Abstract
Background
Treatment of sickle cell anemia is a challenging task and despite the well understood genetic and biochemical pathway of sickle hemoglobin, current therapy continues to be limited to the symptomatic treatment of pain, supplemental oxygen, antibiotics, red blood cell transfusions and hydroxyurea. SANGUINATE is a carbon monoxide releasing molecule and oxygen transfer agent under clinical development for the treatment of sickle cell anemia and comorbidities.
Methods
An open-label randomized Phase Ib study was performed in adult sickle cell anemia patients. Two dose levels of SANGUINATE were compared to hydroxyurea in 24 homozygotes for Hb SS. Twelve subjects received either a low dose (160 mg/kg) of SANGUINATE or 15 mg/kg hydroxyurea. Another 12 subjects received either a high dose (320 mg/kg) of SANGUINATE or 15 mg/kg hydroxyurea. The primary endpoint was the safety of SANGUINATE versus hydroxyurea in sickle cell anemia patients. Secondary endpoints included determination of the plasma pharmacokinetics and assessment of hematologic measurements.
Results
Musculoskeletal related adverse events were the most common. Transient troponin I levels increased in three patients, one of whom had an increase in tricuspid regurgitant velocity; however, no clinical signs were noted. Following an assessment of vital signs, tricuspid regurgitant velocity, electrocardiogram, serum biochemistry, hematology, urinalysis, and analysis of reported adverse events, SANGUINATE was found to be safe in stable sickle cell anemia patients.
Conclusions
The clinical trial met its primary objective of demonstrating an acceptable safety profile for SANGUINATE in patients with sickle cell anemia. This trial established the safety of SANGUINATE at both dose levels and permitted its advance to Phase II trials.
Keywords: Sickle cell disease, SANGUINATE, Safety, Clinical trial
Introduction
Treatment of sickle cell anemia (SCA) is a challenging task and there is only one drug, hydroxyurea, approved by the Food and Drug Administration (FDA) of the United States. Pain is the most frequent manifestation of SCA and is thought to be a consequence of vaso-occlusion. Vaso-occlusive crises are the primary cause of frequent hospitalization of SCA patients and are a major cause of death in adult SCA patients.1, 2 Despite the well-understood genetic and biochemical pathway of sickle hemoglobin and many discoveries of biomarkers of sickle cell pathophysiology, modern-day therapy continues to be limited to symptomatic treatment of pain, supplemental oxygen, antibiotics therapy, red blood cell transfusions, and hydroxyurea. SCA is an inherited blood disorder caused by a defective hemoglobin protein. The polymerization of sickle hemoglobin is associated with many abnormal downstream processes, but no single pathway has been shown to play a primary or critical role in complications occurring in SCA patients. This cascade of events is responsible for the development of comorbidities associated with SCA such as vaso-occlusion, stroke, leg ulcers, and acute chest syndrome.
SANGUINATE (pegylated bovine carboxyhemoglobin) represents a novel approach to treating acute exacerbations of SCA by targeting the underlying inflammation and causes of hypoxia. SANGUINATE is a carbon monoxide releasing/oxygen transfer agent being developed for the treatment of anemic and ischemic hypoxia that result from congenital or acquired hemoglobinopathies and vasculopathies, such as SCA and thalassemia, and from cerebrovascular or peripheral vascular diseases.
As SANGUINATE contains a hemoglobin core, there are particular concerns regarding potential vasoactivity. Trials performed with previous hemoglobin-based oxygen carriers reported adverse events such as hypertension, cardiac arrhythmias/conduction disorders, gastrointestinal symptoms, abnormal liver function tests and hemorrhage/anemia.3 The underlying pathophysiological mechanisms for these cardiac effects are not precisely known but they have been attributed to the scavenging of nitric oxide.4 SANGUINATE, due to the modification by polyethylene glycol and the anti-vasoconstrictive activity of carbon monoxide, has demonstrated no vasoactivity in vivo.5 An ascending dose study of three cohorts of eight healthy volunteers found no serious adverse events at doses of 80, 120 or 160 mg/kg.6 This Phase Ib trial revealed no serious treatment-related adverse effects and showed dose-proportional pharmacokinetics. As SCA patients suffer from inflammation and anemia, which cause a number of comorbidities, an open label, randomized Phase Ib trial was undertaken to ensure the safety of SANGUINATE in clinically stable patients who are homozygous for SCA.
