Abstract
Background
Adult T-cell leukemia/lymphoma is a peripheral disease associated with human T-cell lymphotropic virus type 1. Treatment is carried out according to clinical type with watchful waiting being recommended for less aggressive types. Aggressive adult T-cell leukemia/lymphoma is generally treated with chemotherapy and/or antivirals. The objective of this study was to correlate the survival of patients diagnosed in Bahia, Brazil, with the therapeutic approaches employed and to evaluate what issues existed in their treatment processes.
Methods
Eighty-three adult T-cell leukemia/lymphoma patients (26 smoldering, 23 chronic, 16 acute, 13 lymphoma and five primary cutaneous tumoral) with available data were included in this study.
Results
Complete response was achieved in seven smoldering patients with symptomatic treatment, in two with chronic disease using antivirals/chemotherapy, in one with acute disease using antivirals and in one lymphoma using the LSG15 regimen [vincristine, cyclophosphamide, doxorubicin, and prednisolone (VCAP); doxorubicin, ranimustine, and prednisolone (AMP); and vindesine, etoposide, carboplatin, and prednisolone (VECP)]. Smoldering patients who received symptomatic treatment presented longer survival. Favorable chronic patients treated with antivirals presented longer survival compared to the unfavorable subtype. However, for the acute form, first-line chemotherapy was better, albeit without significance, than antivirals. Only one of the patients with lymphoma and primary cutaneous tumors responded.
Conclusions
Watchful waiting associated with phototherapy represents the best option for smoldering adult T-cell leukemia/lymphoma with survival in Bahia being superior to that described in Japan. There was a trend of better results with zidovudine/interferon-alpha in favorable chronic disease. Excellent results were achieved in the lymphoma type treated with the LSG15 protocol. Patients are diagnosed late probably due to lack of knowledge of adult T-cell leukemia/lymphoma by primary healthcare doctors and a Brazilian treatment protocol needs to be established.
Keywords: Adult T-cell leukemia/lymphoma, ATL, Peripheral T-cell leukemia/lymphoma, Human T-cell lymphotropic virus type-1, HTLV-1 infection
Introduction
Human T-cell lymphotropic virus type 1 (HTLV-1) is endemic in southwestern Japan, sub-Saharan Africa, South America and the Caribbean with foci in the Middle East and Australo-Melanesia.1 A seroprevalence study in the general population of Salvador, Bahia, Brazil showed a rate of 1.7% of HTLV-1 infected individuals.2
Although the majority of HTLV-1 carriers remain asymptomatic, around 10% develop serious diseases such as adult T-cell leukemia/lymphoma (ATL), HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), HTLV-1-associated uveitis and infective dermatitis associated with HTLV-1 (IDH).3 ATL is an aggressive lymphoproliferative disease of peripheral T cells characterized by short survival and a poor response to chemotherapy.4
Diagnostic criteria for ATL include positive serology for HTLV-1 and a histologically or cytologically proven peripheral T-cell malignancy. Whenever possible, the HTLV-1 proviral integration analysis should be performed, except in clinically and morphologically straightforward cases when it is unlikely that confirmation of HTLV-1 viral integration is necessary for diagnosis.5, 6 In endemic areas, it is rare that HTLV-1-associated lymphomas do not exist in seropositive patients.5
Due to diverse presentations, ATL is classified into five clinical types: smoldering, chronic, acute, primary cutaneous tumoral (PCT) and lymphoma (Table 1).4, 7
Table 1.
| Forms | Lymphocytosis | Abnormal lymphocytes (%) | LDH levels | Hypercalcemia | Involved organs |
|---|---|---|---|---|---|
| Smolderinga | Absent | <5 or ≥5 | ≤1.5 × N | Absent | With or without skin/lung lesions |
| PCT | Absent | <5 | ≤1.5 × N | Absent | Skin |
| Chronicb | Present | Present | ≤2 × N | Absent | Any organ except bone, GIT and CNS |
| Lymphoma | Absent | ≤1 | Variable | May occur | Lymph nodes and any other organ |
| Acute | Usually present | ≥5 | >1.5 × N | May occur | Any organ |
Subtyped into leukemic (≥5%) and non-leukemic (<5%) according to abnormal lymphocytes percentage.
Subtyped into favorable and unfavorable according to the serum levels of albumin, urea nitrogen, and lactic dehydrogenase (LDH).
