Abstract
Background/Aims
The pathogen Acinetobacter baumannii is increasingly causing healthcare-associated infections worldwide, particularly in intensive care units. Biofilm formation, a factor contributing to the virulence of A. baumannii, is associated with long-term persistence in hospital environments. The present study investigates the clinical impact of biofilm production on colonization and acquisition after patient admission.
Methods
Forty-nine A. baumannii isolates were obtained between August and November 2013 from Keimyung University Dongsan Medical Center, Daegu, Korea. All isolates were obtained from sputum samples of new patients infected or colonized by A. baumannii. The microtiter plate assay was used to determine biofilm formation.
Results
Twenty-four A. baumannii isolates (48%) demonstrated enhanced biofilm formation capacity than that of the standard A. baumannii strain (ATCC 19606). All isolates were resistant to carbapenem, 38 isolates (77%) were collected from patients in an intensive care unit, and 47 isolates (95%) were from patients who had been exposed to antibiotics in the previous month. The median duration of colonization was longer for biofilm-producing isolates than that of the biofilm non-biofilm producing isolates (18 days vs. 12 days, p < 0.05). Simultaneous colonization with other bacteria was more common for biofilm-producing isolates than that for the non-biofilm producing isolates. The most prevalent co-colonizing bacteria was Staphylococcus aureus.
Conclusions
Biofilm-producing isolates seem to colonize the respiratory tract for longer durations than the non-biofilm producing isolates. During colonization, biofilm producers promote co-colonization by other bacteria, particularly S. aureus. Additional research is required to determine possible links between biofilm formation and nosocomial infection.
Keywords: Acinetobacter baumannii, Biofilms, Cross infection
INTRODUCTION
An important risk factor for nosocomial infection is prior colonization by multidrug resistant organisms. Acinetobacter baumannii have emerged as a major cause of hospital-acquired infections, especially for ventilator-associated pneumonia in Asia and Europe [1]. High prevalence of A. baumannii in ventilator-associated pneumonia and hospital-acquired pneumonia in Asian countries is a well-known problem [2]. From an epidemiological point of view, the most remarkable features of A. baumannii are its long-term survival in the environment and development of resistance to most antimicrobial agents [3].
Biofilm formation is one of the virulence factors of A. baumannii associated with long-term survival in a hospital environment [4]. A. baumannii can survive on fingertips, plastics, other environmental surfaces, and even dry surfaces. Biofilm formation contributes to this taxon’s high level of resistance to desiccation and disinfection, facilitating the survival of bacteria in a hospital setting [5]. Moreover, the ability to form biofilm facilitates contact with susceptible patients, leading to outbreaks of medical device-related infections and ventilator-associated pneumonia [6,7].
Previous studies report several risk factors for acquiring A. baumannii after admission. These risk factors are prolonged hospitalization; prior antibiotic usage; residence in an intensive care unit (ICU); presence of foreign devices; high colonization pressure; and prolonged mechanical ventilation [8,9]. However, relatively few studies address the biofilm effect on colonization and acquisition of A. baumannii after admission. The aim of the present study was to determine the impact of biofilm production on colonization and acquisition of A. baumannii.
METHODS
Bacterial strains
A total of 49 A. baumannii isolates from sputum were obtained between August and November of 2013 at Keimyung University Dongsan Medical Center, Daegu, Korea. All isolates were collected from patients newly infected or colonized by A. baumannii. The isolates were identified using conventional biochemical methods and 16S rRNA sequencing. A. baumannii ATCC 19606 (ATCC, Manassas, VA, USA) was used as a positive control in the biofilm formation assay, and Escherichia coli DH-5α (ATCC) was used as a negative control. A. baumannii ATCC 19606, E. coli DH-5α, and the 49 clinical isolates of A. baumannii were maintained on Muller-Hinton agar at 35°C.
Antibiotic susceptibility testing was conducted using a VITEK II system with NH cards (bioMérieux, Marcy-l’Étoile, France), following the methodology and breakpoints defined by the Clinical and Laboratory Standards Institute.
