ABSTRACT
Dysregulated pH is a common characteristic of cancer cells, as they have an increased intracellular pH (pHi) and a decreased extracellular pH (pHe) compared with normal cells. Recent work has expanded our knowledge of how dysregulated pH dynamics influences cancer cell behaviors, including proliferation, metastasis, metabolic adaptation and tumorigenesis. Emerging data suggest that the dysregulated pH of cancers enables these specific cell behaviors by altering the structure and function of selective pH-sensitive proteins, termed pH sensors. Recent findings also show that, by blocking pHi increases, cancer cell behaviors can be attenuated. This suggests ion transporter inhibition as an effective therapeutic approach, either singly or in combination with targeted therapies. In this Cell Science at a Glance article and accompanying poster, we highlight the interconnected roles of dysregulated pH dynamics in cancer initiation, progression and adaptation.
KEY WORDS: Cancer, Metabolism, Metastasis, Migration, pH, Proliferation
Summary: Cancer cells have dysregulated pH dynamics and the increased intracellular pH enables a variety of cancer cell behaviors.
Introduction
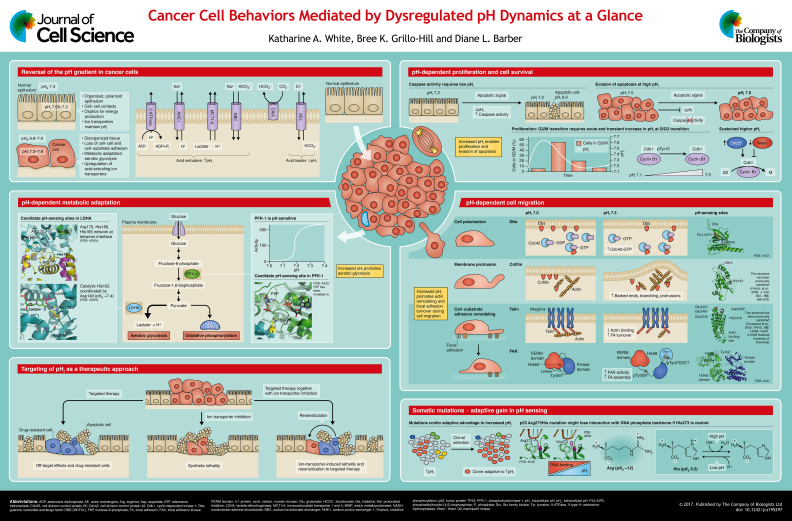
In normal cells, intracellular pH (pHi) is tightly regulated to near-neutral values by ion transport proteins resident in the plasma membrane (Boron, 2004). The activity of these transporters is regulated not only by changes in pHi as a homeostatic mechanism but also by intra- or extra-cellular cues, such as oncogenes (Grillo-Hill et al., 2015; Reshkin et al., 2014), growth factor signaling (Counillon and Pouyssegur, 1995; Clement et al., 2013; Meima et al., 2009; Counillon et al., 2016), metabolic burden (Odunewu and Fliegel, 2013; Wu and Kraut, 2014), hypoxia (Reshkin et al., 2014) and osmolarity (Lacroix et al., 2008; Counillon et al., 2016). In cancer cells, pHi is increased compared to normal cells (∼7.3–7.6 versus ∼7.2), while extracellular pH (pHe) is decreased (∼6.8–7.0 versus ∼7.4; see poster). This reversed pH gradient in cancer cells is an early event in cancer development (Reshkin et al., 2000) and increases during neoplastic progression (Cardone et al., 2005). The higher pHi in cancer cells is paradoxical, considering that metabolic acids are generated through increased metabolism and proliferation; however, an increased pHi is maintained in cancer cells through the increased expression or activity of plasma membrane ion transporters and pHi regulators, including the Na+-H+ exchanger 1 (NHE1) (Cong et al., 2014; Reshkin et al., 2014), carbonic anhydrases (CAs) (Zheng et al., 2015; Gallagher et al., 2015), monocarboxylate transporter 1 and 4 (MCT1 and MCT4, respectively) (Counillon et al., 2016), and Na+-driven HCO3− exchangers (Parks and Pouyssegur, 2015; Lee et al., 2016; Gorbatenko et al., 2014). The dysregulated pH of cancer cells enables cellular processes that are sensitive to small changes in pHi, including cell proliferation, migration and metabolism. These global cell biological effects are produced by the pH-sensitive functions of pH sensors: proteins with activities or ligand-binding affinities that are regulated within the narrow cellular range of pHi dynamics.
In this article and accompanying poster, we describe our current understanding of how the dysregulated pH of cancer enables pH-dependent cancer cell behaviors – with a focus on pHi dynamics. We refer generally to cytosolic pHi, but the pH of various organelles is distinct from cytosolic pH (for review, see Casey et al., 2010) and cytosolic pH can be different in distinct parts of the cell, such as at lamellipodia (Stock et al., 2007) and invadopodia (Beaty et al., 2014). While the decreased pHe of cancer cells will be briefly discussed – as it relates to pHi and its role in enabling cell migration – detailed reviews on how decreased pHe alters cell-matrix remodeling can be found elsewhere (Stock and Schwab, 2009; Brown and Murray, 2015; Yamamoto et al., 2015; Hashim et al., 2011). Where known, we discuss the role of specific pH-sensors in the pHi-dependent cell process and molecular mechanisms for pH sensing. Particular emphasis will be given to how the increased pHi of cancer cells enables their increased proliferation, their ability to evade apoptosis, their migration and invasion, metabolic adaptation, and tumorigenesis. Finally, we briefly present recent advances in limiting cancer progression that were made by inhibiting ion transporters in order to lower pHi, both in clonal cells and animal models, and will propose new views on how altered pHi dynamics may enable adaptive mutations in cancer.
Proliferation and cell survival
From the early work of Pouyssegur and colleagues (Pouyssegur et al., 1984), a higher pHi has been recognized as being a permissive, although not obligatory, signal for increasing cell proliferation. Subsequent work showed that the pHi-dependent proliferative response in cancer cells can be generated by distinct ion transport proteins, including NHE1 (Lauritzen et al., 2012), Na+-driven bicarbonate transporters (Boedtkjer et al., 2013; McIntyre et al., 2016) and the H+/K+-ATPase proton pump (Goh et al., 2014). Additionally, pHi-dependent proliferation has recently been confirmed in vivo (Grillo-Hill et al., 2015).