PCT: primary cutaneous tumoral; N: normal value; GIT: gastrointestinal tract; CNS: central nervous system.
The most aggressive forms of ATL are the acute, lymphoma, PCT and unfavorable chronic forms. Smoldering and the favorable chronic forms of ATL are less aggressive.5
Difficulty in the treatment of ATL is essentially due to chemotherapy resistance and the immune dysregulation caused by HTLV-1 infection making the patients more susceptible to other infections.8, 9
The treatment is performed according to the clinical form. It is recommended to manage patients with less aggressive forms using supportive care, with a watchful waiting approach or antivirals with zidovudine (AZT) and interferon-alpha (IFN-α) being the most used. In aggressive ATL, patients are generally treated with chemotherapy, antivirals and/or bone marrow transplantation. Other treatment protocols are being tested such as monoclonal antibodies and arsenic trioxide.5
Objective
The aim of this study was to correlate survival with treatment approaches for the five different clinical types in Bahia, Brazil and to evaluate what issues existed in their treatment processes.
Methods
Patient characteristics
This was a cohort study of 83 ATL patients whose data were obtained in an ATL database of the Pathology Department of the University Hospital of the Universidade Federal da Bahia (UFBA). The majority of patients were diagnosed, treated and followed-up in the Hematology, Dermatology and Pathology Departments of the hospital. Most of these patients were dependent on the Brazilian National Health System (NHS), but 21 had health insurance plans and came from private hospitals or outpatient services of Salvador, Bahia for pathological reviews and study admission. Patients were diagnosed according to preexistent criteria.5 In patients with more prolonged survival or with less than 19 years of age, HTLV-1 proviral integration was investigated using Southern blot or long-inverse polymerase chain reaction (PCR)10, 11 and all of them presented monoclonality. All patients were human immunodeficiency virus (HIV) negative.
Initially we had 101 patients diagnosed with ATL but 18 were ineligible for the study due to short survival or short treatment duration (< 1 month). Of the 83 selected patients, 55 lived in Salvador and 28 in the interior of Bahia. Mean disease duration (time elapsed from beginning of symptoms until diagnosis) was 24 months, 54.2% of patients were female, median age was 49.4 years (range: 9–84 years) and there was a predominance of Afro-descendants (88%). The study group was composed of 26 smoldering, 23 chronic (16 favorable and seven unfavorable), 16 acute, 13 lymphoma and five PCT patients. All the smoldering patients were non-leukemic, did not have pulmonary involvement and presented skin lesions. ATL association with HAM/TSP occurred in 14 patients (16.9%).
Treatment
Overall, 33 patients received first-line multiagent chemotherapy alone, 27 patients received first-line antiviral therapy alone, and four patients received chemotherapy associated to antiviral therapy as first-line therapy. Nineteen smoldering ATL patients were initially managed with watchful waiting, phototherapy, corticosteroids or radiotherapy. Phototherapy was made with narrow-band ultraviolet B (Nb-UVB) or psoralen and ultraviolet A (PUVA) applied 2–3 times per week, with an average of 109 sessions (minimum of 60 and maximum of 115). The type of phototherapy was chosen according to the degree of infiltration of the skin lesions. Only three smoldering patients received either multiagent chemotherapy or etoposide alone due to generalized exfoliative erythroderma.
Antiviral therapy consisted of a combination of AZT, produced by the Institute of Pharmaceutical Technology (Farmanguinhos/FIOCRUZ), and IFN-α provided by the NHS for home administration. The type of IFN-α, 2a or 2b, given to patients varied over the years. Currently, IFN-α 2a is manufactured by Sheyang Lo Sunshine Pharmaceutical Co., Ltd (China) and IFN-α 2b by the Institute of Technology in Immunobiology Bio-Manguinhos, FIOCRUZ. Both types are lyophilic intramuscular/subcutaneous injections provided in ampoules with 3,000,000 IU.
Chemotherapy consisted of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and CHOP-like regimens with doses adjusted depending on the clinical condition of the patient; one patient was treated with a Japanese protocol of chemotherapy.12 Patients who evolved with disease progression while using antiviral therapy received chemotherapy. Moreover, patients who evolved with disease progression after first-line chemotherapy received either salvage chemotherapy or salvage antiviral therapy. Only one patient was submitted to bone marrow transplantation.