Variables and definitions
The following patient data were collected: age, sex, date of admission, date of discharge, underlying conditions, invasive procedures, antibiotic usage in the previous 1 month, infection or colonization, hospital stay before and after isolation, therapy, and 30-day mortality. Pneumonia was diagnosed according to criteria developed by the Centers for Disease Control and Prevention (United States) [10].
We defined colonization duration as the time from the day of the first A. baumannii-positive culture to the first day when A. baumannii was not detected in culture. Persistent colonization was the sustained detection of A. baumannii until the final culture report. In addition, we required the final culture study to have been conducted within 1 week of the day of discharge or death. Clear-up meant that A. baumannii was no longer reported by any subsequent culture tests. This study was approved by the Institutional Review Board of Keimyung University Dongsan Medical Center (IRB 2013-11-040-004).
Biofilm formation assay
Biofilm formation was assayed by crystal violet staining, as described previously [11]. Fresh bacterial suspensions were prepared from overnight cultures, and each were adjusted to an optical density (OD600) of 0.1. The bacterial suspensions (100 μL) were inoculated in individual wells of a 96-well plate and incubated at 35°C for 24 hours. After an overnight incubation, the plates were gently washed twice with 200 μL phosphate-buffered saline (PBS), air-dried, and stained with 0.1% crystal violet (100 μL) for 15 minutes at room temperature. Plates were gently washed twice with PBS, the stains were solubilized with 99% ethanol, and the OD570 of the supernatant in each well was measured using a Victor 3 microplate reader (PerkinElmer, Waltham, MA, USA). Biofilm-producing isolates were defined as those for which the optical density of solubilized crystal violet in 99% ethanol was higher than the average optical density of solubilized crystal violet for A. baumannii ATCC 19606. Average optical density of solubilized crystal violet for A. baumannii ATCC 19606 was calculated using measurements of the positive control well on each plate. All experiments were performed in triplicate. Each plate included the following controls: media alone, A. baumannii ATCC 19606 (positive control), and E. coli DH-5α (negative control).
Statistical analysis
Data management and statistical analyses were performed with SPSS software version 21.0 (IBM Co., Armonk, NY, USA). All data were first subjected to bivariate analysis. Categorical variables were compared using the chi-square test or Fisher exact test, and continuous variables were compared using the Mann-Whitney U test. Conditions contributing to colonization duration were analyzed using chi-square univariate analysis; those conditions that were significant were included in a subsequent multiple logistic regression analysis to calculate confidence intervals. All tests of significance were two-tailed. We considered tests significant when they had p values of 0.05 or below.
RESULTS
Biofilm mass of A. baumannii
The ability of A. baumannii ATCC 19606 to form a biofilm is established [12]. Of the 49 isolates examined, 24 (48%) exhibited enhanced biofilm formation capacity relative to a standard A. baumannii strain (Fig. 1).
Figure 1.
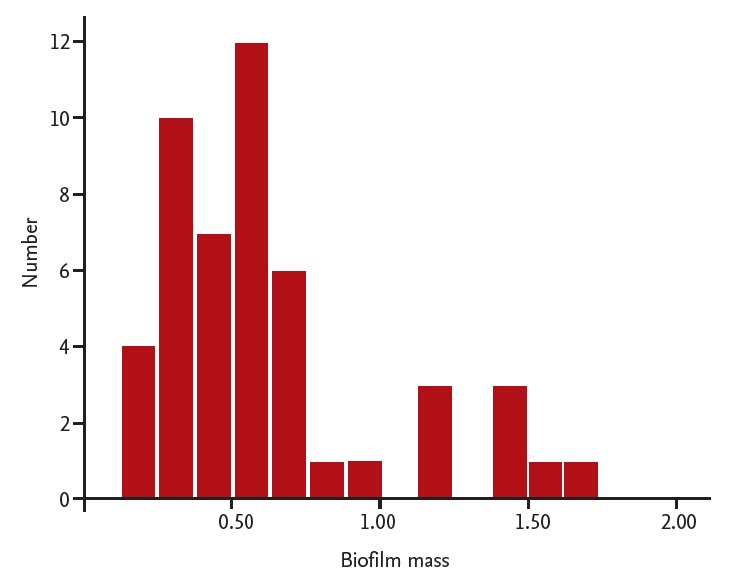
The biofilm mass of Acinetobacter baumannii clinical isolates. To measure the relative amount of the biofilm, the crystal violet stained biofilm was solubilized with ethanol for 5 minutes. Solubilized crystal violet was measured at optical density (OD570nm) using a Victor 3 microplate reader (PerkinElmer). Biofilm-producing isolates were defined as those wells for which the optical density was higher than the average well optical density for A. baumannii ATCC 19606. The average well optical density for the standard strain of A. baumannii ATCC 19606 was 0.513.