Although increased pHi acts downstream of growth factor signaling, precisely how it enables proliferation remains to be determined. In normal interphase cells, a transient increase in pHi at the end of S phase in the cell cycle promotes the G2/M transition (Putney and Barber, 2003) (see poster). Blocking this transient increase in pHi attenuates cyclin B levels and maintains the inhibitory phosphorylation of Cdk1 at Tyr15 (Cdk1-pTyr15) (Putney and Barber, 2003). Yet, how the constitutively higher pHi in cancer cells regulates cell cycle progression remains to be determined. One clue comes from a gene array profile of fibroblast cell lines (Putney and Barber, 2004), which showed that a prolonged higher pHi increases the levels of cyclinB1, increases the levels of Cdc25 phosphatase that dephosphorylates inhibitory Cdk1-pTyr15, and decreases the levels of Wee1 kinase that phosphorylates Cdk1-Tyr15. Collectively, these expression changes increase the amount of active Cdk1, thus promoting G2/M entry and transition.
Additionally, apoptotic cells have a decreased pHi (Gottlieb et al., 1996) (see poster). Programmed cell death is triggered by release of cytochrome c from mitochondria, which subsequently activates catabolic enzymes including caspases. Regulated pH dynamics are also observed during this crucial time, with alkalization of mitochondrial matrix pH and subsequent cytosolic acidification (Matsuyama et al., 2000).
Importantly, decreased pHi has been observed in both apoptosis mediated by death receptors and mitochondria (for review, see Lagadic-Gossmann et al., 2004). In death-receptor-mediated apoptosis, decreased pHi is caspase-dependent (Liu et al., 2000) and precedes DNA fragmentation (Gottlieb et al., 1996). In apoptosis mediated by mitochondria, decreased pHi precedes cytochrome c release from the mitochondria (Matsuyama et al., 2000) and occurs even when caspase inhibitors are used (Zanke et al., 1998), which suggests that decreased pHi is an early signal for caspase activation in apoptosis. Supporting this idea, cytochrome-c-mediated activation of caspases requires cytosolic acidification, with highest caspase activity at pH 6.3–6.8 (Matsuyama et al., 2000). It has also been shown that constitutively increased pHi blocks apoptotic signaling as measured by downstream effects on DNA degradation (Perez-Sala et al., 1995). Although pHi dynamics clearly plays a role in apoptosis, more studies are needed to understand which ion transporters are crucial for pHi regulation during apoptosis and whether increased pHi inhibits responses to different apoptotic signals. Another unresolved question is whether pHi dynamics regulates any non-apoptotic cell death pathways.
Metabolic reprogramming
A metabolic shift to increased aerobic glycolysis and reduced mitochondrial oxidative phosphorylation – often referred to as the Warburg effect – is considered a common feature of most cancers and rapidly proliferating cells (see poster). This metabolic reprogramming confers advantages to cancer cells by enhancing their resistance to hypoxia, thereby allowing a fast conversion of nutrients into biomass to enable cell proliferation, and protecting against damaging mitochondrial reactive oxygen species. Most cancers are confirmed to have increased glucose uptake and lactic acid production. Glycolytic flux increases with alkaline pHi (Peak et al., 1992; Miccoli et al., 1996; Dechant et al., 2010; Dietl et al., 2010), which has been speculated but not experimentally confirmed to be partly dependent on the pH-sensitive activity of some glycolytic enzymes (Damaghi et al., 2013; Reshkin et al., 2014), including lactate dehydrogenase and phosphofructokinase-1 (PFK-1).
Increased expression or activity of lactate dehydrogenase A chain (LDHA), the glycolytic enzyme that converts pyruvate to lactate, occurs in highly aggressive metastatic cancers and, when suppressed, decreases tumor growth (Fantin et al., 2006; Le et al., 2010; Xie et al., 2014). LDHA post-translational acetylation of Lys5, which decreases LDHA activity, is reduced in pancreatic tumors and, accordingly, tumor growth decreases when endogenous LDHA is replaced by an acetylation-mimetic mutant (Zhao et al., 2013). Post-translational modification by protonation or deprotonation also regulates LDHA activity, which increases with physiologically higher pHi (Read et al., 2001). A computational program called pHinder (Isom et al., 2013) identified two potential pH-sensing regions in LDHA, where residues have predicted pKa values to be shifted up or down into the physiological range. The first region involves Asp140 (predicted pKa ∼7.4, solution pKa 3.9), which forms an electrostatic bond with the backbone carbon of His192 in the catalytic site. The second potential pH-sensitive region involves solvent-exposed Lys131 (predicted pKa ∼7.9, solution pKa 10.5) and a network of residues that are located at the tetramer interface: Arg170, His180 and His185. However, the molecular mechanisms of pH-sensing by LDHA remain to be determined.
Although increased lactate is a well-characterized feature of highly proliferative cancer cells, the pathways for upstream carbon flow in glucose metabolism remain unclear. Activity of PFK-1, the first rate-limiting enzyme of glycolysis, is known to be pH sensitive, with a >10-fold increase between pH 7.0 and 7.4 (Trivedi and Danforth, 1966; Frieden et al., 1976; Andres et al., 1990). However, previous studies suggest conflicting roles for PFK-1 in cancer. PFK-1 shows increased protein expression in a broad range of cancers, suggesting that higher PFK-1 activity enables cancer cell phenotypes (Moreno-Sanchez et al., 2012). By contrast, PFK-1 glycosylation, which inhibits enzyme activity, is also increased in cancer (Yi et al., 2012). Additionally, we found that several somatic mutations in PFK-1 that have been identified in human cancers inhibit its enzyme activity (Webb et al., 2015). As with LDHA, the molecular mechanisms for pH-dependent PFK-1 activity remain to be determined, currently limiting attempts to resolve the significance of pH sensing by PFK-1 and its role in metabolic reprogramming in cancer cells. To facilitate understanding of how pH dynamics regulates PFK-1 activity, we recently resolved the crystal structure of the platelet isoform of PFK-1 (Webb et al., 2015). Based on this crystal structure, pKa estimates, and molecular dynamics simulations, His208 was identified as a candidate pH-sensing residue. His208 has an upshifted predicted pKa and is conserved in all three PFK-1 isoforms (liver, muscle, platelet). His208 is located at the bottom of the substrate fructose-6-phosphate (F6P)-binding pocket and, when protonated at low pHi, might be able to disrupt the pocket and reduce F6P binding (Webb et al., 2015). In addition to pH-regulated PFK-1 activity, a higher pHi increases the expression of phosphofructokinase-2 (PFK-2) (Putney and Barber, 2004), which generates fructose-2,6-bisphosphate, an allosteric activator of PFK-1. With pH-sensitive enzymes at proximal (PFK-1 and PFK-2) and distal (LDHA) steps of glycolysis, future studies to resolve pH-regulated mechanisms have substantial promise for the development of therapeutic approaches aimed at suppressing metabolic reprogramming and cancer progression.
