequence in the NCBI nucleotide gene bank (www.ncbi.nlm.nih.gov/BLAST/).
Statistical analysis
Statistical analysis was performed by using SPSS 18.0 software. The data was expressed as frequency and percentage.
Results
In this cross-sectional study, a total of 280 female sandflies were caught in Musian district. All hunted sandflies were P. papatasi that 101 caught in rodent burrows, 109 in indoors and 70 in the outdoors. Among the hunted sandflies, P. papatasi naturally infected with Leishmania major were observed (Table 1). In terms of the hunting place, 4 % of the infected sandflies were caught from rodent burrows, 2.7 % from indoors, and 2.9 % from outdoors respectively (Table 2, 3).
Table 1.
Leishmania species identified in P. papatasi in different villages based on habitats and abdomens
| Village | Mounth Day | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| August 26 | September 14 | September 24 | October 5 | October 16 | October 17 | October 29 | November 1 | November 2 | November 7 | November 8 | ||
| Patak aerab | I | 8 | 5 | |||||||||
| O | 7 | 1 | ||||||||||
| R.B | 6* | |||||||||||
| Chamhendi | I | 7 | ||||||||||
| O | 10* | 5 | ||||||||||
| R.B | 9 | 12* | ||||||||||
| Aynkhosh | I | 2 | 15* | |||||||||
| O | 7* | |||||||||||
| R.B | 19 | 2 | ||||||||||
| Borom | I | 20* | 18 | |||||||||
| O | 15 | 1 | ||||||||||
| R.B | 22 | 25* | ||||||||||
| Nahre Anbar | I | 9 | 23 | |||||||||
| O | 9* | 2 | ||||||||||
| R.B | 4* | 2 | ||||||||||
| Nasr | I | |||||||||||
| O | 4 | |||||||||||
| B | ||||||||||||
| Fath | I | 2 | ||||||||||
| O | 9 | |||||||||||
| R.B | ||||||||||||
| Total | 15 | 47 | 12 | 24 | 57 | 44 | 22 | 27 | 17 | 4 | 11 | |
I indoor, O outdoor, R.B rodent burrow
* Cases positive
Table 2.
Frequency of natural Leishmania infected P. papatasi based on abdominal position and collected areas
| Village | P. papatasi | |||
|---|---|---|---|---|
| Abdomen position | ||||
| FF | G | SG | EM | |
| Patak aerab | 7 | 4 | 1 | 15 (1)+ |
| Chamhendi | 7 (1)+ | 5 (1)+ | 1 | 30 |
| Aynkhosh | 13 (1)+ | 2 | 2 | 28 (1)+ |
| Borom | 55 (1)+ | 10 | 2 | 33 (1)+ |
| Nahre Anbar | 32 (1)+ | 6 | 2 (1)+ | 9 |
| Nasr | 2 | 1 | 1 | |
| Fath | 6 | 1 | 1 | 3 |
| Total | 122 | 30 | 10 | 118 |
FF full fed, G gravid, SG semi gravid, Em empty
+Cases positive
Table 3.
Frequency of natural Leishmania infected P. papatasi based on abdominal position and collected areas
| P. papatasi | ||||
|---|---|---|---|---|
| Location | Cases positive | |||
| Number | % | Number | % | |
| Indoor | 109 | 39 | 2 | 2.7 |
| Outdoor | 70 | 25 | 3 | 2.9 |
| R. Burrow | 101 | 36 | 4 | 4 |
| Total | 280 | 100 | 9 | 3.2 |
R.B rodent burrow
The species with high frequency were caught in all three places in all regions.