Antibiotic resistance of A. baumannii
Antibiotic resistances were similar between the biofilm-producer group and the non-producer group. All isolates were carbapenem resistant A. baumannii. Thirty-eight isolates (77%) were collected from patients in the ICU and a total of 47 patients (95%) were exposed to antibiotics within the previous month. Resistance to aminoglycosides and tigecycline seemed to be higher in the biofilm non-producer group than in the biofilm producer group, with no statistical significance (Table 1).
Table 1.
Antimicrobial resistance rates of biofilm-producing and non-producing Acinetobacter baumannii
| Variable | Biofilm producer (n = 24) | Biofilm non-producer (n = 25) | p value |
|---|---|---|---|
| Amikacin | 1 (4.2) | 5 (20) | 0.189 |
| Ampicillin/Sulbactam | 24 (100) | 23 (92) | 0.490 |
| Aztreonam | 24 (100) | 25 (100) | |
| Cefepime | 24 (100) | 23 (92) | 0.490 |
| Cefotaxime | 24 (100) | 24 (96) | 1.000 |
| Ceftazidime | 24 (100) | 24 (96) | 1.000 |
| Ciprofloxacin | 24 (100) | 24 (96) | 1.000 |
| Colistin | 0 | 0 | |
| Gentamicin | 24 (100) | 24 (96) | 1.000 |
| Imipenem | 24 (100) | 24 (96) | 1.000 |
| Meropenem | 24 (100) | 24 (96) | 1.000 |
| Minocycline | 4 (16.7) | 6 (24) | 0.725 |
| Piperacillin | 24 (100) | 24 (96) | 1.000 |
| Piperacillin/Tazobactam | 24 (100) | 24 (96) | 1.000 |
| Ticarcillin/Clavulanic | 24 (100) | 24 (96) | 1.000 |
| Tigecycline | 2 (8.3) | 6 (24) | 0.247 |
| TMP/SMX | 24 (100) | 23 (92) | 0.490 |
Values are presented as number (%).
TMP/SMX, trimethoprim/sulfamethoxazole.
Clinical characteristics of biofilm producer hosts and biofilm non-producer hosts
The average patient age was lower for the biofilm producer group (66.5 vs. 75, p = 0.017). There was no statistical difference in the underlying diseases of patients harboring biofilm producers versus those harboring biofilm non-producers. Twenty-seven patients (55%) had an endotracheal tube and 17 (34%) were on mechanical ventilation. No underlying patient disease was more common to the biofilm producer group (Table 2).
Table 2.