Migration and metastasis
Abundant evidence indicates that increased pHi is necessary for directed cell migration, including the remodeling of actin filaments and cell-substrate adhesions critical for motile cells (Choi et al., 2010; Frantz et al., 2008; Clement et al., 2013; Denker and Barber, 2002; Meima et al., 2009). Decreased pHe enables cell migration (Stock and Schwab, 2009; Stock et al., 2005) and invasion (Estrella et al., 2013), in part by increasing the activity of acid-activated matrix metalloproteinases (MMPs) that dissolve cell-substrate adhesions (Brown and Murray, 2015; Yamamoto et al., 2015). Evidence suggests that NHE1 is a significant contributor to matrix degradation through local extracellular acidification (Greco et al., 2014) or effects on MMP expression and localization (Lin et al., 2012; Putney and Barber, 2004). Increased pHi resulting from ion transporter activity has been shown to enable cancer cell migration in oncogene-transformed mammary cells (Lauritzen et al., 2012), in patient-derived glioma cell lines (Cong et al., 2014) and in cervical cancer cell lines that do not exhibit hallmarks of metabolic adaptation (De Saedeleer et al., 2014). Furthermore, increased pHi due to higher ion transporter activity has been linked to cell invasion phenotypes (Lin et al., 2012; Grillo-Hill et al., 2015). For a detailed review of which specific ion transporters have been linked to normal and pathological migration effects, see (Stock and Schwab, 2015).
During normal cell migration, dynamic changes in pHi enable both cytoskeletal and focal adhesion remodeling, with increased pHi decreasing the stability of focal adhesions (Srivastava et al., 2008) and increasing overall cell migratory rates (Choi et al., 2010) (see poster). Importantly, decreasing pHi or increasing pHe inhibits cell migration (Parks and Pouyssegur, 2015; Cong et al., 2014; Frantz et al., 2008; Denker and Barber, 2002). We recently described how protonation and deprotonation regulate selective pH sensors involved in cell migration (Schönichen et al., 2013). Through biochemical, molecular dynamics and NMR approaches, we and others have determined the molecular basis for how a higher pHi increases the activity of guanine nucleotide exchange factors (GEFs) for cell polarity (Frantz et al., 2007), cofilin for actin polymerization and membrane protrusion (Pope et al., 2004; Gorbatyuk et al., 2006; Frantz et al., 2008), and talin binding to actin filaments for focal adhesion remodeling (Srivastava et al., 2008; Gingras et al., 2008) (see poster for structures and mechanisms). These proteins have distinct mechanisms for pH regulation, including the deprotonation of His residues at higher pHi, which decreases their binding affinity for negatively charged phosphatidylinositolphosphates (PIPs) in the plasma membrane [His843 in the GEF Dbs (also known as MCF2L), and His133 in cofilin] and allosterically regulated conformational changes (His2418 in talin).
We have recently identified another mechanism of pH sensing through the focal adhesion kinase (FAK) (Choi et al., 2013). FAK includes a four-point-one protein, ezrin, radixin, moesin (FERM) domain for its targeting to focal adhesions and a kinase domain that catalyses activity of downstream kinases, as well as a linker between these two domains containing Tyr397. Increased autophosphorylation of Tyr397 (to pTyr397) is the first step in FAK activation and requires deprotonation of His58 in the FERM domain that occurs above pH 7.4. In this state, protonated His58 maintains a complex electrostatic network that keeps FAK inactive by rendering Tyr397 inaccessible for autophosphorylation. Neutral His58 disrupts this electrostatic network and concomitant conformational changes then allow autophosphorylation of Tyr397. The established roles of FAK in cancer progression (Sulzmaier et al., 2014) make it a promising target for therapeutics designed to selectively maintain His58 in its protonated state.
Importantly, constitutive increases in pHi have been linked to increased migration in cancer cell models (Amith et al., 2015, 2016a; Parks and Pouyssegur, 2015; Lin et al., 2012; Cong et al., 2014), and we have recently shown that increased pHi enables cancer cell invasion in an animal model (Grillo-Hill et al., 2015). Importantly, in these studies, decreasing pHi inhibits the migration or invasion phenotype, suggesting that lowering the pHi of cancer cells as part of cancer therapy can limit metastasis.
Tumorigenesis
As we describe above, dysregulated pHi enables diverse cancer cell behaviors and can be considered a distinguishing feature of cancer. This leads to a growing consensus in the field of cancer research that approaches targeting increased pHi are clinically promising (Neri and Supuran, 2011; Kopecka et al., 2015; Pedersen and Stock, 2013), particularly in cases where other targeted therapies have failed (Gillies et al., 2012; Alfarouk et al., 2015). Supporting this idea, lowering pHi through genetic knockdown or inhibition of ion transporters reduces cell proliferation and migration (Andersen et al., 2016; Amith et al., 2016a; Le Floch et al., 2011), cell invasion (Yang et al., 2010), and suppresses tumorigenesis in xenograft models (Amith et al., 2015; Lagarde et al., 1988; Sonveaux et al., 2008; Colen et al., 2011; Chiche et al., 2012). Additionally, we have recently shown in Drosophila animal models and human clonal cells that, by lowering the pHi, we can induce synthetic lethality with expression of activated oncogenes – notably Raf and Ras – possibly by limiting the efflux of metabolically generated acids (Grillo-Hill et al., 2015). Moreover, increasing pHi in the absence of oncogenes is sufficient to induce hyperproliferation and dysplasia (Grillo-Hill et al., 2015; Petzoldt et al., 2013). One strength of using model organisms, such as flies (Drosophila melanogaster) and zebrafish (Danio rerio), is the ability to use genetic screens to reveal previously unknown signaling pathways and molecular mechanisms that mediate pH-dependent cancer cell behaviors.
Recent work suggests that intratumoral pHi is heterogeneous, which adds to the complexity of studying and interpreting pHi dynamics in xenograft and animal models. Hypoxia has been shown to increase pHi (Reshkin et al., 2014), suggesting that anoxic cells at a tumor center can have a higher pHi than peripheral cells. However, a recent study of rat brain gliomas found that NHE1 and MCT1 are more abundant at the tumor edge (Grillon et al., 2011), although pHi was not determined. Furthermore, increased pHi has recently been proposed to be necessary for epithelial-to-mesenchymal transition (Amith et al., 2016b), and epithelial-to-mesenchymal transition is implicated in initiating metastasis. Finally, we have recently shown that the pHi in stem cells is lower than in differentiated daughter cells (Ulmschneider et al., 2016), which raises the possibility that tumor-initiating cells have a lower pHi than neighboring cells. An important new direction will be to measure intratumoral pHi by using genetically encoded tools, such as tags of pHluorin protein, a GFP variant with near-neutral pKa (Miesenbock, 2012). Another understudied area is how dysregulated pH in stromal cells contributes to maintaining dysregulated tumor pH and supports or enables tumor growth and progression (see Box 1 for a brief review). Mapping spatial differences in intratumoral and stromal pHi could improve our understanding of the heterogeneity of tumor properties and behaviors.
Box 1. Stromal cells and dysregulated pH.
