Abstract
In 2001, highly evolved type 1 circulating vaccine-derived poliovirus (cVDPV) was isolated from three acute flaccid paralysis patients and one contact from three separate communities in the Philippines. Complete genomic sequencing of these four cVDPV isolates revealed that the capsid region was derived from the Sabin 1 vaccine strain but most of the noncapsid region was derived from an unidentified enterovirus unrelated to the oral poliovirus vaccine (OPV) strains. The sequences of the cVDPV isolates were closely related to each other, and the isolates had a common recombination site. Most of the genetic and biological properties of the cVDPV isolates were indistinguishable from those of wild polioviruses. However, the most recently identified cVDPV isolate from a healthy contact retained the temperature sensitivity and partial attenuation phenotypes. The sequence relationships among the isolates and Sabin 1 suggested that cVDPV originated from an OPV dose given in 1998 to 1999 and that cVDPV circulated along a narrow chain of transmission. Type 1 cVDPV was last detected in the Philippines in September 2001, and population immunity to polio was raised by extensive OPV campaigns in late 2001 and early 2002.
Immunization with the oral poliovirus vaccine (OPV) is the cornerstone of the World Health Organization's program for the global eradication of poliomyelitis (15, 44, 55, 56, 65). The attenuated OPV strains of the three poliovirus serotypes (Sabin 1, 2, and 3) replicate in the gut of OPV recipients and can efficiently induce type-specific humoral and mucosal immunity (55), mimicking natural infection. However, replication of OPV in humans is frequently accompanied by genetic change of the vaccine virus, including reversion of key attenuating mutations (5, 42), introduction of other mutations throughout the genome, and intertypic recombination among OPV strains (7, 16). The phenotypic reversion of the OPV strains to neurovirulence is the underlying mechanism for the rare cases of vaccine-associated paralytic poliomyelitis among OPV recipients or their close contacts (41, 54, 55). Cases of vaccine-associated paralytic poliomyelitis in immunocompetent persons are generally associated with poliovirus types 2 and 3 and very rarely with type 1 (54). The large majority of OPV isolates from healthy individuals, the environment, or patients with vaccine-associated paralytic poliomyelitis are closely related to the original OPV strain (Sabin-like), diverging by <1.0% of nucleotide sequences encoding the major capsid protein VP1 (8, 9, 39). The low nucleotide sequence diversities from the respective OPV strains are consistent with the short duration of most poliovirus infections (1) and the usually restricted spread of OPV virus (3).
It is now apparent that in areas with widening gaps in population immunity to poliovirus, especially where wild poliovirus circulation has ceased, viruses derived from OPV may circulate within a population, cause cases of paralytic poliomyelitis, and accumulate further mutations. OPV-derived polioviruses with 1 to 15% sequence divergence from the Sabin strains are now defined as vaccine-derived polioviruses (VDPVs) (8, 9), with the extent of divergence roughly proportional to the duration of viral replication (2, 27, 37) or circulation (25, 30, 66) since administration of the initiating OPV dose. In 2000 to 2001, a poliomyelitis outbreak associated with circulating VDPVs (cVDPVs) occurred on the island of Hispaniola (which is divided into the Dominican Republic and Haiti), underscoring the risk of using OPV at low rates of coverage in polio-free areas (15, 25, 28, 30, 44). Furthermore, retrospective genetic studies identified the endemic circulation of a type 2 cVDPV in Egypt from about 1983 to 1993 (66). More recently, intensified acute flaccid paralysis and laboratory surveillance led to the identification of cVDPV outbreaks in the Philippines in 2001 (type 1) (58, 62) and Madagascar in 2001 to 2002 (type 2) (51).
In this report, we describe the circulation of the type 1 cVDPV in the Philippines in 2001 and the genetic and biological properties of the four cVDPV isolates. Three of the isolates were from children with acute flaccid paralysis, a common clinical manifestation of poliomyelitis. The first case was identified in March 2001 on the southern island of Mindanao in the Philippines, and two additional cases were identified in July 2001, situated about 800 km to the north on the island of Luzon (Fig. 1). The fourth isolate was from a healthy contact of one of the poliomyelitis patients in Cavite province, close to metropolitan Manila. Sequence comparisons revealed that all four cVDPVs were closely related to each other (≈99% VP1 nucleotide sequence identity), divergent from Sabin 1 (≈97% of VP1 nucleotide sequence identity), and independent of type 1 VDPVs heretofore found elsewhere. Recombinant genomes as all noncapsid sequences downstream of a common crossover site in the 2B region were derived from an as yet unidentified enterovirus. Most of the biological properties of the Philippines cVDPVs were indistinguishable from those of wild-type 1 polioviruses. Thus, the biological and genetic characteristics of the type 1 VDPVs from acute flaccid paralysis cases in the Philippines were similar to those of the cVDPVs reported from Hispaniola, Egypt, and Madagascar.
FIG. 1.
Distribution of acute flaccid paralysis cases associated with type 1 cVDPVs in the Philippines in 2001. Three acute flaccid paralysis cases, in Misamis Oriental (isolate Mindanao-01-1), in Laguna (isolate Luzon-01-1), and in Cavite (isolate Luzon-01-2), are mapped by open circles. Isolate Luzon-01-2c was from a contact of the Cavite case. Shading indicates approximate population densities.
MATERIALS AND METHODS
Virus isolation and identification.
