Abstract
The identity of pro- and anti-inflammatory cytokines in the neuropathogenesis of prion diseases remains undefined. Here we have investigated the role of anti-inflammatory cytokines on the progression of prion disease through the use of mice that lack interleukin-4 (IL-4), IL-10, IL-13, or both IL-4 and IL-13. Collectively our data show that among these anti-inflammatory cytokines, IL-10 plays a prominent role in the regulation of prion disease. Mice deficient in IL-10 are highly susceptible to the development of prion disease and show a markedly shortened incubation time. In addition, we have correlated cytokine gene expression in prion-inoculated IL-10−/− mice to wild-type-inoculated animals. Our experiments show that in the absence of IL-10 there is an early expression of tumor necrosis factor alpha (TNF-α). In wild-type prion-inoculated mice, the expression of TNF-α mRNA occurs at a later time point that correlates with the extended incubation time for terminal disease development in these animals compared to those that lack IL-10. Elevated levels of IL-13 mRNA are found at early time points in the central nervous system of prion-inoculated IL-10−/− mice. At terminal disease, the brains of wild-type mice inoculated with RML or ME7 are characterized by elevated levels of mRNA for the proinflammatory cytokines TNF-α and IL-1β, together with the anti-inflammatory cytokines IL-10, IL-13, and transforming growth factor beta. Our data are consistent with a role for proinflammatory cytokines in the initiation of pathology during prion disease and an attempt by anti-inflammatory cytokines to regulate the ensuing, invariably fatal pathology.
Prion diseases, such as Creutzfeldt-Jakob disease of humans, bovine spongiform encephalopathy of cattle, and scrapie of sheep are a group of fatal, neurodegenerative, transmissible conditions. During prion disease, PrPSc, an abnormal isomer of a host protein termed PrPC, accumulates in proteolysis-resistant aggregates. The protein-only hypothesis (21, 50) predicts that the transmissible prion agent comprises solely proteinaceous material. As a consequence, it is proposed that PrPSc forms part or all of the transmissible prion agent and that this abnormal isomer is responsible for the modification of the structure of PrPC. Prion diseases are also characterized by the accumulation of PrPSc in the absence of an overt inflammatory response. A major reason for the apparent lack of inflammation is that the affected host appears immunologically tolerant to PrPSc and possibly other abnormal conformations of the host protein PrPC. As a result, immune-mediated inflammatory responses are not readily evident. Furthermore, the central nervous system appears innately refractory to the development of acute inflammatory responses that typically occur in peripheral tissues. However, inflammation, albeit an atypical response (49), is now regarded as a cardinal sign of prion disease. It remains to be established whether this inflammatory response is responsible for induction or maintenance of the pathology seen during prion disease or is merely a product of the condition.
The normal homeostasis of neurons within the central nervous system is supported by glial cells (51). Both astrocytes (11) and oligodendrocytes (6, 17) are sources of specific inflammatory mediators and may contribute to the inflammation seen during prion disease. However, the primary glial cell type involved in this response appears to be microglia, resident and infiltrating cells of the monocyte/macrophage lineage. Activated microglia, evidenced by their altered morphology and increased expression of cell surface markers, including major histocompatibility complex class II and CR3, have been demonstrated in brains from humans with Creutzfeldt-Jakob disease (4, 20, 44, 52), cattle with bovine spongiform encephalopathy (37), and mice with experimental prion disease (9, 64). Activated microglia are indistinguishable from macrophages in peripheral tissues and are capable of synthesis and release of inflammatory molecules (24). Microglial activation occurs early in the prion disease response (8), and cytokine production by these cells precedes the apoptotic loss of hippocampal neurons in brains from prion-infected mice (47).
Cytokines may be classified as either proinflammatory (for example, interleukin-1 [IL-1], IL-6, IL-12, and tumor necrosis factor alpha [TNF-α]), or anti-inflammatory (for example, IL-4, IL-10, IL-13, and transforming growth factor beta [TGF-β]), based on their actions in peripheral tissues and the central nervous system (1, 65). Several previous studies have attempted to address which of these cytokines may play a role in prion disease. Immunohistochemical studies have reported that proinflammatory cytokines including IL-1β, IL-6, and TNF-α are expressed by astrocytes in early and late prion disease (63). PCR and RNase protection assays have been used to demonstrate the expression of IL-1α, IL-1β, and TNF-α (5, 10, 30, 32). However, the results of other studies have not supported a role for IL-6 or TNF-α alone in prion-infected animals following intracerebral inoculation (13, 36), although the influence of anti-inflammatory cytokines was suggested by increased TGF-β expression (13). Other mediators of inflammation apart from cytokines have been reported in individuals with prion disease. The prostaglandin synthetic enzyme cyclo-oxygenase-2 is induced in the ME7 model of prion disease (61). In addition, prostaglandin E2 has been demonstrated in the cerebrospinal fluid of Creutzfeldt-Jakob disease patients, the brains of ME7-infected mice (41), and the plasma of bovine spongiform encephalopathy-affected cattle (45).
The balance of proinflammatory and anti-inflammatory cytokine expression in the central nervous system is tightly regulated, and sustained inflammation will be detrimental to the host (1, 2). Lack of appropriate anti-inflammatory cytokines during prion disease may accelerate the appearance of pathology if an ongoing proinflammatory response plays a significant role in these conditions. In this study we have tested the hypothesis that anti-inflammatory cytokines can regulate the progression of prion disease through the use of mice that lack genes encoding these molecules. Collectively, our data show that IL-10 plays a prominent role in prion disease regulation. Mice deficient in IL-10 are highly susceptible to the development of prion disease and show a markedly shortened incubation time. These experiments also show that lack of IL-10 leads to early expression of TNF-α that appears to sensitize the mice to prion-induced pathology. In wild-type mice, TNF-α expression occurs later in the disease progression and this correlates with the extended incubation time in these animals compared to those that lack IL-10.
MATERIALS AND METHODS
Mice.
IL-10−/− mice on a 129Sv background were generated as previously described (33). IL-13−/−, IL-4/IL-13−/− mice were generated as described (39, 40) and together with IL-4−/− mice (34) were backcrossed onto BALB/c mice. Equivalently backcrossed BALB/c control mice were bred in-house. The tga20 mouse strain (19) was obtained from Charles Weissmann. 129Sv, CD-1 Swiss, and C57BL/6 wild-type mice were purchased from Harlan, United Kingdom. 129Sv mice were used as controls for the IL-10−/− mice, and BALB/c mice were used as controls for all other cytokine-deficient mice. All regulated procedures involving experimental animals were carried out under Project and Personal license authority issued in accordance with the Animals (Scientific Procedures) Act of 1986.
Prion inoculations.
The prion inoculum used in these experiments was either RML 5.0, which was derived by fivefold serial passage in CD-1 mice of a Chandler mouse-adapted scrapie strain originally donated by Stanley Prusiner (San Francisco, Calif.), or ME7.1, which was prepared at the Centre for Veterinary Science, Cambridge, United Kingdom, from terminally sick C57BL/6 mice previously inoculated with ME7 acquired from the Transmissible Spongiform Encephalopathy Resource Centre, Institute for Animal Health, Compton, United Kingdom, supplied by the Neuropathogenesis Unit (Edinburgh, United Kingdom) (58). The infectivity of stock RML 5.0 is 1010.6 50% lethal dose (LD50)/g of brain tissue and is equivalent to a titer of 109.6 LD50/ml of 10% (wt/vol) brain homogenate; the infectivity of stock ME7.1 is 109.4 LD50/g of brain tissue and is equivalent to a titer of 108.4 LD50/ml of 10% (wt/vol) brain homogenate.
Mice, either male or female, at 5 to 6 weeks of age were inoculated by intracerebral injection into the right parietal lobe at a depth of 4 to 5 mm with a 20-μl volume of a 10−1 (2 × 106.6 LD50 of RML 5.0; and 2 × 105.4 LD50 of ME7.1) (hereafter referred to as 10−1) or a 10−4 dilution (2 × 103.6 LD50 of RML 5.0; and 2 × 102.4 LD50 of ME7.1) of 10% (wt/vol) brain homogenate (hereafter referred to as 10−4). Mice injected by the intraperitoneal route received the same 10−1 or 10−4 dilution of brain homogenate in a final volume of 100 μl. Mice inoculated by oral gavage received a 200-μl volume of a 10% (wt/vol) ME7.1 brain homogenate (2 × 106.4 LD50). ME7.1 was diluted in phosphate-buffered saline (PBS) and RML 5.0 was diluted in PBS plus 5% bovine serum albumin (BSA). Original 10% (wt/vol) brain homogenates were made up in 0.32 M sucrose prior to serial 10-fold dilutions. Control mice received uninfected C57BL/6 or CD-1 brain homogenate. Inoculated mice were monitored daily for clinical signs of mouse prion disease. The diagnosis of prion disease (58) was based upon that of Dickinson et al. (15). Mice were euthanized at the point of neurological disease and dysfunction. Results are shown as mean incubation time in days ± standard deviation.