Methods
This open label randomized Phase Ib trial was conducted in two countries and four medical centers in Central and South America. These studies were conducted in compliance with the US Food and Drug Administration regulations. The trial was registered as NCT01848925. Protocols were designed together with the investigators. Approvals were granted by the Ethics Committees and Regulatory Authorities. The authors had access to primary clinical trial data.
Adult patients with confirmed SCA were enrolled. Eligibility criteria included age of 18 years or older, baseline hemoglobin level >6 or <10 g/dL, taking hydroxyurea or not, but must have been dose stabilized for at least three months and able to discontinue hydroxyurea for seven days prior to randomization. Female patients of childbearing age were required to use a medically acceptable form of contraception during both studies and negative serum pregnancy tests were required at enrollment and prior to infusion. Patient exclusion criteria included individuals on a chronic transfusion program (defined as regular transfusions every 2–8 weeks), acute chest syndrome, serious infections, allergies to hydroxyurea, history of clinically significant diseases and electrocardiogram (ECG) abnormalities. Moreover patients were excluded if they had had more than six Emergency Room visits/hospitalizations per year for SCA-related pain events, renal or liver dysfunction, troponin I >0.31 ng/mL, amylase or lipase >1.1 × upper limit of normal, treatment with investigational drug within 60 days or intention to begin new drug therapy during the study period.
Preparation and dosing
SANGUINATE™ (Prolong Pharmaceuticals, LLC, South Plainfield, New Jersey) 40 mg/mL for intravenous infusion was provided in 500 mL ethylene vinyl acetate blood bags which were shipped in heat-sealed Mylar, gas impermeable foil over-pack bags containing an inert gas, which served to protect the product. SANGUINATE was stored under secure conditions in a locked, limited-access refrigerator, at 2–8 °C. Droxia® (hydroxyurea; Bristol Myers Squibb, Princeton NJ) as 100 mg capsules was obtained through a commercial pharmacy and provided unaltered as per the product labeling.
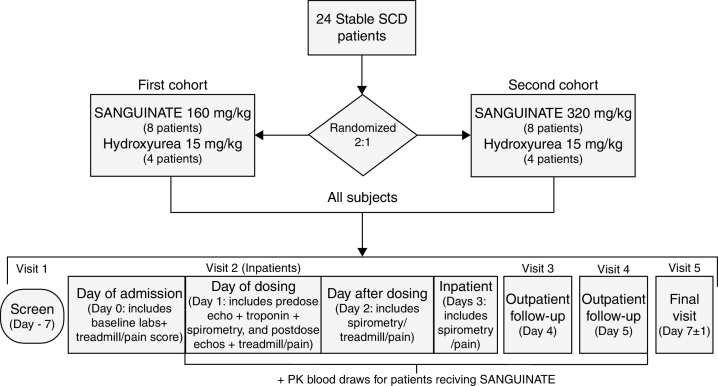
Twenty-four adult SCA patients (Hb SS) were randomized 2:1 to receive, unblinded, either a single 2-h intravenous infusion of SANGUINATE, or a standard dose of hydroxyurea (HU) with 2 h at resting stage. The first 12 patients were to receive either 160 mg/kg of SANGUINATE (eight patients) or 15 mg/kg of hydroxyurea (four patients), and the second 12 patients were to receive either 320 mg/kg of SANGUINATE (eight patients) or 15 mg/kg of hydroxyurea (four patients). The overall study design is presented in Figure 1.
Figure 1.
Overall study design.
Safety assessment
Safety was evaluated throughout the study by assessments over a 7-day period. Patients remained in the study center for a minimum of 48 h after the start of dosing to complete a series of safety assessments including blood and urine lab tests, ECG, and physical exam compared to pre-dosing baseline values. A 24-h urine collection was performed as a measure of dehydration. Urine was collected over 48 h and creatinine clearance was tested to assess kidney function every 24 h. Vital signs as well as evaluations of signs or symptoms of adverse events were conducted periodically by physical exam and patient interview.
