Abstract
Objective:
The associations between duration of second stage of labor, pushing time and risk of adverse neonatal outcomes are not fully established. Therefore, we aimed to examine such relationships.
Study design:
A population-based cohort study including 42 539 nulliparous women with singleton infants born in cephalic presentation at ⩾37 gestational weeks, using the Stockholm-Gotland Obstetric Cohort, Sweden, and the Swedish Neonatal Quality Register, 2008 to 2013. Poisson regression was used to analyze estimated adjusted relative risks (RRs), with 95% confidence intervals (CIs). Outcome measures were umbilical artery acidosis (pH <7.05 and base excess <−12), birth asphyxia-related complications (including any of the following conditions: hypoxic ischemic encephalopathy, hypothermia treatment, neonatal seizures, meconium aspiration syndrome or advanced resuscitation after birth) and admission to neonatal intensive care unit (NICU).
Results:
Overall rates of umbilical artery acidosis, birth asphyxia-related complications and admission to NICU were 1.08, 0.63 and 6.42%, respectively. Rate of birth asphyxia-related complications gradually increased with duration of second stage: from 0.42% at <1 h to 1.29% at ≥4 h (adjusted RR 2.46 (95% CI 1.66 to 3.66)). For admission to NICU, corresponding rates were 4.97 and 9.45%, and adjusted RR (95% CI) was 1.80 (95% CI 1.58 to 2.04). Compared with duration of pushing <15 min, a duration of pushing ⩾60 min increased rates of acidosis from 0.57 to 1.69% (adjusted RR 2.55 (95% CI 1.51 to 4.30)).
Conclusion:
Prolonged durations of second stage of labor and pushing are associated with increased RRs of adverse neonatal outcomes. Clinical assessment of fetal well-being is essential when durations of second stage and pushing increases.
Introduction
Second stage of labor is defined as duration from fully dilated cervix until delivery of the infant. It includes the passive phase, with passive descent of the fetal head, and the active phase, also known as expulsive phase, bearing down or pushing. The active phase starts when contractions become expulsive or when the woman actively starts pushing.1 Previously, the definition of prolonged second stage of labor in nulliparous women was >2 h without and 3 h with epidural analgesia.2 However, recent recommendations often include longer durations in some cases, that is, that management is individualized depending on progress of labor, epidural analgesia, fetal position and interventions.1, 3
Optimal obstetric management of second stage is an ongoing challenge to reduce rates of emergency cesarean deliveries and to avoid adverse maternal and neonatal outcomes. With prolonged duration of second stage, the proportion of spontaneous vaginal deliveries decreases and maternal morbidity increases.4, 5, 6, 7, 8, 9, 10 The relation between duration of second stage and risk of neonatal morbidity has been investigated in numerous studies with conflicting results. Several studies found no association between prolonged second stage and neonatal adverse outcomes.5, 8, 9, 11, 12 Other studies demonstrated that prolonged second stage increased risks of admission to neonatal intensive care unit (NICU), birth asphyxia, birth trauma, low 5 min Apgar score, sepsis or perinatal mortality.4, 6, 7, 13, 14
Most studies have investigated the entire second-stage duration and neonatal morbidities. Only a few studies have, with mixed results, investigated neonatal outcomes by duration of pushing.15, 16, 17 A recent large study found a long duration of pushing increased risks of neonatal complications, but the absolute risk differences were low.17 Two systematic reviews concluded that further studies regarding management and duration of second stage are warranted to guide evidence-based clinical practice.18, 19
Using detailed prospectively collected clinical data from 42 539 nulliparous women and their first-born neonates, the study objective was to investigate associations between durations of second stage and pushing phase, and adverse neonatal outcomes, after accounting for maternal characteristics, delivery management and infant characteristics.
Materials and methods
Data sources
Detailed prospectively collected information on maternal, pregnancy, delivery and neonatal infant characteristics were obtained from the population-based database of the Stockholm-Gotland Obstetric Cohort. This area covers about one-fourth of all deliveries in Sweden (~25 000 annual births). The database includes electronically transferred information directly from the electronic medical record system used for all antenatal, delivery and postnatal care units. Births between 1 January 2008 and 31 December 2013 were included.
In order to include detailed information on neonatal outcomes for infants admitted to NICU, we also used information from the Swedish Neonatal Quality Register.20 This register contains prospectively collected data on infants admitted for any level of neonatal care in Sweden, including all neonatal units in the counties of Stockholm and Gotland. We used information on admitted infants born until 31 December 2013. Individual linkage across the registers was made by using the personal identity number assigned to each citizen at birth or immigration to Sweden.21 This study was approved by the regional ethical committee at Karolinska Institutet, Stockholm, Sweden, numbers 2009/275–31 and 2012/365–32.
Study population
From 2008 through 2013, the database included information on 149 298 mothers and their singleton live-born infants. There were 61 794 nulliparous women, that is, women delivering their first-born infant, in cephalic presentation at 37 gestational weeks or later. We excluded elective cesarean deliveries (n=2647), emergency cesareans during first stage of labor (n=6299) and deliveries without labor partograph or notation on complete dilation of the cervix (n=3750). Of the seven delivery units within the Stockholm-Gotland region, one unit did not routinely take umbilical blood samples and deliveries from this unit were therefore excluded (n=6559). The final study population included 42 539 births. In the analyses of the outcome of acidosis, births without arterial cord values for pH or base excess were excluded (n=9110).
Exposures
Information from the labor partograph was used to measure the main exposure, duration of second stage of labor, defined (and recorded) as time, in minutes, from the first notation on complete dilation of the cervix until delivery of the fetus. Duration of second stage was categorized into five groups: 0 to 59 min (<1 h, reference), 60 to 119 min (1 to <2 h), 120 to 179 min (2 to <3 h), 180 to 239 min (3 to <4 h) and ⩾240 min (⩾4 h).