Histology and immunocytochemistry.
Brain tissue from representative mice from all treatment groups was fixed in 4% (vol/vol) buffered formalin in PBS for 24 h, inactivated for 1 h with 98% (vol/vol) formic acid, soaked in buffered formalin for a further 72 h, and subsequently embedded in paraffin wax. Paraffin sections (5 μm in thickness) were subjected to conventional staining with hematoxylin and eosin (data not shown). Brain sections from terminally sick mice were examined histologically to confirm scrapie pathology (microvacuolation). Reactive gliosis was confirmed by immunohistochemistry for glial fibrillary acidic protein (GFAP) with cow anti-GFAP diluted 1:200 (Dako, Glostrup, Denmark) and developed with an avidin-biotin labeling kit (Vector Labs, Peterborough, United Kingdom).
Western blot analysis.
Brain stem homogenates were made to 10% (wt/vol) with either homogenate buffer (0.5% Nonidet-P40 and 0.5% sodium deoxycholate in PBS) for RML 5.0 samples or lysis buffer (0.5% Nonidet-P40, 0.5% sodium deoxycholate, 100 mM NaCl, 10 mM EDTA and 10 mM Tris-HCl [pH 7.4]) for ME7.1 samples. Samples were treated with proteinase K at a final concentration of 25 μg/ml for 25 min at 37°C. Digestion was terminated by the addition of phenylmethylsulfonyl fluoride. From 40 to 50 μg of total protein was loaded and electrophoresed through a sodium dodecyl sulfate-16% polyacrylamide minigel. Proteins were transferred to nitrocellulose membranes by semidry blotting.
Membranes were blocked with Tris-buffered saline containing Tween 20 (TBS-T) (10 mM Tris HCl [pH 7.8], 100 mM NaCl, 0.05% Tween 20) plus 5% nonfat milk and subsequently incubated first with rabbit polyclonal anti-PrP serum XN (diluted 1:1,000) (43) for 1 h followed by biotin-conjugated goat anti-rabbit immunoglobulin G (IgG) (catalog no. B-7389; Sigma) (diluted 1:1,000) and finally extravidin-horseradish peroxidase (catalog no. E-2886; Sigma) (diluted 1:1,000). All dilutions of antibodies were in 1% nonfat milk in TBS-T. PrP bands were detected by enhanced chemiluminescence.
Tga20 bioassay.
In the tga20 mouse bioassay, 20 μl of a 1% (vol/vol) brain or spleen homogenate from primary inoculated mice prepared in PBS plus 5% BSA was inoculated intracerebrally into five indicator mice per sample analyzed. Inoculated mice were monitored daily for clinical signs of mouse prion disease (58). Mice were sacrificed at the point of neurological disease and dysfunction. Brain or lymphoid tissue prion titers, expressed as log10 LD50 per gram of tissue, were calculated according to the formula y = −0.184x + 9.42, where y is the log10 dilution of the stock RML 5.0 infectivity and x is the incubation time (in days) (58).
Semiquantitative real-time RT-PCR.
Whole brains were removed from IL-10−/− and 129Sv mice on days 0, 15, 30, and 45 post-intracerebral inoculation of 20 μl per mouse of a 10−1 dilution of 10% (wt/vol) RML 5.0 (n = 4). Terminal brain samples were also collected from ME7.1-inoculated C57BL/6 mice, RML 5.0-inoculated CD-1- mice, and their respective control mice that were inoculated with the relevant normal brain homogenate (n = 3). Whole brains were snap frozen in liquid nitrogen and stored at −80°C until processed. Total RNA was extracted from brain samples with 100 mg of tissue per 1 ml of Trizol reagent (Invitrogen, Paisley, United Kingdom). Tissue samples were homogenized in the Trizol reagent as quickly as possible and then 200 μl of chloroform was added and shaken vigorously for 15 s. Samples were placed on ice for 15 min, centrifuged at 16,000 × g for 15 min at 4°C, the aqueous phase was removed to a clean tube, and an equal volume of isopropanol was added and incubated for 16 h at −20°C.
Samples were centrifuged at 16,000 × g for 15 min at 4°C, the pellet was washed in 750 μl of 75% ice-cold ethanol in diethylpyrocarbonate-treated water and recentrifuged at 16,000 × g for 5 min at 4°C. The ethanol was removed, the tube was pulse-centrifuged, and residual ethanol was removed. The pellet was air dried for 2 min and then dissolved in 20 μl of diethylpyrocarbonate-treated water. One to three micrograms of target RNA was used per reverse transcription (RT) reaction to generate cDNA with 20 U of SuperScriptII reverse transcriptase (catalog no. 18064-022; Invitrogen, Paisley, United Kingdom) at 42°C for 60 min in the presence of 50 mM Tris-HCl [pH 8.3], 75 mM KCl, 3 mM MgCl2, 5 mM dithiothreitol, 0.5 mM deoxynucleoside triphosphates, 1 U of RNase H (catalog no. M4281; Promega, Southampton, United Kingdom), and 5 ng of oligo(dT)15 (catalog no. C1101; Promega, Southampton, United Kingdom). For every reaction set, one RNA sample was performed without SuperScriptII reverse transcriptase to provide a negative control in subsequent PCRs.
Primers and probes.
PCR primers were used for the following cytokines: IL-4, IL-10, IL-13, TGF-β, TNF-α, IL-1β, and IL-12p35 (46). The nonextendable fluorogenic probe was an oligonucleotide dually labeled with a reporter dye (6-carboxyfluorescein [FAM]) covalently attached at the 5′ end and a quencher dye (6-carboxytetramethylrhodamine [TAMRA]) covalently attached at the 3′ end (46). To compensate for variations in amounts of input RNA and efficiency of the reverse transcription, an endogenous housekeeping gene (hypoxanthine phosphoribosyltransferase [HPRT]) was also quantified, and results were normalized to these values.
PCR amplification.
PCRs were performed in an ABI Prism 7700 sequence detector (TaqMan; Applied Biosystems) with a 96-well microamp optical reaction plate format. PCR amplifications were performed in a total volume of 25 μl, containing 5 μl of cDNA sample, 2.5 μl of forward primer, 2.5 μl of reverse primer, 2.5 μl of fluorescent probe, and 12.5 μl of TaqMan Universal PCR master mix (catalog no. 4304437; Applied Biosystems, Cheshire, United Kingdom). Each set of primers and probes were optimized and validated independently to ensure accurate amplification. Identical thermal cycling conditions were used for all cytokines tested once the individual optimizations were completed. Each PCR amplification was performed in triplicate wells, with the following conditions: 2 min at 50°C and 10 min at 95°C, followed by a total of 45 two-temperature cycles (15 s at 95°C and 1 min at 60°C). Quantification of signal was achieved by setting thresholds within the logarithmic phase of the PCR for HPRT and the target gene and determining the cycle number at which the threshold was reached (Ct). The Ct for the target gene was subtracted from the Ct for the equivalent HPRT sample. The relative amount of target gene transcript was calculated according to the formula 2 − (Ct1 [minus] Ct2), where Ct1 is the adjusted control samplet value and Ct2 is the adjusted test sample value.
Statistical analysis.
Statistical analysis of the data was performed by one-way analysis of variance, together with Tukey highly significant difference (HSD) for post hoc analysis (Fig. 4 and 5). The data in Table 1 and Fig. 4 and 6 were analyzed with the paired samples t test.
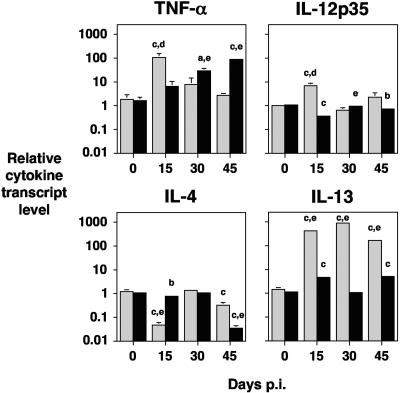
FIG. 4.