Viruses were isolated from stool specimens on the RD (human rhabdomyosarcoma cells; ATCC CCL-136) and L20B (mouse fibroblast cells expressing the human poliovirus receptor) cell lines by standard methods (64). Polioviruses were identified by microneutralization with type-specific antisera. HEp-2C cells (human cervical carcinoma cells) were also used for preparation of the virus stocks.
Intratypic differentiation of polioviruses.
Two different methods, one based on genetic properties (nucleic acid probe hybridization or PCR) and the other based on antigenic properties by enzyme-linked immunosorbent assay (ELISA), were used for intratypic differentiation to distinguish between vaccine and wild polioviruses of the poliovirus isolates (8, 9). Concordance of the intratypic differentiation results by two methods may indicate a vaccine or wild poliovirus. Isolates with discordant intratypic differentiation results (vaccine-like by one method and non-vaccine-like by the other) are further characterized by genomic sequencing.
For the probe hybridization, poliovirus RNA was inactivated by formaldehyde and immobilized on two nylon membranes for probing with digoxigenin-labeled oligonucleotides (14). One probe targeted highly conserved sequences within the 5′ nontranslated region (5′-NTR) of enteroviruses, while the other was an OPV strain-specific probe directed to variable VP1 sequences. Alternatively, viral RNA was amplified by reverse transcription-PCR (RT-PCR) with oligonucleotide primers directed to the same genomic regions targeted by the nucleic acid probes. The results identify a virus isolate as either vaccine-like or wild. The intratypic differentiation-ELISA used serotype-specific polyclonal antisera raised against intact virus particles of the Sabin OPV strains or a reference wild poliovirus strain of each serotype (60). The antiserum was cross-adsorbed against the heterologous strain of the same serotype to remove antibodies directed to shared antigenic sites. Four patterns of reactivity may be observed: vaccine-like, non-vaccine-like, double-reactive, and nonreactive. An isolate with any of the last three reactivity patterns could be either a wild poliovirus or an antigenically drifted OPV-derived poliovirus having a mutation(s) in a capsid surface determinant(s).
Sequencing.
Viral RNA was extracted from the virus stocks with the Qiagen viral RNA kit (Qiagen K.K., Tokyo, Japan) according to the manufacturer's instructions and used for RT-PCR amplification by standard procedures. The RT-PCR products were purified with the QiaQuick PCR system (Qiagen K.K.). The entire VP1 or full-length genome sequence of the purified PCR products of both strands were determined with oligonucleotide primers with an ABI Prism 310 genetic analyzer (Applied Biosystems Japan Ltd., Tokyo, Japan).
The sequencing primers were designed according to the genome sequence of Sabin 1 or by primer walking. The 5′-end sequence was determined with the 5′-rapid amplification of cDNA ends (RACE) system, version 2.0 (Invitrogen, Tokyo, Japan) according to the manufacturer's instructions. Briefly, the CAV-650 (5′-TACTTAGAGTAAACACACTC-3′) primer was used for reverse transcription, and the PV3S-460A (antisense; 5′-GGTTAGGAATTAGCCGCATTC-3′) and Abridged Anchor (sense; provided by the manufacturer) primers were used for PCR amplification. The PV3S-300A primer (antisense; 5′-GGCCCAAGCTACACTCCGGG-3′) was used to determine the 5′-terminal sequence. The 3′-end RT-PCR amplicon was produced with the Access RT-PCR system (Promega K.K., Tokyo, Japan). The PV1S-6530S (sense; 5′-AGTTTGAATGACTCAGTGGC-3′) and 3′-end (antisense; 5′-GACCACGCGTATCGATGTCGACTTTTTTTTTTTTTTTTV-3′, where V is a mixture of A, G, and C) primers were used for PCR amplification, and the sequence was determined with the PV1S-7080S primer (sense; 5′-TTGAAACAGTCACATGGGAG-3′). The resultant chromatogram data were analyzed with Sequencher software (Hitachi Software Engineering Co., Ltd., Kanagawa, Japan).
Phylogenetic analysis.
The entire VP1 sequences were determined for the initial genetic characterization of poliovirus isolates with discordant intratypic differentiation results. Sequence alignments were performed with the Clustal W software (57), and phylogenetic trees were constructed by the neighbor-joining method with Kimura's two-parameter method (52). The reliability of the tree was estimated with 1,000 bootstrap replicates. The tree was displayed with the program TreeView (49).
Comparative analysis of the genome sequences of VDPVs.
The full-length genome sequences of the reference strains of polioviruses, cVDPVs, and species C enteroviruses were obtained from the GenBank database. Pairwise comparisons among nucleotide sequences in each genome region and multiple sequence alignments were analyzed with Genetyx software (Software Development Co., Ltd., Tokyo, Japan). The numbering of nucleotide positions follows that of the original sequence report of Sabin 1 (45).
Neurovirulence test in TgPVR-21 mice.
ICR-PVR-Tg21 mice, carrying the human poliovirus receptor gene, were purchased from the Central Laboratory of Experimental Animals (Kanagawa, Japan) and used for neurovirulence tests as described previously (32, 66). Briefly, six mice (three males and three females) were inoculated intracerebrally with 30 μl of each virus dilution of the type 1 cVDPV, attenuated (Sabin 1) and virulent wild (Mahoney) reference strains per mouse. The mice were observed daily for 14 days, and the dose causing paralysis or death in 50% of the mice (PD50) was calculated by the Kärber formula.
Single-step growth kinetics.