Secondary exposure was duration of pushing, defined as time in minutes from notation of active pushing in the delivery record until delivery. Duration of pushing was categorized into 5 groups: 0 to 14 (reference), 15 to 29, 30 to 44, 45 to 59 and ⩾60 min. In Sweden, the clinical practice is ‘delayed pushing,' that is, to start pushing when the woman either feels a strong urge to push or, in case of lacking urge to push, to actively push when the fetal vertex has reached or almost reached the pelvic floor. According to the National Swedish recommendations, initiation of augmentation with oxytocin is recommended after 30 min of pushing if expected normal progress has ceased.22 If lack of progress thereafter, the clinical routine is to contact an obstetrician after 45 to 60 min.
Combined analyses were also performed where duration of passive phase of second stage, that is, time from notation on fully dilated cervix until start of active pushing, was analysed together with duration of pushing. Passive phase was categorized into: 0 to <1 h, 1 to <2 h, 2 to <3 h and ⩾3 h. Pushing was categorized into: <45 and ⩾45 min. The dichotomization of pushing time in the combined analyses was based on the clinical routines together with the results in the analyses of duration of pushing and adverse neonatal outcomes.
Outcomes
Adverse neonatal outcomes were defined as follows: (a) umbilical artery acidosis, (b) birth asphyxia-related complications and (c) admission to NICU. Information on umbilical artery acidosis was retrieved from the birth record and was defined as a pH value of <7.05 and base excess <−12 mmol l−1 in the umbilical artery.23 If values for either umbilical artery pH or base excess were missing, blood gas samples were considered missing. Neonatal diagnosis were coded using the International Classification of Diseases, tenth revision (ICD-10) at discharge from the delivery hospital and/or the NICU. The diagnoses were retrieved either from the Stockholm-Gotland Obstetric Cohort or the Swedish Neonatal Quality Register. Birth asphyxia-related complications included any of the following diagnoses or procedures: hypoxic ischemic encephalopathy (checkbox in NICU record or ICD-10 diagnosis: P 91.6), hypothermia treatment (checkbox in NICU record or procedure code: DV034), neonatal seizures (checkbox in NICU record or ICD-10 diagnosis: P90.9), meconium aspiration syndrome (checkbox in NICU record or ICD-10 diagnosis: P24.0) and resuscitation in delivery room with heart compressions and/or intubation (checkboxes in birth or NICU records). Diagnoses of infant malformations (ICD-10 diagnoses: Q) were coded in the delivery or NICU records. Admission to NICU was defined as admission to any neonatal unit.
Covariates
The Stockholm-Gotland Obstetric Cohort holds information on maternal characteristics, such as height, body mass index (BMI) and self-reported smoking that were recorded at the first antenatal care visit. Smoking was also self-reported at an antenatal visit in gestational week 30 to 32. Concurrent maternal diseases are reported as check boxes in the standardized antenatal care record or as ICD-10 diagnoses coded during the pregnancy or at discharge from the delivery hospital. Hypertensive diseases include pre-gestational hypertension (ICD-10 codes I10-I15, O10, O11), gestational hypertension (ICD-10 code O13) and preeclampsia (ICD-10 codes O14-O15). Diabetic diseases include pre-gestational (ICD-10 codes E10-E14 and O240-O243) and gestational diabetes (ICD-10 code O244).
Delivery characteristics, such as onset of labor, vaginal examinations (time and cervical dilation), epidural analgesia, use and notation on start of oxytocin for labor augmentation, time of active pushing, mode of delivery and time of birth were obtained from the partograph and from the standardized delivery records.
Information on gestational age was based on the following: (a) date of conception before embryo transfer (4,8%), (b) early second trimester ultrasound offered to all women (93.4%), (c) date of last menstrual period reported at the first antenatal visit (1.8%) and (d) postnatal assessment (<1%). Variables were categorized according to Tables 1a and 1b.
Table 1a. Maternal characteristics and umbilical artery acidosis, birth asphyxia-related complications and admission to NICU.
|
Acidosisa |
Birth asphyxia-related complicationsb |
Admission to NICU |
||||||
|---|---|---|---|---|---|---|---|---|
| Maternal characteristics | N totalc | N | % | N total | N | % | N | % |
| 33 429 | 360 | 1.08 | 42 539 | 269 | 0.63 | 2733 | 6.42 | |
| Age (years) | ||||||||
| <25 | 5980 | 56 | 0.94 | 7721 | 33 | 0.43 | 403 | 5.22 |
| 25–29 | 11 026 | 117 | 1.06 | 14 102 | 78 | 0.55 | 898 | 6.37 |
| 30–34 | 11 557 | 118 | 1.02 | 14 571 | 102 | 0.70 | 936 | 6.42 |
| ⩾35 | 4866 | 69 | 1.42 | 6145 | 56 | 0.91 | 496 | 8.07 |
| Height (cm) | ||||||||
| ⩽154 | 880 | 11 | 1.25 | 1162 | 14 | 1.20 | 93 | 8.00 |
| 155–164 | 11 201 | 146 | 1.30 | 14 331 | 103 | 0.72 | 1002 | 6.99 |
| 165–174 | 17 139 | 171 | 1.00 | 21 739 | 129 | 0.59 | 1 331 | 6.12 |
| ⩾175 | 3880 | 24 | 0.62 | 4890 | 19 | 0.39 | 279 | 5.71 |
| Missing | 329 | 417 | ||||||
| BMI (kg m−2) | ||||||||
| <18.5 | 1113 | 9 | 0.81 | 1 450 | 5 | 0.34 | 66 | 4.55 |
| 18.5–24.9 | 22 789 | 230 | 1.01 | 28 950 | 181 | 0.63 | 1746 | 6.03 |
| 25–29.9 | 6077 | 72 | 1.18 | 7758 | 54 | 0.70 | 572 | 7.37 |
| ⩾30 | 2126 | 30 | 1.41 | 2681 | 23 | 0.86 | 235 | 8.77 |
| Missing | 1324 | 1700 | ||||||
| Daily smoking | ||||||||
| Non-smoker | 31 581 | 349 | 1.11 | 40 198 | 259 | 0.64 | 2613 | 6.50 |
| Smoker | 1807 | 9 | 0.50 | 2287 | 9 | 0.39 | 114 | 4.98 |
| Missing | 41 | 54 | ||||||
| Hypertensive disease | ||||||||
| No | 31 370 | 334 | 1.06 | 39 976 | 249 | 0.62 | 2 516 | 6.29 |
| Pre-gest hypertension | 181 | 2 | 1.10 | 234 | 0 | 0 | 17 | 7.26 |
| Gestational hypertension | 737 | 10 | 1.36 | 916 | 6 | 0.66 | 76 | 8.30 |
| Preeclampsia | 1141 | 14 | 1.23 | 1 413 | 14 | 0.99 | 124 | 8.78 |
| Diabetes | ||||||||
| No | 33 166 | 348 | 1.05 | 42 215 | 263 | 0.62 | 2666 | 6.32 |
| Pre-gestational | 118 | 6 | 5.08 | 147 | 4 | 2.72 | 36 | 24.49 |
| Gestational | 145 | 6 | 4.14 | 177 | 2 | 1.13 | 31 | 17.51 |
Abbreviations: BE, base excess; BMI, body mass index; HIE, hypoxic ischemic encephalopathy; MAS, meconium aspiration syndrome; NICU, neonatal intensive care unit.