Cytokine response during prion disease in IL-10−/− and 129Sv mice. IL-10−/− and 129Sv mice were inoculated by the intracerebral route with a 20-μl volume of a 10−1 dilution of a 10% (wt/vol) brain homogenate of RML 5.0. Four mice per time point were euthanized on days 0, 15, 30, and 45 postinfection, and brain tissue was snap frozen in liquid nitrogen. RNA extraction was performed on the tissue with Trizol, and cDNA was subsequently generated for use in semiquantitative real-time RT-PCR. Cytokines were normalized to the level of HPRT transcript. Results are shown as relative cytokine transcript level ± standard deviation. The grey bar represents IL-10−/− mice; the black bar represents 129Sv mice. Statistical analysis was carried out with analysis of variance and Tukey HSD (a to c) or the paired samples t test (d and e). a P < 0.05 (in comparison with day 0). b P < 0.01 (in comparison with day 0). c P < 0.001 (in comparison with day 0). d P < 0.02 (comparison between IL-10−/− and 129Sv mice). e P < 0.005 (comparison between IL-10−/− and 129Sv mice).
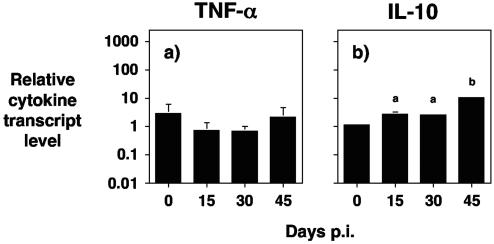
FIG. 5.
Role for IL-10 in prion disease. (a) IL-10−/− mice were inoculated by the intracerebral route with a 20-μl volume of a 10−1 dilution of a 10% (wt/vol) normal brain homogenate. (b) 129Sv mice were inoculated by the intracerebral route with a 20-μl volume of a 10−1 dilution of a 10% (wt/vol) brain homogenate of RML 5.0. In both cases, four mice per time point were euthanized on days 0, 15, 30, and 45 postinfection, and brain tissue was snap frozen in liquid nitrogen. RNA extraction was performed on the tissue with Trizol, and cDNA was subsequently generated for use in semiquantitative real-time RT-PCR. Cytokines were normalized to the level of HPRT transcript. The cytokines shown are (a) TNF-α and (b) IL-10. Results are shown as relative cytokine transcript level ± standard deviation. Statistical analysis was with analysis of variance and Tukey HSD. (a) P < 0.05 (in comparison with day 0). (b) P < 0.001 (in comparison with day 0).
TABLE 1.
Incubation time for prion disease in various mouse strains inoculated with RML 5.0 by different routesa
| Inoculation route | Genotype | 10−1 inoculum
|
10−4 inoculum
|
||
|---|---|---|---|---|---|
| No. of mice with disease (n = 5) | Mean incubation time (days) ± SD | No. of mice with disease (n = 5) | Mean incubation time (days) ± SD | ||
| Intracerebral | IL-10−/− | 5 | 58 ± 14*** | 5 | 112 ± 7*** |
| 129Sv | 5 | 140 ± 2 | 5 | 153 ± 4 | |
| IL-4−/− | 5 | 137 ± 23 | 5 | 166 ± 2** | |
| IL-13−/− | 5 | 141 ± 13 | 5 | 156 ± 14** | |
| IL-4/IL-13−/− | 5 | 148 ± 3 | 5 | 180 ± 5** | |
| BALB/c | 5 | 145 ± 1 | 5 | 195 ± 0 | |
| Peripheral | IL-10−/− | 5 | 119 ± 0*** | 5 | 138 ± 0*** |
| 129Sv | 5 | 186 ± 0 | 5 | 200 ± 2 | |
| IL-4−/− | 5 | 165 ± 29 | 5 | 183 ± 34 | |
| IL-13−/− | 5 | 162 ± 0** | 5 | 204 ± 0 | |
| IL-4/IL-13−/− | 5 | 173 ± 0* | 5 | 219 ± 4 | |
| BALB/c | 5 | 203 ± 15 | 5 | 220 ± 37 | |
IL-4−/−, IL-10−/−, IL-13−/−, IL-4/IL-13−/−, and control BALB/c and 129Sv mice (n = 5 per group) were inoculated with a 10−1 or 10−4 dilution of 10% (wt/vol) RML 5.0. Inoculated mice were monitored daily for clinical signs of mouse prion disease. Mice that succumbed to terminal prion disease were euthanized at the point of neurological disease and dysfunction. Statistical analysis was carried out with the paired samples t test. *, P ≤ 0.01 (comparison with BALB/c mice); **, P ≤ 0.003 (comparison with BALB/c mice); ***, P ≤ 0.001 (comparison with 129Sv mice).
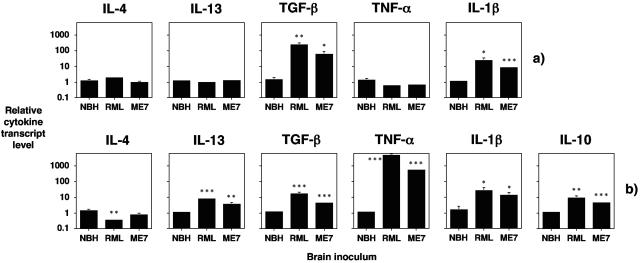
FIG. 6.
Cytokine expression in terminal brains from prion inoculated mice. IL-10−/− (a) or 129Sv mice (b) were inoculated by the intracerebral route with a 20-μl volume of a 10−1 dilution of a 10% (wt/vol) homogenate of normal CD-1 brain or C57BL/6 (normal brain homogenate, NBH), RML 5.0 (RML), or ME7.1 (ME7). Terminal brain samples from three mice per group were collected and snap frozen in liquid nitrogen. Representative data are shown for normal brain homogenate (C57BL/6). RNA extraction was performed on the tissue with Trizol, and cDNA was subsequently generated for use in semiquantitative real-time RT-PCR. Cytokines were normalized to the level of HPRT transcript. Results are shown as relative cytokine transcript level ± standard deviation. Statistical analysis carried out with the paired samples t test. *, P < 0.075 (in comparison with normal brain homogenate). **, P < 0.05 (in comparison with normal brain homogenate). ***, P < 0.025 (in comparison with normal brain homogenate).
RESULTS
Accelerated prion disease in the absence of IL-10.
The effect of lack of the anti-inflammatory cytokines IL-4, IL-10, and IL-13 on the pathogenesis of murine prion disease was investigated in mice rendered deficient in these cytokines through gene ablation. The incubation time for the development of prion disease was measured in these cytokine-deficient mice following intracerebral or intraperitoneal inoculation with RML 5.0 prion inoculum (Table 1). Mice that lacked IL-10 showed a rapid development of prion disease. All of the intracerebrally prion-inoculated IL-10−/− mice developed typical early signs of mouse prion disease, such as head tilt, hyperactive behavior, and plastic tail within 45 days. These signs rapidly progressed to clinical signs typically associated with terminal prion disease, such as progressive proprioceptive deficits, rolling gait, and progressive ataxia. By 72 days postinfection, all of the IL-10−/− mice inoculated with the high dose (10−1) of prion inoculum had succumbed to terminal disease (P ≤ 0.001).
The high susceptibility of IL-10−/− mice to prion disease was seen when either a 10−1 or 10−4 dose of RML 5.0 prion inoculum was used. IL-10−/− mice inoculated with the low dose (10−4) of RML 5.0 inoculum by the intracerebral route also succumbed to terminal prion disease and did so with an incubation time of 112 ± 7 days (P ≤ 0.001). IL-10−/− mice were not overtly susceptible to intracerebral inoculation as these mice inoculated with normal mouse brain homogenate did not succumb to any clinical signs of trauma or central nervous system disease and remained healthy for the duration of the experiment (data not shown). For controls, we inoculated the 129Sv strain of mice that is the genetic background for the IL-10−/− mice. These control mice did not show any signs of prion disease until 130 days post-intracerebral inoculation, and terminal disease was not reached until 140 ± 2 or 153 ± 4 days postinfection for either the high or low dose of RML 5.0 inoculum, respectively. Control 129Sv mice did not show any clinical signs following intracerebral inoculation with normal mouse brain homogenate (data not shown).
When IL-10−/− mice were inoculated with prion inoculum through the intraperitoneal route, these mice showed a similar shortened incubation time to first clinical signs and terminal disease compared to 129Sv control mice. IL-10−/− mice reached terminal disease after only 119 ± 0 days (P ≤ 0.001) and 138 ± 0 days (P ≤ 0.001) compared to 186 ± 0 and 200 ± 2 days in control 129Sv mice inoculated by the same route with the high and low dose of RML 5.0 inoculum, respectively (Table 1).