The growth kinetics of the cVDPV isolates at 39.5°C in HeLa S3 cells (ATCC CCL-2.2) was compared with those of the type 1 reference strains as described previously (66). Virus yields were examined at various times after inoculation (input multiplicity = 10 PFU/cell) in HeLa S3 cell suspensions. Virus titers were determined in duplicate in plaque assays on monolayers of HeLa cells (ATCC CCL-2) at 37°C.
Neutralization titer against monoclonal antibodies.
The antigenic properties of the cVDPV isolates were analyzed by a microneutralization assay, with monoclonal antibodies specific to Sabin 1 (8a034 and 8a057) or the Mahoney strain (11m071) (24). Fifty microliters of the monoclonal antibodies was diluted twofold and incubated with an equal volume of the challenge virus (≈100 cell culture infective doses per 50 μl) for 2 h at 36°C. Then, 100 μl of a suspension of HEp-2C cells per well was added, and cytopathic effects were examined for 7 days. The neutralization titers were determined by the Kärber formula.
Nucleotide sequence accession numbers.
The VP1 nucleotide sequences of the type 1 wild polioviruses determined in this study have been submitted to GenBank under accession numbers AB180058 to AB180069. The other VP1 sequences were obtained from the GenBank database. The complete genomic sequences of the four cVDPVs from the Philippines have been submitted under accession numbers AB180070 to AB180073.
RESULTS
Epidemiologic and clinical background.
Wild poliovirus types 1 and 3 were endemic in the Philippines until 1993 (26, 50), and the Philippines, along with the other countries in the Western Pacific Region, were certified as free of indigenous wild poliovirus in 2000 (63). Continued acute flaccid paralysis and poliovirus surveillance in the Philippines has not detected any wild poliovirus infections after 1993. Polio-free status has thus been sustained in the Philippines.
However, from March to July 2001, three acute flaccid paralysis cases associated with cVDPVs were reported in the Philippines (Table 1). The first patient was an 8-year-old boy from the province of Misamis Oriental in northern Mindanao, an island 800 km south of Manila (Fig. 1). The patient had a history of three doses of OPV, and the onset of paralysis occurred on 15 March 2001. The second patient, a 3-year-old girl from the province of Laguna, close to metropolitan Manila on Luzon Island, had a history of three doses of OPV, with onset of meningitis on 21 July 2001. The third patient was a 14-month-old boy from Cavite province (75 km north of the second case), who had a history of two doses of OPV and onset of paralysis on 26 July 2001. Only the third case had residual paralysis after 60 days from the date of onset of symptoms (Table 1). None of the patients had any history of travel outside of their resident areas since their birth, and no direct epidemiological link has been found among the three acute flaccid paralysis cases. A stool sample was collected from a healthy contact of patient 3 in Cavite on 23 September 2001.
TABLE 1.
Acute Flaccid paralysis and contact cases associated with type 1 cVDPV in the Philippines
| Virusa | Area/province | Age (yr)/sex | Sourceb | No. of OPV doses | Date of onset | Date of sampling | Residual paralysis |
|---|---|---|---|---|---|---|---|
| Mindanao-01-1 | Mindanao/Misamis Oriental | 8/M | AFP | 3 | 15 March 2001 | 28 March 2001 | No |
| Luzon-01-1 | Luzon/Laguna | 3/F | AFP; aseptic meningitis | 3 | 21 July 2001 | 28 July 2001 | No |
| Luzon-01-2 | Luzon/Cavite | 1/Mc | AFP | 2 | 26 July 2001 | 5 August 2001 | Yes |
| Luzon-01-2c | Luzon/Cavite | 3/F | Contact | Not available | Not applicable | 23 September 2001 | No |
Designations indicate geographic origin of specimen-year of isolation-case number and if the isolate was obtained from a contact (c).
AFP, acute flaccid paralysis.
14-month-old child.
To increase population immunity against polio and to interrupt cVDPV circulation, the Department of Health of the Philippines conducted local and nationwide OPV campaigns from December 2001 to March 2002 (50, 62). Consequently, no cVDPV has been identified in the Philippines since October 2001 under the intensified field and laboratory surveillance activities.
Virus isolation and identification.
The isolate from the first acute flaccid paralysis case, Mindanao-01-1, was identified as type 1 poliovirus by microneutralization. Probe hybridization and PCR identified the isolate as Sabin 1-like, but it was found to have non-vaccine-like antigenicity by intratypic differentiation-ELISA (Table 2). Nucleotide sequence analysis of the VP1 region revealed that the isolate was 3.1% divergent from the parental Sabin 1 strain, and it was thus classified as a VDPV (Table 2).
TABLE 2.
Initial characterization of Philippines type 1 cVDPVs
| Virus | Identification by antiserum neutralizationa | Intratypic differentiation methodb
|
Nucleotide diversity from Sabin 1 (%) | |
|---|---|---|---|---|
| Nucleic acid probe hybridization and/ or PCR | ELISA | |||
| Mindanao-01-1 | P1 | SL | NSL | 3.1 |
| Luzon-01-1 | P1 | SL | NSL | 3.4 |
| Luzon-01-2 | P1 | SL | NSL | 3.1 |
| Luzon-01-2c | P1 | SL | NSL | 3.5 |
P1, poliovirus type 1.
SL, Sabin-like; NSL, non-Sabin-like.
