Abstract
As the AIDS epidemic continues unabated, the development of a human immunodeficiency virus (HIV) vaccine is critical. Ideally, an effective vaccine should elicit cell-mediated and neutralizing humoral immune responses. We have determined the in vitro susceptibility profile of sexually transmitted viruses from 91 patients with acute and early HIV-1 infection to three monoclonal antibodies, 2G12, 2F5, and 4E10. Using a recombinant virus assay to measure neutralization, we found all transmitted viruses were neutralized by 4E10, 80% were neutralized by 2F5, and only 37% were neutralized by 2G12. We propose that the induction of 4E10-like antibodies should be a priority in designing immunogens to prevent HIV-1 infection.
The global human immunodeficiency virus (HIV)/AIDS epidemic marches on, with an estimated 5 million new infections occurring in 2003 (25). Demand for an effective HIV-1 vaccine remains unanswered, and there remains a lack of consensus on which immune responses a successful vaccine should induce (6). Enthusiasm for the potential of inducing neutralizing antibodies as an effective vaccine strategy waned early during the epidemic when it became clear that the neutralization profiles of field isolates were markedly different from laboratory-adapted strains (14, 21). Coupled with the observed relationship between host cell-mediated immune responses and plasma viremia in vivo (19), the vaccine effort swung toward the design of immunogens such as DNA and recombinant viral vectors, theoretically capable of stimulating cell-mediated responses (24, 26). More recently however, it is clear that in the simian immunodeficiency virus model, these immunogens may at best alter early events and clinical course but may not provide adequate protection from clinically significant infection (16, 17). Thus, interest in immunogens capable of inducing neutralizing antibodies has once again emerged.
Our initial interest in studying neutralizing antibodies was mainly therapeutic and stimulated by passive immunotherapy studies in which HIV-1 or simian immunodeficiency virus infection could be prevented or the clinical course ameliorated (5, 7-9, 13, 15). The identification and availability of broadly reactive human monoclonal antibodies (MAbs) offer a novel therapeutic modality, perhaps as a potential adjunct to highly active antiretroviral therapy. We have previously hypothesized that subjects identified during acute and early infection and treated promptly and aggressively with highly active antiretroviral therapy would have minimal residual viral burdens after 2 to 3 years of therapy, a viral burden that may be amenable to control with adjunctive therapies that may assist the host in immunologically controlling the infection if treatment is interrupted or discontinued (12).
Three neutralizing MAbs, 2G12, 2F5, and 4E10, have been produced commercially and are available for experimental use both prophylactically (5, 7, 9, 13, 15) and therapeutically (1, 22). 2G12 recognizes a cluster of carbohydrate residues on gp120. This is an unusual antibody with a unique structure capable of binding to clusters of oligomannose-type sugars and interfering with viral binding and entry (3). There are two additional MAbs, 2F5 and 4E10, that recognize adjacent but distinct epitopes on the membrane-proximal region of the gp41 ectodomain and probably act by inhibiting the fusion process. The antibody 2F5 binds to the ELDKWA motif on the ectodomain of gp41 (18), whereas 4E10 likely recognizes an ordered helical peptide structure in gp41 created in part by the epitope NWFDIT slightly upstream from the 2F5 binding site (4, 23, 27).
As a prelude to recruiting patients for a phase I trial of these MAbs, we examined the neutralization profiles of newly transmitted viruses to 2G12, 2F5, and 4E10 and compared this to the profiles of NL4-3, an X-4-tropic laboratory-adapted strain of HIV-1, and JRCSF, an R5-tropic strain of HIV-1. Plasma from 91 newly infected subjects (Table 1), defined by the presence of HIV-1 viremia with either a negative or indeterminate serology or a negative detuned enzyme-linked immunosorbent assay (10), was chosen for susceptibility testing.
TABLE 1.