Mice that lacked either or both IL-4 or IL-13, which were all on a BALB/c background, showed a similar incubation time for the development of terminal prion disease compared to that seen in BALB/c control mice following intracerebral inoculation of the high dose of RML 5.0 inoculum (Table 1). IL-4−/− mice and IL-13−/− mice showed some reduction in incubation time compared to BALB/c control mice with the low dose of RML 5.0 inoculated intracerebrally but not to the same extent as the difference seen between IL-10−/− and 129Sv control mice. There was some reduction in the time course of prion disease following intraperitoneal inoculation in IL-4−/−, IL-13−/−, and IL-4/IL-13−/− mice, but this was not seen with the low dose of RML 5.0 inoculated by the peripheral route.
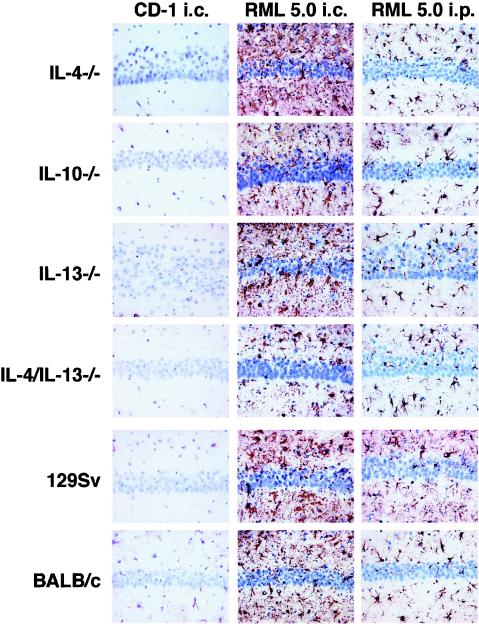
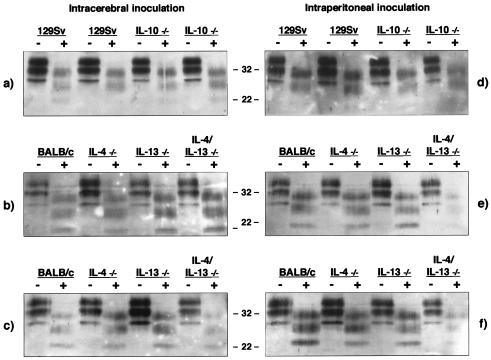
The characteristic features of prion disease were confirmed in all mice that displayed terminal signs of this neurodegenerative disorder by histopathological examination, the presence of GFAP detected by immunocytochemistry, and the presence of PrPSc in brain tissue by Western blot. Figure 1 shows that all mice inoculated by either the intracerebral or the intraperitoneal route with RML 5.0 inoculum displayed typical reactive gliosis in the hippocampal region. In addition, sections of brain tissue from all strains of mice at terminal disease were subjected to conventional staining with hematoxylin and eosin and examined histologically to confirm prion pathology by the presence of microvacuolation (data not shown). Interestingly, although there was a markedly shortened incubation time in IL-10−/− mice, there was no apparent difference in prion pathology in these animals compared to wild-type-prion-infected animals. The presence of prion disease was also confirmed by the detection of PrPSc in brain stem homogenates from mice with terminal prion disease. Following proteinase K digestion, all samples from mice with terminal prion disease showed the presence of typical PrPSc bands of 27 to 30 kDa (Fig. 2). It was interesting that samples from those mice inoculated with the lower dose of RML 5.0 inoculum appeared to accumulate more PrPSc than those that received the higher dose. This may reflect the longer period of time available to the animals to accumulate the abnormal isomer of the prion protein.
FIG. 1.
Immunocytochemistry of cytokine-deficient mice. IL-4−/−, IL-10−/−, IL-13−/−, and IL-4/IL-13−/− together with control 129Sv and BALB/c mice were inoculated by the intracerebral or intraperitoneal route with a 10−1 or 10−4 dilution of 10% (wt/vol) of control CD-1 brain homogenate or RML 5.0. Reactive gliosis analyzed by immunohistochemistry for glial fibrillary acidic protein was detected in the hippocampal region of all mice. The negative control mice were sampled at the latest time point for comparison. Representative sections are shown. Magnification, ×250. Column 1: 10−1 dilution of a 10% (wt/vol) normal CD-1 brain homogenate intracerebrally inoculated. Column 2: 10−4 dilution of a 10% (wt/vol) homogenate of RML 5.0 intracerebrally inoculated. Column 3: 10−4 dilution of a 10% (wt/vol) homogenate of RML 5.0 intraperitoneally inoculated.
FIG. 2.
Western blot analysis of PrPSc from RML 5.0-inoculated mice with terminal prion disease. Aliquots of 10% (wt/vol) brain stem homogenates prepared from RML 5.0-inoculated mice that had succumbed to terminal prion disease were treated with proteinase K followed by Western blotting with rabbit polyclonal anti-PrP serum XN and enhanced chemiluminescence. From 40 to 50 μg of total protein was loaded into each track. The positions of molecular mass markers (in kilodaltons) are indicated between the gels. Samples were digested with proteinase K (+) or not digested (−). The left-hand panel represents intracerebrally inoculated mice, and the right-hand panel represents intraperitoneally inoculated mice. (a and d) Lanes 1, 2, 5, and 6 are samples from mice inoculated with a 10−1 dilution; lanes 3, 4, 7, and 8 are samples from mice inoculated with a 10−4 dilution. (b and e) Samples from mice inoculated with a 10−1 dilution; (c and f) samples from mice inoculated with a 10−4 dilution.
These data clearly indicate that mice that lack IL-10 are highly susceptible to the development of prion disease. In order to verify if the rapid progression towards terminal prion disease in IL-10−/− mice correlated with a rapid accumulation of prion infectivity, brain and spleen homogenates from RML 5.0-inoculated animals were transmitted to tga20 indicator mice. The data in Table 2 show that despite the rapid onset and progression to terminal prion disease by IL-10−/− mice inoculated by the intracerebral route, these animals accumulated as much infectivity as control inoculated mice that succumbed to terminal disease in approximately twice as long an incubation period. In addition, IL-10−/− mice inoculated by the intraperitoneal route showed significant levels of infectivity in the spleen.
TABLE 2.
Prion infectivity in brain and spleen samples of IL-10−/− and 129Sv mice inoculated with RML 5.0a
| Genotype | Inoculum of RML | Intracerebral route
|
Peripheral route
|
||||
|---|---|---|---|---|---|---|---|
| Brain tissue
|
Brain tissue
|
Spleen tissue
|
|||||
| Mean incubation time (days) ± SD | Titer | Mean incubation time (days) ± SD | Titer | Mean incubation time (days) ± SD | Titer | ||
| IL-10−/− | 10−1 | 71 ± 0 | 7.0 | 80 ± 27 | 5.3 | 85 ± 5 | 4.4 |
| 70 ± 1 | 7.1 | 91 ± 19 | 3.3 | 97 ± 14 | 2.2 | ||
| 10−4 | 58 ± 2 | 9.4 | 65 ± 9 | 8.1 | 62 ± 3 | 8.6 | |
| 59 ± 2 | 9.2 | 71 ± 9 | 7.0 | 61 ± 2 | 8.8 | ||
| 129Sv | 10−1 | 62 ± 2 | 8.6 | 80 ± 1 | 5.3 | 75 ± 6 | 6.2 |
| 61 ± 3 | 8.8 | 81 ± 12 | 5.1 | 83 ± 6 | 4.8 | ||
| 10−4 | 64 ± 1 | 8.2 | 85 ± 3 | 4.4 | 72 ± 2 | 6.8 | |
| 62 ± 2 | 8.6 | 87 ± 2 | 4.0 | 74 ± 4 | 6.4 | ||
Twenty-microliter volumes of 1% (wt/vol) brain or spleen homogenates prepared from IL-10−/− or 129Sv mice that had succumbed to terminal prion disease were inoculated intracerebrally into tga20 indicator mice. Inoculated mice were monitored daily for clinical signs of mouse prion disease. Mice that succumbed to terminal prion disease were euthanized at the point of neurological disease and dysfunction. Prion titers were calculated according to the formula y = −0.184x + 9.42, where y is the log10 dilution of the stock RML 5.0 infectivity and x is the incubation time (in days) (58). Prion titers are given as log10 LD50 per gram of tissue (n = 5).
