Abstract
Hepatitis C virus (HCV) infections represent a global health problem and are a major contributor to end-stage liver disease including cirrhosis and hepatocellular carcinoma. An improved understanding of the parameters involved in disease progression is needed to develop better therapies and diagnostic markers of disease manifestation. To better understand the dynamics of host gene expression resulting from persistent virus infection, DNA microarray analyses were conducted on livers from 10 chimpanzees persistently infected with HCV. A total of 162 genes were differentially regulated in chronically infected animals compared to uninfected controls. Many genes exhibited a remarkable consistency in changes in expression in the 10 chronically infected animals. A second method of analysis identified 971 genes altered in expression during chronic infection at a 99% confidence level. As with acute-resolving HCV infections, many interferon (IFN)-stimulated genes (ISGs) were transcriptionally elevated, suggesting an ongoing response to IFN and/or double-stranded RNA which is amplified in downstream ISG expression. Thus, persistent infection with HCV results in a complex and partially predictable pattern of gene expression, although the underlying mechanisms regulating the different pathways are not well defined. A single genotype 3-infected animal was available for analysis, and this animal exhibited reduced levels of ISG expression compared to levels of expression with genotype 1 infections and increased expression of a number of genes potentially involved in steatosis. Gene expression data in concert with other observations from HCV infections permit speculation on the regulation of specific aspects of HCV infection.
Although clinically silent for decades in most cases, an estimated 20% of individuals chronically infected with hepatitis C virus (HCV) progress to serious liver disease, including cirrhosis and hepatocellular carcinoma. The mechanisms leading to viral persistence and disease progression are not understood and are difficult to study within the human population. Fortunately, the risk of acquiring transfusion-transmitted HCV has been significantly reduced by the screening of the blood supply, and combination therapy with pegylated alpha interferon (IFN-α) and ribavirin has improved response rates for sustained viral clearance to 42 and 82% for genotypes 1 and 2/3, respectively (58). However, a significant proportion of the population still develops serious disease as a consequence of HCV infection (2). HCV infection is the leading cause for liver transplantation in the United States (1, 15). As such, a considerable effort has been made to understand the course of infection and disease progression in humans and chimpanzees, the only animal model for HCV infection (47). HCV persists despite humoral and cellular immune responses to viral proteins, although differences in T-cell responses have been documented among individuals with resolving and persistent infections (5, 16, 34, 52, 69, 81, 82, 85). Little is understood regarding the factors leading to viral clearance or persistence despite the currently held belief that early events during the acute stages of viral infection are probably influential in determining the outcome.
Gene expression analyses on liver biopsy samples from chimpanzees that experienced acute-resolving HCV infections have been performed previously (6, 77). The most notable changes in gene expression occurred in the IFN-stimulated genes (ISGs), although unique patterns of gene expression were observed in each animal. The expression levels of many ISGs tended to coincide with viral load. One potential interpretation of our studies consistent with findings in several systems is that an ongoing IFN-α/β response limits virus replication and spread in the liver until virus-infected hepatocytes can be cleared by a specific T-cell response. The importance of IFN-α in HCV clearance, i.e., (i) the high rate of sustained viral clearance of chronic infections following combined therapy with pegylated IFN and ribavirin (58), (ii) the near 100% viral clearance rate when traditional IFN monotherapy is used in acutely infected individuals (42), and (iii) the sensitivity of HCV replicons to IFN-α (7, 32, 35, 49, 55), has been demonstrated in several studies. Thus, a likely scenario exists whereby the innate and adaptive immune responses cooperate to eliminate virus-infected hepatocytes.
Here, we have used DNA microarray analysis to characterize changes in liver gene expression in 10 chimpanzees chronically infected with HCV. These studies have allowed the simultaneous comparison of transcriptional changes of up to 22,000 genes and have demonstrated a remarkable pattern of consistency in the expression patterns of individual genes among the different animals. As with changes in gene expression during acute infection, the chronically infected animals exhibited a sustained elevation in ISGs, indicating an ongoing response to IFN and/or double-stranded RNA (dsRNA) or possibly other cytokines. Changes in some genes were unique to individual animals and were possibly correlated with infection with different genotypes, age, and/or duration of infection. The data provide a window into the global changes in gene expression occurring in vivo during chronic HCV infection using an animal model that provides an opportunity to study the virus-host interaction in the absence of other underlying variables implicit in human cohorts.
MATERIALS AND METHODS
Chimpanzees, alanine aminotransferase (ALT), and antibody.
Chimpanzees were housed at the Southwest National Primate Research Center at the Southwest Foundation for Biomedical Research. The animals were cared for by members of the Department of Comparative Medicine in accordance with the Guide for the Care and Use of Laboratory Animals, and all protocols were approved by the Institutional Animal Care and Use Committee. The profiles of the 10 animals used in this study are listed in Table 1. Genotyping was determined by an INNO-LiPA HCV II kit for HCV genotyping by a line probe assay (Immunogenetics). Antibody response to HCV proteins was determined by using the ELISA Testing System 3.0 (Ortho Diagnostic Systems, Raritan, N.J.). Serum and liver needle biopsies were taken simultaneously in the morning following fasting to avoid diurnal and postprandial changes in liver metabolism.
TABLE 1.
Chimpanzee cohort for microarray analyses of chronic HCV infectiona
| Chimpanzee ID | Sex | Age (yr) | No. of yr infected | ALT level (U/liter) | HCV RNA
|
||
|---|---|---|---|---|---|---|---|
| Genotype | Liver level (ge/μg) | Serum level (ge/ml) | |||||
| 4X0081 | M | 24 | 20 | 73 | 1b | 2.9 × 105 | 5.0 × 107 |
| 4X0119 | M | 24 | 10 | 106 | 3a | 6.5 × 103 | 2.6 × 105 |
| 4X0123 | F | 24 | 20 | 43 | 1a | 4.3 × 104 | 9.7 × 105 |
| 4X0130 | F | 32 | 23 | 42 | 1a | 1.4 × 103 | 6.6 × 104 |
| 4X0183 | M | 23 | 19 | 94 | 1b | 6.9 × 104 | 2.1 × 107 |
| 4X0304 | F | 22 | 15 | 42 | 1a | 1.3 × 105 | 5.9 × 105 |
| 4X0497 | M | 20 | 14 | 100 | 1a | 5.4 × 104 | 3.1 × 106 |
| 4X0498 | M | 19 | 13 | 96 | 1a | 2.2 × 105 | 2.0 × 107 |
| 4X0500 | F | 14 | 11 | 56 | 1a | 2.1 × 105 | 7.5 × 106 |
| 4X0501 | F | 11 | 7 | 118 | 1b | 4.3 × 104 | 4.2 × 106 |
The animals used in this study are described with respect to sex, age, duration of infection, and ALT levels. The viral genotype and the levels of viral RNA in the liver and serum are presented.
TaqMan analyses.
Total RNA prepared from liver biopsies was used to perform microarray analyses and to monitor cellular and viral RNAs by quantitative reverse transcription-PCR (RT-PCR) (50). HCV RNA and cellular transcripts were quantified by real-time 5′ exonuclease RT-PCR (TaqMan) assays with an ABI 7700 sequence detector, and the primers and probes for specific genes were selected by using the Primer Express software (PE Biosystems, Foster City, Calif.). The TaqMan probe-primer set for HCV has been described previously (50). Genotype 3a was detected by using universal primers and a probe that detected all HCV genotypes (forward primer [nucleotides 125 to 144], 5′-CCTICCGIGAGAGCCATAGT-3′; reverse primer [nucleotides 314 to 295], 5′-GCACTCGCAAGCACCCTATC-3′; and probe [nucleotides 173 to 151], 5′-TTCCGGTGTACTCACCGGTTCCG-3′). The primers were obtained from Life Technologies (Gaithersburg, Md.). The fluorogenic probe was labeled with 6-carboxyfluorescein and 6-carboxytetramethylrhodamine and was obtained from Synthegen (Houston, Tex.). TaqMan assays were multiplexed with a probe-primer set for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA (PDAR kit; PE Biosystems) for normalization in some assays. HCV RNA values are reported as genome equivalents (ge) per milliliter of serum or per microgram of total liver RNA.
Microarray analyses.
All RNA and DNA preparations were made according to Affymetrix (Santa Clara, Calif.) protocols, and hybridizations and data analyses were also performed by using Affymetrix protocols, equipment, and software as previously described (6). Microarray hybridizations were performed at the gene expression core facility of the University of Texas Medical Branch, Galveston, Tex., or at Genomic Explorations (Memphis, Tenn.) by using the U133A microarray chip from Affymetrix, representing approximately 22,000 human genes. Since many of these animals have been persistently infected with HCV for over 10 years, the use of preinfected samples as baselines was not possible. Therefore, six samples from animals not infected with HCV were used as the baselines for comparison. These “normal” samples included two pooled samples of total liver RNA from three or nine uninfected animals and four individual samples. Data analyses were facilitated by using the Microarray Suite version 5.0 and Data Mining Tool version 3.0 software from Affymetrix. The samples were analyzed by using a default scaling factor of 250. Statistical (Student's t test) and self-organizing map (SOM) clustering analyses were performed with Affymetrix DMT software. The data were filtered by using the criteria I, MI, D, and MD (I, increasing; D, decreasing; M, minimally, with a change of P < 0.002 for I, P < 0.003 for MI, P > 0.998 for D, and P > 0.997 for MD). Genes were categorized into “up,” “down,” or “no change” categories with respect to the six baselines in each experiment by using the Student's t test. Clustering analyses were performed on the filtered set. The changes in gene expression relative to the baseline samples were recorded as signal log ratios (SLR) that were converted to relative severalfold changes (FCs) (see Fig. 2 and 3) by using the calculation 2n = FC, where n equals the SLR, since the log scale used is base 2. Additional data analyses were facilitated by using several web-based programs from the Affymetrix NETAFFX Analysis Center (http://www.affymetrix.com) (54), Cytokines Online Pathfinder Encyclopedia (http://www.copewithcytokines.de [by Horst Ilbelgauft]), and GeneCards (http://bioinfo.weizmann.ac.il/cards/) (68).