In response to the detection of a VDPV in Mindanao, surveillance for acute flaccid paralysis cases was intensified in the affected community and in other parts of the Philippines. Thirty-four poliovirus isolates were identified from 22 acute flaccid paralysis cases between March 2001 and December 2003. All isolates were determined to have vaccine-like genetic properties by nucleic acid probe hybridization or PCR. All but six of the poliovirus isolates tested had vaccine-like antigenic properties by intratypic differentiation-ELISA. Three type 1 isolates (Luzon-01-1 and Luzon-01-2 from acute flaccid paralysis cases and Luzon-01-2c from a contact of an acute flaccid paralysis case in Cavite) were identified from different patients in 2001 and resembled Mindanao-01-1 in having non-vaccine-like antigenic reactivity (Tables 1 and 2). Consequently, the four type 1 isolates with non-vaccine-like antigenic properties were analyzed further. A double reactive result by intratypic differentiation-ELISA occurred with two poliovirus type 1 isolates in 2002 and one type 3 isolate in 2003. However, sequencing subsequently determined that the type 1 isolates had <0.4% nucleotide variation from the VP1 sequence of Sabin 1, with no evidence of recombination in the noncapsid region (data not shown). Based upon the sequencing results, these isolates were identified as Sabin-like.
Nucleotide sequence analysis.
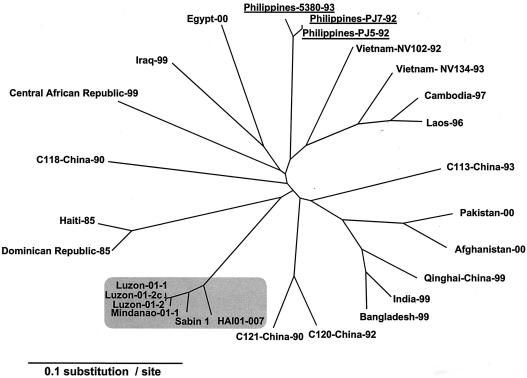
The four type 1 isolates with non-Sabin-like antigenic reactivity by intratypic differentiation-ELISA were initially characterized by analysis of their VP1 nucleotide sequences. The four isolates were found to be closely related to Sabin 1, differing by 3.1% to 3.5% of VP1 nucleotides (Table 2), indicating that all were VDPVs. The Philippines cVDPVs were unrelated (<82% VP1 nucleotide identity) to the wild type 1 polioviruses previously endemic in the Philippines (PJ5-92, PJ7-92, and 5380-93) (26, 50), to those in neighboring countries (11, 23, 31, 67), or those circulating until recently in other parts of the world (Fig. 2) (25, 29). When the VP1 nucleotide sequence relationships were summarized on a phylogenetic tree, the Philippines isolates clustered with Sabin 1 and a representative isolate (HAI01-007) from the Hispaniola cVDPV outbreak (Fig. 2).
FIG. 2.
Phylogenetic analysis of type 1 cVDPVs and the wild type 1 polioviruses based on VP1 nucleotide sequences. The unrooted radial neighbor-joining tree was drawn with the TreeView software. Type 1 cVDPVs (four from the Philippines and one from Haiti, HAI01-007) and Sabin 1 are shaded. Three type 1 wild polioviruses from the Philippines are underlined.
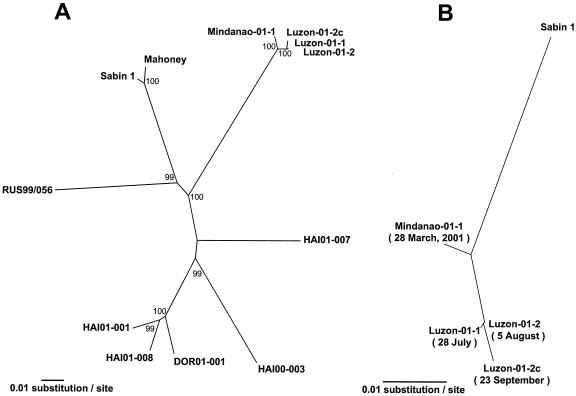
More detailed phylogenetic analysis of the complete genomic sequence relationships among the type 1 VDPV isolates revealed that the Philippines and Hispaniola cVDPVs followed independent pathways of divergence from Sabin 1 (Fig. 3A). The cVDPVs from both outbreaks were distinct from a sporadic type 1 VDPV isolated in Russia in 1999 (10). The Philippines cVDPV isolates were much more closely related to each other (99.0% to 99.9% complete genomic nucleotide identity) than were the Hispaniola cVDPV isolates (25) (Fig. 3A). When the GenBank database was screened with the BLAST search software, the virus determined to be most closely related to the Philippines cVDPV isolates was Sabin 1 (data not shown).
FIG. 3.
Phylogenetic analysis of type 1 VDPV isolates. (A) Complete genomic sequence relationships among type 1 VDPV isolates. cVDPV isolates are from the Philippines, Haiti (HAI), and Dominican Republic (DOR) (25). RUS99-056 is a type 1 VDPV from a sporadic acute flaccid paralysis case in Russia (10). Bootstrap values of >80% for each cluster are shown at the branch nodes. (B) Sequence relationships among Philippines type 1 cVDPV isolates based on the nucleotide sequences derived from Sabin 1 (5′-NTR, capsid, and 2A regions, nucleotides 1 to 3927).
Recombination.
All of the cVDPV isolates from Hispaniola (type 1), Egypt (type 2), and Madagascar (type 2) had highly evolved capsid sequences as well as recombinant noncapsid sequences (25, 51, 66). Although the exact donors of the noncapsid sequences of the cVDPV isolates were not identified, their noncapsid sequences are considered to be derived from some species C enteroviruses (see Discussion).