Lack of IL-10 involvement from immune cells.
Our data are consistent with a protective role mediated by IL-10 in prion disease. IL-10 is a cytokine that was first described in association with cells of the immune system and more recently with those of the central nervous system. In order to examine which system was responsible for the production of IL-10, various immune deficient mice were inoculated with RML 5.0 prion inoculum by the intracerebral route. Accordingly, we inoculated μMT (B-cell deficient), TCRβδ (T-cell deficient), and RAG-1−/− (T- and B-cell deficient) mice with the same dose of RML 5.0 inoculum. In all cases, the duration from prion inoculation to the appearance of clinical signs and to terminal disease was similar in μMT, TCRβδ, and RAG-1−/− and in control C57BL/6 mice, which are the genetic control mice for the various immunodeficient strains. All strains of mice showed incubation times in the range of 130 to 159 days with a high dose of RML 5.0 inoculated intracerebrally (our unpublished observations). From these data we conclude that lymphocytes are not the source of IL-10 that appears to be involved in the regulation of prion disease.
A combination of in situ hybridization and immunocytochemistry of brain tissue, together with RT-PCR analysis of leukocytes isolated from brain parenchyma, has been used to identify the cellular location of IL-10 expression in the central nervous system (35, 54). Neurons are a major cellular location in the central nervous system for IL-10 mRNA, particularly those in the cortex. IL-10 immunoreactivity is also seen in choroid plexus epithelial cells, and weakly on some glial cells (54). Isolated cultures of microglia and astrocytes constitutively express IL-10 receptor 1 mRNA, and these cells, when activated, for example by lipopolysaccharide, can be induced to express IL-10 mRNA and protein (35). Collectively, these observations suggest that IL-10 produced by neurons, activated microglia, and astrocytes modulate glial-mediated inflammatory responses.
Efficient neuroinvasion in IL-10−/− mice following oral inoculation.
The oral route is considered the most likely mode of inoculation in natural transmissible spongiform encephalopathy disease such as scrapie (23), bovine spongiform encephalopathy, or variant Creutzfeldt-Jakob disease (12). However, this is the most inefficient route for prion neuroinvasion, as seen by the extended incubation time for the development of experimental prion disease in comparison with other routes of inoculation. Conditions that alter the integrity of the gastrointestinal tract, such as gastrointestinal inflammation, may lead to a greater efficiency of neuroinvasion by prions that enter by the oral route and hence shorter incubation times.
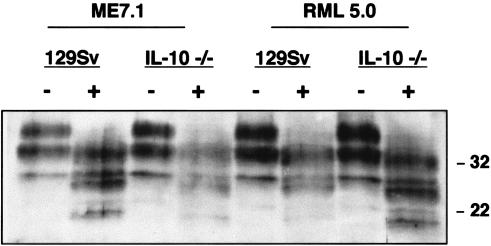
IL-10−/− mice develop chronic enterocolitis due to uncontrolled immune responses stimulated by enteric antigens (33). We have investigated whether these animals show enhanced prion disease progression as a consequence of this condition. IL-10−/− and control mice were orally inoculated with either RML 5.0 or ME7.1 prion inoculum, and the time to terminal disease was measured (Table 3). All mice orally inoculated with prions succumbed to terminal disease. When terminal brains from all of these mice were assessed by Western blot, typical band patterns for PrPSc were detected, indicating that these animals had succumbed to prion disease (Fig. 3). The incubation time for IL-10−/− mice to reach terminal disease was approximately 30% of the time required for the control animals to reach terminal disease. When a similar comparison was made for intraperitoneal inoculation with RML 5.0 inoculum between IL-10−/− and wild-type animals, the difference in incubation time was only approximately 50% for IL-10−/− mice (Table 1). These data suggest that oral transmission in IL-10−/− mice provides a more efficient mode of peripheral prion infection than that normally seen for this route of inoculation. We attribute this enhanced neuroinvasion to the fact that IL-10−/− mice have a gut pathology that probably leads to a greater uptake or leakage of prions across the gut wall.
TABLE 3.
Prion incubation time in mice following high-dose oral inoculationa
| Genotype | ME7.1
|
RML 5.0
|
||
|---|---|---|---|---|
| No. of mice with disease/no. in group | Mean incubation time (days) ± SD | No. of mice with disease/no. in group | Mean incubation time (days) ± SD | |
| IL-10−/− | 5/5 | 85 ± 10 | 5/5 | 81 ± 1 |
| 129Sv | 5/5 | 271 ± 3 | 5/5 | 217 ± 3 |
IL-10−/− and 129Sv mice were inoculated by the oral route with a 200-μl volume of a 10−1 dilution of 10% (wt/vol) ME7.1 diluted in PBS or RML 5.0 diluted in PBS plus 5% BSA. Inoculated mice were monitored daily for clinical signs of mouse prion disease. Mice that succumbed to terminal prion disease were euthanized at the point of neurological disease and dysfunction.
FIG. 3.
Western blot analysis of PrPSc from mice with terminal prion disease following oral inoculation. Aliquots of 10% (wt/vol) brain stem homogenates prepared from 129Sv and IL-10−/− mice that had succumbed to terminal prion disease following oral inoculation were treated with proteinase K followed by Western blotting with rabbit polyclonal anti-PrP serum XN and enhanced chemiluminescence. From 40 to 50 μg of total protein was loaded into each track. The positions of the molecular mass markers (in kilodaltons) are indicated on the right. Samples were digested with proteinase K (+) or not digested (−). 129Sv and IL-10−/− mice were inoculated by the oral route with a 200-μl volume of a 10−1 dilution of 10% (wt/vol) ME7.1 diluted in PBS or 10% (wt/vol) RML 5.0 diluted in PBS plus 5% BSA.
Pro- and anti-inflammatory cytokine transcripts in the brain during prion disease.
Semiquantitative RT-PCR was carried out on brain tissue of RML 5.0-inoculated IL-10−/− mice and 129Sv wild-type mice (Fig. 4) during a time course experiment in order to investigate the central nervous system anti-inflammatory and proinflammatory cytokine profiles during the development of prion disease. The most significant alteration in proinflammatory cytokine mRNA levels in IL-10−/− mice was seen at 15 days post-prion inoculation, when the brains of these mice showed a >60-fold increase in TNF-α mRNA (P < 0.001 in comparison with day 0). This was accompanied by a >10-fold increase in IL-12p35 mRNA level (P < 0.001 in comparison with day 0). At 30 days post-prion inoculation there was a >10-fold increase in TNF-α mRNA level compared to prechallenge levels. IL-1β did not show any significant changes over this time course compared to prechallenge levels (data not shown).
Although IL-10−/− mice succumb rapidly to prion infection, these animals do mount a significant anti-inflammatory response during the development of prion disease. The increase in TNF-α transcript levels following prion inoculation was accompanied by a dramatic rise in IL-13 mRNA level, which showed >500-fold increase on day 30 post-prion inoculation compared to prechallenge levels (P < 0.001). IL-4 transcript levels were depressed at the time of peak TNF-α transcript levels (day 15 postinfection) (P < 0.001 in comparison with day 0), while TGF-β showed no significant variation in these mice over this time course (data not shown). TNF-α mRNA levels in the brains of prion inoculated 129Sv mice were significantly increased compared to prechallenge levels. They did however have a delayed time course consistent with the extended incubation period of these animals to terminal disease compared to IL-10−/− mice. In 129Sv mice at day 45 post-prion challenge, IL-4 transcript levels were significantly decreased in comparison with prechallenge levels (P < 0.001), which coincided with the time of peak TNF-α transcript levels for these animals (P < 0.001).
Cytokine mRNA levels in IL-10−/− mice were directly compared with 129Sv control mice at each of the time points tested following prion inoculation (Fig. 4). On day 15 post-prion inoculation there was a significant increase in TNF-α (P < 0.02), IL-12p35 (P < 0.02) and IL-13 (P < 0.005) with a significant decrease in IL-4 mRNA (P < 0.005) in IL-10−/− mice compared to 129Sv control mice. IL-10−/− mice also showed a significant increase in IL-13 mRNA levels at days 30 and 45 postinfection (P < 0.005) compared to 129Sv control mice. On day 30 postinfection the level of TNF-α and IL-12p35 mRNA in 129Sv control mice was significantly greater (P < 0.005) than IL-10−/− mice. Furthermore, at day 45 postinfection, the level of TNF-α mRNA in 129Sv control mice was significantly greater than that in IL-10−/− mice (P < 0.005), as was the decrease in IL-4 mRNA (P < 0.005). Collectively, these data strongly suggest that prion infection in IL-10−/− mice results in a much more rapid proinflammatory and compensatory anti-inflammatory cytokine response compared to 129Sv control mice.