FIG. 2.
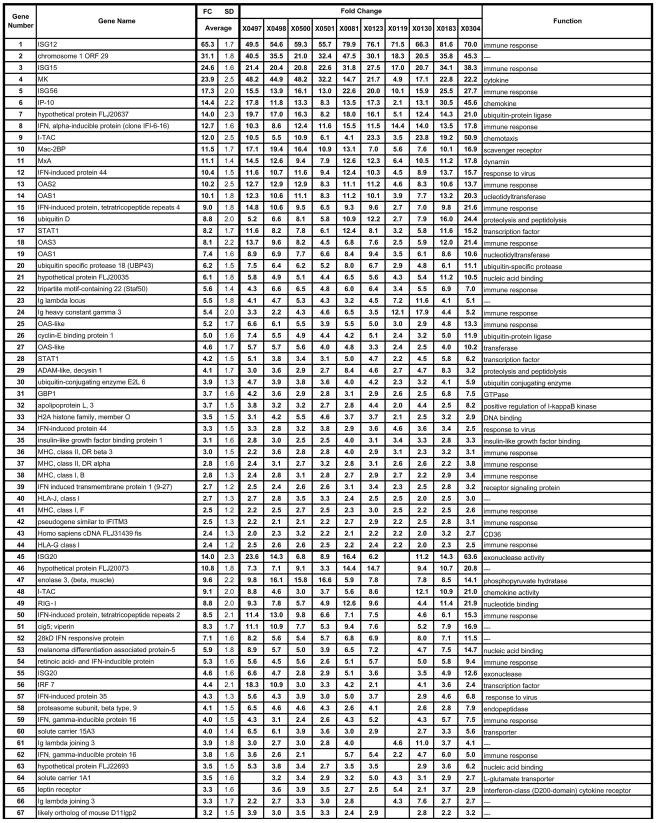
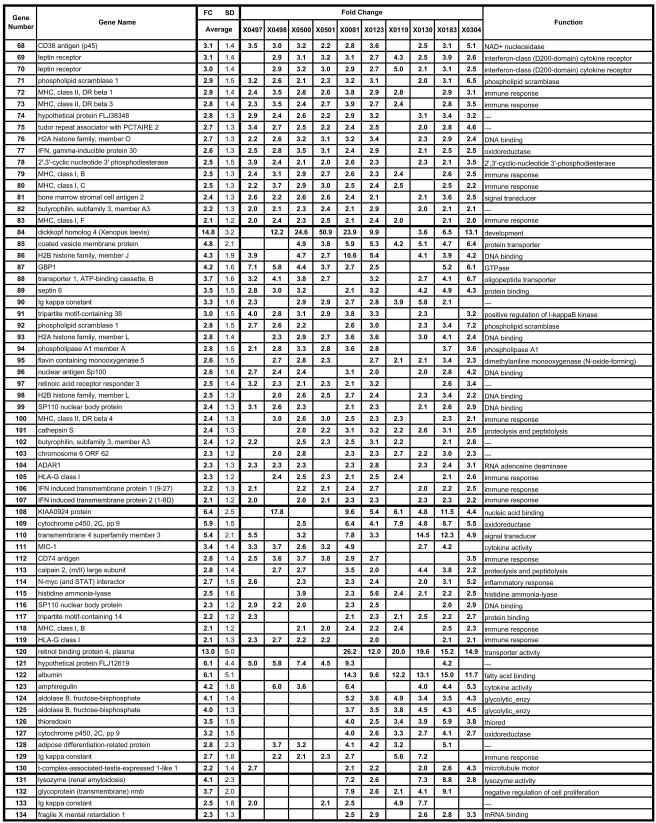
Genes upregulated during persistent HCV infection. Genes that were elevated 2.0-fold or greater in at least 5 of the 10 HCV-infected animals are included. The animal identification number is given at the top, the gene name is indicated at the left, and the gene function is given at the right. The first section includes 39 genes that exhibited changes in all 10 animals, with each subsequent section (separated by heavy lines) representing genes with changes in progressively fewer animals. Genes in each section are listed according to the magnitude of the average expression change with the average being calculated from all 10 animals, even when all 10 did not exhibit an FC of >2.0. Several genes are represented on the array more than once with different probe set identifications. SD, standard deviation. An expanded table is available with the accession numbers, complete gene names, gene symbols, probe ID, standard deviations for all genes for all animals, and functional data with regard to the Gene Ontology Biological Process and Molecular Function (see the supplemental material).
FIG. 3.
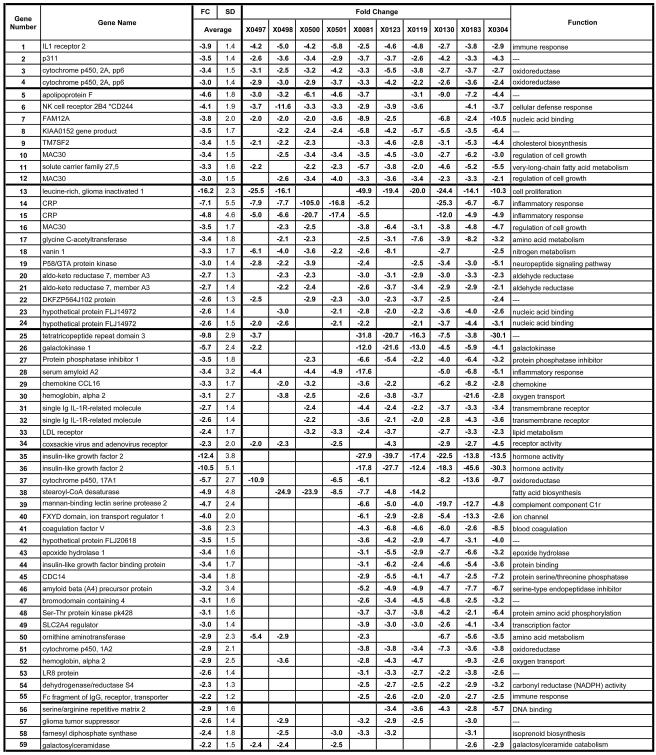
Genes downregulated during persistent HCV infection. Liver genes that were decreased by −2.0-fold or more in 5 of the 10 animals are included. The first section includes six genes that exhibited changes in all 10 animals, with each subsequent section representing genes with changes in progressively fewer animals. Genes in each section are listed according to the magnitude of the average expression change with the average being calculated from all 10 animals, even when all 10 did not exhibit an FC of >2.0. The animal identification number is given at the top, the gene name is indicated at the left, and the function is given on the right. SD, standard deviation. An expanded table is available with the accession numbers, complete gene names, gene symbols, probe ID, standard deviations for all genes for all animals, and functional data with regard to the Gene Ontology Biological Process and Molecular Function (see the supplemental material).
An additional analysis was performed by using two-way analysis of variance. First, two sets of filtering criteria were imposed: all probe sets with an absent call in all samples were removed, and all probe sets with a change of less than twofold in all possible pairwise comparisons were removed. Spotfire was then used to perform Student's t test for probe sets at 95 and 99% confidence levels and to generate heat maps with two-dimensional hierarchical clustering.
Two approaches were used to examine differences in gene expression between the genotype 3 animal and the nine genotype 1 animals. In one approach, the nine genotype 1 animals were used as the baselines for comparison to the genotype 3 animal irrespective of the uninfected animals. Genes were selected that were increased or decreased in expression by 2.0-fold or more in the genotype 3 animal compared to all nine genotype 1 animals (see Fig. 5). In the other analysis, a group of genes selectively upregulated in genotype 3 were identified by SOM cluster analysis (data not shown). Many genes in this set overlapped with the genes identified in the first analysis.
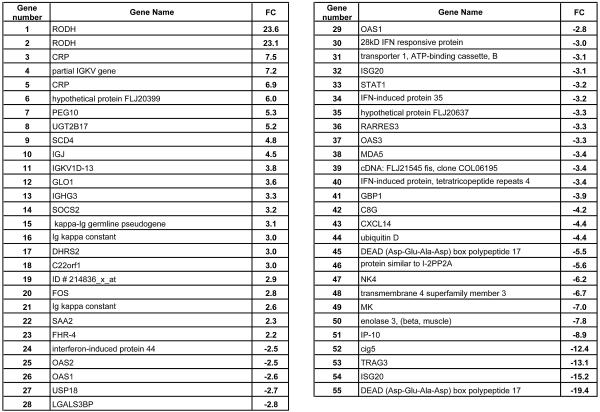
FIG. 5.