Pharmacokinetic assessment
Plasma SANGUINATE concentrations were quantified using a validated high performance liquid chromatography (HPLC) method. Serial blood samples for pharmacokinetic analysis were collected at baseline and at 0.5, 1, 1.5, 2, 4, 6, 8, 12, 24, 36, 48, 72 and 96 h from the start of infusion. Non-compartmental pharmacokinetic methods were used to determine the pharmacokinetic parameters, which included maximum and minimum measured plasma concentration (Cmax and Cmin, respectively), time to reach maximum plasma concentration (tmax), the area under the plasma concentration versus time curve from time zero to the time of the last measurable plasma concentration [area under curve (AUC) 0 → τ], area under the plasma concentration versus time curve from time zero to Infinity (AUC 0 → ∞), terminal half-life (t1/2) and the apparent elimination rate constant (λz).
Results
Demographics and disposition
Overall, the majority of patients were female (15) and of race ‘other’ (23 reported as Hispanic/Latino, and Black or mixed/multiracial). Patients in the 160 mg/kg SANGUINATE treatment group were on average slightly younger than the other treatment groups, but this group also included the oldest patient in the trial (54 years). There was no significant difference in average height and weight between the hydroxyurea-treated and the SANGUINATE-treated patients. There were no remarkable differences between treatment groups in medical history and there were no unexpected findings (not related to SCA) for any patient in the baseline physical examination.
A total of 24 stable SCA patients from clinical centers in Colombia and Panama were enrolled. Of the 24 patients, 22 received their assigned study medication and completed the study as per protocol. Fifteen patients received SANGUINATE and seven patients received hydroxyurea. Two patients discontinued before receiving medications.
Pharmacokinetics
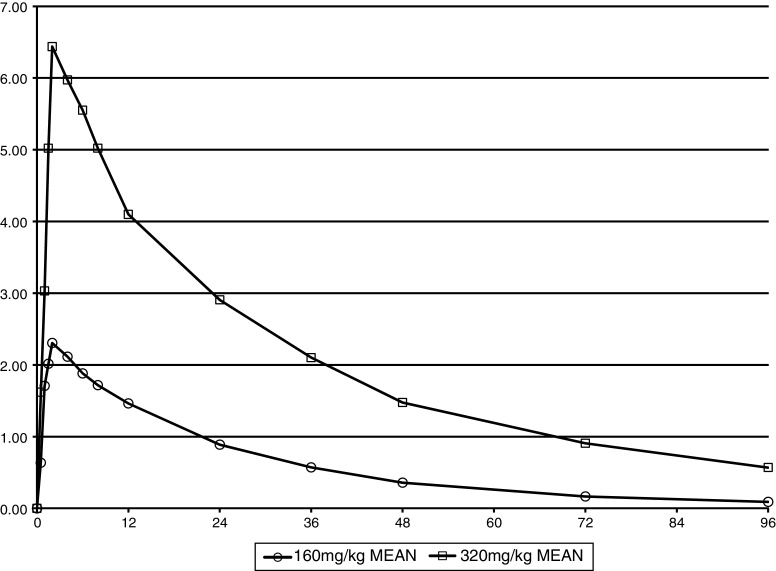
The pharmacokinetics of SANGUINATE were dose-dependent. The mean blood levels of SANGUINATE for the two dose groups are shown in Figure 2. For patients receiving the 160 mg/kg dose of SANGUINATE, the mean peak concentration (Cmax) was 2.59 mg/mL and the mean peak concentration with the 320 mg/kg dose was 6.46 mg/mL. Both maximums occurred at 2 h (completion of the infusion). Doubling the dose from 160 mg/kg to 320 mg/kg led to a mean 150% increase in peak blood level of SANGUINATE in this SCA patient population. In a previous study of healthy subjects receiving a single infusion of 160 mg/kg SANGUINATE, all of the six subjects had blood levels below the level of detection at 96 h after the infusion.6
Figure 2.