The changes in proinflammatory cytokine mRNA levels in the central nervous system of prion inoculated IL-10−/− mice were not simply due to mechanical trauma or general inflammation as a consequence of intracerebral inoculation. Injection of normal brain homogenate into IL-10−/− mice did not result in similar increases in the levels of TNF-α mRNA (Fig. 5a) or IL-1β and IL-12p35 (data not shown) as that seen following prion inoculation. Furthermore, IL-10 would appear to play a role in regulating the development of prion disease in normal animals since mRNA levels of this cytokine were significantly increased in prion inoculated 129Sv mice (Fig. 5b). The increase in IL-10 mRNA at day 45 post-prion inoculation in 129Sv mice was >10-fold compared to the prechallenge level (P < 0.001).
Cytokine mRNA levels were assessed in the brains of IL-10−/− and 129Sv wild-type mice at the time of terminal prion disease in RML 5.0- or ME7.1-inoculated animals (Fig. 6) In IL-10−/− mice significant levels of IL-1β and TGF-β were detected in both ME7.1- and RML 5.0-inoculated animals compared to brains of age-matched mice inoculated with normal brain homogenate (Fig. 6a). In terminal prion diseased brains of 129Sv mice (Fig. 6b), significantly elevated levels of TNF-α, IL-1β, IL-10, IL-13 and TGF-β transcripts were detected in both RML 5.0- and ME7.1-inoculated mice. Collectively, these data are consistent with a role for TNF-α and IL-1β at terminal prion disease in wild-type mice, while IL-10, IL-13, and TGF-β contribute to the regulation of these proinflammatory cytokines.
DISCUSSION
In this study we have investigated whether the absence of anti-inflammatory cytokines alters the progression of prion disease. Various cytokine-deficient mice were assessed for their response to prion inoculation. Most noticeably, IL-10−/− mice displayed considerably shorter incubation times than control animals following either intracerebral or peripheral prion inoculation. The shortest incubation period in prion-inoculated IL-10−/− mice was seen following intracerebral prion infection, where terminal disease was reached in less than 75 days when a high dose of RML 5.0 inoculum was used. This was approximately 50% of the incubation time seen for the corresponding control animals. Similarly, oral inoculation of prions was effective in IL-10−/− mice. This route of inoculation is an efficient portal of entry for prions but is normally associated with an extended incubation time for the onset of terminal disease. However, IL-10−/− mice orally inoculated with either RML 5.0 or ME7.1 prions displayed an incubation period that was reduced to approximately 30% of that seen in 129Sv control mice inoculated in the same manner.
The relatively rapid development of prion disease in orally inoculated IL-10−/− mice may be a consequence of the gastrointestinal inflammation exhibited by these animals (33) that allows increased uptake of prions across the gut wall and subsequent enhanced neuroinvasion. The uptake of prions across the gut wall of IL-10−/− mice may be aided by increased expression of PrPC at this site, which is reported to occur during gastrointestinal inflammation (48). These observations support the suggestion that the physiological state of the gastrointestinal tract may contribute to the outcome of prion infection following oral inoculation (59). IL-4−/− and IL-13−/− mice showed an increased susceptibility to prion disease when inoculated with a low dose of RML 5.0 by the intracerebral route or a high dose by the intraperitoneal route. However, mice that lacked both IL-4 and IL-13 did not show an increased susceptibility to prion disease, indicating that cytokine regulation in these conditions is complex. Collectively, these data showed that in the absence of anti-inflammatory cytokines, in particular IL-10, the progression of prion disease occurred with an accelerated pace. This is strong evidence that the inflammatory response that occurs during prion disease contributes significantly to the pathology observed during these conditions.
The dominant proinflammatory cytokine mRNA detected during the development of prion disease in both IL-10−/− and 129Sv wild-type mice was TNF-α. Central nervous system transcripts for TNF-α in IL-10−/− mice were elevated approximately 100-fold shortly after prion inoculation, while at the same time there was a modest increase of IL-12p35 transcripts and little change in IL-1β mRNA levels. A similar trend was seen in 129Sv wild-type mice, although at later time points, indicating that the TNF-α response modeled in IL-10−/− mice was representative of that seen in normal animals. The different cellular responses to TNF-α occur through its binding to tumor necrosis factor receptor (TNFRI) or TNFRII and subsequent engagement of adaptor proteins; the proapoptotic role of TNF-α is mediated by recruitment of TNF receptor-associated death domain and Fas-associated death domain adaptor proteins, whereas cell survival and inflammatory reactions are transduced by TNF receptors through TNF receptor-associated factors (14).
Activation of TNFRI leads to degradation of IκBα, followed by translocation of NF-κB from the cytoplasm to the nucleus (26, 42), which in neurons is deleterious as it appears to favor apoptosis (55, 66). Similarly, neuronal cell death occurs when NF-κB is activated in neurons following stress events (7) including the response to toxic concentrations of glutamate, a proposed mediator of prion disease (22, 28). This effect of NF-κB involves an increase in pro-apoptotic protein activity including caspase-3, which has been detected early in the pathology of mouse prion disease (27). Sphingolipids constitute another important class of TNF mediators. TNF-α signaling can be mediated through the activation of sphingomyelinases, which act on membrane sphingomyelin and release the second messenger ceramide (25, 31).
Several mechanisms may account for the action of IL-10 in modulating the onset of clinical prion disease mediated by proinflammatory cytokines such as TNF-α. First, IL-10 directly blocks proinflammatory cytokine release by inhibition of NF-κB translocation (62). Second, IL-10 decreases the expression of proinflammatory cytokine receptor expression (53). Third, IL-10 regulates activation of proinflammatory cytokine receptors through induction of suppressor of cytokine synthesis 3 (16). Fourth, IL-10 blocks the glutamate-mediated increase in caspase-3 activity and can block glutamate-mediated apoptosis by preventing the increase in NF-κB binding activity evoked by glutamate (3). In our experiment with IL-10−/− mice, IL-13 appeared to be the major anti-inflammatory mediator in response to prion infection as there was a dramatic increase in IL-13 mRNA transcript level that coincided with the increase in TNF-α transcripts.
Both IL-10 and IL-13 are capable of rapid activation of phosphatidylinositol-3-kinase, thereby reducing the level of second-messenger ceramide (47). However, IL-13 may well contribute to prion disease pathology as this cytokine can induce profound fibrosis (18). The rapid progression to terminal prion disease in IL-10−/− mice implies that IL-10 may be expected to play an important role in limiting the onset of disease in wild-type mice. This was the case, as wild-type mice showed elevated levels of IL-10 transcript expression approximately one-third into the incubation time for terminal disease in these animals. The importance of IL-10 in regulating the progression of prion disease was seen by the fact that transcript levels of this cytokine were elevated at terminal disease in wild-type mice inoculated with different prion strains, RML 5.0 or ME7.1. Similarly, a prominent role for IL-13 was also seen in prion-inoculated wild-type mice, as IL-13 transcript levels were elevated at terminal disease in both ME7.1- and RML 5.0-inoculated mice. Other studies have suggested that TGF-β plays a dominant role in prion disease (13). In our current studies, we have found that TGF-β transcript levels were elevated in wild-type mice at the time of terminal disease but remained reasonably stable during disease progression. Our data would seem to indicate that IL-10 has a more important role in the regulation of prion disease as transcripts for this cytokine appear early during the response to prion infection whereas an increase in TGF-β mRNA expression occurs at a later time point, around the time of clinical and terminal disease.
Our suggestion that TNF-α may play a significant role in prion disease in both IL-10−/− and wild-type mice is not supported by studies in TNF-α−/− mice which did not show any difference from wild-type mice following intracerebral inoculation with prion inoculum (36). Investigations that rely on the perturbation of ligand receptor interaction to identify the role of a single protein in complex multicomponent signaling pathways may not always result in discernible responses such as those shown here. This may be particularly relevant to some of the actions of TNF-α which appear to be closely linked to the neurotrophic factor insulin-like growth factor-I (60). It may also be the case that some of the actions of TNF-α can be compensated by other cytokines, whereas this would not appear to be the case with IL-10.