Genes differentially regulated in genotype 3 infection. The nine genotype 1 animals were used as baselines compared to animal 4X0119 of genotype 3. Genes increased or decreased by >2.0-fold in 4X119 compared to expression levels of all genotype 1 animals are listed.
RESULTS AND DISCUSSION
Chimpanzees.
The animals in this study are described in Table 1 and included five males and five females. The minimum duration of HCV infection for the group was greater than 7 years and the maximum was 23 years at the time of this study. One animal was infected with HCV genotype 3a, three animals were infected with HCV genotype 1b, and six were infected with HCV genotype 1a. All of the animals were HCV antibody positive, and most had elevated or slightly elevated liver enzyme levels; three animals had normal liver enzyme levels. All of the animals had exhibited fluctuations in ALT levels that were above normal during the course of their infections (data not shown). Clustering analyses did not reveal different patterns of gene expression based upon normal or elevated ALT levels. Viral RNA levels differed by >200-fold in the liver (1.4 × 103 to 2.9 × 105 ge/μg of liver RNA) and by 758-fold in the serum (6.6 × 104 to 5.0 × 107 ge/ml of serum), providing an opportunity to examine the impact of viral load on changes in liver gene expression. Animal 4X0119 was selected for study based upon infection with HCV genotype 3a. Animals 4X0119, 4X0123, 4X0130, and 4X0304 exhibited mild abnormalities in liver histology at the time of biopsy for microarray analysis. Chimpanzee 4X0119 exhibited minimal hepatitis at the time of biopsy, 4X0123 exhibited mild lymphocytic infiltration in scattered portal areas and disruption of hepatic sinusoids, 4X183 exhibited moderately severe hepatitis in portal and parenchymal areas, 4X0130 exhibited mild to moderate hepatitis coupled with hemosiderosis and indications of minimal interstitial fibrosis in portal areas, 4X0081 exhibited moderate to severe hemosiderosis without lymphocytic infiltrate, 4X0304 exhibited mild lymphocytic infiltrate in portal areas and disruption of hepatic sinusoids, and 4X0500 and 4X0501 exhibited essentially normal liver tissue. Animals 4X0497 and 4X0498 did not have accompanying histopathology reports.
Baseline samples.
Due to the duration of HCV infection in these animals (greater than 20 years in some cases), preinfection liver biopsies were not available as baselines. Therefore, comparisons were made between the infected animals and a panel of uninfected controls including four individual samples and two pools consisting of three and nine samples each. The baselines were remarkably similar as illustrated in the scatter graph comparison of normal samples (Fig. 1A) and in contrast to the comparison of normal and infected animals (Fig. 1B). Furthermore, duplicate analyses of several uninfected samples were nearly identical (data not shown). The use of six baselines in the analyses facilitated the exclusion of spurious changes associated with individual comparisons.
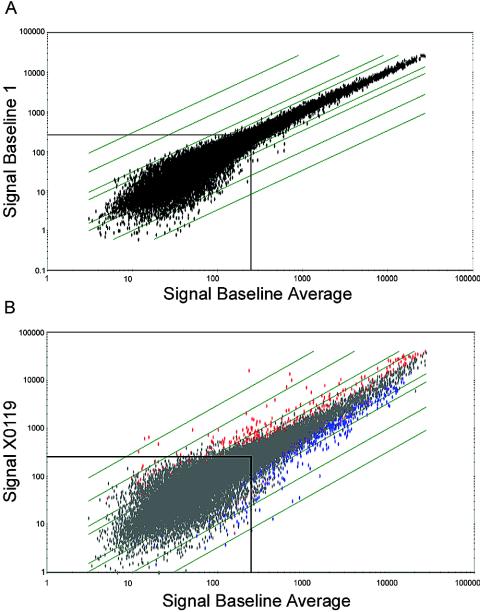
FIG. 1.
Scatter plots of signal intensity comparisons of baselines and infected samples. (A) Log scale plot of signal intensities of genes hybridizing to the baseline 1 array compared to the average signal intensities of all six baselines. (B) Log scale plot of signal intensities of HCV-infected sample 4X0119 compared to the average signal intensities of the six baselines. The diagonal lines indicate changes of 2-, 5-, 10- and 30-fold. Genes with signal intensities below 250 exhibit considerably more scatter (boxed area). In the comparison of baseline samples (A), no significant variation occurs for genes with signal intensities of >250. In contrast, in the comparison of infected and baseline samples (B), numerous genes with signal intensities of >250 exhibit FCs of >2.0. Genes with positive or negative changes (n-fold) are represented by red and blue dots, respectively.
Gene expression patterns.
The microarray data from this study are based on the analyses of 10 animals with the 22,000-gene U133A chip. The HCV-infected animals were compared to six baselines to identify changes in gene expression associated with HCV chronic infection. Those genes exhibiting a statistically significant change in expression relative to each of the baselines were followed further. Data analysis using Affymetrix software revealed that approximately 31 to 43% of the cellular transcripts (22,000 genes) were designated as present. The average SLR was determined for each gene relative to the six baselines, and the direction of change (up or down) in signal intensity was determined statistically by using the Student's t test. The average SLR values were converted to average, relative changes (n-fold) as described above. We determined the proportion of the animals in which each of these genes was differentially regulated and show that those genes exhibit FCs of ≥2.0 in 50% or more of the animals (Fig. 2 and 3). Genes that are represented on the array more than once by different probe sets usually exhibited concordance in FC variations, further substantiating the results. Using these criteria, transcripts from 162 genes exhibited an increasing (I) or decreasing (D) change in expression in the infected animals (Fig. 2 and 3). Overall, 107 genes had increased and 55 genes had decreased levels of expression in at least 5 of the 10 animals in the study. Of these changes, 39 genes exhibiting an FC of ≥2 were elevated in all 10 chronically infected animals (Fig. 2), and 5 genes were decreased in all 10 HCV-infected animals relative to baseline levels (Fig. 3). Expanded versions of Fig. 2 and 3 are available online (see Fig S2 and S3 in the supplemental material) and contain the accession numbers, full gene names, gene symbols, probe set identification numbers (ID), standard deviations for the changes in each gene, and functional information from Gene Ontology.
An independent analysis was performed by using a different approach. Initially, two sets of filtering were performed: genes absent in all samples were omitted and genes with an FC of <2.0 in all possible pairwise combinations were removed. A list of 2,110 genes was generated at the 95% confidence level, and a list of 971 genes was generated at the 99% confidence level. A heat map with two-dimensional hierarchical clustering (Fig. 4) illustrates that the 971 genes clearly segregate between infected and uninfected animals and between genes increasing and decreasing in expression. A complete list of the genes with the gene symbol, probe set ID, accession number and functional description are available (see supplemental Fig. S4 in the supplemental material). Despite the increased number of genes in this list, 48 of the genes present in Fig. 2 and 3 were not included, illustrating the value of approaching microarray analysis by independent methodologies.
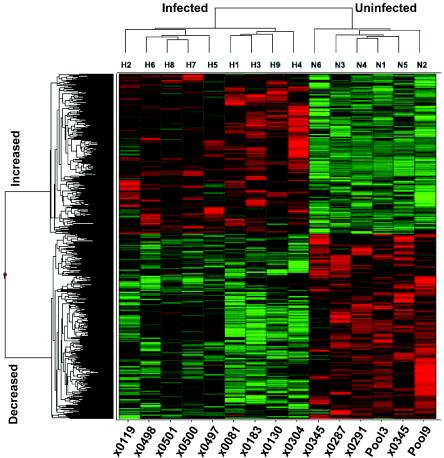
FIG. 4.
Hierarchical clustering of genes with altered expression during HCV infection. A heat map is shown illustrating two-dimensional hierarchical clustering of 971 genes identified as differentially regulated in infected versus noninfected animals by analysis of variance at a 99% confidence level. Animals with HCV infection cluster separately from animals without HCV infection (top cluster diagram). Genes increased or decreased during HCV infection cluster separately (cluster diagram to the left). Increased and decreased expression of specific genes is illustrated by red and green, respectively, while black indicates no change.
A discussion of all of the genes exhibiting changes in expression is beyond the scope of a single paper; however, many of the overall changes occurred in genes encoding enzymes, immunoglobulins (Ig), histones, ISGs, and cytokines. At this time, it is not possible to attribute relevance to the variations between different animals with regard to specific gene expression levels; however, it is clear that both qualitative and quantitative differences exist in the intrahepatic abundance of transcripts derived from these genes in the infected animals versus uninfected animals, and some of these changes are discussed below.
IFN-stimulated and dsRNA pathway genes.
