Abstract
Dynamic interaction between tumor cells and the microenvironment is critical for tumorigenesis, and cancer immunosurveillance plays an important role in the tumor evolution. In some tumors (such as esophageal cancer, pancreatic cancer and colorectal cancer), studies have shown that the number of tumor-infiltrating lymphocytes (TILs) has a significant relationship with the prognosis, but there is little research on the prognosis of TILs and non-small cell lung cancer (NSCLC) has been performed. Therefore, it is necessary to discover the relationship between the TILs and cytokines with NSCLC prognosis and metastasis in patients. Tumor samples were carefully examined for tissue preservation and complete follow-up. A total of 107 tumor samples from NSCLC patients with radical surgical resection were enrolled for the analysis. All samples were subjected to immunohistochemistry for detection of CD3, CD4, CD8, CD28, forkhead box protein P3 (Foxp3), cytotoxic T lymphocyte-associated protein-4, cyclooxygenase2 (COX-2), transforming growth factor β 1, interleukin-2 (IL-2), interleukin-6, interleukin-10, interleukin-12 receptor and hypoxia inducible factor 1a (HIF-1a). The number, function and location of the targets were analyzed to determine their correlation with disease-free survival (DFS) and overall survival (OS). Immunhistochemical results from 107 samples indicated that the FoxP3+ regulatory TIL (HR=1.336, P=0.031), IL-2 (HR=0.595, P=0.007) and HIF-1a (HR=1.510, P=0.002) levels in tumor cells closely correlated with DFS in a COX analysis model. FoxP3+ regulatory TILs (HR=1.566, P=0.002) significantly correlated with OS and tumor node metastasis staging. The patients were divided into two groups due to the coexpression pattern of the IL-2, FoxP3+ and HIF-1a. The high-risk group had an overall worse survival than those at low risk. We confirmed that Foxp3 expression in lymphocyte and IL-2 expression in tumor cells were associated with recurrence or transfer. Furthermore, we also observed that HIF-1a expression significantly correlated with DFS and OS.
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related deaths in Asian countries. Surgical resection is the frontline treatment of this disease, and combination therapy with surgery and chemoradiotherapy have continued to develop in recent years. After surgery and postoperative adjuvant therapy, about 65% of the NSCLC patients will still have persistent disease or have distant metastasis. The 5-year survival rate is ~10%, thus, highlighting a critical need for improved treatment options and better understanding of the pathophysiology underlying recurrent NSCLC.1, 2, 3
Studies have demonstrated a correlation between tumor-infiltrating lymphocytes (TILs) with disease recurrence or survival in patients with NSCLC. A higher degree of TILs within large node-negative NSCLC correlates with decreased risk of disease recurrence and improved disease-free survival (DFS).4 Additionally, high levels of intratumoral TILs are associated with improved recurrence-free survival in stage 1A NSCLC patients as well as a reduced likelihood of systemic recurrence.5 Immunological CD8+ and FOXP3+ regulatory T cells (Tregs) infiltrate within tumor environment is predictive of response to platinum-based neoadjuvant chemotherapy in advanced NSCLC patients. The understanding of the clinical relevance of the immunological milieu in the tumor microenvironment might provide important insights for the identification of novel strategies in cancer immunotherapy.6 Expression of transforming growth factor β (TGF-β) in TILs or in tumor cells was reported to significantly reduce postoperative survival time, especially in patients with squamous cell carcinoma. In these patients, the levels of transforming growth factor β-positive TILs were an immunologic predictor with prognostic significance as determined by multivariate analysis.7
Recent progress in the field indicated that TILs might have prognostic value in NSCLC for recurrence and metastasis. In this study, additional subgroups of T lymphocyte and cytokines in the tumor microenvironment were assessed according to published meta-analyses studies and literature. The results were separately analyzed based on the number, function and location to determine the correlation between early stage NSCLC recurrence and metastasis. Furthermore, differentiation between patients at high and low risk for disease recurrence or metastatic disease was also evaluated. These results will provide additional insight for improved anti-tumor strategies of chemotherapy in combination with immunotherapy for NSCLC.
Materials and Methods
Study population
Archival tumor samples were derived from NSCLC patients who underwent radical surgical resection. Samples were obtained from the Chinese PLA General Hospital between January 1998 and December 2007 and diagnosed at the Institute for Pathology. Inclusion criteria for this study were patients with histological-proven NSCLC stage I–IV, who had complete clinic pathologic data and follow-up date, including gender, age, pathological diagnosis, differentiation status, tumor node metastasis (TNM) staging and DFS. Tumor stage was evaluated according to the guidelines set forth by the American Joint Committee on Cancer TNM classification system (7th edition). All enrolled patients had no previous anti-neoplastic treatment with either chemotherapy or radiotherapy, and had no other primary renal tumors in 5 years. In total, 107 patients met inclusion criteria. All specimens including tumorous and para cancerous tissues were formalin-fixed and paraffin-embedded on a routine basis.