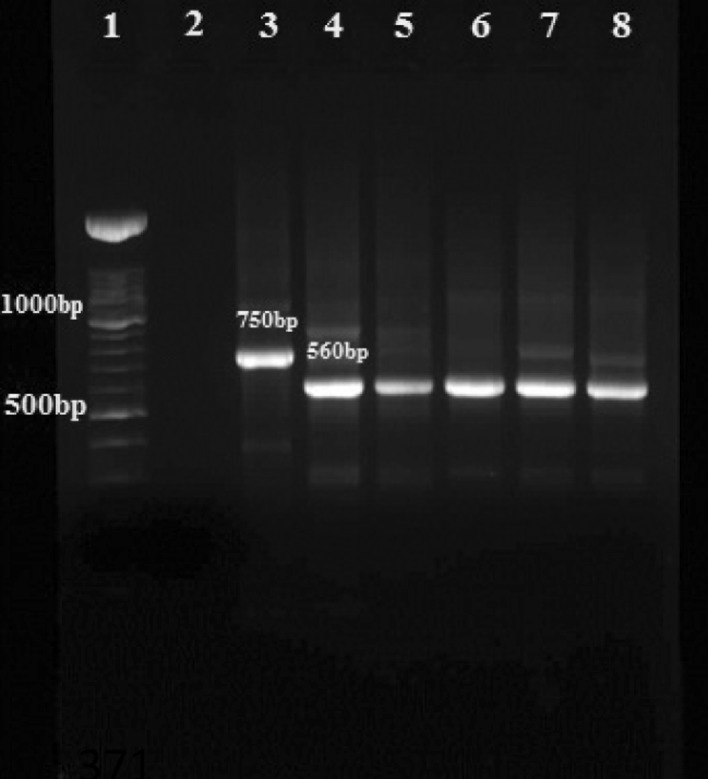
In the Nested-PCR molecular method, on 1.5 % agarose gel bands with size of 560, 750, 680 bp showed as L. major, L. tropica and L. infantum respectively.
PCR results confirm that 11 female sandflies were infected with Leishmania parasites and by using Nested-PCR method all cases were detected as L. major (Fig. 2).
Fig. 2.
Agarose gel electrophoresis of Leishmania isolates from sandflies in nested PCR using the primers 13Z and LiR in the second step: Lane 1 DNA size marker 100 bp; Lane 2 negative control; Lane 3 L. tropica (positive control 750 bp); Lane 4 L. major (positive control 560 bp); Lanes 5–8 L. major isolates obtained from sandflies
To confirm and complete the identification of samples, 4 randomly selected PCR products were sequenced and submitted in the GenBank database (http://www.ncbi.nlm.nih.gov/nuccore/) with accession numbers: LC014642.1, LC014641.1, LC014640.1 and LC014639.1. Multiple sequence alignment revealed conserved nucleotides among the obtained sequences (Fig. 3).
Fig. 3.
Multiple alignments of nucleotide sequences of 4 isolates submitted in gene bank as: LC014642.1, LC014641.1, LC014640.1 and LC014639.1. Dashes indicate computer-generated gaps. Asterisks indicate identical nucleotides
Discussion
Controlling leishmaniasis in endemic areas requires knowledge of the ecology and epidemiology of the parasite, the reservoir host and vector of the disease. In this regard, identifying reservoirs and seeking infected carriers is one of the fundamental problems of those responsible for disease control. Finding sandflies infected with parasites is an essential step to identify the vector species and the potential disease transmission in endemic areas. In terms of low parasite infection in most centers because of Leishmaniasis, to find the Leptomonad in sandfly body, tend to eat the human blood, it is enough to introduce it as a potential vector of the disease. In this study, all caught female sandflies were P. papatasi, that is the dominant species in this region because in faunistic survey (by Kaveri-Zadeh et al.) in the area this species has almost included 74 % of the total caught sandflies that P. papatasi includes 92 % of female sandflies caught in the study.
Phlebotomus papatasi had the highest frequency in indoor, outdoor and nests of rodents, so this study is in agreement with other studies in all three locations at a rate of 36 % in rodent burrows, 25 % in outdoors and 39 % in indoors (Killick-Kendrick 1999; Nadim et al. 1968a, b).
Two conventional methods microscopic and injection into sensitive lab animals and isolating parasites through medium are done for determining and estimating the rate of infection of the vector insects and disease reservoirs. Both methods are difficult, long-term and imprecise, and on the other hand, in terms of morphological similarity of species and subspecies, the diagnostic value of these methods is low and additional studies such as isoenzyme analysis or (Monoclonal antibody) mAbs are required to identify species and subspecies of isolated parasites.
Meanwhile, problems related to medium infection and removing isolated parasites and expensive and time consuming isoenzyme analysis are the major problems faced by the classical laboratory studies (Javadian et al. 1977).
New PCR-based molecular methods have none of these problems. They are generally enhanced the DNA-based molecular techniques to determine the species of Leishmania parasites to detect infection in the past decades. Using molecular methods in endemic and hyper endemic areas is particularly useful in identifying infection of vectors.
In this study, all the sandflies identified with molecular methods and 9 isolates (3.2 %) were diagnosed as positive Leishmania parasites. Molecular results showed that 100 % of cases were only infected with L. major. Comparison of the sequence of PCR results with other sequences submitted in World Gene Bank has also confirmed the L. major. These findings show the difference in other studies obtained in the rest of the country.