Nulliparous women with singleton births, in cephalic presentation at ⩾37 gestational weeks in the Stockholm-Gotland Obstetric Cohort, Sweden 2008–2013.
Acidosis: umbilical artery acidosis, pH <7.05 and BE< −12.
Birth asphyxia-related complications include any of the following conditions: HIE, hypothermia treatment, neonatal seizures, MAS or advanced resuscitation after birth (heart compressions or intubation).
Missing data on pH and/or BE: N=9110.
Table 1b. Delivery and infant characteristics and umbilical artery acidosis, birth asphyxia-related complications and admission to NICU.
|
Acidosisa |
Birth asphyxia-related complicationsb |
Admission to NICU |
||||||
|---|---|---|---|---|---|---|---|---|
| Delivery and infant characteristics | N totalc | N | % | N total | N | % | N | % |
| 33 429 | 360 | 1.08 | 42 539 | 269 | 0.63 | 2733 | 6.42 | |
| Onset of delivery | ||||||||
| Spontaneous | 27 260 | 284 | 1.04 | 34 913 | 209 | 0.60 | 2132 | 6.11 |
| Induction | 6169 | 76 | 1.23 | 7626 | 60 | 0.79 | 601 | 7.88 |
| Oxytocin | ||||||||
| No use | 9258 | 89 | 0.96 | 12 387 | 46 | 0.37 | 584 | 4.71 |
| Use | ||||||||
| Start before retracted cervix | 16 316 | 186 | 1.14 | 20 292 | 170 | 0.84 | 1 509 | 7.44 |
| Start at/after retracted cervix | 7855 | 85 | 1.08 | 9860 | 53 | 0.54 | 640 | 6.49 |
| Start at/after retracted cervix before pushing | 4561 | 46 | 1.01 | 5765 | 28 | 0.49 | 351 | 6.09 |
| Start at/after pushing | 2642 | 29 | 1.10 | 3271 | 16 | 0.49 | 224 | 6.85 |
| Missing information on time for pushing | 652 | 824 | ||||||
| Gestational length at birth (w) | ||||||||
| 37 | 1476 | 7 | 0.47 | 1901 | 6 | 0.32 | 231 | 12.15 |
| 38 | 3410 | 19 | 0.56 | 4391 | 27 | 0.61 | 339 | 7.72 |
| 39 | 7513 | 62 | 0.83 | 9722 | 38 | 0.39 | 521 | 5.36 |
| 40 | 10 655 | 127 | 1.19 | 13 568 | 82 | 0.60 | 790 | 5.82 |
| 41 | 7595 | 101 | 1.33 | 9553 | 88 | 0.92 | 620 | 6.49 |
| ⩾42 | 2780 | 44 | 1.58 | 3 404 | 28 | 0.82 | 232 | 6.82 |
Abbreviations: BE, base excess; HIE, hypoxic ischemic encephalopathy; MAS, meconium aspiration syndrome; NICU, neonatal intensive care unit.
Nulliparous women with singleton births, in cephalic presentation at ⩾37 gestational weeks in the Stockholm-Gotland Obstetric Cohort, Sweden 2008–2013
Acidosis: umbilical artery acidosis, pH <7.05 and BE< −12.
Birth asphyxia-related complications include any of the following conditions: HIE, hypothermia treatment, neonatal seizures, MAS or advanced resuscitation after birth (heart compressions or intubation).
Missing data on pH and/or BE: N=9110.
Statistical analyses
Poisson regression analyses with robust variance, estimated adjusted relative risks (RRs), with 95% confidence intervals.24 All analyses were adjusted for maternal age, height, BMI, smoking, hypertensive and diabetic diseases, onset of labor (spontaneous or induced) and gestational week. Analyses of duration of second stage were also adjusted for oxytocin augmentation that started before fully dilation of the cervix. Analyses of duration of pushing only included vaginal deliveries and were additionally adjusted for oxytocin augmentation that started before pushing and the duration of second stage before pushing (that is, duration of passive phase of second stage). In analyses of duration of pushing, we considered oxytocin augmentation initiated after the beginning of pushing and mode of vaginal delivery to be mediators rather than confounders. Hence, we did not adjust for these factors. Finally, we performed stratified analyses for epidural analgesia and mode of vaginal delivery. Observations with missing values were excluded from the multivariable analysis. Statistical analyses were performed using the SAS software version 9.4.
Availability of data and material
In the ethical approval of the study and in informed consent from the caregivers in Stockholm County Council, we were given access to data to conduct the study but were not given permission to share data. However, statistical analysis code is available on request from the corresponding author.