Immunohistochemistry
Formalin-fixed and paraffin-embedded tissue specimens were cut into 4 μm sections, mounted on poly-lysine-coated slides and incubated with blocking buffer (normal goat or mouse serum) at 37 °C for 20 min. The samples were then incubated with serum and primary antibody that was diluted in phosphate-buffered saline for 2 h at 37 °C. The secondary anti-mouse antibody was applied at 37 °C for 50 min. Staining was performed by incubation with 3,3'-diaminobenzidine chromogen. The slides were then counter-stained with hematoxilin. Specific antibodies were then added and diluted for detection of CD3, CD4, CD8, forkhead box protein P3 (FOXP3), cytotoxic T lymphocyte-associated protein-4 (CTLA-4), cyclooxygenase 2 (COX-2), TGF-β1, interleukin-2 (IL-2), interleukin-10 (IL-10) and interleukin-12 receptor (IL-12R), according to the manufacturer's instructions (Table 1). Negative control slides were treated similarly as described above, but the antibody was replaced by phosphate-buffered saline.
Table 1. Criteria of the level of positive cell.
| Index |
The level of positive |
|||
|---|---|---|---|---|
| − | + | ++ | +++ | |
| CD3 | <25 | 25–50 | 50–75 | ⩾75 |
| CD4 | <25 | 25–50 | 50–75 | ⩾75 |
| CD8 | <10 | 10–30 | 30–60 | ⩾60 |
| CD28 | <25 | 25–50 | 50–75 | ⩾75 |
| Foxp3 | <5 | 5–15 | 15–25 | ⩾25 |
| CTLA-4 | <25 | 25–50 | 50–75 | ⩾75 |
| COX-2 | <25 | 25–50 | 50–75 | ⩾75 |
| TGF-β1 | <10 | 10–30 | 30–60 | ⩾60 |
| IL-2 | <25 | 25–50 | 50–75 | ⩾75 |
| IL-10 | <25 | 25–50 | 50–75 | ⩾75 |
| IL-12R | <25 | 25–50 | 50–75 | ⩾75 |
| HIF-1a | <25 | 25–50 | 50–75 | ⩾75 |
Immunohistochemical evaluation
Positively stained tumor-infiltrating cells were independently counted by two pathologists to assess interobserver agreement. The pathologists were blinded to the clinical and prognosis of all patients. Positive cells were defined by tan particles displayed in the cytoplasm and nucleus or cell membrane. To ensure representativeness and homogeneity, five different fields were selected with the most positive cells at × 40 magnification. The cells were counted until a difference of >5% was observed. The positive rate was obtained by using the integral measuring method.8 According to the positive staining, cells (staining intensity) were divided into negative (−), weakly positive (+, one point), positive (++, two points) and strongly positive (+++, three points). According to the number of positive cell number (percentage of positive cells), they were divided into negative (−), weakly positive (+, one point), positive (++, two points) and strongly positive (+++, three points). The specific classification standard referred to the expression levels of each antigen as identified by relevant literature (Table 1). The computational formula for scoring was calculated as immunoreactivescore=staining intensity × percentage of positive cells. Scores <2 were identified as (−), 2–4.9 as (+), 5–8.9 as (++) and >9 as (+++).
Statistical analysis
Statistical calculations were performed using SPSS 17.0 software. Mann–Whitney U test was used for determining single-factor significance. Correlation analysis of clinicopathologic and immunohistochemical parameters was performed using the Spearman test corrected for multiple testing. Kaplan–Meier curves were calculated for cumulative survival rate and cumulative survival time. Univariate and multivariate survival analysis was performed using the COX regression model. P values <0.05 were considered significant. Two-sided tests were used throughout the study.
Results
Patient characteristics
We identify 107 cases with complete medical records and follow-up. The overall DFS was 67.3%, 47.7% and 33.5% at 1 year, 3 years and 5 years, respectively. The overall survival (OS) rates were reported at 91.6%, 63.6% and 55.0%, respectively. The median follow-up time was 60 months (range, 7–163 months), whereas the median survival time was 67.7 months.