In similar studies conducted in other areas of Iran, the dominant speciesism P. papatasi. In a study by Rassi et al. (2005), the main vector of cutaneous Leishmania in South of Iran is called P. papatasi. The researchers have analyzed the sandflies caught with Nested-PCR method. The results showed that about 2.7 % of P. Papatasi were naturally infected by L. Major.
The study by Roshan-Ghalb and Parvizi (2012) in the north east of Iran with molecular methods has also confirmed the L. Major as agent and P. papatasi as the main vector of ZCL (Roshan-Ghalb and Parvizi 2012).
Sharbatkhori et al. 2014 in a similar study in the North East of Iran have obtained different species, P. caucasicus, P. mongolensis in addition to P. papatasi. The researchers also have mentioned the most vectors as P. papatasi.
One of the differences between our results with similar studies in other parts of Iran is that only L. major was detected using molecular methods. Contrary to our results, Sharbatkhori et al. 2014 in addition to finding L. major have found L. turanica in vectors and disease reservoirs in the North East of Iran. The researchers believed that non-pathogenic Leishmania sp. such as Leishmania turanica and Leishmania Jerbili play a major role in the survival of L. major in vectors and reservoirs. (Sharbatkhori et al. 2014).
Sequences from this study were consistent with L. major recorded in the World Gene Bank in West of Iran (Mehran Ilam) with the access numbers KM555286.1, to KM555295.1.
Given the high frequency of P. papatasi in indoor, outside and nests of rodents and the introduction of the species as the certain vector of this diseases in Khuzestan Province (Province neighboring the area) and separating the L. major from adjacent urban areas patients such as Dehloran, it seems that the species is the main and certain vector of the disease in the region. The type of disease in the area is zoonotic cutaneous Leishmaniasis (ZCL).
Statistical analysis of CL over the years in Dehloran suggests that Musian District in the city is one of the areas that are mostly infected. The trend of the disease during the mentioned years shows that Dehloran and especially Musian is one of the endemic foci of cutaneous Leishmaniasis. In terms of public health in the border region, it is quite significant (Yaghoobi-Ershadi 2012; Yaghoobi-Ershadi and Javadian 1996; Killick-Kendrick 1990).
Phelobotumus papatasi highest infection rate was in October, which is in accordance with the second activity peak of the mosquito and it seems that most transmission occurs this month. So, it is recommended to protect oneself to prevent sandfly bites more than other months. Also, it is recommended that people in affected areas not to leave their homes during the evening and night when the sandflies are active unless it is necessary. Farmers, workers and soldiers who must necessarily be present outdoors when the sandflies are active should have enough cover and use insect repellent to prevent bites. And it is recommended to use screens and mosquito nets impregnated with pesticide to prevent disease (World Health Organization 1993; Yaghoobi-Ershadi et al. 2000; Desjeux 1991).
Examining the natural contamination and determining the identity of Leishmania parasite in sandflies of subgenus Phlebotomus, the only the dominant species in this area is P. papatasi as the certain and main vector of disease, in fact, the first step is to determine the vectors of the disease in this area.
Considering that detecting vectors to run control programs is important, it is hoped that using the results of this study and examining the rodents of this area that are considered as the disease reservoir to help the regional planning to control the disease in the area.