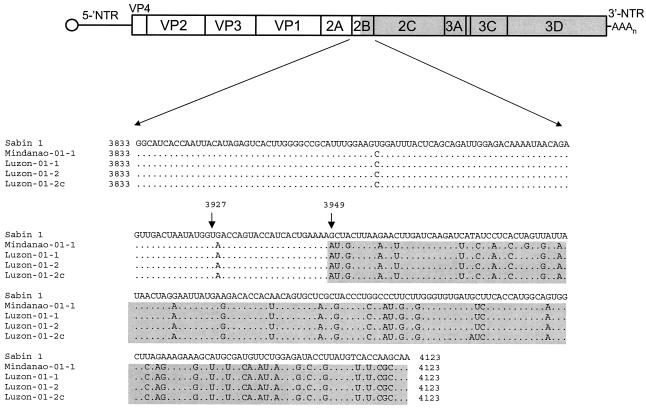
Sequencing of the full-length genomes of the four Philippines cVDPV isolates revealed that all were recombinants between Sabin 1 and non-Sabin viruses (Table 3). Sequence identities between Sabin 1 and Mindanao-01-1 were >96% in the 5′-NTR, capsid, and 2A regions but <85% in the remaining noncapsid regions. The other cVDPV isolates showed very similar sequence differences from Sabin 1, and the four shared ≈99% nucleotide sequence identities with each other along their entire genomes, indicative of circulation within the Philippines (Table 3). Furthermore, the four isolates had a common recombination site located in the middle of the 2B region (between nucleotides 3927 and 3949; Fig. 4).
TABLE 3.
Comparison of the entire genome of Mindanao-01-1 to that of other VDPVs and reference enterovirus strains
| Virus | GenBank accession no. | Nucleotide sequence identity to Mindanao-01-1a (%)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5′-NTR | VP4 | VP2 | VP3 | VP1 | 2A | 2B | 2C | 3A | 3B | 3C | 3D | 3′-NTR | ||
| Luzon-01-1 | AB180071 | 99.1 | 99.5 | 98.9 | 99.4 | 98.8 | 98.4 | 99.7 | 99.1 | 99.6 | 100 | 99.6 | 99.4 | 100 |
| Luzon-01-2 | AB180072 | 99.1 | 99.5 | 98.9 | 99.4 | 99.1 | 98.4 | 99.7 | 99.1 | 100 | 100 | 99.6 | 99.4 | 100 |
| Luzon-01-2c | AB180073 | 98.7 | 99.5 | 98.5 | 99.4 | 98.7 | 97.8 | 99.3 | 99.0 | 99.6 | 100 | 99.5 | 99.2 | 100 |
| Sabin 1 | V01150 | 97.7 | 96.6 | 98.4 | 98.2 | 96.9 | 97.1 | 84.2 | 82.1 | 75.9 | 80.3 | 80.0 | 84.1 | 97.2 |
| Sabin 2 | X00595 | 85.2 | 78.3 | 72.8 | 75.5 | 68.1 | 82.1 | 77.7 | 80.9 | 75.9 | 74.2 | 80.3 | 83.7 | 97.2 |
| Sabin 3 | K00043 | 81.7 | 78.3 | 71.2 | 73.6 | 68.2 | 80.3 | 75.6 | 78.9 | 73.2 | 81.8 | 80.9 | 84.0 | 98.6 |
| DOR00-013 | AF405690 | 96.5 | 94.2 | 95.8 | 96.4 | 95.3 | 93.3 | 77.3 | 79.9 | 77.4 | 81.8 | 79.4 | 85.4 | 98.6 |
| DOR00-041c1 | AF405682 | 96.0 | 92.8 | 95.5 | 95.9 | 95.4 | 93.1 | 77.0 | 79.8 | 77.4 | 81.8 | 79.2 | 84.5 | 100 |
| HAI 01-003 | AF405669 | 95.8 | 96.1 | 95.8 | 96.8 | 94.6 | 94.6 | 77.0 | 78.9 | 75.5 | 77.3 | 79.6 | 83.7 | 100 |
| HAI 01-007 | AF405666 | 96.4 | 94.7 | 95.0 | 95.5 | 94.3 | 93.7 | 79.0 | 80.7 | 77.0 | 75.8 | 79.4 | 84.2 | 100 |
| CAV11 | AF499635 | 87.2 | 75.9 | 69.0 | 69.2 | 64.3 | 77.4 | 81.8 | 78.7 | 76.6 | 75.8 | 79.6 | 84.9 | 97.2 |
| CAV17 | AF499638 | 85.8 | 73.4 | 69.6 | 73.0 | 67.6 | 80.8 | 75.9 | 79.4 | 77.0 | 83.3 | 81.2 | 85.5 | 98.6 |
| CAV20 | AF499642 | 88.4 | 68.6 | 69.1 | 71.8 | 66.9 | 79.6 | 79.3 | 80.5 | 77.8 | 77.3 | 81.6 | 84.1 | 98.6 |
GenBank accession no. AB180070.
FIG. 4.
Putative recombination crossover site in the 2B noncapsid region of the Philippines type 1 cVDPVs. (A) Schematic diagram of the poliovirus genome. (B) Nucleotide sequence alignment. Sequences derived from Sabin 1 are unshaded, while those derived from the unidentified species C enterovirus are shaded.