The similarities in gene expression among the 10 animals suggest the involvement of specific pathways during persistence. Most of the changes in gene expression common to all animals occurred in known ISGs indicating an ongoing IFN and/or dsRNA response to the virus. These data are consistent with those of previous studies demonstrating increased ISG expression in HCV-infected patients (21, 43, 56, 64, 88). At least 30% of the genes shown in Fig. 2 are known ISGs (20, 70) and/or dsRNA response genes (20, 28, 41), and representative genes in this class include OAS (genes 13, 14, 18, and 19 of Fig. 2), MxA (gene 11), ISG15 (gene 15), ISG20 (gene 45), interferon regulatory factor 7 (IRF-7) (gene 56), STAT1α/β (gene 17,28), IFIT4 (gene 15), and ADAR1 (gene 104). Increases in expression patterns of these genes closely matched those of a previous study in an acutely infected animal (6) that cleared HCV genotype 1a, although some notable differences in the pattern of ISG expression were observed. A number of genes with well-established antiviral effects were increased in expression, including OAS (genes 13, 14, 18, and 19) and MxA (gene 11). RIG-I (retinoic acid-inducible gene I) was upregulated 21.9-fold (gene 49), is an ISG, and has recently been shown to induce type I IFN. RIG-I is a dsRNA-binding RNA helicase that activates IRF-3 and NF-κB through a caspase recruitment domain and results in the induction of type I IFN (87). Cig5 or viperin was increased by 16.9-fold (gene 51) and has antiviral activity for human cytomegalovirus when expressed constitutively in fibroblasts (13). ISG15 encodes a ubiquitin-like protein that is secreted and may act as a cytokine enhancing the proliferation and cytolytic activity of NK cells (18, 19). In acute infection, ISG15 expression increased by >100-fold by 7 days postinfection. The rapid increase in expression during the acute infection may reflect the activation of NK cells that aids in limiting virus spread early after infection. Elevated expression levels of ISG15 (and other ISGs) during chronic infection, however, may reflect multiple stimuli in addition to IFN that affect liver gene expression. The increase in ISG expression levels during both acute and chronic infections suggests that similar mechanisms may limit viral replication and the percentage of infected hepatocytes during both types of infections (discussed below).
Real-time RT-PCR was used to confirm the microarray data for several ISGs, cytokines, T-cell markers, and IFN genes (Table 2). For most upregulated genes, RT-PCR yielded data very similar to those of the microarray analysis. Two ISGs not significantly elevated and thus not shown in Fig. 2 were examined by RT-PCR: PKR and IRF-1. PKR was scored as present on the arrays and exhibited a marginally increased signal intensity (a trend of two- to threefold increase in all chronically infected animals) but was below the statistical cutoff for the designation of increased expression. Real-time RT-PCR analyses revealed a similar profile in PKR gene expression (Table 2). IRF-1 mRNA was also scored as present on the array; however, a significant increase in IRF-1 mRNA (2.5-fold) was detected in only one animal (4X0500), and marginally increased expression was detected in two others (4X0497 and 4X0304 [1.5- and 1.6-fold, respectively]). Again, RT-PCR analyses revealed similar data indicating that IRF-1 is not substantially upregulated in the chronically infected animals.
TABLE 2.
RT-PCR analyses of ISGs in chronically infected animalsa
| Gene | Change (fold) in gene expression for chimpanzee
|
||||
|---|---|---|---|---|---|
| 4X0119 | 4X0123 | 4X0130 | 4X0258 | 4X0304 | |
| ISG15 | 11.6 | 13.8 | 20.8 | 13.9 | 32.5 |
| ISG12 | 55.0 | 50.2 | 106.6 | 40.8 | 56.5 |
| ISG20 | 2.1 | 2.1 | 8.6 | 12.0 | 17.7 |
| STAT1α | NC | 2.2 | 4.4 | 4.5 | 3.6 |
| STAT1β | 2.0 | 2.7 | 3.5 | 3.2 | 4.6 |
| MK | 28.4 | 119.0 | 153.5 | 18.8 | 99.9 |
| IP-10 | NC | 14.7 | 48.2 | 13.7 | 62.4 |
| PKR | NC | NC | 3.3 | 3.7 | 2.0 |
| IRF-1 | NC | −2.7 | NC | NC | 2.1 |
| IFN-α | NC | NC | −6.1 | NC | −3.3 |
| IFN-β | NC | −2.6 | −7.2 | NC | −3.3 |
| IFN-γ | NC | −2.6 | −2.3 | 2.6 | −2.5 |
| CD3 | NC | NC | 5.6 | 3.8 | NC |
| CD8 | NC | −2.3 | NC | 2.2 | −2.6 |
| TNF-α | −2.0 | 2.2 | NC | NC | NC |
| iNOS | NC | NC | −3.8 | NC | 2.0 |
Changes (n-fold) over an average of six baseline (uninfected) samples determined by real-time RT-PCR (TaqMan) are indicated. Changes of <2.0-fold are listed as no change (NC). TNF-α, tumor necrosis factor alpha; iNOS, inducible nitric oxide synthase.
Increases were not detected for IFN-β, IFN-α2, and IFN-γ genes by microarray or by TaqMan. Furthermore, all IFN-α genes represented on the U133A array exhibited a decreasing trend in expression relative to the six baselines used in the comparisons (below the statistically significant cutoff of the software). The relevance of these data is uncertain, as the relative expression levels of ISGs in all animals was similar despite the expression levels of various IFN genes. The potency of IFNs suggests that a low level of up-regulation in a subset of the cells may be undetectable by microarray or RT-PCR yet may have substantial biological consequences.
Potential modulators of immune cell activity.
Among the genes listed in Fig. 2 and 3 are several genes that are of known importance in T-cell and/or NK cell regulation or function and include HLA-G and NK cell activation protein 2B4 (CD244). HLA-G is a nonclassical major histocompatibility complex (MHC) gene that encodes splice variants (40) of a membrane-bound or soluble protein which functions to promote maternal tolerance during pregnancy and possibly graft tolerance in transplants (4, 11, 51). HLA-G has been shown to inhibit lytic activity of NK cells (22) and antigen-specific cytotoxic T lymphocytes (3, 23) and to inhibit allogeneic proliferative responses (53). HLA-G may shift the balance in Th1/Th2 responses and thus modulate cytokine expression in various normal and/or pathological conditions (4, 11, 51). HLA-G1 is the full-length transcript from the HLA-G gene (encoding the membrane-bound form) and is represented three times on the array by different probe sets (genes 44, 105, and 119). HLA-G1 transcription was elevated in all 10 animals for all three probe sets, suggesting that it may be important in modulating cellular immune responses during HCV chronicity. Increased transcription of HLA-G was detected by real-time RT-PCR in these and other animals chronically infected with HCV as well (C. B. Bigger and R. E. Lanford, unpublished data). Further work is required to confirm that this up-regulation in mRNA abundance is consistent with elevated levels of HLA-G1 protein and to determine the importance of increases in HLA-G1 with respect to function.
CD244 (NK cell activation protein 2B4) is a cell surface glycoprotein of the CD2 family and is expressed on all NK cells, CD8+ T cells, monocytes, and basophils. The activation of CD244 leads to NK cytolytic activity and IFN-γ secretion (8, 61, 62). CD244 was decreased in expression in 9 of 10 animals, with an average decrease of −4.0-fold (gene 6 [Fig. 3]). Thus, the combination of increased expression of HLA-G and decreased expression of an NK activation marker may partially explain the “stunned” phenotype of immune cells during HCV persistence and the reduction or absence of IFN-γ expression in these animals (33, 86). Previous studies have shown that CD81 ligation by the HCV E2 glycoprotein inhibits NK cell function (17, 83), revealing yet another mechanism by which HCV may down-regulate NK and T-cell activity in the liver.
Cytokine-chemokine gene expression.
Several cytokine-chemokine transcripts were elevated in the majority of the chronically infected animals, including MK (gene 4), Mac-2-binding protein (Mac-2BP) (gene 10), macrophage inhibitory cytokine 1 (MIC-1) (gene 111), IFN-inducible protein 10 (IP-10) (gene 6), and I-TAC (genes 9 and 48). Differences between animals in the magnitude of expression of most chemokines did not correlate with the viral load, inflammatory changes, or the serum ALT levels. For example, MK differs by 10-fold across the 10 animals (Fig. 2), but these changes were not reflective of viral load (compare 4X0081 and 4X0130 with highest and lowest viral loads [Fig. 2 and 6]). Increases of IP-10 (average FC of 14.4), and I-TAC (average FC of 12.0) (both of which are CXCR3 Th1 chemokines) were detected in all of the animals. IP-10 and I-TAC are both expressed by the liver in response to IFN and are important chemokines for the recruitment of activated T and NK cells to the liver. IP-10 (49) and I-TAC (38) are expressed by hepatocyte cell lines in vitro, and I-TAC was detected in hepatocytes in HCV-infected human livers by immunological staining (38). One of the changes observed in acute-resolving infections but not in chronic infections involved macrophage inflammatory protein 1 beta (MIP-1β). MIP-1β is a CCR chemokine that is induced by interleukin-1β and is a chemoattractant for macrophages, T cells, and NK cells. MIP-1β increased during acute infection at the time of viral clearance but was not up-regulated in chronic infection. The significance of this observation will not be clear until a larger number of acute-resolving infections have been examined since some variation is observed in the profiles of these animals. Monokine induced by gamma interferon (MIG) is another CXCR3 chemokine that is a chemoattractant for activated T cells and a sensitive indicator of IFN-γ expression (9). MIG rapidly increases in parallel with IFN-γ at the time of viral clearance (48) but was detected in only two of the chronically infected animals at low levels (4X0501 and 4X0130).
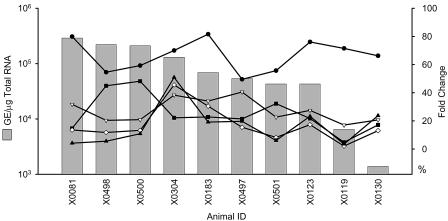
FIG. 6.