Clinical and laboratorial exams, as well as imaging tests (ultrasound or computed tomography, X-ray or chest computed tomography) were used to evaluate treatment response.
Statistical analysis
The data were analyzed using the IBM Statistical Program for the Social Sciences (SPSS) Software v20.0. The median survival time (MST) was calculated by the Kaplan–Meier method, and the log-rank method was used to compare the differences between the various groups. For statistical purposes, a p-value <0.05 was considered significant.
Ethical aspects
The Research Ethics Committee (CEP) of the University Hospital of the Universidade Federal da Bahia (UFBA) approved the research project.
Results
First-line treatment
Complete response was achieved in seven smoldering patients with symptomatic treatment, in two favorable chronic patients (one treated with antiviral therapy alone and one with chemotherapy alone), in one acute case treated with antiviral alone and in one lymphoma case treated with a Japanese protocol.12
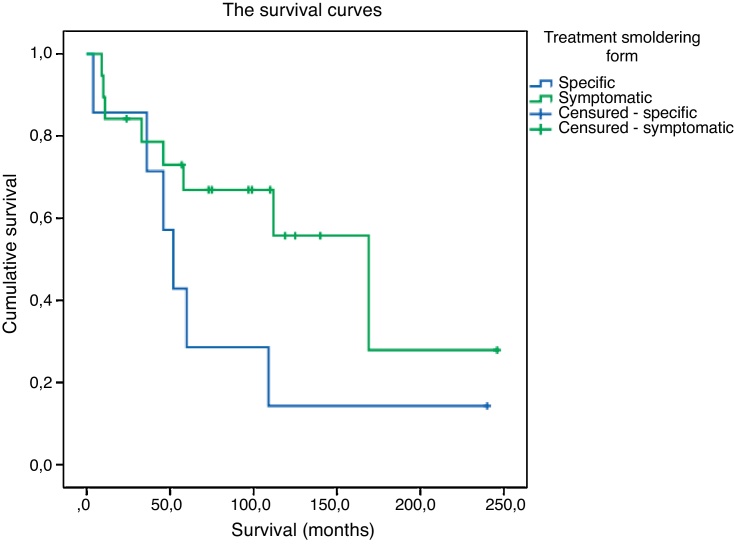
On comparing the 19 smoldering patients who initially were submitted to symptomatic treatment alone, with the seven others who received specific treatment for ATL, the former presented a longer survival (169 months vs.52 months; p-value = 0.1164) (Figure 1). Of those who received symptomatic treatment, only 12 were submitted to phototherapy, seven of whom achieved complete response and four partial response. The smoldering patient who received multiagent chemotherapy had a shorter survival in relation to those who received antivirals or etoposide alone (Table 2). The patient who received etoposide for three months and later AZT/IFN-α or just IFN-α, irregularly for another seven years achieved complete remission with a survival of 240 months. The other patient who received etoposide during 12 months and, due to disease progression, was treated with AZT/IFN-α had partial response and survived 36 months.
Figure 1.
Survival curves of specific and symptomatic treatments for smoldering adult T-cell leukemia/lymphoma.
Table 2.
First-line therapy and survival in 83 patients with adult T-cell leukemia/lymphoma according to clinical type.
| Clinical type | First-line therapy | n | MST |
|---|---|---|---|
| Smoldering | Skin directed therapies | 19 | 169 |
| AZT/IFN-α | 4 | 52 | |
| Chemoa | 1 | 4 | |
| Ectoposide alonea | 2 | 36 | |
| Total | 26 | 109 | |
| Favorable chronic | AZT/IFN-α | 9 | 44 |
| Chemo | 6 | 36 | |
| Chemo + AZT/IFN-α | 1 | 22 | |
| Total | 16 | 42 | |
| Unfavorable chronic | AZT/IFN | 4 | 6 |
| Chemo | 2 | 11 | |
| Chemo + AZT/IFN-α | 1 | 6 | |
| Total | 7 | 10.5 | |
| Acute | AZT/IFN-α | 7 | 6 |
| Chemo | 8 | 11 | |
| Chemo + AZT/IFN-α | 1 | 2 | |
| Total | 16 | 6 | |
| Lymphoma | Chemob | 12 | 7 |
| AZT/IFN-α | 1 | 15 | |
| Total | 13 | 9 | |
| Primary cutaneous tumoral | AZT/IFN-α | 2 | 4 |
| Chemo | 2 | 15 | |
| Chemo + AZT/IFN-α | 1 | 28 | |
| Total | 5 | 20 | |
| Total | 83 | 18 | |
MST: median survival time; AZT: zidovudine; IFN-α: interferon-alpha; Chemo: multiagent chemotherapy.