Results
Information on maternal, delivery and infant characteristics and rates of acidosis, birth asphyxia-related complications, and admission to NICU are summarized in Tables 1a and 1b. Rates of all adverse neonatal outcomes generally increased with maternal age and BMI, and with decreasing maternal stature. Rates were also increased among infants of non-smokers and among mothers with gestational hypertension, preeclampsia and diabetes (Table 1a). Adverse neonatal outcomes were slightly more common after induction of labor and use of oxytocin. Rates of acidosis increased with gestational age (from 37 to ⩾42 weeks). There highest rate of admission to NICU was obtained at 37 weeks and the lowest rate at 39 weeks (Table 1b).
The median duration of second stage of labor was 93 min and 95% were delivered within 272 min (4.5 h). There were 360 infants (1.08%) with umbilical artery acidosis, 269 infants (0.63%) with birth asphyxia-related complications (defined as any of the following conditions: hypoxic ischemic encephalopathy n=97, hypothermia treatment n=23, neonatal seizures n=95, meconium aspiration syndrome n=68 and resuscitation in delivery room with heart compressions n=46 or intubation n=71) and 2733 infants (6.42%) were admitted to NICU (Tables 1a, 1b and 2).
Table 2. Duration of second stage of labor and rates of specific birth asphyxia-related complications.
|
Birth asphyxia-related complications (N=269)a |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total |
HIE |
Hypothermia treatment |
Neonatal seizures |
Meconium aspiration |
Heart compressions |
Intubation |
|||||||
| N | % | N | % | N | % | N | % | N | % | N | % | ||
| 42 539 | 97 | 0.23 | 23 | 0.05 | 95 | 0.22 | 68 | 0.16 | 46 | 0.11 | 71 | 0.17 | |
| Second stage (h) | |||||||||||||
| <1 | 13 558 | 18 | 0.13 | 3 | 0.02 | 20 | 0.15 | 15 | 0.11 | 8 | 0.06 | 12 | 0.09 |
| 1 to <2 | 12 225 | 25 | 0.20 | 5 | 0.04 | 23 | 0.19 | 16 | 0.13 | 9 | 0.07 | 16 | 0.13 |
| 2 to <3 | 7710 | 17 | 0.22 | 5 | 0.06 | 23 | 0.30 | 19 | 0.25 | 6 | 0.08 | 16 | 0.21 |
| 3 to <4 | 5238 | 15 | 0.29 | 3 | 0.06 | 12 | 0.23 | 8 | 0.15 | 13 | 0.25 | 14 | 0.27 |
| ⩾4 | 3808 | 22 | 0.58 | 7 | 0.18 | 17 | 0.45 | 10 | 0.26 | 10 | 0.26 | 13 | 0.34 |
| P-value | <0.0001 | 0.0047 | 0.0055 | 0.0648 | 0.0001 | 0.0021 | |||||||
Abbreviation: HIE, hypoxic ischemic encephalopathy.
Infants had one or several diagnoses.
Six infants died before 27 days of age. When analyzing rates of specific birth asphyxia-related complications, rates generally increased with longer durations of second stage (Table 2). Rates and adjusted RRs of compound birth asphyxia-related complications and admission to NICU generally increased gradually with duration of second stage. Compared with duration of second stage of <1 h, duration of at least 4 h was associated with almost a 2.5-fold increased risk of birth asphyxia-related complications and an 80% increased risk of admission to NICU. There was no association between duration of second stage and risk of acidosis (Table 3).
Table 3. Duration of second stage of labor and rates, and risks of umbilical artery acidosis, birth asphyxia-related complications and admission to NICU.
|
Acidosisa (N=360) |
Birth asphyxia-related complicationsb (N=269) |
Admission to NICU (N=2733) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N totalc | % | aRRd | (95% CI) | N total | % | aRRd | (95% CI) | % | aRRd | (95% CI) | |
| 33 429 | 1.08 | 42 539 | 0.63 | 6.42 | |||||||
| Second stage (h) | |||||||||||
| <1 | 10 518 | 0.99 | 1.00 | Reference | 13 558 | 0.42 | 1.00 | Reference | 4.97 | 1.00 | Reference |
| 1 to <2 | 9690 | 1.12 | 1.10 | (0.84–1.45) | 12 225 | 0.53 | 1.19 | (0.83–1.70) | 6.02 | 1.22 | (1.10–1.35) |
| 2 to <3 | 6130 | 1.27 | 1.19 | (0.88–1.60) | 7710 | 0.77 | 1.59 | (1.10–2.31) | 7.11 | 1.41 | (1.26–1.57) |
| 3 to <4 | 4115 | 0.92 | 0.77 | (0.52–1.14) | 5238 | 0.74 | 1.56 | (1.04–2.35) | 7.92 | 1.57 | (1.39–1.77) |
| ⩾4 | 2976 | 1.04 | 0.84 | (0.55–1.28) | 3808 | 1.29 | 2.46 | (1.66–3.66) | 9.45 | 1.80 | (1.58–2.04) |
Abbreviations: aRR, adjusted relative risk; CI, confidence interval; HIE, hypoxic ischemic encephalopathy; MAS, meconium aspiration syndrome; NICU, neonatal intensive care unit.
Acidosis: umbilical artery acidosis, pH <7.05 and BE <−12.
Birth asphyxia-related complications include any of the following conditions: HIE, hypothermia treatment, neonatal seizures, MAS or advanced resuscitation after birth (heart compressions or intubation).
Missing data on pH and/or BE: N=9110.
Adjusted for: maternal characteristics: maternal age, height, BMI, smoking, hypertensive disease and diabetes. Delivery and fetal characteristics: onset of delivery (spontaneous or induction), oxytocin before retracted cervix and gestational length at birth.
The median duration of pushing among vaginal deliveries was 32 min and 95% were delivered within 78 min (missing 6.2%). Rates and adjusted RRs of acidosis among vaginal deliveries increased with duration of pushing. Compared with duration of pushing of <15 min, pushing for 60 min or longer was associated with a 2.5-fold increased risk of acidosis. Rates of birth asphyxia-related complications and admission to NICU were increased after 45 min (Table 4).