Mean plasma concentration of SANGUINATE (mg/mL) by dose.
Safety
More adverse experiences were reported in the SANGUINATE groups than reported in the hydroxyurea groups. Of the 44 reported adverse events in SANGUINATE-treated patients, 16 were from only two of the 15 patients. In addition, 46 of the 51 reported adverse events involving pain (including headache). Musculoskeletal and connective tissue disorder-related adverse events were the most commonly reported, with arthralgia accounting for ten (nine SANGUINATE, one hydroxyurea) of the 51 reports (Table 1).
Table 1.
Summary of adverse events.
| Body system | Hydroxyurea | Sanguinate | Sanguinate |
|---|---|---|---|
| 160 mg/kg | 320 mg/kg | ||
| Cardiac disorders | 0 | 0 | 1 |
| Tricuspid valve incompetence | 0 | 0 | 1 |
| Ear and labyrinth disorders | 0 | 1 | 0 |
| Ear pain | 0 | 1 | 0 |
| Gastrointestinal disorders | 0 | 1 | 4 |
| Abdominal pain | 0 | 0 | 2 |
| Abdominal pain upper | 0 | 0 | 1 |
| Nausea | 0 | 1 | 1 |
| General disorders/administration site conditions | 1 | 2 | 1 |
| Chest pain | 0 | 1 | 0 |
| Feeling cold | 0 | 0 | 1 |
| Infusion site erythema | 0 | 1 | 0 |
| Non-cardiac chest pain | 1 | 0 | 0 |
| Infections and infestations | 1 | 2 | 1 |
| Influenza | 0 | 2 | 0 |
| Upper respiratory tract infection | 0 | 0 | 1 |
| Viral infection | 1 | 0 | 0 |
| Injury/procedural complications | 0 | 1 | 0 |
| Contusion | 0 | 1 | 0 |
| Investigations | 1 | 0 | 1 |
| Oxygen saturation decreased | 1 | 0 | 0 |
| Troponin I increased | 0 | 0 | 1 |
| Musculoskeletal/connective tissue disorders | 3 | 10 | 9 |
| Arthralgia | 1 | 4 | 5 |
| Back pain | 1 | 1 | 0 |
| Bone pain | 0 | 0 | 1 |
| Musculoskeletal chest pain | 0 | 1 | 0 |
| Musculoskeletal pain | 0 | 1 | 2 |
| Neck pain | 0 | 0 | 1 |
| Pain in extremity | 1 | 3 | 0 |
| Nervous system disorders | 1 | 2 | 2 |
| Dizziness | 0 | 0 | 1 |
| Headache | 1 | 2 | 1 |
| Respiratory, thoracic, mediastinal disorders | 0 | 0 | 2 |