Dysregulated pH can also affect stromal cells including fibroblasts and endothelial cells (for review see Andersen et al., 2014). Metabolic links between tumor cells and stromal cells are well established (for review see Draoui and Feron, 2011). Monocarboxylate transporters (MCTs) have an important role in shuttling lactate produced in tumor cells to stromal cells where it is converted to pyruvate and then processed through oxidative phosphorylation (Koukourakis et al., 2006). Endothelial cells can also take up lactate from the stroma, which can drive angiogenesis (Vegran et al., 2011). Although there are no reports describing a direct measurement of pH in stromal cells in vivo, recent work by Hulikova and colleagues has shown that stromal myofibroblasts can act as proton reservoirs, thereby mitigating extracellular acidification, buffering protons, and transmitting acids across a stromal syncytium (Hulikova et al., 2016). Given the complex interactions between cancer cells and stromal cells, more work is needed to investigate the contributions of stromal cells in enabling or maintaining dysregulated pH in cancer cells.
Future outlook for dysregulated pH dynamics and cancer
Recent work suggests that dysregulated pHi contributes to chemotherapy resistance (Daniel et al., 2013; Alfarouk et al., 2015; Harguindey et al., 2005) and radiotherapy resistance (Huber et al., 2015). Several groups have shown that lowering pHi by suppressing the expression of ion transporters or using selective inhibitors of ion transporters is sufficient to re-sensitize a chemoresistant cell line (Lauritzen et al., 2010; Zheng et al., 2015) or xenografts (Amith et al., 2015; Nath et al., 2015) to chemotherapeutic treatment. Furthermore, targeting increased pHi in combination with other chemotherapeutic drugs holds promise as a combinatorial therapeutic approach (see poster). Synergistic inhibition of 3D colony growth and invadopodia formation has been reported in various pancreatic ductal adenocarcinoma (PDAC) cell lines in response to a combination of NHE1 inhibition and erlotinib, an inhibitor of the epidermal growth factor receptor (EGFR) pathway (Cardone et al., 2015). Additionally, based on findings that acidic pHe attenuates immune response (Lardner, 2001), neutralizing pHe has recently been shown to improve responses to cancer immunotherapies (Pilon-Thomas et al., 2016).
These promising results, and the variety of cell lines, tumor types and animal models in which inhibition of ion transporters has been successful, suggest that clinical applications are more generally applicable than other targeted therapies. Future work might reveal new combination therapies that use treatments targeted towards a genetic signature or histological identity in combination with general ion transporter inhibitors. Moreover, designing therapeutics that alter the protonation state of pH sensors regulating cancer cell behaviors is a promising, but challenging, future direction.
Finally, ongoing work in our lab investigates a new idea with substantial impact: that some recurrent mutations are adaptive to the increased pHi of cancer cells. Of particular interest are Arg→His and His→Arg mutations. Arg residues, with a pKa of ∼12, would be constitutively charged; however, His residues have a pKa of ∼6.5 and might be able to titrate between the higher pHi of cancer cells and the lower pHi of untransformed cells. One example is the recurrent Arg273His mutation in p53 (also known as TP53). In wild-type p53, Arg273 forms electrostatic interactions with the DNA phosphate backbone, which might be retained by protonated His273 but not neutral His273 (Joerger et al., 2005) (see poster). Other examples include the EGFR-Arg776His mutation for gain in pH sensing, and PIK3CA-His1047Arg for loss of pH sensing. Determining whether changes in the pH-sensitive function of mutant proteins can confer a fitness advantage to the higher pHi of cancer cells might improve our understanding of how mutations drive cancer phenotypes.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was support by the National Institutes of Health [grant numbers: CA177085 (to K.A.W.) and GM116384 (to D.L.B.)]. Deposited in PMC for release after 12 months.
Cell science at a glance
A high-resolution version of the poster and individual poster panels are available for downloading at http://jcs.biologists.org/lookup/doi/10.1242/jcs.195297.supplemental
References
- Alfarouk K. O., Stock C.-M., Taylor S., Walsh M., Muddathir A. K., Verduzco D., Bashir A. H. H., Mohammed O. Y., Elhassan G. O., Harguindey S. et al. (2015). Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. 15, 71 10.1186/s12935-015-0221-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amith S. R., Wilkinson J. M., Baksh S. and Fliegel L. (2015). The Na(+)/H(+) exchanger (NHE1) as a novel co-adjuvant target in paclitaxel therapy of triple-negative breast cancer cells. Oncotarget 6, 1262-1275. 10.18632/oncotarget.2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amith S. R., Wilkinson J. M. and Fliegel L. (2016a). KR-33028, a potent inhibitor of the Na+/H+ exchanger NHE1, suppresses metastatic potential of triple-negative breast cancer cells. Biochem. Pharmacol. 118, 31-39. 10.1016/j.bcp.2016.08.010 [DOI] [PubMed] [Google Scholar]
- Amith S. R., Wilkinson J. M. and Fliegel L. (2016b). Na+/H+ exchanger NHE1 regulation modulates metastatic potential and epithelial-mesenchymal transition of triple-negative breast cancer cells. Oncotarget 7, 21091-21113. 10.18632/oncotarget.8520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen A. P., Moreira J. M. and Pedersen S. F. (2014). Interactions of ion transporters and channels with cancer cell metabolism and the tumour microenvironment. pHilos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130098 10.1098/rstb.2013.0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen A. P., Flinck M., Oernbo E. K., Pedersen N. B., Viuff B. M. and Pedersen S. F. (2016). Roles of acid-extruding ion transporters in regulation of breast cancer cell growth in a 3-dimensional microenvironment. Mol. Cancer 15, 45 10.1186/s12943-016-0528-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres V., Carreras J. and Cussó R. (1990). Regulation of muscle phosphofructokinase by physiological concentrations of bisphosphorylated hexoses: effect of alkalinization. Biochem. Biophys. Res. Commun. 172, 328-334. 10.1016/S0006-291X(05)80213-X [DOI] [PubMed] [Google Scholar]