Table 4. Duration of pushing in vaginal deliveries and risks of umbilical artery acidosis, birth asphyxia-related complications and admission to NICU.
|
Acidosisa (N=339) |
Birth asphyxia-related complicationsb (N=250) |
Admission NICU (N=2577) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N totalc | % | aRRd | (95% CI) | N total | % | aRRd | (95% CI) | % | aRRd | (95% CI) | |
| 32 417 | 1.05 | 41 340 | 0.60 | 6.23 | |||||||
| Pushing (min) | |||||||||||
| 0 to <15 | 3520 | 0.57 | 1.00 | Reference | 4565 | 0.42 | 1.00 | Reference | 5.39 | 1.00 | Reference |
| 15 to <30 | 9949 | 0.81 | 1.41 | (0.86–2.34) | 12 820 | 0.46 | 1.07 | (0.64–1.80) | 4.90 | 0.94 | (0.81–1.09) |
| 30 to <45 | 8082 | 1.03 | 1.80 | (1.09–2.98) | 10 126 | 0.49 | 1.03 | (0.60–1.76) | 5.93 | 1.11 | (0.96–1.29) |
| 45 to <60 | 4701 | 1.15 | 1.93 | (1.13–3.28) | 5943 | 0.81 | 1.65 | (0.95–2.85) | 7.47 | 1.43 | (1.22–1.67) |
| ⩾60 | 4208 | 1.69 | 2.55 | (1.51–4.30) | 5338 | 0.73 | 1.60 | (0.91–2.81) | 7.96 | 1.54 | (1.31–1.80) |
| Missing | 1957 | 1.53 | 2548 | 1.37 | 9.18 | ||||||
Abbreviations: BE, base excess; BMI, body mass index; HIE, hypoxic ischemic encephalopathy; MAS, meconium aspiration syndrome; NICU, neonatal intensive care unit.
Acidosis: umbilical artery acidosis, pH<7.05 and BE< −12.
Birth asphyxia-related complications include any of the following conditions: HIE, hypothermia treatment, neonatal seizures, MAS or advanced resuscitation after birth (heart compressions or intubation).
Missing data on pH and/or BE: N=8923.
Adjusted for maternal characteristics: maternal age, height, BMI, smoking, hypertensive disease and diabetes. Delivery and fetal characteristics: onset of delivery (spontaneous or induction), oxytocin before pushing phase, duration of second stage before pushing phase and gestational length at birth.
We also performed analyses stratified by use of epidural analgesia. The risks of birth asphyxia-related complications and admission to NICU generally increased with duration of second stage in both the non-epidural and epidural groups. However, the analyses were to some extent hampered by statistical power (Supplementary Table S1). In stratified analyses by mode of vaginal delivery, the adjusted RRs of birth asphyxia-related complications and admission to NICU increased with duration of second stage in non-instrumental deliveries but not in instrumental vaginal deliveries (Supplementary Table S2).
In additional sensitivity analyses, deliveries with infants diagnosed with any congenital malformation were excluded. Overall rates of acidosis were 1.05%, birth asphyxia-related complications 0.61% and admission to NICU 6.01%, and the analyses of RRs demonstrated the same patterns as for the whole study population, with generally slightly lower rates of adverse neonatal outcomes (Supplementary Table S3).
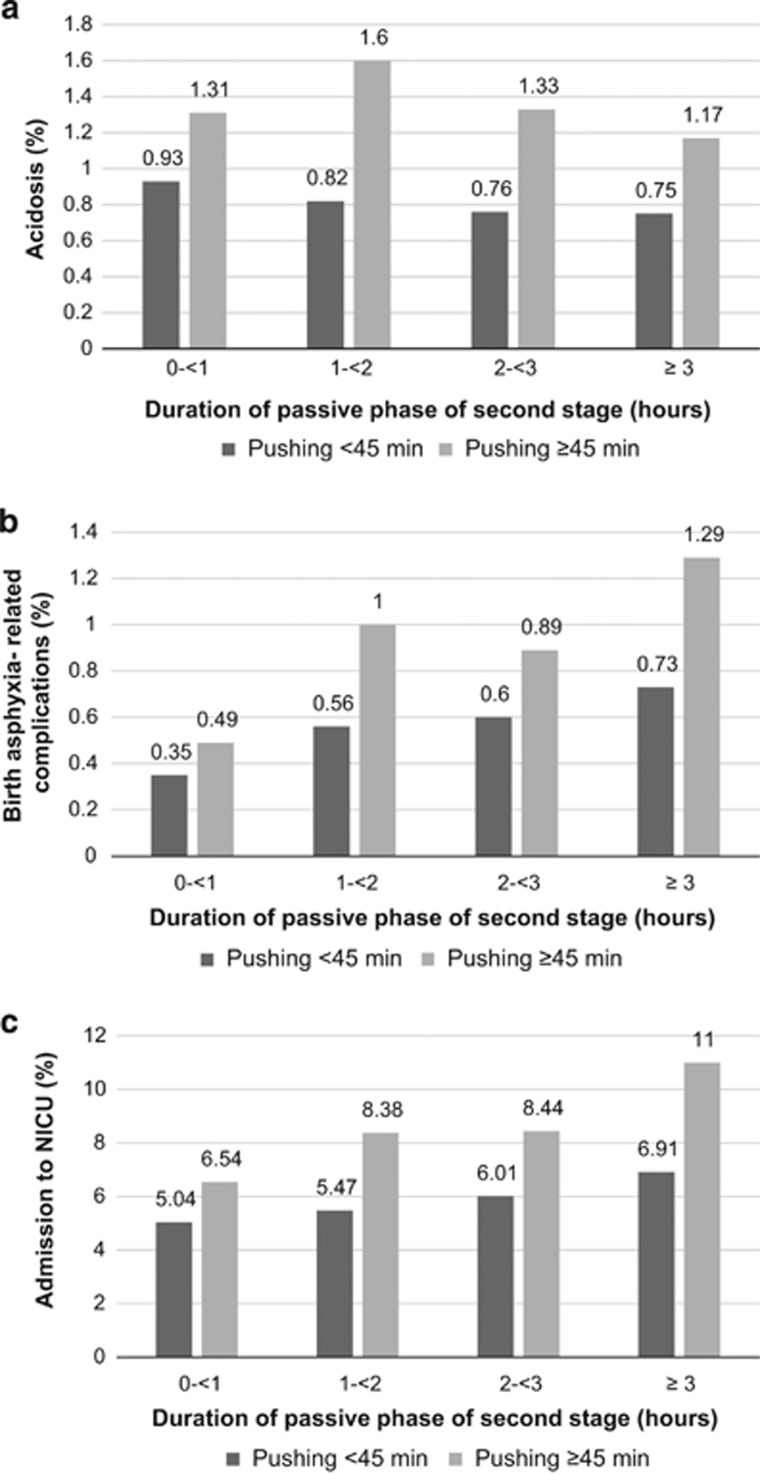
Figures 1a–c demonstrates duration of passive phase of second stage by duration of pushing (<45 min or ⩾45 min) and rates of adverse neonatal outcomes in vaginal deliveries. Rates of acidosis were increased in deliveries with long pushing time, whereas duration of passive phase did not have a major impact (Figure 1a). Rates of birth asphyxia-related complications generally increased with both durations of passive phase of second stage and pushing time, and especially in combination of both. Compared with duration of passive phase <1 h combined with pushing <45 min, a passive phase of ⩾3 h combined with pushing ⩾45 min had an almost fourfold increased rate of birth asphyxia-related complications (Figure 1b). Rates of admission to NICU also gradually increased with increasing durations of passive phase and pushing. However, in deliveries with longer duration of pushing, the rates increased more with duration of passive phase. The highest rate of admission to NICU was observed in deliveries with duration of passive phase of at least 3 h combined with pushing for ⩾45 min (Figure 1c).
Figure 1.
Durations of passive phase and pushing during second stage of labour and (a) acidosis, (b) birth asphyxia-related complications and (c) admission to NICU.
Discussion