| Pulmonary hypertension | 0 | 0 | 1 |
| Tachypnoea | 0 | 0 | 1 |
| Skin and subcutaneous tissue disorders | 0 | 1 | 1 |
| Erythema | 0 | 1 | 0 |
| Pruritus | 0 | 0 | 1 |
| Vascular disorders | 0 | 1 | 1 |
| Endothelial dysfunction | 0 | 0 | 1 |
| Orthostatic hypotension | 0 | 1 | 0 |
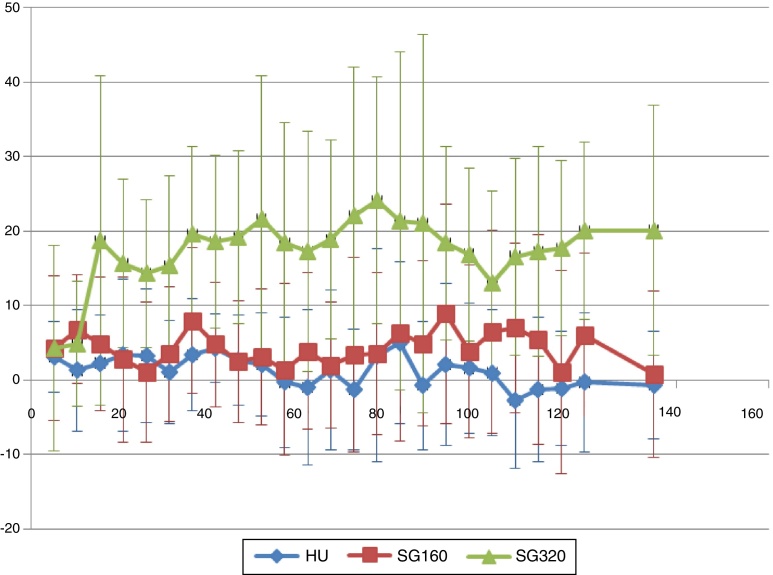
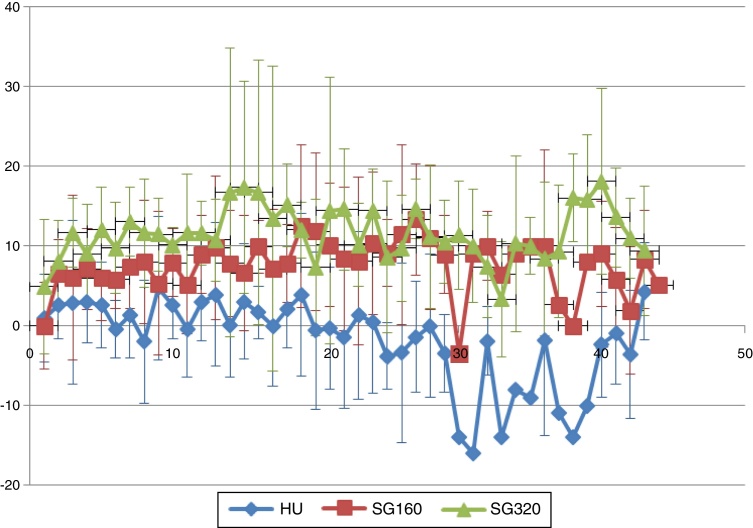
Mean increases in systolic and diastolic arterial blood pressures (transient) were expected following SANGUINATE infusions due to its plasma expansion properties in the bloodstream. These increases do not appear to be dose-dependent. The mean systolic blood pressure following infusion of 320 mg/kg is not appreciably different from the 160 mg/kg group (Figure 3). The chart of mean diastolic pressures (Figure 4) supports the suggestion that the oncotic effect may not be dose-dependent.
Figure 3.
Mean systolic blood pressure.
Figure 4.
Diastolic blood pressure.
Many of the laboratory parameters that were abnormal at baseline remained outside of the normal range for the duration of the study, and for many laboratory parameters, there were no meaningful differences between treatment groups. Mean values for hemoglobin and hematocrit over time show that treatment with SANGUINATE does not provide an appreciable increase in quantity or concentration of hemoglobin in these patients.
However, the levels of direct (conjugated) bilirubin (Table 2) demonstrated a clear mean decrease on the day of dosing relative to baseline levels for the patients receiving SANGUINATE, which was not seen in the hydroxyurea treatment group. Notably, the mean level following infusion of 320 mg/kg SANGUINATE approximates the upper limit of the laboratory's normal range. This mean treatment difference is not apparent in the level of total bilirubin, but is closely mirrored, albeit always within the normal range, in the levels of gamma-glutamyl transpeptidase.
Table 2.
Direct Bilirubin and gamma-glutamyl transpeptidase (GGT).