ISG and cytokine gene expression as a function of viral load. The viral load in the livers of the 10 chronically infected animals in this study was plotted relative to the magnitude of expression changes in ISGs (ISG12 [•] and ISG15 [▿]) and cytokines (I-TAC [♦], IP-10 [▴], and midkine [▪]). The viral RNA level in the liver is given as genome equivalents per microgram of total cell RNA.
Cytokines that exhibited a decreasing trend but were not above the signal intensity cutoff for a score of present included RANTES (CCR3 Th2 cytokine) in three of the animals and Gro-β in four animals. Cytokines that exhibited increasing trends in some of the animals included Exodus-1, -2, and -3; platelet-derived endothelial cell growth factor (PD/EDCGF); and CYR61. Exodus chemokines are involved in lymphocyte trafficking and adhesion or NK cell migration, and CYR61 belongs to the CCN family of growth factors. Although the expression levels of many of these gene products may be below detectable levels in some of the samples, the absence of detection does not negate a potential function in the liver for these cytokines.
Ig genes and MHC-related proteasome components.
A number of the genes that were elevated in the chronically infected animals encode immunoglobulin or MHC-related polypeptides (Fig. 2). Elevations in immunoglobulin heavy and lambda light chains may reflect the humoral immune response to a constantly changing viral quasispecies. Since the elevated levels in mRNA do not reveal the antigenic determinant of the target protein(s), qualitative assessments of the gene products cannot be made from these data alone. Alternatively, the elevations in Ig genes may also suggest ongoing autoimmune pathologies (e.g., mixed cryoglobulinemia) that are often observed in HCV-infected individuals (59, 71). Members of class I and class II MHC genes were moderately up-regulated, as were components of the proteasome and antigen-processing pathways.
Lipid metabolism and fatty acid biosynthesis.
A previous microarray study of HCV-infected chimpanzees revealed that genes involved in lipid metabolism were selectively up-regulated at early times after infection in correlation with the increases in viremia in two animals with either sustained or transient clearance of viremia (77). In addition, inhibition of fatty acid biosynthesis in the HCV replicon model suppressed replicon RNA levels (77), suggesting that lipid metabolism is important in viral RNA replication. Several of the genes that were elevated in that study included UDP-glucose ceramide glucosyltransferase (UGCG); lipase A (LiPA), ATP citrate lyase, serum response binding protein cleavage-activated protein (SCAP), and fatty acid synthase (FAS), while hepatic lipase C expression decreased in the same animals. In agreement with data reported by Su et al. (77), LiPA mRNA expression was increased in both acute-resolving animals that we examined but was also elevated in two of the chronically infected animals. ATP citrate lyase also exhibited increasing trends in the two acute-resolving infections. Some of the genes identified in the previous studies as correlating with increasing viremia in resolving animals did not show consistent increases in chronically infected animals. UGCG was decreased in three of the chronic animals, while FAS was decreased in four of the persistently infected animals. SCAP was not changed in the chronically infected animals. These data imply that up-regulation of genes associated with fatty acid biosynthesis is not absolutely required to maintain viral persistence and that these genes are not regulated in concordance with the levels of viremia during chronic infection. These findings are not inconsistent with the previous observations since they involve observations from acute and chronic infections, respectively.
Cluster analyses and genotype 3-associated changes.
HCV genotype 3 infections are associated with both a positive outcome for IFN therapy (58) and a strong association with the development of steatosis (31, 60, 67). The genotype 3-infected animal (4X0119) exhibited transcript levels that were either below the average of all animals or below the cutoff of a FC of 2.0 for 83 of the 107 genes (Fig. 2). This was especially evident for the ISG and chemokine genes. Considering that most genes shown in Fig. 2 were expressed at lower levels in the genotype 3 animal, the increased expression level of a small group of genes in the genotype 3 animal compared to the nine genotype 1 animals (Fig. 5) invites speculation on their significance to a genotype 3-specific phenotype, including the development of steatosis. Of particular interest was the 4.8-fold elevation of stearoyl coenzyme A desaturase 4 (SCD4) (Fig. 5). SCD is a rate-limiting enzyme in the synthesis of monounsaturated fats, and the pharmacologic manipulation of SCD has been proposed as a therapy for hepatic steatosis (14, 72, 75). Additionally, one of the genes belonging to the genotype 3 SOM cluster was CDP-diacylglycerol synthase, which catalyzes the conversion of phosphocholine to phosphatidylcholine. The depletion of phosphocholine is directly associated with the development of steatosis (10, 63, 73, 89). Other genes in this cluster are associated with, or are affected by, changes in androgen metabolism. Changes in androgen metabolism can significantly affect liver metabolism (e.g., lipogenesis) and are associated with the development of liver diseases, including steatosis (46, 84). These genes include RODH (oxidative 3 alpha hydroxysteroid dehydrogenase), GPSN2 (trans-2,3-enoyl-coenzyme A reductase synaptic glycoprotein 2), and LOX (lysyl oxidase). LOX is an early, key proteinase responsible for cross-linking collagen in the extracellular matrix during fibrogenesis and has been proposed as a target for chemotherapeutic intervention of fibrosis (44, 74) and may be associated with the development of fibrosis that is accelerated by steatosis in genotype 3-infected humans. However, all of the changes discussed for the genotype 3 animal must be interpreted with caution, since only one genotype 3 animal was available for this study.
Relevance of ISG gene expression in HCV infection.
The increased expression of a subset of ISGs suggests that they may be similarly regulated. Indeed, many of these genes are induced by both type I and type II IFNs as well as dsRNA (20, 70). Significant changes in IFN-α, IFN-β, and IFN-γ transcripts were not detected by TaqMan (Table 2) or microarray analyses. Additionally, increases in serum levels of type I IFNs were not detectable by enzyme-linked immunosorbent assay (data not shown). However, due to the potency of these cytokines, even undetectable increases in expression may result in increased ISG expression due to paracrine and autocrine amplification of the signal. It should be noted that the data are presented as FCs in expression levels relative to baseline values. IFN-α mRNA was scored as present in all of the samples, including the baseline samples. A small increase in transcription over baseline in a subset of hepatocytes or invading lymphocytes may be biologically significant yet may not result in a significant change in total liver RNA. Alternatively, other mechanisms may be involved in ISG expression in the chronically infected animals. Increases in the expression of specific genes possessing multiple promoter elements (e.g., IFN-stimulated response elements, gamma activation sequences, NF-kB, IRF-3, and p53) (39, 70) maybe due to multiple stimuli (e.g., IFN-α/β/γ and/or dsRNA). In some cases, it may be possible to identify the pathway being induced by the differential expression of specific transcripts, although considerable cross talk exists between these pathways (28, 41, 70). Many of these stimuli also act synergistically as well, leading to even greater increases in gene expression (36, 37, 45).
Reconciliation of the data—a model.
Several fundamental and interrelated questions remain unresolved by the gene expression studies: (i) what is the stimulus involved in the induction of the ISG response during acute and chronic HCV infection (dsRNA, type I IFN, and type II IFN), (ii) does the virus limit the host IFN or ISG response, (iii) what are the primary cell types in the liver involved in IFN production and ISG expression, and (iv) does the ISG response limit the replication and spread of the virus?
Several HCV-encoded proteins (NS5A, NS3, and E2) have been shown to alter the IFNα/β response. NS5A has been shown to inhibit PKR function in multiple systems (26, 27, 29, 30, 65, 79) and to inhibit the IFN pathway by PKR-independent mechanisms as well (30, 66, 78). E2 has been shown to modulate both PKR and CD81 activity (17, 79, 80, 83). Recently, Foy and coworkers demonstrated that NS3/NS4a protease function inhibited virus-induced IRF-3 phosphorylation and induction of IFN-β transcription (24). The cells in the liver involved in ISG and IFN expression are not presently defined. Specific aspects of this pathway may be blocked by viral proteins in infected cells, while uninfected cells may become resistant to infection due to IFN induction of the ISG pathways. This would account for the observations that viral proteins appear to inhibit the IFN pathway in vitro, yet multiple studies have observed elevated ISG expression levels in HCV-infected livers (this study and references 56, 64, 76, 77, and 88). Similarly, it may be that ISGs are produced predominantly by reactive, nonpermissive cells of the immune system. Studies of human cirrhotic livers suggested that thousands of genes may differ in expression in comparison to normal liver, but a high level of variation exists from one patient to the next due in part to advanced disease status (76). Of a selected set of 241 genes known to be altered in vitro during viral infections, 24 were altered in the majority of comparisons between cirrhotic and normal liver, and many of these genes were among the ISGs identified in the present study (76).