Immunohistochemistry
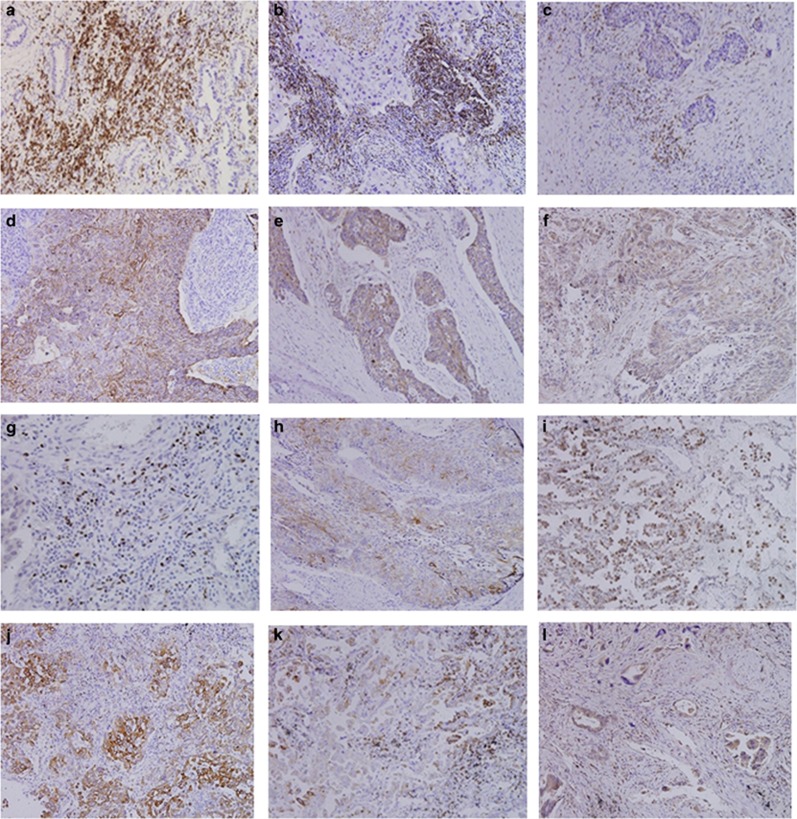
CD3+, CD4+:CD3 and CD4 expression were observed and localized to TILs. Qualitative immunohistochemical data are shown in Figure 1.
Figure 1.
Immunohistochemical detection of TIL and tumor cells in NSCLC (× 20 magnification). (a) Expression of CD3+ TILs. (b) Expression of CD4+ TILs. (c) Expression of CD8+ TILs. (d) Expression of CD28+ TILs. (e) Expression of CTLA-4+ TILs. (f) Expression of Foxp3+ TILs. (g) Expression of COX-2+ tumor cells. (h) Expression of TGF-β1+ tumor cells. (i) Expression of HIF-1a+ tumor cells. (j) Expression of IL-2+ tumor cells. (k) Expression of IL-10+ tumor cells. (l) Expression of IL-12R+ tumor cells.
The number of samples in each group was dissimilar due to the poor quality of staining in several samples (Table 2).
Table 2. Immunohistochemical staining results.
| Parameter | Cases | L (lymphocyte) or T (tumor cell) |
Results |
|||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| CD3 | 107 | L | 14 | 42 | 36 | 15 |
| T | 0 | 0 | 0 | 0 | ||
| CD4 | 107 | L | 15 | 38 | 41 | 13 |
| T | 0 | 0 | 0 | 0 | ||
| CD8 | 105 | L | 65 | 32 | 6 | 2 |
| T | 22 | 31 | 12 | 40 | ||
| CD28 | 101 | L | 0 | 0 | 0 | 0 |
| T | 30 | 36 | 24 | 11 | ||
| Foxp3 | 102 | L | 54 | 26 | 17 | 5 |
| T | 0 | 0 | 0 | 0 | ||
| CTLA-4 | 99 | L | 27 | 29 | 26 | 17 |
| T | 86 | 6 | 6 | 1 | ||
| COX-2 | 103 | L | 35 | 21 | 18 | 29 |
| T | 0 | 0 | 0 | 0 | ||
| TGF-β1 | 103 | L | 68 | 28 | 7 | 0 |
| T | 0 | 0 | 0 | 0 | ||
| IL-2 | 102 | L | 26 | 51 | 20 | 5 |
| T | 0 | 0 | 0 | 0 | ||
| IL-10 | 96 | L | 60 | 23 | 10 | 3 |
| T | 0 | 0 | 0 | 0 | ||
| IL-12R | 99 | L | 40 | 34 | 22 | 3 |
| T | 0 | 0 | 0 | 0 | ||
| HIF-1a | 95 | L | 33 | 31 | 25 | 6 |
| T | 0 | 0 | 0 | 0 | ||
Rank-sum test
Correlations between the clinical and pathological characteristics of patients and the result of TILs measured by different parameters were performed using the Mann–Whitney U test. As a caveat, this test cannot rule out possible interaction between various other variables. To avoid possible loss of valid data, P values <0.1 were considered significant. We found that the smoking status, lymph node metastasis, distant metastasis and TNM staging were significantly associated with DFS. OS correlated with lymph node metastases, pathological type and TNM staging. On the other hand, significant associations were observed between DFS and the density of CD3+TILS, CD8+, IL-2+, IL-12R+ or HIF-1a+. There was also a significant association between OS and Foxp3+TILs, CTLA-4+TILs, IL-12R+ or HIF-1a+ (Table 3).