| Units | Statistic | Hydoxyurea |
Sanguinate (160 mg/kg) |
Sanguinate (320 mg/kg) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline result | Visit result | Difference | Baseline RESULT | Visit result | Difference | Baseline result | Visit result | Difference | |||
| Bilirubin | mg/dL | n | 7 | 7 | 7 | 8 | 8 | 8 | 7 | 7 | 7 |
| Mean | 0.7 | 0.7 | 0 | 1.2 | 0.5 | −0.7 | 0.7 | 0.2 | −0.5 | ||
| Std. dev. | 0.2 | 0.2 | 0.1 | 0.7 | 0.4 | 0.3 | 0.2 | 0.1 | 0.1 | ||
| Median | 0.7 | 0.6 | 0 | 1.2 | 0.4 | −0.7 | 0.6 | 0.2 | −0.4 | ||
| Minimum | 0.3 | 0.5 | −0.2 | 0.4 | 0.1 | −1.2 | 0.5 | 0 | −0.7 | ||
| Maximum | 1.1 | 1.2 | 0.2 | 2.6 | 1.4 | −0.3 | 1 | 0.4 | −0.3 | ||
| GGT | U/L | n | 7 | 7 | 7 | 8 | 8 | 8 | 7 | 7 | 7 |
| Mean | 41 | 40.4 | −0.6 | 55.5 | 33.5 | −22 | 49.1 | 26.6 | −22.6 | ||
| Std. dev. | 29.3 | 30.4 | 1.9 | 53 | 40 | 16.5 | 46.1 | 36.3 | 11.9 | ||
| Median | 42 | 41 | −1 | 35.5 | 22.5 | −19 | 24 | 9 | −17 | ||
| Minimum | 10 | 10 | −3 | 22 | 3 | −59 | 14 | 1 | −42 | ||
| Maximum | 95 | 98 | 3 | 181 | 122 | −4 | 141 | 99 | −11 | ||
The mean results of the chemical urinalysis test for presence of blood in the urine show a treatment-specific (though not apparently dose-specific) difference following infusion with SANGUINATE. The mean red blood cell levels seen upon microscopic analysis approximately mirror this finding, which may be the result of the increased colloid-osmotic pressure produced by the infusion, causing an increase in forced glomerular filtration. A similar result was seen for urinary protein.
There were brief but substantial increases in troponin I levels in three patients receiving SANGUINATE (320 mg/kg) and in one patient receiving HU. The increases were of short duration and not accompanied by any clinically identified or patient reported adverse experiences. One of the three patients with elevated troponin levels also had a reported serious adverse event due to an increase in tricuspid regurgitant velocity (TRV) which was labeled as a sign of moderate pulmonary hypertension; however, associated symptoms of pulmonary hypertension were not present after angiography. The other two patients had baseline values near or above the upper limit of the laboratory reference range for troponin I (0.028 ng/mL). The highest measured level was also the shortest lasting: the serum troponin I level of one patient was assayed at nearly 16-times the upper limit at 1 h after the end of SANGUINATE infusion. Yet, despite reading above the limit at baseline, troponin I was down to the low level of normal (0.007) by the time of the next measurement 5 h later, where it remained for the duration of the study. Because of the short duration, and because it was not accompanied by any clinically-identified or patient-reported adverse experiences, these were not reported by the investigators as serious adverse events. One patient receiving hydroxyurea also had a brief increase in troponin I level (0.045 ng/mL) above the upper limit of the laboratory reference range at 72 h after dosing.
Discussion
The pathobiology of SCA is characterized by inflammation and oxygen deprivation. While hydroxyurea therapy has decreased the painful episodes of SCA, poor compliance and non-response by a significant proportion of SCA patients results in the development of vaso-occlusive crises and other acute comorbidities. SANGUINATE is a novel construct of hemoglobin that contains carbon monoxide and pegylated bovine carboxyhemoglobin. It has been shown to reduce infarct volume in animal models7, 8 and has been administered under multiple emergency Investigational New Drugs (eINDs).9, 10 In vitro studies have demonstrated the ability of SANGUINATE to return SCA blood cells to a more normal morphology11 and demonstrated the transfer of oxygen to hypoxic cells.
This first in-patients study assessed the safety-related effects of SANGUINATE in SCA patients. Since only 15 patients received SANGUINATE and seven patients received hydroxyurea, the size of the study population was too small to allow a calculation of statistical significance between treatment groups. It was observed that many of the laboratory parameters being measured were abnormal at baseline in this population as expected. The damage that has developed in these patients from a lifetime of hemolytic anemia and ischemic hypoxia means that they present an extremely diverse range of possible physiological responses that may be undetectable using the parameters and methods of this study.