An understanding of the stimuli involved in ISG expression during HCV infection may help resolve the cell types involved as well. Infected hepatocytes are presumably the initial source of the ISG response, with dsRNA inducing transcription from ISREs; however, due to the potential inhibition of this pathway by viral proteins, it is not clear whether infected hepatocytes produce IFN-α/β in response to viral dsRNA. However, one can envision a scenario in which newly infected cells produce IFN-α/β before the levels of viral proteins accumulate to their critical inhibitory levels. A high level of cell turnover could provide newly infected hepatocytes even during the chronic infection. Dendritic cells may also be involved in IFN-α production due to the interaction of dsRNA with Toll-like receptor 3. The magnitude of the ISG response is the only evidence for the production of type I IFN, since the type I IFN transcripts themselves do not increase significantly. However, if only a portion of hepatocytes are infected, and only newly infected hepatocytes express IFN-α/β prior to the accumulation of NS3 and/or NS5A, the lack of a significant increase in IFN transcripts over the baseline level in total liver RNA would not be surprising. A comparison of the viral load in both the serum and liver of chronically infected chimpanzees (Table 1) indicates that the ISG response is not proportional to the amount of viral RNA present in the liver at the time of biopsy (Fig. 2 and 6). In contrast, during acute infections, the ISG response increased and decreased as the viral RNA levels peaked and then declined. Although the present study was cross-sectional in nature, the abundance of viral RNA in the serum and liver of these animals has been remarkably consistent at multiple sampling times (data not shown), suggesting that this observation is valid.
We have performed an extrapolation to estimate the percentage of infected hepatocytes based on the level of viral RNA in total liver RNA. Several assumptions were made that are subject to error; however, the 200-fold variation in viral RNA in the liver supports the conclusion that the percentage of infected hepatocytes must vary substantially among different animals. The alternative hypothesis that the viral genome copy number per infected cell varies over a 200-fold range seems inconsistent with the historical difficulty in detecting viral antigen in infected livers. The stability of viral RNA levels over time in each animal suggests that this set point is characteristic of the infection in that animal. The percentage of infected hepatocytes was based upon the extrapolation that an infected cell must contain, at minimum, 10 copies of viral RNA (1 to 2 copies of negative-strand RNA and 8 to 9 copies of positive-strand RNA) to maintain a productive infection and that 1 μg of cell RNA represents approximately 105 cells. Thus, 105 ge/μg of liver RNA indicates that a maximum of 10% of the hepatocytes are infected. If an infected cell contains more than 10 copies of viral RNA, the estimated percentage declines even further, while if only 104 hepatocytes are represented in 1 μg of cell RNA, all cells could be infected. Certainly, in the animals with the highest levels of viral RNA in the liver, most or even all hepatocytes may be infected. Nonetheless, based on our assumptions, the estimated percentage of infected cells in the 10 chronically infected animals ranged from 30% for 4X0081 to 0.1% for 4X0130, yet the magnitude of ISG expression (ISG12 and ISG15) and even cytokine gene mRNA levels (I-TAC, IP-10, and MK) were relatively similar (Fig. 2 and 6). These data may suggest that dsRNA in infected hepatocytes is not the only stimulus for ISG induction during chronic infection or, alternatively, that a very low level of IFN production in the liver results in maximal ISG stimulation.
Several observations suggest that the IFN and ISG response may be important for limiting viral replication and spread in the liver. The antiviral efficacy of pharmacologic doses of IFN is supported by the dramatically improved sustained clearance rate with the introduction of pegylated IFN (58), the near 100% clearance rate for treatment of acute HCV infection with standard IFN monotherapy (42), and the potent antiviral activity of IFN in the replicon system (7, 32, 35, 49, 55). The speculation that many or most hepatocytes are not infected during acute (6) or chronic infection suggests that they may be resistant to infection. Although hepatocytes differ in their levels of differentiation within the liver, a requirement for a specific differentiation status does not explain the 200-fold variation in the levels of viral RNA in the liver (and presumably infected cells) observed in the chronically infected animals. One possible explanation is that type I IFN expression induces an antiviral state in uninfected cells. The adaptive immune response may limit the percentage of infected cells as well. In addition to the direct killing of infected cells, the T-cell response may limit infection by the secretion of antiviral cytokines. IFN-γ has antiviral activity in the replicon system (12, 25, 49) and is elevated during viral clearance in acute infections (48, 57, 77, 81). However, recent studies of chronically HCV-infected chimpanzees immunodepleted in CD8+ T cells showed no significant change in the level of viremia (Chris Walker, personal communication). Thus, the factors regulating the broad range of viremic set points observed during chronic infection are currently unknown.
It is possible to envision a model of HCV infections that is consistent with the microarray data from acute and chronic infections, the ability of viral proteins to block the ISG-IFN responses, and the characteristics of the T-cell responses during acute and chronic infections. During acute infection, viral spread in the liver would occur rapidly until the secretion of type I IFN renders uninfected cells resistant to infection. A percentage of hepatocytes becomes infected; this may be 10% or less in most infections. The dsRNA response results in the induction of ISGs in infected cells. Presumably, IFN is then secreted by infected cells, and the secreted IFN induces zones of resistant cells. This results in a loss of available replication space in the liver and a decrease in viral spread with no further increase in viremia. Infected cells quickly lose the ability to secrete IFN due to the accumulation of viral proteins that block this response. During acute-resolving infection, a T-cell response emerges and aids in the clearance of infected cells. IFN-γ may help mediate clearance by suppressing viral replication and possibly contribute to noncytolytic clearance of viral RNA in some cells. The primary difference between acute and chronic infections may be the success of the T-cell response in eliminating infected cells, and the emergence of escape mutants may play an important role in determining whether the T-cell response is successful. In the absence of viral clearance, a dynamic equilibrium between newly infected cells, IFN secretion, inhibition of IFN secretion by viral proteins, cell death, cell division, and new susceptible cells continues indefinitely.
Currently, we understand little of this process. Ultimately, the complex interplay among the different cell types in the liver and infiltrating immune cells modulate the host response to HCV. These studies have provided a window into virus-host interactions at the organ level in vivo during HCV chronicity. An understanding of the cytokine pathways that are activated during persistent infection in addition to delineating specific genes that are consistently elevated in chronically infected animals should provide insight and avenues for future studies aimed at defining the mechanism(s) of HCV persistence.
Supplementary Material
Acknowledgments
This work was funded in part by NIH grants U19 AI40035 and P51 RR13986.
We thank Brad Pfieffer and Debbie Chavez for technical assistance and Silvia Geedman and April Hopstetter for assistance with manuscript preparation. We also thank Tom Wood, director of the Molecular Genomics Core for processing of microarray chips and Mala Sinha of the Bioinformatics Program for Spotfire analysis and generation of heat maps, both at the University of Texas Medical Branch.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Alter, H. J., and M. Houghton. 2000. Hepatitis C virus and eliminating post-transfusion hepatitis. Nat. Med. 6:1082-1086. [DOI] [PubMed] [Google Scholar]
- 2.Alter, H. J., and L. B. Seeff. 2000. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin. Liver Dis. 20:17-35. [DOI] [PubMed] [Google Scholar]
- 3.Aractingi, S., N. Briand, C. Le Danff, M. Viguier, H. Bachelez, L. Michel, L. Dubertret, and E. D. Carosella. 2001. HLA-G and NK receptor are expressed in psoriatic skin: a possible pathway for regulating infiltrating T cells? Am. J. Pathol. 159:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bainbridge, D., S. Ellis, P. Le Bouteiller, and I. Sargent. 2001. HLA-G remains a mystery. Trends Immunol. 22:548-552. [DOI] [PubMed] [Google Scholar]
- 5.Bassett, S. E., B. Guerra, K. Brasky, E. Miskovsky, M. Houghton, G. R. Klimpel, and R. E. Lanford. 2001. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology 33:1479-1487. [DOI] [PubMed] [Google Scholar]