In this large population-based cohort study, increasing duration of second stage was associated with increased RRs of birth asphyxia-related complications and admission to NICU, also after adjusting for maternal and infant characteristics, and delivery management. Longer duration of pushing also increased the risk of umbilical artery acidosis and admission to NICU. However, the outcomes were relatively rare and, hence, the absolute risk difference for birth asphyxia-related complications and acidosis was small. Furthermore, combined analyses demonstrated that rates of birth asphyxia-related complications and admission to NICU increased with duration of passive phase of second stage and were even higher when combined with longer pushing. Duration of pushing, but not duration of passive phase, was related to increased rates of umbilical artery acidosis.
Given the large sample size of nulliparous women with detailed delivery data, we were able to investigate how durations of second stage and pushing were associated with important immediate neonatal morbidities in separate analyses. A strength, of high clinical relevance, was that we were able to investigate the impact of the total duration of second stage and duration of pushing both separately and in combined analyses of passive phase of second stage and pushing. We were also able to adjust risk estimates for important factors, such as maternal BMI, use of oxytocin and birthweight for gestational age, and to stratify analyses on epidural analgesia and mode of vaginal delivery. Still, unmeasured factors related to prolonged durations and obstetric management may contribute to the associations between duration of second stage and increased risks of neonatal morbidities. For example, chorioamnionitis, occiput posterior position and duration of first stage of labor are associated with prolonged second stage and may lead to neonatal adverse outcomes, and such data were not considered in the present study.4, 7, 25, 26, 27 However, some of these factors were probably related to augmentation with oxytocin before second stage of labor, which we adjusted for. We did neither have information on intrapartum fetal heart rate tracing and related actions. Gestational age was mainly based on an early second trimester sonogram, which can have up to 14 days difference from last menstrual period. Finally, information on umbilical artery blood gas values was missing for 21.4% of the deliveries. If missing values were more common among the most depressed neonates, such selection bias may potentially underestimate association between duration of second stage and risk of acidosis.
Prolonged second stage of labor was recently reported to be associated with an increased risk of low 5 min Apgar score for neonates of nulliparous women in a Swedish study from our research group.14 Similar results were also presented in two studies from Canada and the United States.4, 7 The US study also found an increased risk of severe asphyxia and admission to NICU for second-stage duration longer than 3 h compared with <3 h in nulliparous women with epidural analgesia.4 The Canadian study reported that regardless of mode of delivery, there was an increased risk of birth depression (that is, birth asphyxia-related conditions) and admission to NICU, with second-stage durations beyond 2 h compared with <2 h in nulliparous women.7 However, our findings are also in contrast to several studies finding no association between second stage duration and infant risks.5, 8, 9, 11, 12 The conflicting results might be due to differences in duration of time limits,8, 12 restriction to vaginal deliveries12 and lack of or differences in control for confounders.5, 6, 8, 9, 11, 12 Further, some of these studies were rather small and probably afflicted by limited statistical power.6, 8, 12
Contemporary studies report longer durations of second stage for nulliparous women than suggested by previous guidelines.7, 13, 28, 29 Obstetric practice has developed considerably over the last 50 years and is now characterized by extensive use of epidural analgesia and oxytocin augmentation, lower rates of instrumental vaginal deliveries and higher cesarean sections rates. Furthermore, child-bearing women of today are older and have higher BMI.29 Thus, previous results can be misleading, unless maternal and management factors are taken into account. In a review by Altman et al.,18 it was emphasized that several factors made it difficult to evaluate the impact of duration of second stage and maternal and neonatal outcomes. Such factors include, but are not limited to, different and oversimplified definitions of prolonged second stage, various study population characteristics, lack of confounder control and not taking mode of delivery into account.