Acknowledgments
This study was financially supported by grants No: 91114 from Ahvaz Jundishapur Medical Sciences. The authors would like to thank Mr. Akhavan A. A. of Tehran University and health centers of Musian and Dasht Abbas for their help in sample collection.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Davami MH, Motazedian MH, Sarkari B. The changing profile of cutaneous Leishmaniasis in a focus of the disease in Jahrom district, southern Iran. Ann Trop Med Parasitol. 2010;104:377–382. doi: 10.1179/136485910X12786389891083. [DOI] [PubMed] [Google Scholar]
- Desjeux P (1991) Information on the epidemiology and control of the Leishmaniasis by country or territory, 2nd edn. WHO/Leish/91.30, WHO, Geneva
- Hajjaran H et al (2013) Molecular identification and polymorphism determination of cutaneous and visceral leishmaniasis agents isolated from human and animal hosts in Iran. Hindawi Publishing Corporation BioMed Research International, Volume 2013 Article ID 789326, p 7 [DOI] [PMC free article] [PubMed]
- Hajjaran H, Mohebali M, Alimoradi S, Abaei MR, Edrissian GhH. Isolation and characterization of pathogenic Leishmania turanica from Nesokia indica (Rodentia, Muridae) by PCR-RFLP and ITS1 sequencingin Iran. Trans R Soc Trop Med Hyg. 2009;103:1177–1179. doi: 10.1016/j.trstmh.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Islamic Republic of Iran Ministry of Heath and Medical Education (2007) [Instruction of Leishmaniasis control]. Tehran: Center for disease control 2007:78(Persian)
- Javadian E, Mesghali A. Studies on cutaneous leishmaniasis in Khuzestan, Iran. Part I. The leptomonad infection of sandflies. Bull Soc Pathol Exot. 1974;67:513–516. [PubMed] [Google Scholar]
- Javadian E, Tesh R, Saidi S, Nadim A. Studies on the epidemiology of sandfly fever in Iran. III. Host-feeding patterns of Phlebotomus papatasi in an endemic area of the disease. Am J Trop Med Hyg. 1977;26:294–302. doi: 10.4269/ajtmh.1977.26.294. [DOI] [PubMed] [Google Scholar]
- Javadian E, Jalali-Galousang A, Seyedi Rashti MA. Sandflies of Ilam province, west of Iran with description of two new species from the Genus Phlebotomus: Ph. ilami and Ph. nadimi I. Iran J Public Health. 1997;26:13–20. [Google Scholar]
- Kavarizadeh F, Vazirianzadeh B, Rassi Y, Jalali Glusang A, Moravvej SA. A faunistic study of sandflies of Musian district southwestern of Iran. Pak J Zool. 2013;45:549–554. [Google Scholar]
- Killick-Kendrick R. Phlebotomine vectors of the leishmaniasis: a review. Med Vet Entomol. 1990;4:1–24. doi: 10.1111/j.1365-2915.1990.tb00255.x. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick R. The biology and control of phlebotominae sandflies. Med Vet Entomol. 1999;17:279–289. [Google Scholar]
- Lewis DJ. A taxonomic review of the genus Phlebotomus (Diptera: Psychodidae) Bull Br Mus Nat Hist Entomol Ser. 1982;45:121–209. [Google Scholar]
- Mansoori AM, Kafravi G, Sharifim . Geography of Ilam province. th. Tehran: Company of Iran Text Book Printing and Publishing; 2009. [Google Scholar]
- Mirzaei A, Rouhani S, Taherkhani H, Farahmand M, Kazemi B, Hedayati M, et al. Detection and isolation of Leishmania species in naturally infected Rhombomis opimus, are reservoir host of zoonotic cutaneous leishmaniasis in Turkemen Sahara, north east of Iran. Exp Parasitol. 2011;29:375–380. doi: 10.1016/j.exppara.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Muller GC, Schlein Y. Different methods of using attractive sugar baits (ATSB) for the control of Phlebotomus Papatasi. Ann Trop Med Parasitol. 2011;36:64–70. doi: 10.1111/j.1948-7134.2011.00113.x. [DOI] [PubMed] [Google Scholar]
- Nadim A, Javadian E. Key for the species identification of sandflies (Diptera Phlebotominae) of Iran. Iran J Public Health. 1976;5:25–28. [Google Scholar]
- Nadim A, Seyedi-Rashti MA. Brief review of the epidemiology of various types of leishmaniasis in Iran. Acta Med Iran. 1971;14:99–106. [Google Scholar]
- Nadim A, Seyedi-Rashti MA, Mesgali A. Epidemiology of cutaneous leishmaniasis in Turkemen Sahara Iran. J Trop Med Hyg. 1968;71:238–239. [PubMed] [Google Scholar]
- Nadim A, Mesghali A, Amini H. Epidemiology of cutaneous leishmaniasis in Isfahan province of Iran. III. The vector. Trans R Soc Trop Med Hyg. 1968;62:543–549. doi: 10.1016/0035-9203(68)90141-7. [DOI] [PubMed] [Google Scholar]