- Beaty B. T., Wang Y., Bravo-Cordero J. J., Sharma V. P., Miskolci V., Hodgson L. and Condeelis J. (2014). Talin regulates moesin-NHE-1 recruitment to invadopodia and promotes mammary tumor metastasis. J. Cell Biol. 205, 737-751. 10.1083/jcb.201312046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boedtkjer E., Moreira J. M., Mele M., Vahl P., Wielenga V. T., Christiansen P. M., Jensen V. E., Pedersen S. F. and Aalkjaer C. (2013). Contribution of Na+,HCO3(-)-cotransport to cellular pH control in human breast cancer: a role for the breast cancer susceptibility locus NBCn1 (SLC4A7). Int. J. Cancer 132, 1288-1299. 10.1002/ijc.27782 [DOI] [PubMed] [Google Scholar]
- Boron W. F. (2004). Regulation of intracellular pH. Adv. Physiol. Educ. 28, 160-179. 10.1152/advan.00045.2004 [DOI] [PubMed] [Google Scholar]
- Brown G. T. and Murray G. I. (2015). Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 237, 273-281. 10.1002/path.4586 [DOI] [PubMed] [Google Scholar]
- Cardone R. A., Casavola V. and Reshkin S. J. (2005). The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat. Rev. Cancer 5, 786-795. 10.1038/nrc1713 [DOI] [PubMed] [Google Scholar]
- Cardone R. A., Greco M. R., Zeeberg K., Zaccagnino A., Saccomano M., Bellizzi A., Bruns P., Menga M., Pilarsky C., Schwab A. et al. (2015). A novel NHE1-centered signaling cassette drives epidermal growth factor receptor-dependent pancreatic tumor metastasis and is a target for combination therapy. Neoplasia 17, 155-166. 10.1016/j.neo.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. R., Grinstein S. and Orlowski J. (2010). Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 11, 50-61. 10.1038/nrm2820 [DOI] [PubMed] [Google Scholar]
- Chiche J., Le Fur Y., Vilmen C., Frassineti F., Daniel L., Halestrap A. P., Cozzone P. J., Pouysségur J. and Lutz N. W. (2012). In vivo pH in metabolic-defective Ras-transformed fibroblast tumors: key role of the monocarboxylate transporter, MCT4, for inducing an alkaline intracellular pH. Int. J. Cancer 130, 1511-1520. 10.1002/ijc.26125 [DOI] [PubMed] [Google Scholar]
- Choi C.-H., Patel H. and Barber D. L. (2010). Expression of actin-interacting protein 1 suppresses impaired chemotaxis of Dictyostelium cells lacking the Na+-H+ exchanger NHE1. Mol. Biol. Cell 21, 3162-3170. 10.1091/mbc.E09-12-1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C.-H., Webb B. A., Chimenti M. S., Jacobson M. P. and Barber D. L. (2013). pH sensing by FAK-His58 regulates focal adhesion remodeling. J. Cell Biol. 202, 849-859. 10.1083/jcb.201302131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement D. L., Mally S., Stock C., Lethan M., Satir P., Schwab A., Pedersen S. F. and Christensen S. T. (2013). PDGFRalpha signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2-ERK1/2-p90RSK and AKT signaling pathways. J. Cell Sci. 126, 953-965. 10.1242/jcs.116426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colen C. B., Shen Y., Ghoddoussi F., Yu P., Francis T. B., Koch B. J., Monterey M. D., Galloway M. P., Sloan A. E. and Mathupala S. P. (2011). Metabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: an in vivo study. Neoplasia 13, 620-632. 10.1593/neo.11134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong D., Zhu W., Shi Y., Pointer K. B., Clark P. A., Shen H., Kuo J. S., Hu S. and Sun D. (2014). Upregulation of NHE1 protein expression enables glioblastoma cells to escape TMZ-mediated toxicity via increased H(+) extrusion, cell migration and survival. Carcinogenesis 35, 2014-2024. 10.1093/carcin/bgu089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counillon L. and Pouysségur J. (1995). Structure-function studies and molecular regulation of the growth factor activatable sodium-hydrogen exchanger (NHE-1). Cardiovasc. Res. 29, 147-154. 10.1016/0008-6363(96)88562-2 [DOI] [PubMed] [Google Scholar]
- Counillon L., Bouret Y., Marchiq I. and Pouysségur J. (2016). Na(+)/H(+) antiporter (NHE1) and lactate/H(+) symporters (MCTs) in pH homeostasis and cancer metabolism. Biochim. Biophys. Acta 1863, 2465-2480. 10.1016/j.bbamcr.2016.02.018 [DOI] [PubMed] [Google Scholar]
- Damaghi M., Wojtkowiak J. W. and Gillies R. J. (2013). pH sensing and regulation in cancer. Front. Physiol. 4, 370 10.3389/fphys.2013.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C., Bell C., Burton C., Harguindey S., Reshkin S. J. and Rauch C. (2013). The role of proton dynamics in the development and maintenance of multidrug resistance in cancer. Biochim. Biophys. Acta 1832, 606-617. 10.1016/j.bbadis.2013.01.020 [DOI] [PubMed] [Google Scholar]
- Dechant R., Binda M., Lee S. S., Pelet S., Winderickx J. and Peter M. (2010). Cytosolic pH is a second messenger for glucose and regulates the PKA pathway through V-ATPase. EMBO J. 29, 2515-2526. 10.1038/emboj.2010.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker S. P. and Barber D. L. (2002). Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J. Cell Biol. 159, 1087-1096. 10.1083/jcb.200208050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Saedeleer C. J., Porporato P. E., Copetti T., Pérez-Escuredo J., Payen V. L., Brisson L., Feron O. and Sonveaux P. (2014). Glucose deprivation increases monocarboxylate transporter 1 (MCT1) expression and MCT1-dependent tumor cell migration. Oncogene 33, 4060-4068. 10.1038/onc.2013.454 [DOI] [PubMed] [Google Scholar]
- Dietl K., Renner K., Dettmer K., Timischl B., Eberhart K., Dorn C., Hellerbrand C., Kastenberger M., Kunz-Schughart L. A., Oefner P. J. et al. (2010). Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J. Immunol. 184, 1200-1209. 10.4049/jimmunol.0902584 [DOI] [PubMed] [Google Scholar]
- Draoui N. and Feron O. (2011). Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis. Model Mech. 4, 727-732. 10.1242/dmm.007724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrella V., Chen T., Lloyd M., Wojtkowiak J., Cornnell H. H., Ibrahim-Hashim A., Bailey K., Balagurunathan Y., Rothberg J. M., Sloane B. F. et al. (2013). Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 73, 1524-1535. 10.1158/0008-5472.CAN-12-2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin V. R., St-Pierre J. and Leder P. (2006). Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9, 425-434. 10.1016/j.ccr.2006.04.023 [DOI] [PubMed] [Google Scholar]