Epidemiologic and predisposing factors for colonization with biofilm-producing or non-producing Acinetobacter baumannii
| Variable | Biofilm producer (n = 24) | Biofilm non-producer (n = 25) | p value |
|---|---|---|---|
| Age, yr | 66.5 (55.5–74.75) | 75 (68.5–79) | 0.017 |
| Male sex | 19 (79.2) | 16 (64) | 0.345 |
| No underlying disease | 14 (58.3) | 4 (16) | 0.003 |
| Malignancy | 5 (20.8) | 4 (16) | 0.725 |
| Liver cirrhosis | 1 (4.2) | 4 (16) | 0.349 |
| Cardiovascular disease | 9 (37.5) | 14 (56) | 0.256 |
| Neurologic disease | 9 (37.5) | 7 (28) | 0.551 |
| Diabetes mellitus | 5 (20.8) | 5 (20) | 1.000 |
| Chronic lung disease | 3 (12.5) | 5 (20) | 0.702 |
| Urinary catheter | 18 (75) | 20 (80) | 0.742 |
| Central venous catheter | 9 (37.5) | 16 (64) | 0.089 |
| Parenteral nutrition | 12 (50) | 14 (56) | 0.778 |
| Endotracheal tube | 13 (54.2) | 14 (56) | 1.000 |
| Mechanical ventilator | 7 (29.2) | 10 (40) | 0.551 |
Values are presented as median (interquartile range) or number (%).
Impact of biofilm on colonization of A. baumannii
Thirty-seven isolates (75%) had persistently colonized their host patient’s respiratory tract. The median duration of colonization was longer for biofilm-producing isolates than it was for biofilm non-producing isolates. Simultaneous colonization with other bacteria was more common for biofilm-producing isolates. The most prevalent co-colonizing bacteria were Staphylococcus aureus. The median timespan from admission to acquisition seemed to be shorter for the biofilm-producing group; however, this difference was not statistically significant (Table 3).
Table 3.
The impact of biofilm on colonization by Acinetobacter baumannii
| Variable | Biofilm producer (n = 24) | Biofilm non-producer (n = 25) | p value |
|---|---|---|---|
| Persistence | 18 (75) | 19 (76) | 1.000 |
| Clear-up | 1 (4.2) | 1 (4.0) | 1.000 |
| Duration, day | 18 (8–47.75) | 12 (2.5–23) | 0.044 |
| Admission to colonization, day | 9 (4–22.5) | 12 (7.5–15.75) | 0.299 |
| Pathogen | 2 (8.3) | 5 (20) | 0.417 |
| Colonization | 22 (91.7) | 20 (80) | |
| Concomitant bacteria | 18 (75) | 11 (44) | 0.042 |
| Staphylococcus aureus | 12 (66.7) | 5 (45.5) | 0.438 |
| Pseudomonas aeruginosa | 5 (27.8) | 2 (18.32) | 0.677 |
| Klebsiella pneumoniae | 5 (27.8) | 3 (27.3) | 1.000 |
Values are presented as number (%) or median (interquartile range).
Conditions associated with colonization duration
We conducted a multivariate analysis to confirm the relationship of biofilm formation and colonization duration. In the multivariate analysis, isolates with colonization durations of more than 2 weeks are significantly associated with biofilm and ICU stays of more than 2 weeks (Table 4).
Table 4.
Multivariate analysis for conditions contributing to colonization duration
| Variable | Colonization duration > 2 wk (n = 22) | Colonization duration < 2 wk (n = 27) | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |||
| Biofilm | 16 (72.7) | 8 (29.6) | 6.333 (1.814–22.107) | 0.004 | 7.809 (1.305–46.736) | 0.024 |
| ICU stay > 2 wk | 15 (83.3) | 6 (30) | 11.667 (2.438–55.833) | 0.001 | 12.363 (1.927–79.310) | 0.008 |
| Endotracheal tube | 15 (68.2) | 12 (44.4) | 2.679 (0.827–8.675) | 0.149 | 2.080 (0.355–12.202) | 0.417 |
Values are presented as number (%).
OR, odds ratio; CI, confidence interval; ICU, intensive care unit.
DISCUSSION
In the present study, we evaluated the impact of biofilm on host acquisition and colonization for A. baumannii. The biofilm-producing isolates seem to colonize the respiratory tract for longer durations than isolates not producing biofilm. During the colonization, biofilm producers also facilitate co-colonization by other bacteria, particularly S. aureus.