Table 3. Rank-sum test of OS and DFS.
| Parameters |
DFS |
OS |
||
|---|---|---|---|---|
| Z value | P value | Z value | P value | |
| Age (⩾60 vs <60) | −1.121 | 0.262 | −0.914 | 0.361 |
| Gender (woman vs man) | −0.538 | 0.590 | −0.572 | 0.567 |
| Smoking status (yes vs no) | −1.664 | 0.096* | −1.287 | 0.198 |
| Pathological type | ||||
| Adenocarcinoma/non | −1.170 | 0.242 | −1.955 | 0.051* |
| Squamous carcinoma/non | −0.238 | 0.812 | −0.670 | 0.503 |
| Differentiation (G1/G2/G3) | −0.261 | 0.794 | −0.194 | 0.846 |
| T staging (T1/T2/T3/T4) | −0.812 | 0.417 | −0.906 | 0.365 |
| N staging (N0/N1/N2/N3) | −2.322 | 0.020* | −2.670 | 0.008* |
| M staging (M0/M1) | −1.633 | 0.103* | −1.033 | 0.302 |
| PTNM staging (I/II/III/IV) | −2.152 | 0.031* | −2.535 | 0.011* |
| Lymphocyte | ||||
| CD3 | −2.159 | 0.031* | −0.501 | 0.617 |
| CD4 | −0.842 | 0.400 | −0.410 | 0.682 |
| CD8 | −0.918 | 0.359 | −0.096 | 0.924 |
| CTLA-4 | −0.848 | 0.397 | −2.122 | 0.034* |
| Foxp3 | −1.253 | 0.210 | −2.848 | 0.004* |
| Tumor cells | ||||
| CD8 | −1.638 | 0.101* | −0.224 | 0.823 |
| COX-2 | −0.524 | 0.601 | −0.866 | 0.386 |
| TGF-β1 | −0.305 | 0.761 | −0.199 | 0.842 |
| IL-2 | −1.953 | 0.051* | −1.453 | 0.146 |
| IL-10 | −0.561 | 0.575 | −0.462 | 0.644 |
| IL-12R | −2.774 | 0.006* | −2.411 | 0.016* |
| CTLA-4 | −0.442 | 0.658 | −0.414 | 0.679 |
| HIF-1a | −2.474 | 0.013* | −2.012 | 0.044* |
| CD28 | −0.145 | 0.884 | −0.398 | 0.691 |
*P<0.01.
Survival analysis
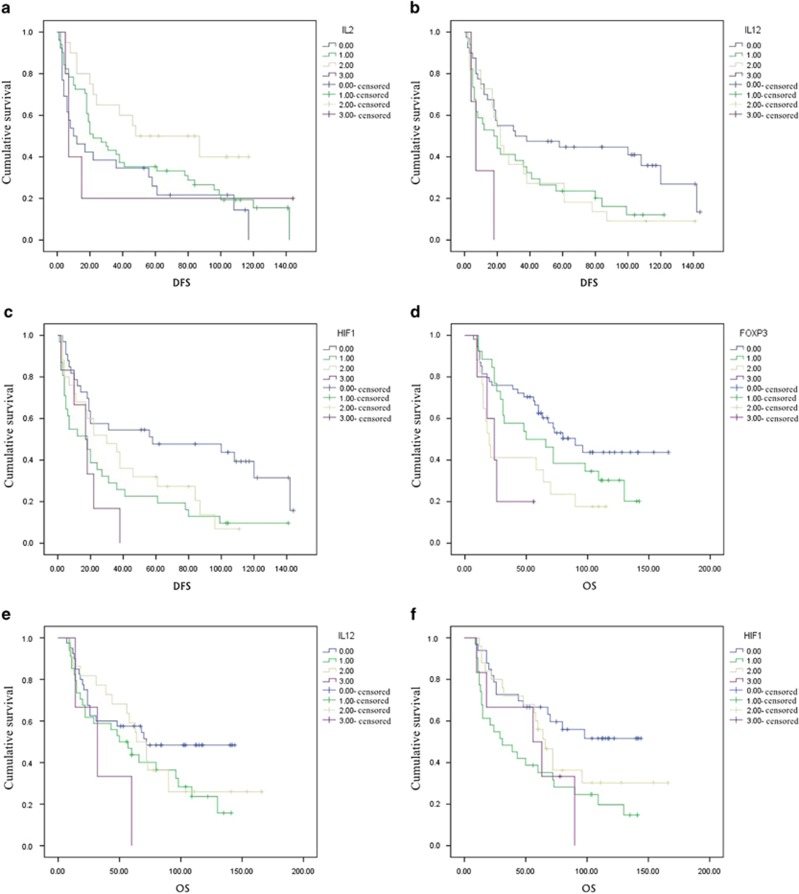
A univariate analysis was used to determine various clinical parameters as predicted by a rank-sum test (P<0.01). The correlation between the clinical characteristics and DFS or OS was consistent with the rank-sum test. IL-2+, HIF-1a+ and IL-12R expression correlated with OS, whereas Foxp3TIL+ and HIF-1a+ were associated with DFS (Figure 2).