Erythrodermic patients.
One used the Japanese chemotherapy protocol.
The overall MST was 18 months with the acute, lymphoma, PCT and unfavorable chronic forms having shorter survival (Table 2).
The MST of the favorable chronic patients who received first-line antiviral therapy (44 months) was longer, albeit without statistical significance, to those who received first-line chemotherapy (36 months) or first-line antivirals associated to 22 months of chemotherapy (p-value = 0.465, p-value = 0.0069 and p-value = 0.177, respectively). In contrast, unfavorable chronic patients who received 11 months of first-line chemotherapy had a longer MST, though non-significant, than those who received six months of first-line antivirals or six months of chemotherapy associated with antivirals (p-value = 0.198, p-value = 0.157 and p-value = 0.639).
In the acute form, the MST of the patients who received first-line chemotherapy was superior to those who received simultaneously chemotherapy and AZT/IFN-α, with a statistically significant difference (11 months vs. two months; p-value = 0.005). Comparing the MST of the patients who used only antiviral treatment with those who used only chemotherapy, no statistically significant difference was found (six months vs. 11 months; p-value = 0.109). Notwithstanding, the patient with acute ATL treated during ten months exclusively with AZT/IFN-α achieved a complete response. This patient was submitted to bone marrow transplantation but died with post-transplant complications.
Patients with PCT and lymphoma initially received chemotherapy, AZT/IFN-α or combinations of the two, without response, except for one patient who used six cycles of the Japanese original LSG15 (VCAP-AMP-VECP) regimen. This regimen consists of vincristine, cyclophosphamide, doxorubicin, and prednisolone (VCAP); doxorubicin, ranimustine, and prednisolone (AMP); and vindesine, etoposide, carboplatin, and prednisolone (VECP) with intrathecal administration of methotrexate and prednisone as prophylaxis against central nervous system relapse.12 As ranimustine does not exist in Brazil, it was substituted by carmustine.
Fifteen (65.2%) of the chronic patients progressed to the acute form (71.4% of the unfavorable and 62.5% of the favorable chronic patients). Four patients with the smoldering form progressed to chronic and one to the PCT type. Two patients with lymphoma progressed to the acute form of the disease.
Among the smoldering patients who progressed to the chronic form, one was treated with chemotherapy followed by antiviral treatment and died after 169 months due to cerebral hemorrhage and disease progression.13 A second patient of this group who used only antivirals is still alive after 110 months in complete remission and is refusing further treatment. A third patient has refused specific treatment until now and is alive after 246 months with stable disease.14 Finally, the fourth patient, alive with a survival of 57 months, used phototherapy with complete remission of the skin lesions after five months with this treatment being halted three years ago without relapse. The patient who progressed to the PCT form received antiviral treatment followed by chemotherapy after progression and continued to alternate these treatments until death after 46 months.
Radiotherapy was used in two smoldering patients after progression to the chronic form and PCT with isolated cutaneous lesions. In one case, the irradiated lesions regressed completely leaving scars, even after 20 years of follow-up.14 The other patient had only a partial response, with later progression and death.13
The favorable chronic patients who only took AZT/IFN-α during the course of the disease showed higher MST when compared to those who took AZT/IFN-α followed by chemotherapy and those who took these therapies simultaneously (p-value = 0.016 and p-value = 0.046, respectively). In the unfavorable chronic and acute patients, the comparisons in the sequence of different treatments showed no statistically significant differences.
The mortality was 81.9% (68 cases), with 15 patients alive at the end of the study. The cause of death was due to disease in 72% of the patients, to other causes in 17.6% and to other infections in 7.3%. Deaths due to ATL occurred in 17 (80.9%) chronic (75% of the favorable and 83.3% of the unfavorable subtypes), in 15 (93.75%) acute, in all cases of lymphoma and PCT and in three (11.5%) smoldering patients. Two of the smoldering patients died due to disease progression and the other due to complications of exfoliative erythroderma resistant to treatment.
Discussion
ATL comprises a set of different clinical forms that have different evolutions and therapeutic indications, hence the great importance of their proper classification before any guidance on therapeutic approaches.