Patient characteristics in this study
| Characteristic | Result |
|---|---|
| Sex | Male, 90; female, 1 |
| Risk factor | MSMa (90) |
| Mean CD4+ T-cell count in cells/mm3 (range) | 471 (119-1353) |
| Mean log HIV-1 RNA copies/ml of plasma (range) | 6.56 (2.77-7.87) |
| Estimated median duration of infection (days)b | 39 |
MSM, men who have sex with men.
The approximate date of infection was calculated as 2 weeks prior to the onset of acute retroviral illness (93%). In cases where no acute retroviral illness symptoms were experienced, the midpoint between the last negative antibody result and first positive Western blot result was determined.
A previously described recombinant viral assay was used to measure virus-antibody neutralization (20). In brief, nucleic acid derived from HIV-positive plasma was amplified by reverse transcription-PCR and incorporated into an expression vector (pCXAS) by conventional cloning methods. Recombinant HIV-1 stocks expressing patient virus envelope proteins were prepared by cotransfecting HEK293 cells with a replication-defective, luciferase expression cassette containing HIV-1 genomic viral vector and an appropriate envelope expression vector. Pseudotyped recombinant viruses were harvested 48 h posttransfection and incubated for 1 h at 37°C with serial fourfold dilutions of the three MAbs and plasma controls. U87 cells that express CD4, CCR5, and CXCR4 were inoculated with virus-antibody dilutions. Luciferase activity determined 72 h postinoculation was used as the indicator of infectivity. Neutralizing activity was displayed as the percent inhibition of luciferase production at each antibody concentration compared to that of an antibody-negative control. The 50% inhibitory concentration (IC50) is defined as the concentration of MAb required to inhibit virus infectivity by 50%. For the purposes of this study, viruses were classified as susceptible to neutralization if the IC50 for a particular antibody was ≤50 μg/ml.
All 91 viruses tested were neutralized by 4E10, whereas 74 of 91 (81%) were susceptible to neutralization to 2F5. In contrast, only 38 of 91 (38%) of the transmitted isolates were susceptible to neutralization by 2G12 (Table 2). Thirty-three of 91 subjects (36%) harbored transmitted virus susceptible to neutralization by all three MAbs, and 78 of 91 (86%) isolates were susceptible to at least two MAbs.
TABLE 2.
Susceptibility profile of transmitted viruses from 91 newly infected patients to MAbs 2F5, 2G12, and 4E10
| Strain | Result fora:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2F5
|
4E10
|
2G12
|
|||||||
| % of viruses neutralized (no. neutralized/ total) | Mean IC50 (μg/ml) | Median IC50 (μg/ml) | % of viruses neutralized (no. neutralized/ total) | Mean IC50 (μg/ml) | Median IC50 (μg/ml) | % of viruses neutralized (no. neutralized/ total) | Mean IC50 (μg/ml) | Median IC50 (μg/ml) | |
| Patient HIV-1 | 81 (74/91) | 7.64 (0.6-26.7) | 5.54 | 100 (91/91) | 9.74 (0.8-46.8) | 6.76 | 40 (38/91) | 12.17 (0.2-49.9) | 5.20 |
| JRCSF | 4.80 | 9.10 | 0.44 | ||||||
| NL4-3 | 1.30 | 4.00 | 0.53 | ||||||
Values in parentheses represent ranges.