The very high degree of noncapsid sequence identity among the Philippines cVDPV isolates contrasted with the cVDPV isolates from Hispaniola (25) and Egypt (66), in which heterogeneous recombinant noncapsid sequences had been generated by successive recombination events. The noncapsid sequences of the Philippines isolates were generally related to (i) other polioviruses (the three Sabin strains and the Hispaniola isolates with different noncapsid sequences) and (ii) species C enteroviruses (e.g., coxsackievirus types A11, A17, and A20) (Table 3), but no sequence with >95% homology was found in GenBank (data not shown). This result suggests that the recombinant noncapsid sequences might be classified into a major species C enterovirus phylogeny, including all polioviruses and many species C enteroviruses, as recently reported by Brown et al. for the reference species C enterovirus strains (6).
Estimated time of initiating OPV dose.
The first cVDPV isolate (Mindanao-01-1, isolated on 28 March 2001) was more closely related to Sabin 1 (96.9% VP1 nucleotide identity) than the last isolate (Luzon-01-2c, isolated on 23 September 2001) (96.5% VP1 nucleotide identity), consistent with a pattern of increasing divergence from Sabin 1 over time (Fig. 3B). Most (≈70%) of the genetic changes in VP1 were substitutions at synonymous sites (data not shown). If one assumes that the rate of VP1 sequence divergence from Sabin 1 was 0.03 substitution per synonymous site per year (17, 25, 27, 35, 37), then we can estimate that the initiating OPV dose was given in early to mid-1999. We further estimate that the four cVDPV isolates were derived from a common ancestral infection that occurred in mid- to late 2000. Such a relationship is evident from the high overall sequence similarity among the four Philippines cVDPV isolates (Fig. 2 and Table 3) and the topology of the tree shown in Fig. 3B.
The Sabin 1-derived genomic sequence of the common ancestral virus is represented by the node joining the sequence of Mindanao-01-1 to the three Luzon isolate sequences (Fig. 3B). The terminal branches extending from that node were shorter than the branch extending back to the Sabin 1 sequence, again suggesting that divergence of the observed cVDPV lineages occurred 1 to 2 years after the initiating OPV dose. The estimated date is based on only four isolates identified over a 6-month period. The earliest isolate was from a March 2001 case which occurred about 2 years after the initiating OPV dose. A more accurate determination could have been made if a greater number of cVDPV isolates had been identified over a longer period.
Genetic properties of the cVDPV isolates.
The complete genomic sequences of the four Philippines cVDPVs were compared with those of Sabin 1 and its neurovirulent parental strain, Mahoney (45). The four cVDPV isolates shared a G-to-A nucleotide substitution at 480 in the domain V of the 5′-NTR, which represents the reversion of a major attenuation determinant of the Sabin 1 OPV strain (Table 4). Two other substitutions at positions 7410 and 7441 of the 3′-NTR distinguished the cVDPV isolates and Mahoney from Sabin 1. The cVDPV isolates also differed from Sabin 1 at nine amino acid reversions to the parental Mahoney strain (Table 4). Among three amino acid reversions in the capsid proteins, two amino acid changes in VP1 (VP1-99 and VP1-106) were located within neutralization antigenic site 1. Four amino acid differences were mapped in the 3Dpol region (3D residues 53, 73, 250, and 362). These reversions in 3Dpol were introduced by recombination and were similar to the amino acid changes found at equivalent positions among the four different recombinant 3Dpol sequences in the Hispaniola cVDPVs despite the highly diverse nucleotide sequences (Tables 3 and 4) (25). Some of these substitutions, alone or in combination, have been found to contribute to the temperature sensitivity and/or attenuation phenotypes of the Sabin 1 strain (5, 12, 19, 20, 40, 48, 59, 61).
TABLE 4.
Genetic and phenotypic characterizations of type 1 cVDPV in the Philippines
| Virus | Nucleotide and amino acid reversionsa
|
Neutralization titer
|
Neuro- virulencec (PD50) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5′-NTR at nt:
|
Capsid
|
Nonstructural proteins
|
3′-NTR at nt:
|
|||||||||||||||
| 26 | 355 | 480 | VP4-65 | VP1-99b | VP1-106b | 2A-134 | 2B-95 | 3D-53 | 3D-73 | 3D-250 | 3D-362 | 7410 | 7441 | 8a034 | 8a057 | 11m071 | ||
| Sabin 1 | G | T | G | S | K | T | T | T | N | H | E | I | C | G | 102,400 | >64,000 | <20 | >8.0 |
| Mindanao-01-1 | A | C | A | A | T | A | S | I | D | Y | K | T | U | A | <20 | 673 | 905 | 2.4 |
| Luzon-01-1 | A | C | A | A | T | A | S | I | D | Y | K | T | U | A | <20 | 800 | 1,810 | ND |
| Luzon-01-2 | A | C | A | A | T | A | S | I | D | Y | K | T | U | A | <20 | 336 | 1,076 | 2.6 |
| Luzon-01-2c | A | C | A | A | T | A | S | I | D | Y | K | T | U | A | ND | ND | ND | 4.9 |
| Mahoney | A | C | A | A | T | A | S | I | D | Y | K | T | U | A | <20 | 450 | 160 | 2.6 |
| HAI00-003d | A | C | A | A | T | A | A | I | D | Y | K | T | U | A | ND | ND | ND | 2.8 |
Nucleotide and amino acid reversions from the Sabin 1 (Genbank accession no. V01150) to Mahoney (VO1149; the neurovirulent parent of Sabin 1) sequences are indicated. Nucleotide substitutions are given only for the 5′- and 3′-NTRs.