Figure 2.
Kaplan-Meier survival curves demonstrate tumor-infiltrating lymphocytes and immune factors is correlated to OS or DFS. (a)Correlation of DFS with IL-2+ cell in NSCLC tissues. Low IL-2+ was associated with reduced DFS in NSCLC patients. (b) Correlation of DFS with IL-12R+ cell in NSCLC tissues. IL-12R+ high was associated with reduced DFS in NSCLC patients. (c) Correlation of DFS with HIF-1a+ cell in NSCLC tissues. HIF-1a high cell was associated with reduced DFS in NSCLC patients. (d) Correlation of OS with Foxp3+ cell in TILs. Foxp3+ high was associated with reduced OS in NSCLC patients. (e) Correlation of OS with IL-12R+ cell in NSCLC tissues. IL-12R+ high was associated with reduced OS in NSCLC patients. (f Correlation of OS with HIF-1a+ cell in NSCLC tissues. HIF-1a+ high was associated with reduced OS in NSCLC patients.
COX multivariate analysis of survival indicated that TNM staging (HR=1.252, P=0.045), Foxp3 expression (HR=1.336, P=0.031), IL-2 (HR=0.595, P=0.007) and HLF-1a expression (HR=1.510, P=0.002) were associated with DFS. Additionally, pathological type ((non)adenocarcinoma) (HR=0.538, P=0.02), TNM staging (HR=1.522, P=0.003) and Foxp3 expression (HR=1.566, P=0.002) were associated with OS (Table 4).
Table 4. COX multivariate analysis.
| Variables |
DFS |
OS |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Smoking status (yes vs no) | 1.052 (0.591–1.873) | 0.864 | ||
| Pathological type (adenocarcinoma/non) | 0.538 (0.320–0.905) | 0.02* | ||
| N staging (N0/N1/N2/N3) | 1.033 (0.591–1.804) | 0.911 | 1.087 (0.669–1.767) | 0.736 |
| M staging (M0/M1) | 0.731 (0.228–2.343) | 0.598 | ||
| PTNM staging (I/II/III/IV) | 1.252 (0.961–1.631) | 0.045* | 1.522 (1.156–2.003) | 0.003** |
| CD3 | 0.779 (0.580–1.045) | 0.096 | ||
| Foxp3 | 1.336 (1.027–1.737) | 0.031* | 1.566 (1.183–2.073) | 0.002** |
| IL-2 | 0.595 (0.408–0.868) | 0.007** | ||
| IL-12R | 1.285 (0.871–1.895) | 0.206 | ||
| HIF-1a | 1.510 (1.160–1.966) | 0.002** | 1.110 (0.836–1.475) | 0.471 |
*P<0.05, **P<0.01
Conjoint analysis of effector and inhibitory factors in the tumor microenvironment
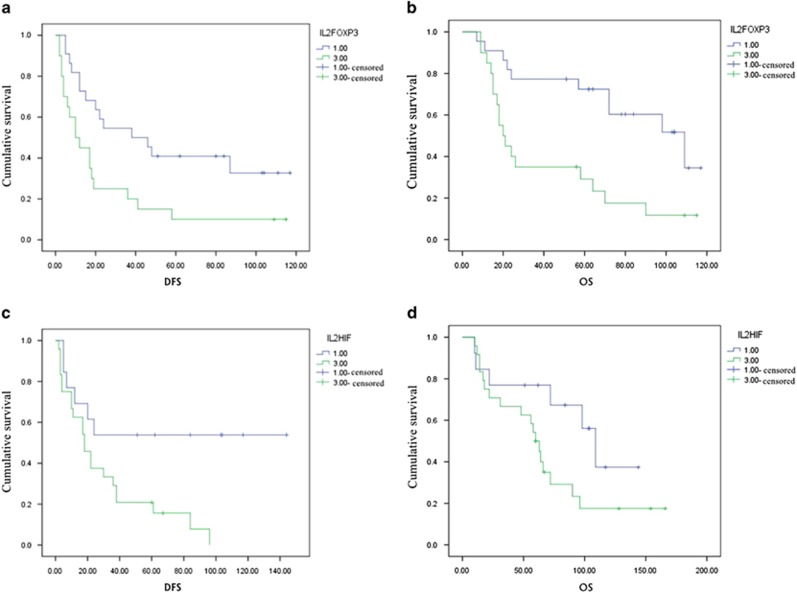