- 6.Bigger, C. B., K. M. Brasky, and R. E. Lanford. 2001. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J. Virol. 75:7059-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. [DOI] [PubMed] [Google Scholar]
- 8.Boles, K. S., S. E. Stepp, M. Bennett, V. Kumar, and P. A. Mathew. 2001. 2B4 (CD244) and CS1: novel members of the CD2 subset of the immunoglobulin superfamily molecules expressed on natural killer cells and other leukocytes. Immunol. Rev. 181:234-249. [DOI] [PubMed] [Google Scholar]
- 9.Brice, G. T., N. L. Graber, S. L. Hoffman, and D. L. Doolan. 2001. Expression of the chemokine MIG is a sensitive and predictive marker for antigen-specific, genetically restricted IFN-gamma production and IFN-gamma-secreting cells. J. Immunol. Methods 257:55-69. [DOI] [PubMed] [Google Scholar]
- 10.Buchman, A. L., M. E. Ament, M. Sohel, M. Dubin, D. J. Jenden, M. Roch, H. Pownall, W. Farley, M. Awal, and C. Ahn. 2001. Choline deficiency causes reversible hepatic abnormalities in patients receiving parenteral nutrition: proof of a human choline requirement: a placebo-controlled trial. JPEN J. Parenter. Enteral Nutr. 25:260-268. [DOI] [PubMed] [Google Scholar]
- 11.Carosella, E. D., P. Moreau, S. Aractingi, and N. Rouas-Freiss. 2001. HLA-G: a shield against inflammatory aggression. Trends Immunol. 22:553-555. [DOI] [PubMed] [Google Scholar]
- 12.Cheney, I. W., V. C. H. Lai, W. D. Zhong, T. Brodhag, S. Dempsey, C. Lim, Z. Hong, J. Y. N. Lau, and R. C. Tam. 2002. Comparative analysis of anti-hepatitis C virus activity and gene expression mediated by alpha, beta, and gamma interferons. J. Virol. 76:11148-11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin, K. C., and P. Cresswell. 2001. Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus. Proc. Natl. Acad. Sci. USA 98:15125-15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen, P., J. M. Ntambi, and J. M. Friedman. 2003. Stearoyl-CoA desaturase-1 and the metabolic syndrome. Curr. Drug Targets Immune Endocr. Metabol. Disord. 3:271-280. [DOI] [PubMed] [Google Scholar]
- 15.Conry-Cantilena, C., M. VanRaden, J. Gibble, J. Melpolder, A. O. Shakil, L. Viladomiu, L. Cheung, A. DiBisceglie, J. Hoofnagle, J. W. Shih, R. Kaslow, P. Ness, and H. J. Alter. 1996. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N. Engl. J. Med. 334:1691-1696. [DOI] [PubMed] [Google Scholar]
- 16.Cooper, S., A. L. Erickson, E. J. Adams, J. Kansopon, A. J. Weiner, D. Y. Chien, M. Houghton, P. Parham, and C. M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10:439-449. [DOI] [PubMed] [Google Scholar]
- 17.Crotta, S., A. Stilla, A. Wack, A. D'Andrea, S. Nuti, U. D'Oro, M. Mosca, F. Filliponi, R. M. Brunetto, F. Bonino, S. Abrignani, and N. M. Valiante. 2002. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J. Exp. Med. 195:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Cunha, J., E. J. Knight, A. L. Haas, R. L. Truitt, and E. C. Borden. 1996. Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc. Natl. Acad. Sci. USA 93:211-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Cunha, J., S. Ramanujam, R. J. Wagner, P. L. Witt, E. J. Knight, and E. C. Borden. 1996. In vitro and in vivo secretion of human ISG15, an IFN-induced immunomodulatory cytokine. J. Immunol. 157:4100-4108. [PubMed] [Google Scholar]
- 20.Der, S. D., A. Zhou, B. R. Williams, and R. H. Silverman. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez, M., J. A. Quiroga, J. Martin, M. Herrero, M. Pardo, M. A. Horisberger, and V. Carreno. 1999. In vivo and in vitro induction of MxA protein in peripheral blood mononuclear cells from patients chronically infected with hepatitis C virus. J. Infect. Dis. 180:262-267. [DOI] [PubMed] [Google Scholar]
- 22.Forte, P., L. Pazmany, U. B. Matter-Reissmann, G. Stussi, M. K. Schneider, and J. D. Seebach. 2001. HLA-G inhibits rolling adhesion of activated human NK cells on porcine endothelial cells. J. Immunol. 167:6002-6008. [DOI] [PubMed] [Google Scholar]
- 23.Fournel, S., M. Aguerre-Girr, X. Huc, F. Lenfant, A. Alam, A. Toubert, A. Bensussan, and P. Le Bouteiller. 2000. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J. Immunol. 164:6100-6104. [DOI] [PubMed] [Google Scholar]
- 24.Foy, E., K. Li, C. F. Wang, R. Sumpter, M. Ikeda, S. M. Lemon, and M. Gale. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300:1145-1148. [DOI] [PubMed] [Google Scholar]
- 25.Frese, M., V. Schwarzle, K. Barth, N. Krieger, V. Lohmann, S. Mihm, O. Haller, and R. Bartenschlager. 2002. Interferon-gamma inhibits replication of subgenomic and genomic hepatitis C virus RNAs. Hepatology 35:694-703. [DOI] [PubMed] [Google Scholar]
- 26.Gale, M., C. M. Blakely, B. Kwieciszewski, S. L. Tan, M. Dossett, N. M. Tang, M. J. Korth, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1998. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol. Cell. Biol. 18:5208-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gale, M. J., M. J. Korth, N. M. Tang, S. L. Tan, D. A. Hopkins, T. E. Dever, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217-227. [DOI] [PubMed] [Google Scholar]
- 28.Geiss, G., G. Jin, J. Guo, R. Bumgarner, M. G. Katze, and G. Sen. 2001. A comprehensive view of regulation of gene expression by double-stranded RNA-mediated cell signaling. J. Biol. Chem. 276:30178-30182. [DOI] [PubMed] [Google Scholar]
- 29.Geiss, G. K., V. S. Carter, Y. He, B. K. Kwieciszewski, T. Holzman, M. J. Korth, C. A. Lazaro, N. Fausto, R. E. Bumgarner, and M. G. Katze. 2003. Gene expression profiling of the cellular transcriptional network regulated by alpha/beta interferon and its partial attenuation by the hepatitis C virus nonstructural 5A protein. J. Virol. 77:6367-6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girard, S., P. Shalhoub, P. Lescure, A. Sabile, D. E. Misek, S. Hanash, C. Brechot, and L. Beretta. 2002. An altered cellular response to interferon and up-regulation of interleukin-8 induced by the hepatitis C viral protein NS5A uncovered by microarray analysis. Virology 295:272-283. [DOI] [PubMed] [Google Scholar]
- 31.Gochee, P. A., J. R. Jonsson, A. D. Clouston, N. Pandeya, D. M. Purdie, and E. E. Powell. 2003. Steatosis in chronic hepatitis C: association with increased messenger RNA expression of collagen I, tumor necrosis factor-alpha and cytochrome P450 2E1. J. Gastroenterol. Hepatol. 18:386-392. [DOI] [PubMed] [Google Scholar]
- 32.Gosert, R., D. Egger, V. Lohmann, R. Bartenschlager, H. E. Blum, K. Bienz, and D. Moradpour. 2003. Identification of the hepatitis C virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J. Virol. 77:5487-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gruener, N. H., F. Lechner, M.-C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grüner, N. H., T. J. Gerlach, M.-C. Jung, H. M. Diepolder, C. A. Schirren, W. W. Schraut, R. Hoffmann, R. Zachoval, T. Santantonio, M. Cucchiarini, A. Cerny, and G. R. Pape. 2000. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J. Infect. Dis. 181:1528-1536. [DOI] [PubMed] [Google Scholar]
- 35.Guo, J. T., V. Bichko, and C. Seeger. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 75:8516-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harcourt, J. L., M. K. Hagan, and M. K. Offermann. 2000. Modulation of double-stranded RNA-mediated gene induction by interferon in human umbilical vein endothelial cells. J. Interferon Cytokine Res. 20:1007-1013. [DOI] [PubMed] [Google Scholar]
- 37.Harcourt, J. L., and M. K. Offermann. 2001. Multiple signaling cascades are differentially involved in gene induction by double stranded RNA in interferon-alpha-primed cells. Eur. J. Biochem. 268:1373-1381. [DOI] [PubMed] [Google Scholar]
- 38.Helbig, K. J., A. Ruszkiewicz, K. Semendirc, H. A. J. Harley, S. R. McColl, and M. R. Beard. 2004. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology 39:1220-1229. [DOI] [PubMed] [Google Scholar]
- 39.Hummer, B. T., X. L. Li, and B. A. Hassel. 2001. Role for p53 in gene induction by double-stranded RNA. J. Virol. 75:7774-7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishitani, A., and D. E. Geraghty. 1992. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc. Natl. Acad. Sci. USA 89:3947-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs, B. L., and J. O. Langland. 1996. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219:339-349. [DOI] [PubMed] [Google Scholar]
- 42.Jaekel, E., M. Cornberg, J. Mayer, J. N. Koerbel, H. Wedemeyer, A. Schueler, M. Zankel, C. Trautwein, and M. P. Manns. 2001. Early treatment of acute hepatitis C infection with interferon-alpha-2B monotherapy prevents development of chronic HCV infection. N. Engl. J. Med. 345:1-6. [DOI] [PubMed] [Google Scholar]
- 43.Ji, X., R. Cheung, S. Cooper, Q. Li, H. B. Greenberg, and X. S. He. 2003. Interferon alfa regulated gene expression in patients initiating interferon treatment for chronic hepatitis C. Hepatology 37:610-621. [DOI] [PubMed] [Google Scholar]
- 44.Kagan, H. M., and W. Li. 2003. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J. Cell. Biochem. 88:660-672. [DOI] [PubMed] [Google Scholar]
- 45.Kolla, V., D. J. Lindner, W. Xiao, E. C. Borden, and D. V. Kalvakolanu. 1996. Modulation of interferon (IFN)-inducible gene expression by retinoic acid. Up-regulation of STAT1 protein in IFN-unresponsive cells. J. Biol. Chem. 271:10508-10514. [DOI] [PubMed] [Google Scholar]