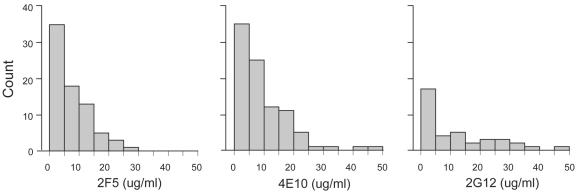
The range of susceptibilities was quite broad, although in general if susceptible, IC50s were below 10 μg/ml (Fig. 1). Mean IC50s of 2F5, 2G12, and 4E10 for susceptible isolates were 7.64, 12.17, and 9.74 μg/ml, respectively. Susceptibility to neutralization by 4E10 and 2F5 was highly correlated (R = 0.667, P < 0.0001), whereas susceptibility to 2G12 did not predict susceptibility to the other two MAbs. Both NL4-3 and JRCSF were on average more susceptible to neutralization by all three MAbs, although both were markedly more susceptible to the effect of 2G12 when compared to the 91 transmitted isolates. It is worth noting that the median values for susceptibility of the 91 isolates to 2F5 and 4E10 were 5.45 and 6.53 μg/ml, respectively, and were quite comparable to the IC50s observed for both NL4-3 and JRCSF (Table 2). We could not demonstrate a relationship between the duration of infection, CD4 cell count, or HIV-1 RNA levels and degree of susceptibility to neutralization.
FIG. 1.
Neutralization profile of each of the three MAbs. The mean IC50 of the three MAbs (micrograms per milliliter), grouped on the x axis, is represented against the number of susceptible viral isolates on the y axis.
These findings have important implications for vaccine development. We have found that essentially all newly acquired isolates screened for susceptibility to three available MAbs were susceptible to neutralization by 4E10. These data are in agreement with data cited by Binley and Burton in a recent editorial (2). Importantly, all 91 viruses tested here were recently transmitted, in the range of weeks to months. It is critical to note that all isolates are clade B and 98% were transmitted to men having sex with men. This would imply that the identification of an immunogen capable of eliciting a 4E10-like antibody response in vivo could well provide an effective vaccine strategy for HIV-1 prophylaxis. This said, it must be emphasized that the viruses studied were not necessarily identical to the viruses transmitted as infection had been established weeks to months prior to presentation and the in vivo generation time of HIV-1 of 2 days (11) would allow for some degree of viral diversity.
The relatively low number of newly transmitted viruses susceptible to 2G12 was somewhat unexpected and differs from a previous report in which 22 of 30 (73%) of clade B isolates were susceptible to neutralization by 2G12 (2). Barring confounding factors, such as assay differences and variation in lots of MAbs, an explanation for this observation is that the isolates tested here were newly transmitted and may be less susceptible to neutralization by 2G12. That the duration of infection did not positively correlate with 2G12 susceptibility argues against this explanation. This said, studies are currently under way to better understand the correlation of envelope structure and 2G12 susceptibility.
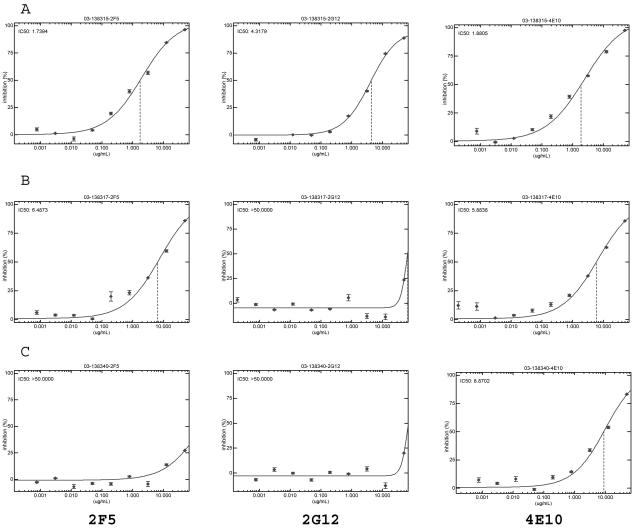
We believe that these data should be interpreted cautiously. It is not clear that the definition of susceptibility (that is an IC50 of ≤50 μg/ml) is clinically relevant. Ideally one would want to achieve ICs in the 99% range, and the susceptibility curves suggest that these antibody concentrations are substantially higher than the IC50s determined (Fig. 2). Furthermore, given the nature of HIV-1 transmission and dissemination, concentrations of neutralizing antibodies in plasma may not necessarily be predictive of such at mucosal surfaces or in tissue compartments. Nevertheless, we believe our unique data set supports the renewed interest in the design of immunogens with the potential to induce neutralizing antibodies, and the design of those with 4E10-like induction capacity should assume the highest priority.