Immune effectors, such as IL-2, hypoxia inducible factor 1a (HIF-1a) and Foxp3, positively correlated with DFS and OS. Particularly, Foxp3 expression was strongly correlated with DFS and OS, as determined by multivariate analysis. An analysis of the prognostic value of COX-2 and Tregs demonstrated that higher expression of immune effectors and IL-2 in lower expression group was associated with worsen DFS or OS. Conversely, lower expression of immune effectors and higher expression of IL-2 were associated with a survival advantage (Figure 3).
Figure 3.
The relationship between the coexpression of IL-2 and immune suppressor with prognosis. P<0.05. (a) The relationship between the coexpression of IL-2 and Foxp3 with DFS in NSCLC tissues. Patients with higher IL-2/FOXP3 relapsed within a shorter period of time than patients with lower IL-2/FOXP3. (b) The relationship between the coexpression of IL-2 and Foxp3 with OS in NSCLC tissues. Patients with higher IL-2/FOXP3 level resulted in shorter survival than those patients with lower IL-2/FOXP3 level. (c) The relationship between the coexpression of IL-2 and HIF-1a with DFS in NSCLC tissues. Patients with higher IL-2/HIF-1a level relapsed within a shorter period than patients with lower IL-2/ HIF-1a level. (d) The relationship between the coexpression of IL-2 and HIF-1a with OS in NSCLC tissues. Patients with higher IL-2/HIF-1a level lived a shorter life than patients with lower IL-2/HIF-1a level.
Discussion
In this study, we confirmed that Foxp3 expression in lymphocyte and IL-2 expression in tumor cells were associated with recurrence or transfer. Furthermore, we also observed that HIF-1a expression significantly correlated with DFS and OS. Currently, there are large research interests regarding the role of TILs as a biological therapy and the association between TIL-induced tumor tissue infiltration, patient prognosis and survival.9 Histopathological analysis of a variety of human tumors showed high concentrations of T lymphocytes is associated with better prognosis in melanoma, breast cancer, prostate cancer, non-Hodgkin lymphoma, head and neck cancer, NSCLC, esophageal cancer and urethral carcinoma.10, 11, 12 In an earlier study of NSCLC, a large number of tumor infiltrating CD8+ T cells were found to induce tumor cell apoptosis.13 CD4+ T and B lymphocytes are a double-edged sword in tumor immunity. Studies by Tesar et al.14 and Moyron-Quiroz et al.15 and by others have reported that CD4+ cells in interstitial infiltrates are associated with improved clinical outcomes.