All guidelines do not separate management of active and passive phases of the second stage of labor. There are also considerable variations in the management of second stage of labor between countries, which might affect the external validity of the results. The Swedish clinical practice is ‘delayed pushing,' that is, to start expulsive efforts when the woman feels an urge to push or to actively push with, for example, the Valsalva technique when the fetal head has reached or almost reached the pelvic floor. Most studies of second stage and adverse neonatal outcomes have not distinguished between immediate (that is, when the cervix is fully dilated) or delayed pushing and the duration of pushing. A Cochrane review from 2015 reports that in women with epidural analgesia, delayed compared with immediate pushing was associated with an increased overall duration of second stage, but a decreased duration of pushing and an increase in spontaneous vaginal delivery. There were no differences between immediate and delayed pushing with respect to admission to NICU, 5 min Apgar score<7 and delivery room resuscitation. However, based on one study, an association between delayed pushing and low umbilical blood pH (arterial <7.10) was reported.30, 31
Studies on the association between pushing and adverse neonatal outcomes show different results. A recent large US study demonstrated higher frequencies of brachial plexus palsy, seizures and hypoxic ischemic encephalopathy, and increased risk of compound adverse neonatal outcome, with increasing duration of pushing.17 A European study reported increased risk of neonatal acidosis already after 15 min of pushing.16 On the contrary, Le Ray et al.15 did not find any association between duration of pushing and adverse neonatal outcomes, including neonatal acidosis, with continuous fetal surveillance during delivery. Piquard et al.32 demonstrated lack of influence on fetal acid–base status during the passive second stage but increasing fetal acidosis during pushing. Other studies have demonstrated a decline in fetal arterial pH and cerebral oxygenation, as well as an increase in lactate with prolonged pushing time.33, 34, 35 These results are consistent with the present study where an association between duration of pushing and umbilical artery acidosis was demonstrated, whereas risk of acidosis was not related to the total duration of second stage.
In sensitivity analyses, risks of birth asphyxia-related complications and admission to NICU were increased with duration of second stage in women with non-instrumental vaginal delivery. This indicates that duration of second stage may be an independent risk factor for these outcomes. Epidural analgesia is associated with increased duration of second stage of labor but not with adverse neonatal outcomes.36 Rates of birth asphyxia-related complications and admission to NICU were slightly higher for epidural users compared with non-users but the RRs were of similar magnitude. Thus, the effects of duration of labor on these outcomes cannot be explained by use of epidural analgesia. The pathophysiology of oxygen deficiency is the same for the fetus regardless of the mother's use of epidural analgesia.
Prolonged duration of second stage of labor was associated with increased RRs of birth asphyxia-related complications and admission to NICU. Umbilical acidosis increased with duration of pushing but not with duration of second stage. However, the absolute risk differences of birth asphyxia-related complications and acidosis were low and the observational study design made it not possible to conclude causal associations. Additional well-powered longitudinal prospective studies with detailed information on population and delivery characteristics, potentially with randomization of interventions, could add further information.
Acknowledgments
This study was supported by grants from the Swedish Research Council (2013–2429 to OS and 2008–5857 to SC) and by grants provided by the Stockholm County Council (ALF project 20130156 to OS and SC). The funders had no role in the conceiving, conduction or publication of the study. All authors were independent researchers from funders. We acknowledge Gunnar Petersson, database manager of the Obstetric database, for creating the data set used in this study.
Footnotes
Supplementary Information accompanies the paper on the Journal of Perinatology website (http://www.nature.com/jp)
The authors declare no conflict of interest.
Supplementary Material
References
- NICE Guidelines: Intrapartum care for healthy women and babies. CG190 (cited: 26 March 2016). December 2014. Available from. https://www.nice.org.uk/guidance/cg190/chapter/recommendations#care-in-established-labour.
- American College of Obstetrics and Gynecology Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin Number 49, December 2003: dystocia and augmentation of labor. Obstet Gynecol 2003; 102(6): 1445–1454. [DOI] [PubMed] [Google Scholar]
- American College of Obstetrics and Gynecologist (College)Society for Maternal-Fetal MedicineAmerican College of Obstetrics and Gynecologist (College), Caughey AB American College of Obstetrics and Gynecologist (College), Cahill AG American College of Obstetrics and Gynecologist (College), Guise JM American College of Obstetrics and Gynecologist (College), Rouse DJ. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol 2014; 210(3): 179–193. [DOI] [PubMed] [Google Scholar]
- Laughon SK, Berghella V, Reddy UM, Sundaram R, Lu Z, Hoffman MK. Neonatal and maternal outcomes with prolonged second stage of labor. Obstet Gynecol 2014; 124(1): 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YW, Hopkins LM, Caughey AB. How long is too long: does a prolonged second stage of labor in nulliparous women affect maternal and neonatal outcomes? Am J Obstet Gynecol 2004; 191(3): 933–938. [DOI] [PubMed] [Google Scholar]
- Rouse DJ, Weiner SJ, Bloom SL, Varner MW, Spong CY, Ramin SM et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol 2009; 201(4): 357 e1–357 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen VM, Baskett TF, O'Connell CM, McKeen D, Allen AC. Maternal and perinatal outcomes with increasing duration of the second stage of labor. Obstet Gynecol 2009 Jun; 113(6): 1248–1258. [DOI] [PubMed] [Google Scholar]