Measurement of infectivity levels in the brains of IL-10−/− mice at terminal prion disease showed that these animals had accumulated the same level of prion infectivity as did control mice, but in approximately half the incubation period. This is a similar phenomenon to that seen in tga20 mice, which overexpress murine PrPC, compared to wild-type mice with normal levels of this protein. We suspect therefore that accelerated prion disease in IL-10−/− mice was not merely a consequence of increased PrP expression, although we cannot totally exclude the possibility of cytokine-driven increased PrPC expression in the central nervous system during the disease process. This would imply that the anti-inflammatory response that normally occurs during prion disease attempts to limit the accumulation of prion infectivity, PrPSc and neurotoxic factors, either directly by promoting their metabolism, or indirectly by limiting the inflammatory response that appears to promote their accumulation. It is interesting that the brains of IL-10−/− mice that succumbed to terminal disease had the same neuropathology as those animals that took longer to reach terminal disease. This would suggest that neurotoxic factors associated with prion disease are handled less well during their rapid rather than slow accumulation and reinforces the suggestion that a principal factor in determining the outcome of prion infection is the rate of conversion of PrPC to PrPSc (59).
In summary, there is an increasing amount of evidence that reveals a role for proinflammatory cytokines in initiating and maintaining the pathology of prion disease. Our data suggest that IL-10 plays a significant role in the hosts attempt to halt the ensuing prion pathology mediated by this inflammatory response and highlights two important features of disease modulation. Firstly, as polymorphisms in cytokine genes have been linked to central nervous system disorders (29, 38), it is reasonable to postulate that genetic factors which predispose to lower levels of IL-10, or its activity, may render a prion-infected individual more susceptible to prion disease pathology. IL-10 gene polymorphisms are associated with decreased levels of serum IL-10 (57) and this may occur at other sites. Secondly, attempts to retard prion diseases through the use of anti-inflammatory compounds may be increased by use of compounds that mimic IL-10 in both its anti-inflammatory and cell survival function (56).
Acknowledgments
This work was supported by the BBSRC.
We thank Charles Weissmann for the tga20 mice. We thank Adriano Aguzzi for the gift of RML 5.0 and XN antibody. We thank Fred Heath for assistance with the statistical analysis.
REFERENCES
- 1.Allan, S. M., and N. J. Rothwell. 2001. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2:734-744. [DOI] [PubMed] [Google Scholar]
- 2.Allan, S. M., and N. J. Rothwell. 2003. Inflammation in central nervous system injury. Phil. Trans. R. Soc. Lond. B Biol. Sci. 358:1669-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachis, A., A. M. Colangelo, S. Vicini, P. P. Doe, M. A. De Bernardi, G. Brooker, and I. Mocchetti. 2001. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J. Neurosci. 21:3104-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker, C. A., Z. Y. Lu, I. Zaitsev, and L. Manuelidis. 1999. Microglial activation varies in different models of Creutzfeldt-Jakob disease. J. Virol. 73:5089-5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker, C. A., D. Martin, and L. Manuelidis. 2002. Microglia from Creutzfeldt-Jakob disease-infected brains are infectious and show specific mRNA activation profiles. J. Virol. 76:10905-10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann, N., and D. Pham-Dinh. 2001. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 81:871-927. [DOI] [PubMed] [Google Scholar]
- 7.Bethea, J. R., M. Castro, R. W. Keane, T. T. Lee, W. D. Dietrich, and R. P. Yezierski. 1998. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J. Neurosci. 18:3251-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betmouni, S., and V. H. Perry. 1999. The acute inflammatory response in CNS following injection of prion brain homogenate or normal brain homogenate. Neuropathol. Appl. Neurobiol. 25:20-28. [DOI] [PubMed] [Google Scholar]
- 9.Betmouni, S., V. H. Perry, and J. L. Gordon. 1996. Evidence for an early inflammatory response in the central nervous system of mice with scrapie. Neuroscience 74:1-5. [DOI] [PubMed] [Google Scholar]
- 10.Campbell, I. L., M. Eddleston, P. Kemper, M. B. Oldstone, and M. V. Hobbs. 1994. Activation of cerebral cytokine gene expression and its correlation with onset of reactive astrocyte and acute-phase response gene expression in scrapie. J. Virol. 68:2383-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, Y., and R. A. Swanson. 2003. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 23:137-149. [DOI] [PubMed] [Google Scholar]
- 12.Collinge, J. 1999. Variant Creutzfeldt-Jakob disease. Lancet 354:317-323. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham, C., D. Boche, and V. H. Perry. 2002. Transforming growth factor beta1, the dominant cytokine in murine prion disease: influence on inflammatory cytokine synthesis and alteration of vascular extracellular matrix. Neuropathol. Appl. Neurobiol. 28:107-119. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey, P. W., S. E. Doyle, J. Q. He, and G. Cheng. 2003. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 14:193-209. [DOI] [PubMed] [Google Scholar]
- 15.Dickinson, A. G., V. M. Meikle, and H. Fraser. 1968. Identification of a gene which controls the incubation period of some strains of scrapie agent in mice. J. Comp. Pathol. 78:293-299. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly, R. P., H. Dickensheets, and D. S. Finbloom. 1999. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 19:563-573. [DOI] [PubMed] [Google Scholar]
- 17.Du, Y., and C. F. Dreyfus. 2002. Oligodendrocytes as providers of growth factors. J. Neurosci. Res. 68:647-654. [DOI] [PubMed] [Google Scholar]
- 18.Fallon, P. G., C. L. Emson, P. Smith, and A. N. McKenzie. 2001. IL-13 overexpression predisposes to anaphylaxis following antigen sensitization. J. Immunol. 166:2712-2716. [DOI] [PubMed] [Google Scholar]
- 19.Fischer, M., T. Rulicke, A. Raeber, A. Sailer, M. Moser, B. Oesch, S. Brandner, A. Aguzzi, and C. Weissmann. 1996. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 15:1255-1264. [PMC free article] [PubMed] [Google Scholar]
- 20.Giese, A., D. R. Brown, M. H. Groschup, C. Feldmann, I. Haist, and H. A. Kretzschmar. 1998. Role of microglia in neuronal cell death in prion disease. Brain Pathol. 8:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffith, J. S. 1967. Self-replication and scrapie. Nature 215:1043-1044. [DOI] [PubMed] [Google Scholar]
- 22.Grilli, M., M. Pizzi, M. Memo, and P. Spano. 1996. Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science 274:1383-1385. [DOI] [PubMed] [Google Scholar]
- 23.Hadlow, W. J., R. C. Kennedy, and R. E. Race. 1982. Natural infection of Suffolk sheep with scrapie virus. J. Infect. Dis. 146:657-664. [DOI] [PubMed] [Google Scholar]
- 24.Hanisch, U. K. 2002. Microglia as a source and target of cytokines. Glia 40:140-155. [DOI] [PubMed] [Google Scholar]
- 25.Hannun, Y. A. 1996. Functions of ceramide in coordinating cellular responses to stress. Science 274:1855-1859. [DOI] [PubMed] [Google Scholar]
- 26.Hsu, H., J. Xiong, and D. V. Goeddel. 1995. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 81:495-504. [DOI] [PubMed] [Google Scholar]
- 27.Jamieson, E., M. Jeffrey, J. W. Ironside, and J. R. Fraser. 2001. Activation of Fas and caspase 3 precedes PrP accumulation in 87V scrapie. Neuroreport 12:3567-3572. [DOI] [PubMed] [Google Scholar]
- 28.Kaltschmidt, C., B. Kaltschmidt, and P. A. Baeuerle. 1995. Stimulation of ionotropic glutamate receptors activates transcription factor NF-kappa B in primary neurons. Proc. Natl. Acad. Sci. USA 92:9618-9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanemoto, K., J. Kawasaki, T. Miyamoto, H. Obayashi, and M. Nishimura. 2000. Interleukin (IL)1beta, IL-1alpha, and IL-1 receptor antagonist gene polymorphisms in patients with temporal lobe epilepsy. Ann. Neurol. 47:571-574. [PubMed] [Google Scholar]