In the smoldering form, the MST was superior to what is described in the literature7, 15, 16 which may be due to the use of the classification proposed by Bittencourt et al.,4 which separates patients with tumors or nodules, in a distinct clinical form, the PCT type, unlike Japanese authors.7, 15, 16, 17 The patients with the smoldering form died mostly due to other causes, contrary to what was observed in the other clinical types, including the PCT. In this form, watchful waiting until disease progression was viewed as the best choice, although there was no statistically significant differences in the MST of this group compared to patients treated with specific therapies. This may be due to the greater number of living patients (censored).
The data of this study are consistent with other authors for whom targeted therapies were generally believed unnecessary for the smoldering form and where watchful waiting was considered the best option.15 It should be remembered that 11 of 19 patients managed with watchful waiting until disease progression were submitted to phototherapy, all with a complete or partial response and that this proved to be an effective conduct in the present study. Although watchful waiting is the most recommended in the literature for the smoldering form,5 phototherapy seems to be the best approach as it leads to a complete or partial remission of the cutaneous lesions, as reported by other authors.15
Patients with the smoldering type who received specific treatment had erythroderma and probably this was the reason for phototherapy. Two took etoposide, with disease progression in one and complete remission in the other. Treatment with etoposide associated with phototherapy in the smoldering form is reported to increase survival.15
The second best prognosis was observed in the chronic form. Some Japanese authors classify the smoldering and chronic forms as indolent.16 However, it is important to remember that the chronic form is classified into favorable and unfavorable subtypes, with different survivals and responses to treatment,5 as noted in this study.
The most aggressive forms, lymphoma, acute, unfavorable chronic, and PCT had lower MST, regardless of the therapeutic approaches employed.
Considering the first-line therapy in chronic and acute patients, no significant differences were observed between treatment with antivirals or multiagent chemotherapy. In contrast to this observation, Bazarbachi et al.8 showed that first-line antiviral treatment induces a high rate of remission and prolongs survival in chronic ATL. Notwithstanding, in acute ATL, only the patients with initial complete response presented a prolonged survival (5-year overall survival: 82%)8 as observed in the present study. The worst option for these ATL types was the simultaneous use of antivirals and chemotherapy, probably due to overlapping side effects.
The only patient in this series who underwent bone marrow transplantation died due to post-transplant complications. Although it is a recommended practice for aggressive forms of ATL, mortality is still high.18, 19
The lymphoma ATL patients did not respond to antivirals and chemotherapy except one who used a Japanese protocol of intensive sequenced chemotherapy followed by antiviral therapy.12 This patient is alive in complete remission after 64 months. In Japan, good results have been obtained with this protocol.5
Analyzing the treatment of the favorable chronic patients, they had a higher survival rate with AZT/IFN-α, while the worst response was with the simultaneous use of chemotherapy and antivirals. In the unfavorable chronic form, chemotherapy gave better results, but the small number of cases precluded analysis.
However, it was difficult to analyze the antiviral treatment response because the treatment was very irregular in at least 50% of patients. It is noteworthy that treatment with antiviral drugs is self-administered at home and subject to poor adherence; a significant number of the patients interrupted treatment either due to their own decisions or due to medical advice because of the excessive side effects, particularly with IFN-α. Sometimes, the treatment was temporarily interrupted owing to problems in getting the drugs that were unavailability in the hospital pharmacy as there is a limited amount of medication available or because patients were unable to travel to Salvador to pick up the medications.
Adherence to therapy hindered by the fact that many patients do not live in Salvador, often have poor socioeconomic status and depend on transportation provided by their local governments, plus the side-effects and, perhaps, the lack of understanding about these effects. On the other hand, there is the possibility that patients did not use adequate refrigeration to store the products.
In contrast, the chemotherapy treatment was administered mainly during hospitalization and to a lesser extent, in outpatient hematologic services. In these cases, only medical personnel interrupted treatment due to lack of response, but another treatment plan was soon established. Thus, patients had continued treatment and assistance. Furthermore, most patients who received chemotherapy received a CHOP-like regimen, with reduced dosage, and hence, fewer side effects.