Previously, we reported higher rates of biofilm production by Korean A. baumannii nosocomial samples [13]. Similarly, biofilm production in the present study varies extensively among strains, and 48% of the A. baumannii clinical isolates exhibit a greater capacity for biofilm formation than A. baumannii ATCC 19606 exhibits. There are well known contributors to A. baumannii acquisition, such as colonization pressure, ICU admission, duration of hospitalization, and prior antibiotic use. Previous studies confirm that acquisition of multidrug resistant A. baumannii positively correlates with colonization pressure [8]. In addition, duration of hospitalization before admission is an important risk factor for infection by multidrug resistant A. baumannii [9]. Longterm care facilities are major reservoirs of multidrug resistant bacteria. Current wound management, in situ medical devices, and pressure ulcers are risk factors for multidrug resistant bacteria colonization in long-term care facilities [14].
In this study, biofilm-producing strains colonize the patient’s respiratory tract for longer durations than biofilm non-producing strains. The longer colonization lasts, the higher the colonization pressure climbs. Heightened colonization pressure means that larger numbers of naive patients acquire multidrug resistant A. baumannii. Previous studies on ventilator-associated pneumonia report that airway colonization, biofilm formation, and pneumonia development have a microbial link [15,16]. Biofilm-producing strains colonize the patient’s respiratory tract for longer and put the patient at high risk of developing pneumonia.
There are many reports of A. baumannii interacting with abiotic surfaces. However, few studies address the interaction of biotic surfaces with other bacteria, or fungi. Previous studies report that S. aureus and Candida albicans can co-exist in biofilm with synergistic effects. The biofilm mass of S. aureus and C. albicans is much higher when they grow together. Scanning electron microscope images reveal extensive adherence of S. aureus to hyphae of C. albicans [17]. In a related study, A. baumannii was co-cultured with C. albicans. This study reveals that A. baumannii 19606 attaches to C. albicans filaments, forming aggregates on the surfaces of the fungal filaments. Deletion of the OmpA gene results in a defect in the interaction of C. albicans and A. baumannii.
Also, A. baumannii interacts with the human alveolar epithelial cell. Attachment of A. baumannii causes human alveolar epithelial cell rounding, loss of cell projections, and their detachment from the plates [18]. The effect of biofilm on interaction with human bronchial epithelial cells is also reported. A. baumannii isolates carrying blaPER-1 show a heightened capacity for epithelial cell adherence and biofilm formation. The results show that biofilm formation correlates with epithelial cell adherence [4]. This is a possible explanation for biofilm forming multidrug resistant A. baumannii correlating with poor outcomes in hospital-acquired pneumonia. In addition, biofilm associated infections are more resistant to antimicrobial agents. Because of such circumstances, we use interventions such as contact precautions, environmental cleaning, active surveillance, and restrictions on administering broad-spectrum antibiotics for controlling A. baumannii.
This study has several limitations. We do not investigate the actual connection to infection from the colonization. Also, most of the isolates were colonization and multidrug resistant organism. We cannot evaluate the effect of biofilm on infection, clinical outcome, or antibiotic resistance. This study is retrospective, and the isolates were collected at a single center over a short duration. Further prospective studies conducted in larger patient populations involving multiple centers are needed. Biofilm-forming strains co-colonize with S. aureus. However, little is known about the interactions between A. baumannii and S. aureus.
These results suggest the need for further investigation of interactions between A. baumannii and other bacteria. Additional research is needed on possible links between colonization of biofilm-producing strains and nosocomial infections.
KEY MESSAGE
1. Biofilm production can vary extensively among the strains. Forty-eight percent of the Acinetobacter baumannii clinical isolates exhibited a greater capacity for biofilm formation than exhibited by A. baumannii ATCC 19606.
2. The median duration of colonization was longer for biofilm-producing isolates than for biofilm non-producing isolates.
3. Simultaneous colonization with other bacteria was more common in biofilm-producing isolates. The most prevalent co-colonizing bacteria were Staphylococcus aureus.