FIG. 2.
Recombinant viral assay to determine the sensitivity of patient-derived virus to the MAbs 2F5, 2G12, and 4E10. (A) Patient sample demonstrating susceptibility to all three MAbs: 2F5 (IC50, 1.74 μg/ml), 2G12 (IC50, 4.32 μg/ml), and 4E10 (IC50, 1.88 μg/ml). (B) Patient sample susceptible to both 2F5 (IC50, 6.49 μg/ml) and 4E10 (IC50, 5.88 μg/ml) but not to 2G12 (IC50, >50 μg/ml). (C) Susceptibility to 4E10 is preserved (IC50, 8.87 μg/ml) in the face of resistance to both 2G12 (IC50, >50 μg/ml) and 2F5 (IC50, >50 μg/ml).
REFERENCES
- 1.Armbruster, C., G. M. Stiegler, B. A. Vcelar, W. Jager, N. L. Michael, N. Vetter, and H. W. Katinger. 2002. A phase I trial with two human monoclonal antibodies (hMAb 2F5, 2G12) against HIV-1. AIDS 16:227-233. [DOI] [PubMed] [Google Scholar]
- 2.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 3.Calarese, D. A., C. N. Scanlan, M. B. Zwick, S. Deechongkit, Y. Mimura, R. Kunert, P. Zhu, M. R. Wormald, R. L. Stanfield, K. H. Roux, J. W. Kelly, P. M. Rudd, R. A. Dwek, H. Katinger, D. R. Burton, and I. A. Wilson. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065-2071. [DOI] [PubMed] [Google Scholar]
- 4.Cardosa, R., M. B. Zwick, R. Kunert, H. Katinger, D. R. Burton, and I. A. Wilson. 2003. Structural insights for 4E10 antibody neutralization on HIV-1. AIDS Vaccine 2003, New York, N.Y. [Online.] http://www.aidsvaccine2003.org.
- 5.Conley, A. J., J. A. Kessler II, L. J. Boots, P. M. McKenna, W. A. Schleif, E. A. Emini, G. E. Mark III, H. Katinger, E. K. Cobb, S. M. Lunceford, S. R. Rouse, and K. K. Murthy. 1996. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J. Virol. 70:6751-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desrosiers, R. C. 2004. Prospects for an AIDS vaccine. Nat. Med. 10:221-223. [DOI] [PubMed] [Google Scholar]
- 7.Ferrantelli, F., R. Hofmann-Lehmann, R. A. Rasmussen, T. Wang, W. Xu, P. L. Li, D. C. Montefiori, L. A. Cavacini, H. Katinger, G. Stiegler, D. C. Anderson, H. M. McClure, and R. M. Ruprecht. 2003. Post-exposure prophylaxis with human monoclonal antibodies prevented SHIV89.6P infection or disease in neonatal macaques. AIDS 17:301-309. [DOI] [PubMed] [Google Scholar]
- 8.Ferrantelli, F., R. A. Rasmussen, R. Hofmann-Lehmann, W. Xu, H. M. McClure, and R. M. Ruprecht. 2002. Do not underestimate the power of antibodies—lessons from adoptive transfer of antibodies against HIV. Vaccine 20(Suppl. 4):A61-A65. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann-Lehmann, R., J. Vlasak, R. A. Rasmussen, B. A. Smith, T. W. Baba, V. Liska, F. Ferrantelli, D. C. Montefiori, H. M. McClure, D. C. Anderson, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, H. Katinger, G. Stiegler, L. A. Cavacini, M. R. Posner, T.-C. Chou, J. Andersen, and R. M. Ruprecht. 2001. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J. Virol. 75:7470-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen, R. S., G. A. Satten, S. L. Stramer, B. D. Rawal, T. R. O'Brien, B. J. Weiblen, F. M. Hecht, N. Jack, F. R. Cleghorn, J. O. Kahn, M. A. Chesney, and M. P. Busch. 1998. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 280:42-48. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz, M., M. Louie, A. Hurley, E. Sun, M. Di Mascio, A. S. Perelson, and D. D. Ho. 2003. A novel antiviral intervention results in more accurate assessment of human immunodeficiency virus type 1 replication dynamics and T-cell decay in vivo. J. Virol. 