The decrease in bilirubin and gamma-glutamyl transpeptidase may be due to the impact of SANGUINATE upon the red blood cell. SANGUINATE has been shown to ‘unsickle’ red blood cells in SCA patients in vitro.12 The improvement in red blood cell morphology and blood flow parameters may reduce hemolysis and thereby impact these biochemical parameters. This hypothesis remains to be confirmed in larger clinical studies.
The clinical significance of the transient increases in troponin I levels seen in some of these patients is also not clear. The transient elevation of troponin I in three of 15 subjects lasted a few hours or a few days, without sequelae or repeated elevations. Current American College of Cardiology Foundation (ACCF)/American Heart association (AHA) guidelines do not define the duration of troponin elevation needed to indicate pathology, and do not recommend their diagnostic or prognostic use alone in heart failure.13 Furthermore, as clarified in the 2007 Joint European Society of Cardiology (ESC)/ACCF/AHA/World Heart Federation (WHF) report “Universal Definition of Myocardial Infarction”, serial measures of cardiac troponin after the onset of clinical symptoms are needed for diagnosis of cardiac ischemia.14 A rapid rise and fall of troponin I does not fit the standard cardiac disease paradigm, and the return of troponin I levels to normal is indicative of response to treatment or spontaneous improvement.
The response of one patient, who received 320 mg/kg SANGUINATE, of multi-day increases in TRV to levels typically associated with moderate-to-severe pulmonary hypertension, substantial increases in systemic blood pressure, and elevated troponin I to nearly 4-fold the upper level of normal, lasting several days, are results which, albeit paradoxical, were associated with no clinically-identified or patient-reported adverse effects. Pulmonary hypertension, defined by persistent TRV values above 3.0 m/s, has been correlated with a significant increased risk of mortality in SCA patients.15 The impact of TRV elevations above 3.0 m/s that last for only a few days, however, occurring in SCA patients with average TRV levels >2.0 m/s has not been determined. Given the lack of clinical adverse effects of the patient, her professed well-being, and the isolated nature of this event, the impact of the reported event on the understanding of risk related to the study drug in SCA patients is very limited.
Infusion of SANGUINATE at both 160 and 320 mg/kg generated an expected increase in arterial pressure due to its colloid osmotic properties, which resolved without apparent sequelae. Mean systolic and diastolic systemic arterial pressure was increased in SANGUINATE-treated patients compared to hydroxyurea-treated patients, but accompanying mean increases in TRV values were not seen in this study. The known transient effect of SANGUINATE on arterial pressure, possibly including pulmonary arterial pressure that was undetectable in this small study, is believed to be due solely to its oncotic effect. The lack of clinically meaningful adverse effects resulting from the pressure increase further supports a transient fluid-volume basis for the mechanism.
Pharmacokinetic results found that SANGUINATE (160 mg/kg) had a prolonged T1/2 in stable SCA patients (19.56 h) compared to that found in healthy volunteers (13.75 h).7 In the Phase I study with healthy volunteers, it was observed that haptoglobin levels were significantly reduced following SANGUINATE administration. Because the core of SANGUINATE is hemoglobin, it has been proposed that the clearance mechanism will be the same as the mechanism used to clear native hemoglobin following hemolysis by the reticuloendothelial system. Because of the widespread hemolysis in SCA patients, this clearance mechanism would be burdened by the patient's extensive cell-free hemoglobin, thus reducing the rate at which this mechanism can clear SANGUINATE, which would lead to a longer circulatory half-life.
Conclusion
This is a first in-patient study of patients with stable SCA with either 160 mg/kg or 320 mg/kg of SANGUINATE. While there were more adverse events in the SANGUINATE arm, they were mild and self-limited. Following assessment of vital signs, echocardiographic measures of TRV, electrocardiogram analysis, laboratory measures of serum biochemistry, hematology and urinalysis, and reported adverse events, no clear evidence of clinically meaningful safety concerns were identified. These results support further development of SANGUINATE for the treatment of SCA comorbidities and initiation of further clinical trials designed to optimize dosing and select appropriate endpoints to assess safety and efficacy.