However, because of the mechanistic complexity of the immune system, it remains a challenge to quantitate the number of TILs in the immuno-microenvironment. TILs are not commonly activated in the local tumors. In vitro studies have demonstrated that cultured TILs remain activated after stimulation and induced apoptosis of tumor cells. The biological mechanism associated with tumor immunosuppression in the local tumor microenvironment remains unknown.16 Immunosuppressive cells in the tumor microenvironment include FoxP3+ Treg17 and Gr-1+ CD1lb+ myeloid suppressor cells.18 Soluble cytokines also found in the tumor microenvironment include IL-10, TGF-β, VEGF and PGE2.19, 20, 21 Indoleamine 2,3-dioxygenase and arginase I (refs 22, 23) are metabolic factors found deregulated in microenvironment, whereas those of immune inhibitory ligands include B7-H4 and PD-1.24, 25 A variety of factors regulate the tumor inhibitory network in the microenvironment. These factors affect the efficacy of current immunotherapies by inhibiting the activation of the immune response. Studies have demonstrated that there are various immunobiomarkers, which could help characterize the tumor immune microenvironment. In this study, immunohistochemistry was used to assess lymphocytes surrounding the tumors and evaluated whether the degree of expression of these immune cells could influence prognosis. In a univariate survival analysis, clinical features associated with DFS included smoking (P=0.079), distant metastases (P=0.034), lymph node metastasis (P=0.006) and TNM staging (P=0.002). Immunohistochemical parameters such as IL-2 (P=0.054), HIF-1a (P=0.007) and IL-12r (P=0.008) expression in tumor cells are also associated with DFS. Clinical features associated with OS include pathologic types (non-adenocarcinoma versus adenocarcinoma) (P=0.063), lymph node metastasis (P=0.002) and TNM staging (P=0.001) and immunohistochemical parameters such as FoxP3 expression in lymphocytes (P=0.001) and HIF-1a expression in tumor cells (P=0.098). Multi-factor survival analysis showed that the factors associated with DFS include TNM staging (HR=1.252, P=1.252), FoxP3 positive lymphocytes (HR=1.336, P=1.336), IL-2 expressing tumor cells (HR=0.595, P=0.595) and HIF-1a expression (HR=1.510, P=1.510). Factors associated with OS including the pathologic types (non-adenocarcinoma vs adenocarcinoma) (HR=0.538, P=0.538), pTNM staging (HR=1.522, P=1.522) and FoxP3 expressing lymphocytes (HR=1.566, P=1.566). Joint analysis of the effectors and inhibitory factors in the tumor microenvironment demonstrated that lower IL-2 expression and higher expression of immune effectors is associated with a poorer DFS and OS. In contrast, higher IL-2 expression and lower expression of the immune effectors is associated with a survival advantage. We also observed that tumor cells contain high levels of CD8 positivity. CD8 T lymphocytes are membrane proteins. Studies26 have reported that ectopic expression of membrane protein in tumor cells may greatly enhance the invasive and recurrence metastasis of tumor cells. However, after careful analysis, we have no meaningful statistical results to confirm this. These results need further verification. Other indicators such as CD3, CD4, CD8, CD28, CTLA-4, COX-2, TGF-β1, IL-10 and IL-12R have no statistical significance and do not have prognostic value.
Compared with the previous results, we found a more stringent counting method can influence the interpretation of study results. We confirmed the Foxp3 expression in lymphocytes and IL-2 expression in tumor cells are associated with recurrence or transfer. Additionally, HIF-1a expression is significantly associated with DFS and OS. This may reflect the test sample of individual differences and the complexity of the immune network system.
IL-2 is an important immune effector. It has been demonstrated that IL-2 expands T-cell populations, which is a property that is exploited for cancer immunotherapies. The infusion of IL-2 at low or high doses at multiple cycles in patients with metastatic melanoma and renal cell carcinoma was the first successful immunotherapy for cancer, proving that the immune system could completely eradicate tumor cells under certain conditions.27, 28 Additionally, Treg cells can inhibit currently almost all known a priori and secondary immune response. Tregs also play a role in inherent, acquired and humoral immunity, as well as in antigen presentation.29, 30 HIF-1a induces hypoxia in mammalian cells and helps to adapt cells to a low oxygen environment. It has been reported that hypoxia can promote the growth of tumor cells. Conjoint analysis of three indicators showed they are associated with the local immune microenvironment, which could assist in identifying high-risk patients with NSCLC.
Infiltrating lymphocytes and inflammatory cytokines make up the complex immune microenvironment observed in NSCLC. The interaction between tumor cells and the tumor microenvironment play a critical role in persistence and recurrence of NSCLC. Therefore, identifying strategies to modulate immunosuppressive factors in the tumor microenvironment may provide insight into additional therapies for NSCLC. This study further characterizes the immune tumor microenvironment and its potential role in relapse and metastatic NSCLC.
Footnotes
The authors declare no conflict of interest.