Among other relevant issues, in Brazil, owing to the difficulty of access to healthcare services and to ignorance of the disease by primary care professionals, there was a large gap (average of 25 months) between the onset of symptoms and diagnosis of ATL. Unfortunately, the management guide on infection by HTLV of the Ministry of Health states that multiagent chemotherapy is the only adjuvant treatment in ATL, contradictorily with the recent international literature, and, moreover, does not indicate the best approach for patients with the smoldering form.20
It is noteworthy that cases of the most severe forms, where survival is short, did not always have time to begin therapy as they often died before treatment began or within a month after starting, aspects that reflect both the gravity of ATL and the delay in diagnosis. In the present series, these facts occurred in 15 patients, all of whom had lymphoma and acute ATL.
The small number of cases makes it difficult to compare the treatment of the five forms of ATL, in particular due to the lack of a pre-established treatment protocol and thus various treatment regimens were used.
It is important to emphasize the need for regular monitoring of ATL patients, as some of them progress to more aggressive forms, even during treatment. In Japan, smoldering ATL progresses to the acute form much more frequently than was seen in the current study.15, 16 In the chronic form, a high number of patients (56.2%) of this study progressed to the acute form as is reported in the literature.16
It was observed that smoldering and favorable chronic ATL can have a long survival time, even after discontinuing specific treatment or phototherapy after remission of disease. This observation was curious because it is considered that antiviral treatment does not cure and treatment should be administered for an indefinite period, even after the disappearance of symptoms.21 Even so, these patients should continue being followed-up.
With this paper, we hope to get the attention of the public health organs in order to improve the care of ATL patients through adequate public health policies.
Conclusions
In the smoldering form, the best approach is watchful waiting associated with phototherapy until progression of disease.
In the favorable chronic form, there was a trend of better results with AZT/IFN-α compared to chemotherapy, highlighting the importance of sub-classifying this form.
Although ATL is always considered a serious disease, there are patients who have a long survival with complete remission.
Probably due to the lack of knowledge of this disease by primary healthcare doctors, it takes time for an ATL patient to be diagnosed.
A Brazilian therapeutic protocol for ATL needs to be established. We believe that more attention of the public health organs should be given to ATL in respect to the vertical transmission of HTLV-1 infection in order to reduce this disease in the future.
Funding
This work was supported by Conselho Nacional de Pesquisa (CNPq) and Fundação de Apoio à Pesquisa no Estado da Bahia (FAPESB).
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors are grateful to the dermatologists and oncologists who provided the follow-up data from their patients.
References
- 1.Gessain A., Gout O., Saal F., Daniel M.T., Rio B., Flandrin G. Epidemiology and immunovirology of human T-cell leukemia/lymphoma virus type I-associated adult T-cell leukemia and chronic myelopathies as seen in France. Cancer Res. 1990;50(17 Suppl.):5692S–5696S. [PubMed] [Google Scholar]
- 2.Dourado I., Alcantara L.C., Barreto M.L., da Gloria Teixeira M., Galvão-Castro B. HTLV-I in the general population of Salvador, Brazil: a city with African ethnic and sociodemographic characteristics. J Acquir Immune Defic Syndr. 2003;34(5):527–531. doi: 10.1097/00126334-200312150-00013. [DOI] [PubMed] [Google Scholar]
- 3.Gonçalves D.U., Proietti F.A., Ribas J.G., Araújo M.G., Pinheiro S.R., Guedes A.C. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev. 2010;23(3):577–589. doi: 10.1128/CMR.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bittencourt A.L., Vieira M.G., Brites C.R., Farre L., Barbosa H.S. Adult T-cell leukemia/lymphoma in Bahia. Brazil: analysis of prognostic factors in a group of 70 patients. Am J Clin Pathol. 2007;128(5):875–882. doi: 10.1309/2YGD1P0QCVCWBLDX. [DOI] [PubMed] [Google Scholar]