- Myles TD, Santolaya J. Maternal and neonatal outcomes in patients with a prolonged second stage of labor. Obstet Gynecol 2003; 102(1): 52–58. [DOI] [PubMed] [Google Scholar]
- Saunders NS, Paterson CM, Wadsworth J. Neonatal and maternal morbidity in relation to the length of the second stage of labor. Br J Obstet Gynaecol 1992; 99(5): 381–385. [DOI] [PubMed] [Google Scholar]
- Stephansson O, Sandstrom A, Petersson G, Wikstrom AK, Cnattingius S. Prolonged second stage of labor, maternal infectious disease, urinary retention and other complications in the early postpartum period. BJOG 2015; 123(4): 608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menticoglou SM, Manning F, Harman C, Morrison I. Perinatal outcome in relation to second-stage duration. Am J Obstet Gynecol 1995; 173(3 Pt 1): 906–912. [DOI] [PubMed] [Google Scholar]
- Janni W, Schiessl B, Peschers U, Huber S, Strobl B, Hantschmann P et al. The prognostic impact of a prolonged second stage of labor on maternal and fetal outcome. Acta Obstet Gynecol Scand 2002; 81(3): 214–221. [DOI] [PubMed] [Google Scholar]
- Cheng YW, Shaffer BL, Nicholson JM, Caughey AB. Second stage of labor and epidural use: a larger effect than previously suggested. Obstet Gynecol 2014; 123(3): 527–535. [DOI] [PubMed] [Google Scholar]
- Altman M, Sandstrom A, Petersson G, Frisell T, Cnattingius S, Stephansson O. Prolonged second stage of labor is associated with low Apgar score. Eur J Epidemiol 2015; 30(11): 1209–1215. [DOI] [PubMed] [Google Scholar]
- Le Ray C, Audibert F, Goffinet F, Fraser W. When to stop pushing: effects of duration of second-stage expulsion efforts on maternal and neonatal outcomes in nulliparous women with epidural analgesia. Am J Obstet Gynecol 2009; 201(4): 361 e1–361 e7. [DOI] [PubMed] [Google Scholar]
- Yli BM, Kro GA, Rasmussen S, Khoury J, Noren H, Amer-Wahlin I et al. How does the duration of active pushing in labor affect neonatal outcomes? J Perinat Med 2012; 40(2): 171–178. [DOI] [PubMed] [Google Scholar]
- Grobman WA, Bailit J, Lai Y, Reddy UM, Wapner RJ, Varner MW et al. Association of the duration of active pushing with obstetric outcomes. Obstet Gynecol 2016; 127(4): 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman MR, Lydon-Rochelle MT. Prolonged second stage of labor and risk of adverse maternal and perinatal outcomes: a systematic review. Birth 2006; 33(4): 315–322. [DOI] [PubMed] [Google Scholar]
- Cheng YW, Caughey AB. Second stage of labor. Clin Obstet Gynecol 2015; 58(2): 227–240. [DOI] [PubMed] [Google Scholar]
- The Swedish Neonatal Quality Register. Cited: 22 August 2016 2016. Available from https://www.medscinet.com/pnq/. In Swedish.
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009; 24(11): 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Board of Health and Welfare. Indication for augmentation with oxytocin during active labor [Indikation för värkstimulering med oxytocin under aktiv förlossning]. Rapport från samarbetsprojektet Nationella Medicinska Indikationer (cited: 16 May 2016) 2011. Available from. https://www.socialstyrelsen.se/SiteCollectionDocuments/nationella-indikationer-varkstimulering-oxytocin.pdf. In Swedish.
- Belfort MA, Saade GR, Thom E, Blackwell SC, Reddy UM, Thorp JM Jr et al. A randomized trial of intrapartum fetal ECG ST-segment analysis. N Engl J Med 2015; 373(7): 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005; 162(3): 199–200. [DOI] [PubMed] [Google Scholar]
- Senecal J, Xiong X, Fraser WD Pushing Early Or Pushing Late with Epidural Study Group. Effect of fetal position on second-stage duration and labor outcome. Obstet Gynecol 2005; 105(4): 763–772. [DOI] [PubMed] [Google Scholar]
- Nelson DB, McIntire DD, Leveno KJ. Relationship of the length of the first stage of labor to the length of the second stage. Obstet Gynecol 2013; 122(1): 27–32. [DOI] [PubMed] [Google Scholar]
- Schiessl B, Janni W, Jundt K, Rammel G, Peschers U, Kainer F. Obstetrical parameters influencing the duration of the second stage of labor. Eur J Obstet Gynecol Reprod Biol 2005; 118(1): 17–20. [DOI] [PubMed] [Google Scholar]
- Zhang J, Landy HJ, Branch DW, Burkman R, Haberman S, Gregory KD et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol 2010; 116(6): 1281–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon SK, Branch DW, Beaver J, Zhang J. Changes in labor patterns over 50 years. Am J Obstet Gynecol 2012; 206(5): 419 e1–419 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos A, Amorim MM, Dornelas de Andrade A, de Souza AI, Cabral Filho JE, Correia JB. Pushing/bearing down methods for the second stage of labor. Cochrane Database Syst Rev 2015; 10: CD009124. [DOI] [PubMed] [Google Scholar]
- Fraser WD, Marcoux S, Krauss I, Douglas J, Goulet C, Boulvain M. Multicenter, randomized, controlled trial of delayed pushing for nulliparous women in the second stage of labor with continuous epidural analgesia. The PEOPLE (Pushing Early or Pushing Late with Epidural) Study Group. Am J Obstet Gynecol 2000; 182(5): 1165–1172. [DOI] [PubMed] [Google Scholar]
- Piquard F, Schaefer A, Hsiung R, Dellenbach P, Haberey P. Are there two biological parts in the second stage of labor? Acta Obstet Gynecol Scand 1989; 68(8): 713–718. [DOI] [PubMed] [Google Scholar]
- Nordstrom L, Achanna S, Naka K, Arulkumaran S. Fetal and maternal lactate increase during active second stage of labor. BJOG 2001; 108(3): 263–268. [DOI] [PubMed] [Google Scholar]
- Aldrich CJ, D'Antona D, Spencer JA, Wyatt JS, Peebles DM, Delpy DT et al. The effect of maternal pushing on fetal cerebral oxygenation and blood volume during the second stage of labor. Br J Obstet Gynaecol 1995; 102(6): 448–453. [DOI] [PubMed] [Google Scholar]
- Wood C, Ng KH, Hounslow D, Benning H. Time–-an important variable in normal delivery. J Obstet Gynaecol Br Common 1973; 80(4): 295–300. [DOI] [PubMed] [Google Scholar]
- Anim-Somuah M, Smyth RM, Jones L. Epidural versus non-epidural or no analgesia in labor. Cochrane Database Syst Rev 2011; (12): CD000331. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.