77:5037-5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markowitz, M., M. Vesanen, K. Tenner-Racz, Y. Cao, J. M. Binley, A. Talal, A. Hurley, X. Jin, M. R. Chaudhry, M. Yaman, S. Frankel, M. Heath-Chiozzi, J. M. Leonard, J. P. Moore, P. Racz, D. F. Nixon, D. D. Ho, and X. Jin. 1999. The effect of commencing combination antiretroviral therapy soon after human immunodeficiency virus type 1 infection on viral replication and antiviral immune responses. J. Infect. Dis. 179:527-537. [DOI] [PubMed] [Google Scholar]
- 13.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mascola, J. R., S. W. Snyder, O. S. Weislow, S. M. Belay, R. B. Belshe, D. H. Schwartz, M. L. Clements, R. Dolin, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, M. C. Walker, K. F. Wagner, J. G. McNeil, F. E. McCutchan, D. S. Burke, et al. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J. Infect. Dis. 173:340-348. [DOI] [PubMed] [Google Scholar]
- 15.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 16.Mortara, L., F. Letourneur, H. Gras-Masse, A. Venet, J.-G. Guillet, and I. Bourgault-Villada. 1998. Selection of virus variants and emergence of virus escape mutants after immunization with an epitope vaccine. J. Virol. 72:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortara, L., F. Letourneur, P. Villefroy, C. Beyer, H. Gras-Masse, J. G. Guillet, and I. Bourgault-Villada. 2000. Temporal loss of Nef-epitope CTL recognition following macaque lipopeptide immunization and SIV challenge. Virology 278:551-561. [DOI] [PubMed] [Google Scholar]
- 18.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Ruker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 20.Petropoulos, C. J., N. T. Parkin, K. L. Limoli, Y. S. Lie, T. Wrin, W. Huang, H. Tian, D. Smith, G. A. Winslow, D. J. Capon, and J. M. Whitcomb. 2000. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 44:920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sattentau, Q. J. 1996. Neutralization of HIV-1 by antibody. Curr. Opin. Immunol. 8:540-545. [DOI] [PubMed] [Google Scholar]
- 22.Stiegler, G., C. Armbruster, B. Vcelar, H. Stoiber, R. Kunert, N. L. Michael, L. L. Jagodzinski, C. Ammann, W. Jager, J. Jacobson, N. Vetter, and H. Katinger. 2002. Antiviral activity of the neutralizing antibodies 2F5 and 2G12 in asymptomatic HIV-1-infected humans: a phase I evaluation. AIDS 16:2019-2025. [DOI] [PubMed] [Google Scholar]
- 23.Stiegler, G., R. Kunert, M. Purtscher, S. Wolbank, R. Voglauer, F. Steindl, and H. Katinger. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 17:1757-1765. [DOI] [PubMed] [Google Scholar]
- 24.Tang, D. C., M. DeVit, and S. A. Johnston. 1992. Genetic immunization is a simple method for eliciting an immune response. Nature 356:152-154. [DOI] [PubMed] [Google Scholar]
- 25.UNAIDS. 2003. AIDS epidemic update 2003. [Online.] http://wwwunaids.org.
- 26.Wang, B., K. E. Ugen, V. Srikantan, M. G. Agadjanyan, K. Dang, Y. Refaeli, A. I. Sato, J. Boyer, W. V. Williams, and D. B. Weiner. 1993. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 90:4156-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. O. Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. H. I. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]