Surface residues forming part of neutralizing antigenic site 1.
ND, not determined.
A representative type 1 cVDPV strain from Hispaniola. Its PD50 value is quoted from a previous report (25).
Biological properties of the cVDPV isolates.
The neurovirulence of the cVDPV isolates in the Philippines was evaluated by intracerebral inoculation of ICR-PVR-Tg21 mice carrying the human poliovirus receptor gene. The Mindanao-01-1 and Luzon-01-2 isolates showed potent neurovirulence (PD50 = 2.4 and 2.6 cell culture infective dose per mouse, respectively) that was comparable to that of the virulent Mahoney strain (PD50 = 2.6 cell culture infective dose per mouse) (Table 4). By contrast, the Luzon-01-2c strain, which was isolated from a contact of the second case in Luzon (Luzon-01-2), exhibited only moderate attenuation or neurovirulence in ICR-PVR-Tg21 mice (PD50 = 4.9 cell culture infective dose per mouse).
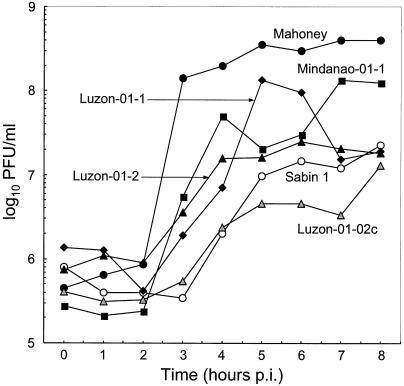
The temperature-sensitive phenotype of impaired virus replication at supraoptimal temperatures is a shared characteristic of all three serotypes of the OPV strains. We evaluated the temperature sensitivity of the Philippines type 1 cVDPV isolates by comparing virus yields in single-step growth experiments at 39.5°C in HeLa S3 cells (66). Three cVDPV isolates (Mindanao-01-1, Luzon-01-1, and Luzon-01-2) exhibited moderate temperature sensitivity with respect to the growth kinetics of temperature-resistant (Mahoney) and temperature-sensitive (Sabin 1) type 1 reference strains, suggesting that they had partially lost the temperature-sensitive phenotype (Fig. 5). The exception was again the last cVDPV isolate, Luzon-01-2c, which had regained the temperature-sensitive phenotype for growth at 39.5°C in HeLa S3 cells (Fig. 5). Both the low neurovirulence in ICR-PVR-Tg21 mice and the temperature sensitivity of Luzon-01-2c were surprising because its nucleotide and amino acid sequences at key sites were identical to those of the other three cVDPV isolates from acute flaccid paralysis cases (Table 4).
FIG. 5.
Single-step growth curves of type 1 Philippines cVDPV isolates, Mahoney, and Sabin 1 strains at 39.5°C in HeLa S3 cells.
The neutralization antigenicities of the Philippines cVDPV isolates were analyzed in neutralization assays with three monoclonal antibodies specific to either Sabin 1 or Mahoney (24). As anticipated from the non-Sabin-like reactivity in the ELISA-intratypic differentiation assay (Table 2), the cVDPV isolates were not neutralized or very weakly neutralized by the Sabin 1-specific monoclonal antibodies (8a304 and 8a057) (Table 4). In a similar manner, the same cVDPVs were efficiently neutralized by a monoclonal antibody specific for the Mahoney strain, 11m071. Amino acid reversions in the capsid proteins (VP1-99 and VP1-106) and/or some additional amino acid substitutions (VP1-66, VP1-90, etc.; data not shown) might be responsible for the altered neutralization antigenicity of the cVDPV isolates. Such amino acid changes have frequently been found in many Sabin 1-derived polioviruses isolated from OPV recipients, vaccine-associated paralytic poliomyelitis patients, and the environment (18, 39, 43).
DISCUSSION
The recent cVDPV outbreak in the Philippines has important implications for the global initiative to eradicate polio. First, it demonstrates that use of OPV with suboptimal coverage rates can lead to the emergence and spread of cVDPVs even in countries where indigenous wild polioviruses have already been eradicated. Second, it again shows that use of OPV at high rates of coverage can prevent further spread of cVDPVs. Third, it reaffirms the importance of maintaining sensitive acute flaccid paralysis and poliovirus surveillance in both polio-free and polio-endemic countries. Finally, it provides additional insights into the conditions permissive for cVDPV emergence and the biological and genetic properties of the emergent viruses.
The cVDPV outbreak in the Philippines differed in key respects from earlier outbreaks reported in Egypt (66) and Haiti in Hispaniola (25) and the subsequent outbreak in Madagascar (51). In the other outbreak countries, OPV coverage rates were particularly low (<50%) in the affected communities and generally low nationwide. Moreover, nearly all of the case patients in the other outbreaks were unimmunized or incompletely immunized children (25, 51, 66). By contrast, nationwide rates of routine coverage with three doses of OPV were reported to have been approximately 80% in the Philippines since the early 1990s (50, 62), and two of the case patients had received three doses of OPV and the third patient had received two doses (Table 1). However, gaps in population immunity probably occurred after 1997, when the mass OPV campaigns in the form of national immunization days were last conducted in the Philippines. Subnational immunization days that covered the urban areas of Manila, Cebu, and Davao (Mindanao) followed in 1998 and 1999 but did not include the three provinces with cVDPV cases (Fig. 1) (62).