- Frantz C., Karydis A., Nalbant P., Hahn K. M. and Barber D. L. (2007). Positive feedback between Cdc42 activity and H+ efflux by the Na-H exchanger NHE1 for polarity of migrating cells. J. Cell Biol. 179, 403-410. 10.1083/jcb.200704169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz C., Barreiro G., Dominguez L., Chen X., Eddy R., Condeelis J., Kelly M. J., Jacobson M. P. and Barber D. L. (2008). Cofilin is a pH sensor for actin free barbed end formation: role of phosphoinositide binding. J. Cell Biol. 183, 865-879. 10.1083/jcb.200804161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden C., Gilbert H. R. and Bock P. E. (1976). Phosphofructokinase. III. Correlation of the regulatory kinetic and molecular properties of the rabbit muscle enzyme. J. Biol. Chem. 251, 5644-5647. [PubMed] [Google Scholar]
- Gallagher F. A., Sladen H., Kettunen M. I., Serrao E. M., Rodrigues T. B., Wright A., Gill A. B., Mcguire S., Booth T. C., Boren J. et al. (2015). Carbonic anhydrase activity monitored in vivo by hyperpolarized 13C-magnetic resonance spectroscopy demonstrates its importance for pH regulation in tumors. Cancer Res. 75, 4109-4118. 10.1158/0008-5472.CAN-15-0857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies R. J., Verduzco D. and Gatenby R. A. (2012). Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat. Rev. Cancer 12, 487-493. 10.1038/nrc3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras A. R., Bate N., Goult B. T., Hazelwood L., Canestrelli I., Grossmann J. G., Liu H., Putz N. S., Roberts G. C., Volkmann N. et al. (2008). The structure of the C-terminal actin-binding domain of talin. EMBO J. 27, 458-469. 10.1038/sj.emboj.7601965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh W., Sleptsova-Freidrich I. and Petrovic N. (2014). Use of proton pump inhibitors as adjunct treatment for triple-negative breast cancers. An introductory study. J. Pharm. Pharm. Sci. 17, 439-446. 10.18433/J34608 [DOI] [PubMed] [Google Scholar]
- Gorbatenko A., Olesen C. W., Boedtkjer E. and Pedersen S. F. (2014). Regulation and roles of bicarbonate transporters in cancer. Front. Physiol. 5, 130 10.3389/fphys.2014.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk V. Y., Nosworthy N. J., Robson S. A., Bains N. P. S., Maciejewski M. W., Dos Remedios C. G. and King G. F. (2006). Mapping the phosphoinositide-binding site on chick cofilin explains how PIP2 regulates the cofilin-actin interaction. Mol. Cell 24, 511-522. 10.1016/j.molcel.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Gottlieb R. A., Nordberg J., Skowronski E. and Babior B. M. (1996). Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification. Proc. Natl. Acad. Sci. USA 93, 654-658. 10.1073/pnas.93.2.654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco M. R., Antelmi E., Busco G., Guerra L., Rubino R., Casavola V., Reshkin S. J. and Cardone R. A. (2014). Protease activity at invadopodial focal digestive areas is dependent on NHE1-driven acidic pHe Oncol. Rep. 31, 940-946. 10.3892/or.2013.2923 [DOI] [PubMed] [Google Scholar]
- Grillo-Hill B. K., Choi C., Jimenez-Vidal M. and Barber D. L. (2015). Increased H(+) efflux is sufficient to induce dysplasia and necessary for viability with oncogene expression. Elife 4 10.7554/elife.03270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon E., Farion R., Fablet K., De Waard M., Tse C. M., Donowitz M., Rémy C. and Coles J. A. (2011). The spatial organization of proton and lactate transport in a rat brain tumor. PLoS ONE 6, e17416 10.1371/journal.pone.0017416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harguindey S., Orive G., Luis Pedraz J., Paradiso A. and Reshkin S. J. (2005). The role of pH dynamics and the Na+/H+ antiporter in the etiopathogenesis and treatment of cancer. Two faces of the same coin--one single nature. Biochim. Biophys. Acta 1756, 1-24. 10.1016/j.bbcan.2005.06.004 [DOI] [PubMed] [Google Scholar]
- Hashim A. I., Zhang X., Wojtkowiak J. W., Martinez G. V. and Gillies R. J. (2011). Imaging pH and metastasis. NMR Biomed. 24, 582-591. 10.1002/nbm.1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. M., Butz L., Stegen B., Klumpp L., Klumpp D. and Eckert F. (2015). Role of ion channels in ionizing radiation-induced cell death. Biochim. Biophys. Acta 1848, 2657-2664. 10.1016/j.bbamem.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Hulikova A., Black N., Hsia L.-T., Wilding J., Bodmer W. F. and Swietach P. (2016). Stromal uptake and transmission of acid is a pathway for venting cancer cell-generated acid. Proc. Natl. Acad. Sci. USA 113, E5344-E5353. 10.1073/pnas.1610954113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom D. G., Sridharan V., Baker R., Clement S. T., Smalley D. M. and Dohlman H. G. (2013). Protons as second messenger regulators of G protein signaling. Mol. Cell 51, 531-538. 10.1016/j.molcel.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger A. C., Ang H. C., Veprintsev D. B., Blair C. M. and Fersht A. R. (2005). Structures of p53 cancer mutants and mechanism of rescue by second-site suppressor mutations. J. Biol. Chem. 280, 16030-16037. 10.1074/jbc.M500179200 [DOI] [PubMed] [Google Scholar]
- Kopecka J., Campia I., Jacobs A., Frei A. P., Ghigo D., Wollscheid B. and Riganti C. (2015). Carbonic anhydrase XII is a new therapeutic target to overcome chemoresistance in cancer cells. Oncotarget 6, 6776-6793. 10.18632/oncotarget.2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukourakis M. I., Giatromanolaki A., Harris A. L. and Sivridis E. (2006). Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 66, 632-637. 10.1158/0008-5472.CAN-05-3260 [DOI] [PubMed] [Google Scholar]
- Lacroix J., Poët M., Huc L., Morello V., Djerbi N., Ragno M., Rissel M., Tekpli X., Gounon P., Lagadic-Gossmann D. et al. (2008). Kinetic analysis of the regulation of the Na+/H+ exchanger NHE-1 by osmotic shocks. Biochemistry 47, 13674-13685. 10.1021/bi801368n [DOI] [PubMed] [Google Scholar]
- Lagadic-Gossmann D., Huc L. and Lecureur V. (2004). Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ. 11, 953-961. 10.1038/sj.cdd.4401466 [DOI] [PubMed] [Google Scholar]
- Lagarde A. E., Franchi A. J., Paris S. and Pouysségur J. M. (1988). Effect of mutations affecting Na+: H+ antiport activity on tumorigenic potential of hamster lung fibroblasts. J. Cell. Biochem. 36, 249-260. 10.1002/jcb.240360306 [DOI] [PubMed] [Google Scholar]
- Lardner A. (2001). The effects of extracellular pH on immune function. J. Leukoc. Biol. 69, 522-530. [PubMed] [Google Scholar]