- 30.Kim, J. I., W. K. Ju, J. H. Choi, E. Choi, R. I. Carp, H. M. Wisniewski, and Y. S. Kim. 1999. Expression of cytokine genes and increased nuclear factor-kappa B activity in the brains of scrapie-infected mice. Brain Res. Mol. Brain Res. 73:17-27. [DOI] [PubMed] [Google Scholar]
- 31.Kolesnick, R., and D. W. Golde. 1994. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell 77:325-328. [DOI] [PubMed] [Google Scholar]
- 32.Kordek, R., V. R. Nerurkar, P. P. Liberski, S. Isaacson, R. Yanagihara, and D. C. Gajdusek. 1996. Heightened expression of tumor necrosis factor alpha, interleukin 1 alpha, and glial fibrillary acidic protein in experimental Creutzfeldt-Jakob disease in mice. Proc. Natl. Acad. Sci. USA 93:9754-9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263-274. [DOI] [PubMed] [Google Scholar]
- 34.Kuhn, R., K. Rajewsky, and W. Muller. 1991. Generation and analysis of interleukin-4 deficient mice. Science 254:707-710. [DOI] [PubMed] [Google Scholar]
- 35.Ledeboer, A., J. J. Breve, A. Wierinckx, S. van der Jagt, A. F. Bristow, J. E. Leysen, F. J. Tilders, and A. M. Van Dam. 2002. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur. J. Neurosci. 16:1175-1185. [DOI] [PubMed] [Google Scholar]
- 36.Mabbott, N. A., A. Williams, C. F. Farquhar, M. Pasparakis, G. Kollias, and M. E. Bruce. 2000. Tumor necrosis factor alpha-deficient, but not interleukin-6-deficient, mice resist peripheral infection with scrapie. J. Virol. 74:3338-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manuelidis, L., W. Fritch, and Y. G. Xi. 1997. Evolution of a strain of CJD that induces BSE-like plaques. Science 277:94-98. [DOI] [PubMed] [Google Scholar]
- 38.McGeer, P. L., and E. G. McGeer. 2001. Polymorphisms in inflammatory genes and the risk of Alzheimer disease. Arch. Neurol. 58:1790-1792. [DOI] [PubMed] [Google Scholar]
- 39.McKenzie, G. J., A. Bancroft, R. K. Grencis, and A. N. McKenzie. 1998. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr. Biol. 8:339-342. [DOI] [PubMed] [Google Scholar]
- 40.McKenzie, G. J., P. G. Fallon, C. L. Emson, R. K. Grencis, and A. N. McKenzie. 1999. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J. Exp. Med. 189:1565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minghetti, L., A. Greco, F. Cardone, M. Puopolo, A. Ladogana, S. Almonti, C. Cunningham, V. H. Perry, M. Pocchiari, and G. Levi. 2000. Increased brain synthesis of prostaglandin E2 and F2-isoprostane in human and experimental transmissible spongiform encephalopathies. J. Neuropathol. Exp. Neurol. 59:866-871. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto, S., M. Maki, M. J. Schmitt, M. Hatanaka, and I. M. Verma. 1994. Tumor necrosis factor alpha-induced phosphorylation of I kappa B alpha is a signal for its degradation but not dissociation from NF-kappa B. Proc. Natl. Acad. Sci. USA 91:12740-12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montrasio, F., R. Frigg, M. Glatzel, M. A. Klein, F. Mackay, A. Aguzzi, and C. Weissmann. 2000. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science 288:1257-1259. [DOI] [PubMed] [Google Scholar]
- 44.Muhleisen, H., J. Gehrmann, and R. Meyermann. 1995. Reactive microglia in Creutzfeldt-Jakob disease. Neuropathol. Appl. Neurobiol. 21:505-517. [DOI] [PubMed] [Google Scholar]
- 45.Murphy, C., C. Breen, M. Rogers, and M. Giese. 2001. Interferon gamma and prostaglandin in BSE-infected cattle. Cytokine 13:169-173. [DOI] [PubMed] [Google Scholar]
- 46.Overbergh, L., D. Valckx, M. Waer, and C. Mathieu. 1999. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 11:305-312. [DOI] [PubMed] [Google Scholar]
- 47.Pahan, K., M. Khan, and I. Singh. 2000. Interleukin-10 and interleukin-13 inhibit proinflammatory cytokine-induced ceramide production through the activation of phosphatidylinositol 3-kinase. J. Neurochem. 75:576-582. [DOI] [PubMed] [Google Scholar]
- 48.Pammer, J., H. S. Cross, Y. Frobert, E. Tschachler, and G. Oberhuber. 2000. The pattern of prion-related protein expression in the gastrointestinal tract. Virchows Arch. 436:466-472. [DOI] [PubMed] [Google Scholar]
- 49.Perry, V. H., C. Cunningham, and D. Boche. 2002. Atypical inflammation in the central nervous system in prion disease. Curr. Opin. Neurol. 15:349-354. [DOI] [PubMed] [Google Scholar]
- 50.Prusiner, S. B. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136-144. [DOI] [PubMed] [Google Scholar]
- 51.Raivich, G., M. Bohatschek, C. U. Kloss, A. Werner, L. L. Jones, and G. W. Kreutzberg. 1999. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res. Brain Res. Rev. 30:77-105. [DOI] [PubMed] [Google Scholar]
- 52.Sasaki, A., J. Hirato, and Y. Nakazato. 1993. Immunohistochemical study of microglia in the Creutzfeldt-Jakob diseased brain. Acta Neuropathol. (Berlin) 86:337-344. [DOI] [PubMed] [Google Scholar]
- 53.Sawada, M., A. Suzumura, H. Hosoya, T. Marunouchi, and T. Nagatsu. 1999. Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J. Neurochem. 72:1466-1471. [DOI] [PubMed] [Google Scholar]
- 54.Schluter, D., N. Kaefer, H. Hof, O. D. Wiestler, and M. Deckert-Schluter. 1997. Expression pattern and cellular origin of cytokines in the normal and Toxoplasma gondii-infected murine brain. Am. J. Pathol. 150:1021-1035. [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider, A., A. Martin-Villalba, F. Weih, J. Vogel, T. Wirth, and M. Schwaninger. 1999. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat. Med. 5:554-559. [DOI] [PubMed] [Google Scholar]
- 56.Strle, K., J. H. Zhou, W. H. Shen, S. R. Broussard, R. W. Johnson, G. G. Freund, R. Dantzer, and K. W. Kelley. 2001. Interleukin-10 in the brain. Crit. Rev. Immunol. 21:427-449. [PubMed] [Google Scholar]
- 57.Suarez, A., P. Castro, R. Alonso, L. Mozo, and C. Gutierrez. 2003. Interindividual variations in constitutive interleukin-10 messenger RNA and protein levels and their association with genetic polymorphisms. Transplantation 75:711-717. [DOI] [PubMed] [Google Scholar]
- 58.Thackray, A. M., M. A. Klein, A. Aguzzi, and R. Bujdoso. 2002. Chronic subclinical prion disease induced by low-dose inoculum. J. Virol. 76:2510-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thackray, A. M., M. A. Klein, and R. Bujdoso. 2003. Subclinical prion disease induced by oral inoculation. J. Virol. 77:7991-7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venters, H. D., S. R. Broussard, J. H. Zhou, R. M. Bluthe, G. G. Freund, R. W. Johnson, R. Dantzer, and K. W. Kelley. 2001. Tumor necrosis factor(alpha) and insulin-like growth factor-I in the brain: is the whole greater than the sum of its parts? J. Neuroimmunol. 119:151-165. [DOI] [PubMed] [Google Scholar]
- 61.Walsh, D. T., V. H. Perry, and L. Minghetti. 2000. Cyclooxygenase-2 is highly expressed in microglial-like cells in a murine model of prion disease. Glia 29:392-396. [PubMed] [Google Scholar]
- 62.Wang, P., P. Wu, M. I. Siegel, R. W. Egan, and M. M. Billah. 1995. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J. Biol. Chem. 270:9558-9563. [DOI] [PubMed] [Google Scholar]
- 63.Williams, A., A. M. Van Dam, D. Ritchie, P. Eikelenboom, and H. Fraser. 1997. Immunocytochemical appearance of cytokines, prostaglandin E2 and lipocortin-1 in the CNS during the incubation period of murine scrapie correlates with progressive PrP accumulations. Brain Res. 754:171-180. [DOI] [PubMed] [Google Scholar]
- 64.Williams, A. E., L. J. Lawson, V. H. Perry, and H. Fraser. 1994. Characterization of the microglial response in murine scrapie. Neuropathol. Appl. Neurobiol. 20:47-55. [DOI] [PubMed] [Google Scholar]
- 65.Wynn, T. A. 2003. IL-13 effector functions. Annu. Rev. Immunol. 21:425-456. [DOI] [PubMed] [Google Scholar]
- 66.Yang, L., K. Lindholm, Y. Konishi, R. Li, and Y. Shen. 2002. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J. Neurosci. 22:3025-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]