- 5.Tsukasaki K., Hermine O., Bazarbachi A., Ratner L., Ramos J.C., Harrington W., Jr. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27(3):453–459. doi: 10.1200/JCO.2008.18.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foucar K. Mature T-cell leukemias including T-prolymphocytic leukemia, adult T-cell leukemia/lymphoma, and Sézary syndrome. Am J Clin Pathol. 2007;127(4):496–510. doi: 10.1309/KWJYBCCGTB90B6AE. [DOI] [PubMed] [Google Scholar]
- 7.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87) Br J Haematol. 1991;79(3):428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 8.Bazarbachi A., Plumelle Y., Carlos Ramos J., Tortevoye P., Otrock Z., Taylor G. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28(27):4177–4183. doi: 10.1200/JCO.2010.28.0669. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho E.M., Bacellar O., Porto A.F., Braga S., Galvão-Castro B., Neva F. Cytokine profile and immuno modulation in asymptomatic human T-lymphotropic virus type 1-infected blood donors. J Acquir Immune Defic Syndr. 2001;27(1):1–6. doi: 10.1097/00126334-200105010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kamihira S., Sugahara K., Tsuruda K., Minami S., Uemura A., Akamatsu N. Proviral status of HTLV-1 integrated into the host genomic DNA of adult T-cell leukemia cells. Clin Lab Haematol. 2005;27(4):235–241. doi: 10.1111/j.1365-2257.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- 11.Etoh K., Tamiya S., Yamaguchi K., Okayama A., Tsubouchi H., Ideta T. Persistent clonal proliferation of human T-lymphotropic virus type I-infected cells in vivo. Cancer Res. 1997;57(21):4862–4867. [PubMed] [Google Scholar]
- 12.Kato K., Akashi K. Recent advances in therapeutic approaches for adult T-cell leukemia/lymphoma. Viruses. 2015;7(12):6604–6612. doi: 10.3390/v7122960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bittencourt A.L., Barbosa H.S., Pimenta A., Farré L. A case of adult T-cell leukemia/lymphoma (ATL) with a survival of more than 13 years. Acta Oncol. 2008;47(5):981–983. doi: 10.1080/02841860701704732. [DOI] [PubMed] [Google Scholar]
- 14.Bittencourt A.L., Barbosa H.S., Requião C., Silva A.C., Vandamme A.M., Van Weyenbergh J. Adult T-cell leukemia/lymphoma with a mixed CD4+ and CD8+ phenotype and a indolent course. J Clin Oncol. 2007;25(17):2480–2482. doi: 10.1200/JCO.2007.11.3043. [DOI] [PubMed] [Google Scholar]
- 15.Sawada Y., Hino R., Hama K., Ohmori S., Fueki H., Yamada S. Type of skin eruption is an independent prognostic indicator for adult T-cell leukemia/lymphoma. Blood. 2011;117(15):3961–3967. doi: 10.1182/blood-2010-11-316794. [DOI] [PubMed] [Google Scholar]
- 16.Takasaki Y., Iwanaga M., Imaizumi Y., Tawara M., Joh T., Kohno T. Long-term study of indolent adult T-cell leukemia-lymphoma. Blood. 2010;115(22):4337–4343. doi: 10.1182/blood-2009-09-242347. [DOI] [PubMed] [Google Scholar]
- 17.Katsuya H., Ishitsuka K., Utsunomiya A., Hanada S., Eto T., Moriuchi Y. ATL–Prognostic Index Project. Treatment and survival among 1594 patients with ATL. Blood. 2015;126(24):2570–2577. doi: 10.1182/blood-2015-03-632489. [DOI] [PubMed] [Google Scholar]
- 18.Bazarbachi A., Ghez D., Lepelletier Y., Nasr R., de Thé H., El-Sabban M.E. New therapeutic approaches for adult T-cell leukaemia. Lancet Oncol. 2004;5(11):664–672. doi: 10.1016/S1470-2045(04)01608-0. [DOI] [PubMed] [Google Scholar]
- 19.Ishida T., Hishizawa M., Kato K., Tanosaki R., Fukuda T., Takatsuka Y. Impact of graft-versus-host disease on allogeneic hematopoietic cell transplantation for adult T cell leukemia-lymphoma focusing on preconditioning regimens: nationwide retrospective study. Biol Blood Marrow Transplant. 2013;19(12):1731–1739. doi: 10.1016/j.bbmt.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Ministério da Saúde. Guia de Manejo Clínico da Infecção pelo HTLV. Brasília: Ministério da Saúde; 2013. 80 pp. Available from: http://www.aids.gov.br/sites/default/files/anexos/publicacao/2014/56099/htlv_manual_final_pdf_25082.pdf.
- 21.Marçais A., Suarez F., Sibon D., Frenzel L., Hermine O., Bazarbachi A. Therapeutic options for adult T-cell leukemia/lymphoma. Curr Oncol Rep. 2013;15(5):457–464. doi: 10.1007/s11912-013-0332-6. [DOI] [PubMed] [Google Scholar]