Acknowledgments
The authors thank Hui-Jung Jung, Hyejin Park, and Yun yi Yang for support and technical assistance in experiments. This study was made possible by the 2013 Samsung Eye Hospital grant.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung DR, Song JH, Kim SH, et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184:1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 3.Talbot GH, Bradley J, Edwards JE, Jr, et al. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 4.Lee HW, Koh YM, Kim J, et al. Capacity of multidrug-resistant clinical isolates of Acinetobacter baumannii to form biofilm and adhere to epithelial cell surfaces. Clin Microbiol Infect. 2008;14:49–54. doi: 10.1111/j.1469-0691.2007.01842.x. [DOI] [PubMed] [Google Scholar]
- 5.Espinal P, Marti S, Vila J. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J Hosp Infect. 2012;80:56–60. doi: 10.1016/j.jhin.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Da Silva G, Dijkshoorn L, van der Reijden T, van Strijen B, Duarte A. Identification of widespread, closely related Acinetobacter baumannii isolates in Portugal as a subgroup of European clone II. Clin Microbiol Infect. 2007;13:190–195. doi: 10.1111/j.1469-0691.2006.01628.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaliterna V, Goic-Barisic I. The ability of biofilm formation in clinical isolates of Acinetobacter baumannii belonging to two different European clones causing outbreaks in the Split University Hospital, Croatia. J Chemother. 2013;25:60–62. doi: 10.1179/1973947812Y.0000000052. [DOI] [PubMed] [Google Scholar]
- 8.Arvaniti K, Lathyris D, Ruimy R, et al. The importance of colonization pressure in multiresistant Acinetobacter baumannii acquisition in a Greek intensive care unit. Crit Care. 2012;16:R102. doi: 10.1186/cc11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheng WH, Liao CH, Lauderdale TL, et al. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int J Infect Dis. 2010;14:e764–e769. doi: 10.1016/j.ijid.2010.02.2254. [DOI] [PubMed] [Google Scholar]
- 10.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Pettit RK, Weber CA, Kean MJ, et al. Microplate Alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob Agents Chemother. 2005;49:2612–2617. doi: 10.1128/AAC.49.7.2612-2617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology. 2003;149(Pt 12):3473–3484. doi: 10.1099/mic.0.26541-0. [DOI] [PubMed] [Google Scholar]
- 13.Kim HA, Ryu SY, Seo I, Suh SI, Suh MH, Baek WK. Biofilm formation and colistin susceptibility of Acinetobacter baumannii isolated from Korean nosocomial samples. Microb Drug Resist. 2015;21:452–457. doi: 10.1089/mdr.2014.0236. [DOI] [PubMed] [Google Scholar]
- 14.Lim CJ, Cheng AC, Kennon J, et al. Prevalence of multidrug-resistant organisms and risk factors for carriage in long-term care facilities: a nested case-control study. J Antimicrob Chemother. 2014;69:1972–1980. doi: 10.1093/jac/dku077. [DOI] [PubMed] [Google Scholar]
- 15.Pneumatikos IA, Dragoumanis CK, Bouros DE. Ventilator-associated pneumonia or endotracheal tube-associated pneumonia? An approach to the pathogenesis and preventive strategies emphasizing the importance of endotracheal tube. Anesthesiology. 2009;110:673–680. doi: 10.1097/ALN.0b013e31819868e0. [DOI] [PubMed] [Google Scholar]
- 16.Gil-Perotin S, Ramirez P, Marti V, et al. Implications of endotracheal tube biofilm in ventilator-associated pneumonia response: a state of concept. Crit Care. 2012;16:R93. doi: 10.1186/cc11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zago CE, Silva S, Sanita PV, et al. Dynamics of biofilm formation and the interaction between Candida albicans and methicillin-susceptible (MSSA) and -resistant Staphylococcus aureus (MRSA) PLoS One. 2015;10:e0123206. doi: 10.1371/journal.pone.0123206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaddy JA, Tomaras AP, Actis LA. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect Immun. 2009;77:3150–3160. doi: 10.1128/IAI.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