- Noyes HA, Reyburn H, Bailey JW, Smith D. A nested PCR-based schizodeme method for identifying Leishmania kinetoplast minicircle classes directly from clinical samples and its application to the study of the epidemiology of Leishmania tropica in Pakistan. J Clin Microbiol. 1998;36:2877–2881. doi: 10.1128/jcm.36.10.2877-2881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvizi P, Mauricio I, Aransay AM, Miles MA, Ready PD. First detection of Leishmania major in peri domestic Phlebotomus Papatasi from Isfahan province, Iran: comparison of nested PCR of nuclear ITS ribosomal DNA and semi-nested PCR of minicircle kinetoplast DNA. Acta Trop. 2005;93:75–83. doi: 10.1016/j.actatropica.2004.09.007. [DOI] [PubMed] [Google Scholar]
- RanjbarKermani M (1989) Evaluating the status of cutaneous leishmaniasis among fighters and determining its main vector in north-western Khuzestan and Dehloran. Thesis for receiving a Master’s degree in Medical Entomology and Vector Control, School of Public Health, Tehran University No. 1690
- Rassi Y, Gassmi MM, Javadian F, Motazedian MH (2005) Confirmation of Phlebotomus papatasi as the main vector of cutaneous leishmaniasis in south of Iran of the third proceeding of the international symposium on phlebotomine sandflies, Tunis, Tunisia, Archives de L Institut Pasteur de Tunis 82:43
- Roshan-Ghalb M, Parvizi P. Isolation and determination of Leishmania major and Leishmania turanica in Phlebotomus papatasi main vector of zoonotic cutaneous leishmaniasis in Turkenmen Sahara, Golestan Province. J Mazand Univ Med Sci. 2012;21:74–83. [Google Scholar]
- Seydei-Rashti MA, Nadim A. The genus Phlebotomus (Diptera: Psychodidae, Phlebotominae) of the countries of the eastern Mediterranean region. Iran J Public Health. 1992;21:11–50. [Google Scholar]
- Sharbatkhori M, Spotin A, Taherkhani H, Roshanghalb M, Parvizi P. Molecular variation in Leishmania parasites from sandflies species of a zoonotic cutaneous leishmaniasis in northeast of Iran. J Vector Borne Dis. 2014;51:16–21. [PubMed] [Google Scholar]
- Strelkova MV, Eliseev LN, Ponirovsky EN, Dergacheva TI, Evans DA. Mixed Leishmania infections in Rhombomys opimus: a key to the persistence of Leishmania major from one transmission season to the next. Ann Trop Med Parasitol. 2001;95:811–819. doi: 10.1080/00034980120111154. [DOI] [PubMed] [Google Scholar]
- Tashakori M, Kuhls K, Al-Jawabreh A, Mauricio I, Schönian G, Farajnia S, et al. Leishmania major: genetic heterogeneity of Iranian isolates by single-strand conformation polymorphism and sequence analysis of ribosomal DNA internal transcribed spacer. Acta Trop. 2006;98:52–58. doi: 10.1016/j.actatropica.2006.01.010. [DOI] [PubMed] [Google Scholar]
- WHO (2010) Control of the leishmaniases. Report of a meeting of the WHO Expert Committee Leishmaniases, on the Control of Geneva 949:22–26
- World Health Organization . Control of tropical diseases, the leishmaniasis. Geneva: WHO/CTD/ICO; 1993. pp. 2–14. [Google Scholar]
- Yaghoobi-Ershadi MR. Phlebotomine sandflies (Diptera: Psychodidae) in Iran and their role on Leishmania transmission. J Arthropod-Borne Dis. 2012;6:1–17. [PMC free article] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Javadian E. Seasonal variation of Leishmania major infection rates in sandflies from rodent burrows in Isfahan province. Iran Med Vet Entomol. 1996;10:181–184. doi: 10.1111/j.1365-2915.1996.tb00726.x. [DOI] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Javadian E. Epidemiological study of reservoir hosts in an endemic area of zoonotic cutaneous leishmaniasis in Iran. Bull World Health Org. 1996;74:587–590. [PMC free article] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Javadian E, Kannani A. Host preference pattern of phlebotominae sandflies of Borkhar rural district, Isfahan province, Iran. Acta Trop. 1995;60:155–158. doi: 10.1016/0001-706X(95)00122-U. [DOI] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Akhavan AA, Mohebali M. Meriones libycus and Rhombomys opimus (Rodentia: Gerbillidae) are the main reservoir hosts in a new focus of zoonotic cutaneous leishmaniasis in Iran. Trans R Soc Trop Med Hyg. 1996;90:503–504. doi: 10.1016/S0035-9203(96)90295-3. [DOI] [PubMed] [Google Scholar]
- Yaghoobi-Ershadi MR, Akhavan AA, Zahraei-Ramezani AR, Javadian E, Motavalli-Emami M. Field trial for the control of zoonotic cutaneous leishmaniasis in Badrood, Iran. Ann Saud Med. 2000;20:386–389. doi: 10.5144/0256-4947.2000.386. [DOI] [PubMed] [Google Scholar]