- Lauritzen G., Jensen M. B. F., Boedtkjer E., Dybboe R., Aalkjaer C., Nylandsted J. and Pedersen S. F. (2010). NBCn1 and NHE1 expression and activity in DeltaNErbB2 receptor-expressing MCF-7 breast cancer cells: contributions to pHi regulation and chemotherapy resistance. Exp. Cell Res. 316, 2538-2553. 10.1016/j.yexcr.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Lauritzen G., Stock C.-M., Lemaire J., Lund S. F., Jensen M. F., Damsgaard B., Petersen K. S., Wiwel M., Ronnov-Jessen L., Schwab A. et al. (2012). The Na+/H+ exchanger NHE1, but not the Na+, HCO3(−) cotransporter NBCn1, regulates motility of MCF7 breast cancer cells expressing constitutively active ErbB2. Cancer Lett. 317, 172-183. 10.1016/j.canlet.2011.11.023 [DOI] [PubMed] [Google Scholar]
- Le A., Cooper C. R., Gouw A. M., Dinavahi R., Maitra A., Deck L. M., Royer R. E., Vander Jagt D. L., Semenza G. L. and Dang C. V. (2010). Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA 107, 2037-2042. 10.1073/pnas.0914433107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Axelsen T. V., Andersen A. P., Vahl P., Pedersen S. F. and Boedtkjer E. (2016). Disrupting Na(+), HCO(3)(−)-cotransporter NBCn1 (Slc4a7) delays murine breast cancer development. Oncogene 35, 2112-2122. 10.1038/onc.2015.273 [DOI] [PubMed] [Google Scholar]
- Le Floch R., Chiche J., Marchiq I., Naiken T., Ilc K., Murray C. M., Critchlow S. E., Roux D., Simon M. P. and Pouyssegur J. (2011). CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl. Acad. Sci. USA 108, 16663-16668. 10.1073/pnas.1106123108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Wang J., Jin W., Wang L., Li H., Ma L., Li Q. and Pang T. (2012). NHE1 mediates migration and invasion of HeLa cells via regulating the expression and localization of MT1-MMP. Cell Biochem. Funct. 30, 41-46. 10.1002/cbf.1815 [DOI] [PubMed] [Google Scholar]
- Liu D., Martino G., Thangaraju M., Sharma M., Halwani F., Shen S. H., Patel Y. C. and Srikant C. B. (2000). Caspase-8-mediated intracellular acidification precedes mitochondrial dysfunction in somatostatin-induced apoptosis. J. Biol. Chem. 275, 9244-9250. 10.1074/jbc.275.13.9244 [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Llopis J., Deveraux Q. L., Tsien R. Y. and Reed J. C. (2000). Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat. Cell Biol. 2, 318-325. 10.1038/35014006 [DOI] [PubMed] [Google Scholar]
- Mcintyre A., Hulikova A., Ledaki I., Snell C., Singleton D., Steers G., Seden P., Jones D., Bridges E., Wigfield S. et al. (2016). Disrupting hypoxia-induced bicarbonate transport acidifies tumor cells and suppresses tumor growth. Cancer Res. 76, 3744-3755. 10.1158/0008-5472.CAN-15-1862 [DOI] [PubMed] [Google Scholar]
- Meima M. E., Webb B. A., Witkowska H. E. and Barber D. L. (2009). The sodium-hydrogen exchanger NHE1 is an Akt substrate necessary for actin filament reorganization by growth factors. J. Biol. Chem. 284, 26666-26675. 10.1074/jbc.M109.019448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miccoli L., Oudard S., Sureau F., Poirson F., Dutrillaux B. and Poupon M.-F. (1996). Intracellular pH governs the subcellular distribution of hexokinase in a glioma cell line. Biochem. J. 313, 957-962. 10.1042/bj3130957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenbock G. (2012). Synapto-pHluorins: genetically encoded reporters of synaptic transmission. Cold Spring Harb Protoc. 2012, 213-217. 10.1101/pdb.ip067827 [DOI] [PubMed] [Google Scholar]
- Moreno-Sánchez R., Marin-Hernández A., Gallardo-Pérez J. C., Quezada H., Encalada R., Rodríguez-Enríquez S. and Saavedra E. (2012). Phosphofructokinase type 1 kinetics, isoform expression, and gene polymorphisms in cancer cells. J. Cell. Biochem. 113, 1692-1703. 10.1002/jcb.24039 [DOI] [PubMed] [Google Scholar]
- Nath K., Nelson D. S., Heitjan D. F., Zhou R., Leeper D. B. and Glickson J. D. (2015). Effects of hyperglycemia on lonidamine-induced acidification and de-energization of human melanoma xenografts and sensitization to melphalan. NMR Biomed. 28, 395-403. 10.1002/nbm.3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri D. and Supuran C. T. (2011). Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 10, 767-777. 10.1038/nrd3554 [DOI] [PubMed] [Google Scholar]
- Odunewu A. and Fliegel L. (2013). Acidosis-mediated regulation of the NHE1 isoform of the Na(+)/H(+) exchanger in renal cells. Am. J. Physiol. Renal. Physiol. 305, F370-F381. 10.1152/ajprenal.00598.2012 [DOI] [PubMed] [Google Scholar]
- Parks S. K. and Pouyssegur J. (2015). The Na(+)/HCO3(-) Co-Transporter SLC4A4 Plays a Role in Growth and Migration of Colon and Breast Cancer Cells. J. Cell. Physiol. 230, 1954-1963. 10.1002/jcp.24930 [DOI] [PubMed] [Google Scholar]
- Peak M., Al-Habori M. and Agius L. (1992). Regulation of glycogen synthesis and glycolysis by insulin, pH and cell volume. Interactions between swelling and alkalinization in mediating the effects of insulin. Biochem. J. 282, 797-805. 10.1042/bj2820797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S. F. and Stock C. (2013). Ion channels and transporters in cancer: pathophysiology, regulation, and clinical potential. Cancer Res. 73, 1658-1661. 10.1158/0008-5472.CAN-12-4188 [DOI] [PubMed] [Google Scholar]
- Perez-Sala D., Collado-Escobar D. and Mollinedo F. (1995). Intracellular alkalinization suppresses lovastatin-induced apoptosis in HL-60 cells through the inactivation of a pH-dependent endonuclease. J. Biol. Chem. 270, 6235-6242. 10.1074/jbc.270.11.6235 [DOI] [PubMed] [Google Scholar]
- Petzoldt A. G., Gleixner E. M., Fumagalli A., Vaccari T. and Simons M. (2013). Elevated expression of the V-ATPase C subunit triggers JNK-dependent cell invasion and overgrowth in a Drosophila epithelium. Dis. Model Mech. 6, 689-700. 10.1242/dmm.010660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Thomas S., Kodumudi K. N., El-Kenawi A. E., Russell S., Weber A. M., Luddy K., Damaghi M., Wojtkowiak J. W., Mule J. J., Ibrahim-Hashim A. et al. (2016). Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 76, 1381-1390. 10.1158/0008-5472.CAN-15-1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope B. J., Zierler-Gould K. M., Kuhne R., Weeds A. G. and Ball L. J. (2004). Solution structure of human cofilin: actin binding, pH sensitivity, and relationship to actin-depolymerizing factor. J. Biol. Chem. 279, 4840-4848. 10.1074/jbc.M310148200 [DOI] [PubMed] [Google Scholar]