Funding
This study was supported by research funding from Prolong Pharmaceuticals.
Conflicts of interest
HM, JB, JB and AA are employees of Prolong Pharmaceuticals and Investigators KMG, LFU, ALH and NRS were funded by Prolong Pharmaceuticals.
References
- 1.Perronne V., Roberts-Harewood M., Bachir D., Roudot-Thoraval F., Delord J.M., Thuret I. Patterns of mortality in sickle cell disease in adults in France and England. Hematol J. 2002;3(1):56–60. doi: 10.1038/sj.thj.6200147. [DOI] [PubMed] [Google Scholar]
- 2.Platt O.S., Brambilla D.J., Rosse W.F., Milner P.F., Castro O., Steinberg M.H. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 3.Lanzkron S., Strouse J.J., Wilson R., Beach M.C., Haywood C., Park H. Systematic review: hydroxyurea for the treatment of adults with sickle cell disease. Ann Intern Med. 2008;148(12):939–955. doi: 10.7326/0003-4819-148-12-200806170-00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverman T.A., Weiskopf R.B. Hemoglobin-based oxygen carriers: current status and future directions. Anesthesiology. 2009;111(5):946–963. doi: 10.1097/ALN.0b013e3181ba3c2c. [DOI] [PubMed] [Google Scholar]
- 5.Cabrales P. Examining and mitigating acellular hemoglobin vasoactivity. Antioxid Redox Signal. 2013;18(17):2329–2341. doi: 10.1089/ars.2012.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J., Cao S., Kwansa H., Crafa D., Kibler K.K., Koehler R.C. Transfusion of hemoglobin-based oxygen carriers in the carboxy state is beneficial during transient focal cerebral ischemia. J Appl Physiol. 2012;113(11):1709–1717. doi: 10.1152/japplphysiol.01079.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Misra H., Lickliter J., Kazo F., Abuchowski A. PEGylated carboxyhemoglobin bovine (SANGUINATE): results of a Phase I clinical trial. Artif Organs. 2014;38(8):702–707. doi: 10.1111/aor.12341. [DOI] [PubMed] [Google Scholar]
- 8.Ananthakrishnan R., Li Q., O'Shea K.M., Quadri N., Wang L., Abuchowski A. Carbon monoxide form of PEGylated hemoglobin protects myocardium against ischemia/reperfusion injury in diabetic and normal mice. Artif Cells Nanomed Biotechnol. 2013;41(6):428–436. doi: 10.3109/21691401.2012.762370. [DOI] [PubMed] [Google Scholar]
- 9.Klaus J.A., Kibler K.K., Abuchowski A., Koehler R.C. Early treatment of transient focal cerebral ischemia with bovine PEGylated carboxy hemoglobin transfusion. Artif Cells Blood Substit Immobil Biotechnol. 2010;38(5):223–229. doi: 10.3109/10731199.2010.488635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alaali Y., Ioco E., Varelas P., Abdelhak T., Mastorodimos V., Mendez M. Use of Sanguinate in acute chest syndrome. J Sickle Cell Dis Hemoglobinopat. 2014;1:1. [Google Scholar]
- 11.Parmar D. A case study of SANGUINATE™ in a patient with a comorbidity due to an underlying hemoglobinopathy. J Sickle Cell Dis Hemoglobinopat. 2014;1:2. [Google Scholar]
- 12.Jubin R., Buontemp P., Yglesias R.A., Abuchowski A., Chen Y., Kazo G. 56th ASH annual meeting abstracts and program. 2014. Rapid reversal of red blood cell sickling promoted by PEGylated carboxyhemoglobin bovine gas transfer properties. Abstract 1371. [Google Scholar]
- 13.Misra H, Buontempo P, Buontempo C, Yglesias, R, Chen Y, Jubin R, et al. Anti-inflammatory activity and rapid reversal of sickle cell morphology by PEG-COHb mediated gas transfer in vitro. Presented in HEMO BRAZIL 2015, Brazilian congress of hematology, hemotherapy, and cell therapy. November 20, 2015.
- 14.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Drazner M.H. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K., Alpert J.S., White H.D., Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]