- 46.Koteish, A., and A. M. Diehl. 2001. Animal models of steatosis. Semin. Liver Dis. 21:89-104. [DOI] [PubMed] [Google Scholar]
- 47.Lanford, R. E., C. Bigger, S. Bassett, and G. R. Klimpel. 2001. The chimpanzee model of hepatitis C virus infections. ILAR J. 42:117-126. [DOI] [PubMed] [Google Scholar]
- 48.Lanford, R. E., B. Guerra, D. Chavez, C. Bigger, K. M. Brasky, X. H. Wang, S. C. Ray, and D. L. Thomas. 2003. Cross-genotype immunity to hepatitis C. J. Virol. 78:1575-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanford, R. E., B. Guerra, H. Lee, D. R. Averett, B. Pfeiffer, D. Chavez, L. Notvall, and C. Bigger. 2003. Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, poly(I)-poly(C), tumor necrosis factor alpha, and ribavirin in hepatitis C virus subgenomic replicons. J. Virol. 77:1092-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanford, R. E., H. Lee, D. Chavez, B. Guerra, and K. M. Brasky. 2001. Infectious cDNA clone of the hepatitis C virus genotype 1 prototype sequence. J. Gen. Virol. 82:1291-1297. [DOI] [PubMed] [Google Scholar]
- 51.Le Bouteiller, P., and A. Blaschitz. 1999. The functionality of HLA-G is emerging. Immunol. Rev. 167:233-244. [DOI] [PubMed] [Google Scholar]
- 52.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lila, N., N. Rouas-Freiss, J. Dausset, A. Carpentier, and E. D. Carosella. 2001. Soluble HLA-G protein secreted by allo-specific CD4+ T cells suppresses the allo-proliferative response: a CD4+ T cell regulatory mechanism. Proc. Natl. Acad. Sci. USA 98:12150-12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu, G., A. E. Loraine, R. Shigeta, M. Cline, J. Cheng, V. Valmeekam, S. Sun, D. Kulp, and M. A. Siani-Rose. 2003. NetAffx: Affymetrix probesets and annotations. Nucleic Acids Res. 31:82-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lohmann, V., F. Körner, J. O. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 56.MacQuillan, G. C., C. Mamotte, W. D. Reed, G. P. Jeffrey, and J. E. Allan. 2003. Upregulation of endogenous intrahepatic interferon stimulated genes during chronic hepatitis C virus infection. J. Med. Virol. 70:219-227. [DOI] [PubMed] [Google Scholar]
- 57.Major, M. E., K. Mihalik, M. Puig, B. Rehermann, M. Nascimbeni, C. M. Rice, and S. M. Feinstone. 2002. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J. Virol. 76:6586-6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, M. H. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358:958-965. [DOI] [PubMed] [Google Scholar]
- 59.Manns, M. P., and E. G. Rambusch. 1999. Autoimmunity and extrahepatic manifestations in hepatitis C virus infection. J. Hepatol. 31(Suppl. 1):39-42. [DOI] [PubMed] [Google Scholar]
- 60.Monto, A., J. Alonzo, J. J. Watson, C. Grunfeld, and T. L. Wright. 2002. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology 36:729-736. [DOI] [PubMed] [Google Scholar]
- 61.Nakajima, H., M. Cella, H. Langen, A. Friedlein, and M. Colonna. 1999. Activating interactions in human NK cell recognition: the role of 2B4-CD48. Eur. J. Immunol. 29:1676-1683. [DOI] [PubMed] [Google Scholar]
- 62.Nakajima, H., and M. Colonna. 2000. 2B4: an NK cell activating receptor with unique specificity and signal transduction mechanism. Hum. Immunol. 61:39-43. [DOI] [PubMed] [Google Scholar]
- 63.Oliveira, C. P., L. C. de Costa Gayotto, C. Tatai, B. I. Della Bina, M. Janiszewski, E. S. Lima, D. S. Abdalla, F. P. Lopasso, F. R. Laurindo, and A. A. Laudanna. 2002. Oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, in rats fed with a choline-deficient diet. J. Cell. Mol. Med. 6:399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patzwahl, R., V. Meier, G. Ramadori, and S. Mihm. 2001. Enhanced expression of interferon-regulated genes in the liver of patients with chronic hepatitis C virus infection: detection by suppression-subtractive hybridization. J. Virol. 75:1332-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pflugheber, J., B. Fredericksen, R. Sumpter, C. F. Wang, F. Ware, D. L. Sodora, and M. Gale. 2002. Regulation of PKR and IRF-1 during hepatitis C virus RNA replication. Proc. Natl. Acad. Sci. USA 99:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Podevin, P., A. Sabile, R. Gajardo, N. Delhem, A. Abadie, P. Y. Lozach, L. Beretta, and C. Brechot. 2001. Expression of hepatitis C virus NS5A natural mutants in a hepatocytic cell line inhibits the antiviral effect of interferon in a PKR-independent manner. Hepatology 33:1503-1511. [DOI] [PubMed] [Google Scholar]
- 67.Poynard, T., J. McHutchison, M. Manns, and J. Albrecht. 2003. Biochemical surrogate markers of liver fibrosis and activity in a randomized trial of peginterferon alfa-2b and ribavirin. Hepatology 38:481-492. [DOI] [PubMed] [Google Scholar]
- 68.Rebhan, M., V. Chalifa-Caspi, J. Prilusky, and D. Lancet. 1998. GeneCards: a novel functional genomics compendium with automated data mining and query reformulation support. Bioinformatics 14:656-664. [DOI] [PubMed] [Google Scholar]
- 69.Rice, C. M., and C. M. Walker. 1995. Hepatitis C virus-specific T lymphocyte responses. Curr. Opin. Immunol. 7:532-538. [DOI] [PubMed] [Google Scholar]
- 70.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schott, P., H. Hartmann, and G. Ramadori. 2001. Hepatitis C virus-associated mixed cryoglobulinemia. Clinical manifestations, histopathological changes, mechanisms of cryoprecipitation and options of treatment. Histol. Histopathol. 16:1275-1285. [DOI] [PubMed] [Google Scholar]
- 72.Sekiya, M., N. Yahagi, T. Matsuzaka, Y. Najima, N. Nakakuki, R. Nagai, S. Ishibashi, J. Osuga, N. Yamada, and H. Shimano. 2003. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology 38:1529-1539. [DOI] [PubMed] [Google Scholar]
- 73.Shronts, E. P. 1997. Essential nature of choline with implications for total parenteral nutrition. J. Am. Diet. Assoc. 97:639-646 [DOI] [PubMed] [Google Scholar]
- 74.Slee, R. B., S. G. Hillier, P. Largue, C. R. Harlow, G. Miele, and M. Clinton. 2001. Differentiation-dependent expression of connective tissue growth factor and lysyl oxidase messenger ribonucleic acids in rat granulosa cells. Endocrinology 142:1082-1089. [DOI] [PubMed] [Google Scholar]
- 75.Sleeman, M. W., K. Garcia, R. Liu, J. D. Murray, L. Malinova, M. Moncrieffe, G. D. Yancopoulos, and S. J. Wiegand. 2003. Ciliary neurotrophic factor improves diabetic parameters and hepatic steatosis and increases basal metabolic rate in db/db mice. Proc. Natl. Acad. Sci. USA 100:14297-14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith, M. W., Z. N. Yue, M. J. Korth, H. A. Do, L. Boix, N. Fausto, J. Bruix, R. Carithers, Jr., and M. G. Katze. 2003. Hepatitis C virus and liver disease: global transcriptional profiling and identification of potential markers. Hepatology 38:1458-1467. [DOI] [PubMed] [Google Scholar]
- 77.Su, A. I., J. P. Pezacki, L. Wodicka, A. D. Brideau, L. Supekova, R. Thimme, S. Wieland, J. Bukh, R. H. Purcell, P. G. Schultz, and F. V. Chisari. 2002. Genomic analysis of the host response to hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 99:15669-15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan, S. L., and M. G. Katze. 2001. How hepatitis C virus counteracts the interferon response: the jury is still out on NS5A. Virology 284:1-12. [DOI] [PubMed] [Google Scholar]
- 79.Taylor, D. R., S. T. Shi, P. R. Romano, G. N. Barber, and M. M. Lai. 1999. Inhibition of the interferon-inducible protein kinase PKR by HCV E2 protein. Science 285:107-110. [DOI] [PubMed] [Google Scholar]
- 80.Taylor, D. R., B. Tian, P. R. Romano, A. G. Hinnebusch, M. M. C. Lai, and M. B. Mathews. 2001. Hepatitis C virus envelope protein E2 does not inhibit PKR by simple competition with autophosphorylation sites in the RNA-binding domain. J. Virol. 75:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thimme, R., J. Bukh, H. C. Spangenberg, S. Wieland, J. Pemberton, C. Steiger, S. Govindarajan, R. H. Purcell, and F. V. Chisari. 2002. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc. Natl. Acad. Sci. USA 99:15661-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tseng, C. T. K., and G. R. Klimpel. 2002. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J. Exp. Med. 195:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Verhoeven, G., and J. V. Swinnen. 1999. Indirect mechanisms and cascades of androgen action. Mol. Cell. Endocrinol. 151:205-212. [DOI] [PubMed] [Google Scholar]
- 85.Walker, C. M. 1997. Comparative features of hepatitis C virus infection in humans and chimpanzees. Springer Semin. Immunopathol. 19:85-98. [DOI] [PubMed] [Google Scholar]
- 86.Wedemeyer, H., X.-S. He, M. Nascimbeni, A. R. Davis, H. B. Greenberg, J. H. Hoofnagle, T. J. Liang, H. Alter, and B. Rehermann. 2002. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 169:3447-3458. [DOI] [PubMed] [Google Scholar]
- 87.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 88.Yu, S. H., K. Nagayama, N. Enomoto, N. Izumi, F. Marumo, and C. Sato. 2000. Intrahepatic mRNA expression of interferon-inducible antiviral genes in liver diseases: dsRNA-dependent protein kinase overexpression and RNase L inhibitor suppression in chronic hepatitis C. Hepatology 32:1089-1095. [DOI] [PubMed] [Google Scholar]
- 89.Zhu, X., J. Song, M.-H. Mar, L. J. Edwards, and S. H. Zeisel. 2003. Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem. J. 370:987-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.