- Pouyssegur J., Sardet C., Franchi A., L'allemain G. and Paris S. (1984). A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc. Natl. Acad. Sci. USA 81, 4833-4837. 10.1073/pnas.81.15.4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney L. K. and Barber D. L. (2003). Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J. Biol. Chem. 278, 44645-44649. 10.1074/jbc.M308099200 [DOI] [PubMed] [Google Scholar]
- Putney L. K. and Barber D. L. (2004). Expression profile of genes regulated by activity of the Na-H exchanger NHE1. BMC Genomics 5, 46 10.1186/1471-2164-5-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read J. A., Winter V. J., Eszes C. M., Sessions R. B. and Brady R. L. (2001). Structural basis for altered activity of M- and H-isozyme forms of human lactate dehydrogenase. Proteins 43, 175-185. [DOI] [PubMed] [Google Scholar]
- Reshkin S. J., Bellizzi A., Caldeira S., Albarani V., Malanchi I., Poignee M., Alunni-Fabbroni M., Casavola V. and Tommasino M. (2000). Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J. 14, 2185-2197. 10.1096/fj.00-0029com [DOI] [PubMed] [Google Scholar]
- Reshkin S. J., Greco M. R. and Cardone R. A. (2014). Role of pHi, and proton transporters in oncogene-driven neoplastic transformation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130100 10.1098/rstb.2013.0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönichen A., Webb B. A., Jacobson M. P. and Barber D. L. (2013). Considering protonation as a posttranslational modification regulating protein structure and function. Annu. Rev. Biophys. 42, 289-314. 10.1146/annurev-biophys-050511-102349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonveaux P., Vegran F., Schroeder T., Wergin M. C., Verrax J., Rabbani Z. N., De Saedeleer C. J., Kennedy K. M., Diepart C., Jordan B. F. et al. (2008). Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 118, 3930-3942. 10.1172/jci36843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava J., Barreiro G., Groscurth S., Gingras A. R., Goult B. T., Critchley D. R., Kelly M. J. S., Jacobson M. P. and Barber D. L. (2008). Structural model and functional significance of pH-dependent talin-actin binding for focal adhesion remodeling. Proc. Natl. Acad. Sci. USA 105, 14436-14441. 10.1073/pnas.0805163105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock C. and Schwab A. (2009). Protons make tumor cells move like clockwork. Pflugers Arch. 458, 981-992. 10.1007/s00424-009-0677-8 [DOI] [PubMed] [Google Scholar]
- Stock C. and Schwab A. (2015). Ion channels and transporters in metastasis. Biochim. Biophys. Acta 1848, 2638-2646. 10.1016/j.bbamem.2014.11.012 [DOI] [PubMed] [Google Scholar]
- Stock C., Gassner B., Hauck C. R., Arnold H., Mally S., Eble J. A., Dieterich P. and Schwab A. (2005). Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. J. Physiol. 567, 225-238. 10.1113/jphysiol.2005.088344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock C., Mueller M., Kraehling H., Mally S., Noël J., Eder C. and Schwab A. (2007). pH nanoenvironment at the surface of single melanoma cells. Cell. Physiol. Biochem. 20, 679-686. 10.1159/000107550 [DOI] [PubMed] [Google Scholar]
- Sulzmaier F. J., Jean C. and Schlaepfer D. D. (2014). FAK in cancer: mechanistic findings and clinical applications. Nat. Rev. Cancer 14, 598-610. 10.1038/nrc3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi B. and Danforth W. H. (1966). Effect of pH on the kinetics of frog muscle phosphofructokinase. J. Biol. Chem. 241, 4110-4112. [PubMed] [Google Scholar]
- Ulmschneider B., Grillo-Hill B. K., Benitez M., Azimova D., Barber D. L. and Nystul N. G. (2016). Increased intracellular pH is necessary for adult epithelial and embryonic stem cell differentiation. J. Cell Biol. 215, 345-355. 10.1083/jcb.201606042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegran F., Boidot R., Michiels C., Sonveaux P. and Feron O. (2011). Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 71, 2550-2560. 10.1158/0008-5472.CAN-10-2828 [DOI] [PubMed] [Google Scholar]
- Webb B. A., Forouhar F., Szu F. E., Seetharaman J., Tong L. and Barber D. L. (2015). Structures of human phosphofructokinase-1 and atomic basis of cancer-associated mutations. Nature 523, 111-114. 10.1038/nature14405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D. and Kraut J. A. (2014). Role of NHE1 in the cellular dysfunction of acute metabolic acidosis. Am. J. Nephrol. 40, 36-42. 10.1159/000364783 [DOI] [PubMed] [Google Scholar]
- Xie H., Hanai J.-i, Ren J.-G., Kats L., Burgess K., Bhargava P., Signoretti S., Billiard J., Duffy K. J., Grant A. et al. (2014). Targeting lactate dehydrogenase--a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 19, 795-809. 10.1016/j.cmet.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Murphy G. and Troeberg L. (2015). Extracellular regulation of metalloproteinases. Matrix Biol. 44-46, 255-263. 10.1016/j.matbio.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Yang X., Wang D., Dong W., Song Z. and Dou K. (2010). Inhibition of Na(+)/H(+) exchanger 1 by 5-(N-ethyl-N-isopropyl) amiloride reduces hypoxia-induced hepatocellular carcinoma invasion and motility. Cancer Lett. 295, 198-204. 10.1016/j.canlet.2010.03.001 [DOI] [PubMed] [Google Scholar]
- Yi W., Clark P. M., Mason D. E., Keenan M. C., Hill C., Goddard W. A. III, Peters E. C., Driggers E. M. and Hsieh-Wilson L. C. (2012). Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science 337, 975-980. 10.1126/science.1222278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanke B. W., Lee C., Arab S. and Tannock I. F. (1998). Death of tumor cells after intracellular acidification is dependent on stress-activated protein kinases (SAPK/JNK) pathway activation and cannot be inhibited by Bcl-2 expression or interleukin 1beta-converting enzyme inhibition. Cancer Res. 58, 2801-2808. [PubMed] [Google Scholar]
- Zhao D., Zou S.-W., Liu Y., Zhou X., Mo Y., Wang P., Xu Y.-H., Dong B., Xiong Y., Lei Q. Y. et al. (2013). Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell 23, 464-476. 10.1016/j.ccr.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G., Peng C., Jia X., Gu Y., Zhang Z., Deng Y., Wang C., Li N., Yin J., Liu X. et al. (2015). ZEB1 transcriptionally regulated carbonic anhydrase 9 mediates the chemoresistance of tongue cancer via maintaining intracellular pH. Mol. Cancer 14, 84 10.1186/s12943-015-0357-6 [DOI] [PMC free article] [PubMed] [Google Scholar]