References
- Tokuda Y, Takigawa N, Kozuki T, Kamei H, Bessho A, Tada A et al. Long-term follow-up of phase II trial of docetaxel and cisplatin with concurrent thoracic radiation therapy for locally advanced non-small cell lung cancer. Acta Oncologica 2012; 51: 537–540. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- Wood SL, Pernemalm M, Crosbie PA, Whetton AD. Molecular histology of lung cancer: from targets to treatments. Cancer Treat Rev 2015; 41: 361–375. [DOI] [PubMed] [Google Scholar]
- Kilic A, Landreneau RJ, Luketich JD, Pennathur A, Schuchert MJ. Density of tumor-infiltrating lymphocytes correlates with disease recurrence and survival in patients with large non-small-cell lung cancer tumors. J Surg Res 2011; 167: 207–210. [DOI] [PubMed] [Google Scholar]
- Horne ZD, Jack R, Gray ZT, Siegfried JM, Wilson DO, Yousem SA et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1 A non-small-cell lung cancer. J Surg Res 2011; 171: 1–5. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang T, Ye J, Li H, Huang J, Li X et al. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol Immunother 2012; 61: 1849–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlacci W, Wolf D, Savic S, Hilbe W, Schmid T, Jamnig H et al. High transforming growth factor beta expression represents an important prognostic parameter for surgically resected non-small cell lung cancer. Hum Pathol 2012; 43: 339–349. [DOI] [PubMed] [Google Scholar]
- Camus M, Tosolini M, Mlecnik B, Pages F, Kirilovsky A, Berger A et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res 2009; 69: 2685–2693. [DOI] [PubMed] [Google Scholar]
- Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest 2006; 116: 3183–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Presti E, Dieli F, Meraviglia S. Tumor-Infiltrating gammadelta T lymphocytes: pathogenic role, clinical significance, and differential programing in the tumor microenvironment. Front Immunol 2014; 5: 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Huang J, Zhang C, Yang H, Qiu H, Li J et al. Infiltration of dendritic cells and T lymphocytes predicts favorable outcome in epithelial ovarian cancer. Cancer Gene Ther 2015; 22: 198–206. [DOI] [PubMed] [Google Scholar]
- Khan H, Pillarisetty VG, Katz SC. The prognostic value of liver tumor T cell infiltrates. J Surg Res 2014; 191: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlan B, Simsa P, Foldi J, Fridman W, Glassy M, McKnight M et al. Immunoglobulin repertoire of B lymphocytes infiltrating breast medullary carcinoma. Hum Antibodies 2003; 12: 113–121. [PubMed] [Google Scholar]
- Tesar B, Chalasani G, Smith-Diggs L, Baddoura F, Lakkis F, Goldstein D. Direct antigen presentation by a xenograft induces immunity independently of secondary lymphoid organs. J Immunol 2004; 173: 4377–4386. [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity 2006; 25: 643–654. [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 2004; 10: 927–934. [DOI] [PubMed] [Google Scholar]
- Oda J, Hirata B, Guembarovski R, Watanabe M. Genetic polymorphism in FOXP3 gene: imbalance in regulatory T-cell role and development of human diseases. J Genet 2013; 92: 163–171. [DOI] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006; 66: 1123–1131. [DOI] [PubMed] [Google Scholar]
- Wulff H, Krieger T, Kruger K, Stahmer I, Thaiss F, Schafer H et al. Cloning and characterization of an adenoviral vector for highly efficient and doxycycline-suppressible expression of bioactive human single-chain interleukin 12 in colon cancer. BMC Biotechnol 2007; 7: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood 1999; 93: 1634–1642. [PubMed] [Google Scholar]
- Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med 2001; 7: 1118–1122. [DOI] [PubMed] [Google Scholar]
- Bauer TM, Jiga LP, Chuang JJ, Randazzo M, Opelz G, Terness P. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl Int 2005; 18: 95–100. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res 2004; 64: 5839–5849. [DOI] [PubMed] [Google Scholar]
- Gibson A, Ogese M, Sullivan A, Wang E, Saide K, Whitaker P et al. Negative regulation by PD-L1 during drug-specific priming of IL-22-secreting T cells and the influence of PD-1 on effector T cell function. J Immunol 2014; 192: 2611–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R et al. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res 2005; 306: 128–141. [DOI] [PubMed] [Google Scholar]
- Jia YL, Shi L, Zhou JN, Fu CJ, Chen L, Yuan HF et al. Epimorphin promotes human hepatocellular carcinoma invasion and metastasis through activation of focal adhesion kinase/extracellular signal-regulated kinase/matrix metalloproteinase-9 axis. Hepatology 2011; 54: 1808–1818. [DOI] [PubMed] [Google Scholar]
- Sim GC, Radvanyi L. The IL-2 cytokine family in cancer immunotherapy. Cytokine Growth Factor Rev 2014; 25: 377–390. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014; 192: 5451–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chao QG, Ping LZ, Xue C, Xia ZY, Qian D et al. The prevalence of FOXP3+ regulatory T-cells in peripheral blood of patients with NSCLC. Cancer Biother Radiopharm 2009; 24: 357–367. [DOI] [PubMed] [Google Scholar]
- Trzonkowski P, Szmit E, Mysliwska J, Dobyszuk A, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol 2004; 112: 258–267. [DOI] [PubMed] [Google Scholar]