It is likely that gaps in OPV coverage developed most rapidly in the slum areas, such as those around metropolitan Manila, and these gaps were aggravated by a temporary shortage of OPV supply in 2000 to 2001. The widening immunity gap, coupled with very high population densities (especially around metropolitan Manila; Fig. 1), poor hygiene or sanitation, and tropical conditions may have established local conditions favoring cVDPV emergence. Once poliovirus circulation starts, three prior OPV doses may not be enough to protect all children from poliomyelitis, particularly in high-risk communities (55). However, overall population immunity appears to have been sufficiently high to restrict cVDPV transmission to a minimally branched chain, in contrast to the pattern of multichain transmission seen in Egypt and Hispaniola (25, 66). The important lesson from the Philippines outbreak is that cVDPVs can emerge even in countries with good rates of OPV coverage nationwide if immunity gaps develop in local areas at highest potential risk for poliovirus circulation.
The detection of the cVDPVs in the Philippines highlights the significant role of poliovirus surveillance in the final stages of global polio eradication. Immediately following the cVDPV outbreak in Hispaniola, intensive screening of cVDPVs was initiated by laboratories within the entire World Health Organization Global Polio Laboratory Network (8, 9). Vaccine-related poliovirus isolates are identified by genetic methods, such as probe hybridization (14), and also characterized for evidence of antigenic divergence from the prototype OPV strains by antigenic tests, such as intratypic differentiation-ELISA (8, 9, 60). The likelihood of antigenic divergence increases with the duration of replication of OPV strains in the human gut (41), and all documented cVDPV isolates have been antigenic variants of the OPV strains (25, 51, 66). Vaccine-related isolates having altered antigenic properties are candidate VDPVs and are characterized further by genomic sequencing. In addition, the World Health Organization is promptly notified of the virologic findings in order to accelerate active surveillance and to prepare for any necessary supplementary immunization campaigns, as described here for the Philippines (8, 9).
The Philippines cVDPV isolates, as with the other cVDPV isolates described so far (25, 51, 66), have recombinant noncapsid sequences derived from other species C enteroviruses (6). Since OPV contains all three serotypes of the Sabin strains, a recombinant poliovirus among heterogeneous strains readily emerges during virus replication in the gut of vaccinees. Nevertheless, recombination among the vaccine strains is known to occur frequently with serotypes 2 and 3 but rarely with type 1 (4, 7, 13, 16, 21, 22, 38). On the other hand, circulating wild polioviruses with a block of sequence derived from Sabin 1 have been described (34, 35). It appears most likely that the donor of the noncapsid sequences to the Philippines type 1 cVDPV isolates was nonpolio enteroviruses, as the sensitive surveillance scheme for cases of acute flaccid paralysis maintained in the Philippines has not detected any indigenous or imported wild polioviruses since 1993. Although the apparent donor of the recombinant noncapsid sequences of cVDPVs has not been identified, growing evidences indicate frequent recombination between polioviruses and species C nonpolio enteroviruses (6, 22, 25, 28, 30, 34, 35, 51, 66) as well as between serotypes within the same nonpolio enterovirus species (33, 36, 46, 47, 53). Further epidemiological studies of species C nonpolio enteroviruses, especially in tropical areas, are needed to understand the conditions favorable for cVDPV recombination.
Although recombination with other enteroviruses appears to be an indicator of poliovirus circulation (25), the possible role of recombination in the phenotypic reversion of OPV is less clear. Genetic determinants of attenuation and temperature sensitivity in Sabin 1 (but not in Sabin 2 and 3) are mapped in the 3Dpol noncapsid region (5, 12, 20, 59), so that recombination may be an efficient mechanism to replace these mutations with consensus wild enterovirus sequences. However, the major determinants of attenuation in Sabin 1 map to the 5′-NTR and capsid regions, which were not replaced by recombination in either the Philippines or Hispaniola cVDPVs.
The partially attenuated and temperature-sensitive phenotypes of the most recently identified cVDPV isolate, Luzon-01-2c, from a healthy contact child, were unexpected in view of the close sequence relationship of the contact isolate to the other three Philippines cVDPV isolates that had biological properties similar to those of wild type 1 polioviruses. The temporal and phylogenetic relationships among the Philippines cVDPV isolates suggest that isolate Luzon-01-2c was derived from a more neurovirulent and less temperature sensitive progenitor, raising the possibility that reversion of the attenuated and temperature-sensitive phenotypes of Sabin 1 is not necessarily irreversible during cVDPV evolution. Although the Luzon-01-2c isolate has the same recombinant properties as the other cVDPV isolates, it does differ from the other three isolates at some specific nucleotide and amino acid substitutions (644, 667, and 720 in the 5′-NTR, VP1-224, 2A-101, 2A-129, 2B-75, and 2C-94). Further virologic, epidemiologic, and reverse genetic studies are needed to understand the role of mutation and recombination in poliovirus evolution and cVDPV emergence.
Acknowledgments
We thank the Philippines local and regional Department of Health staff for their extensive surveillance. We also thank the virologists from the World Health Organization Polio Laboratory Network for contributing to the isolation and identification of wild polioviruses. The single-step growth experiments were performed by Ray Campagnoli and Annalet Martin (CDC). Paul Chenoweth helped prepare the map identifying the source of the cVDPV isolates. We thank Junko Wada (NIID) and Ann Turnbull (VIDRL) for excellent technical assistance.
H.S., A.U., M.A., H.Y., T.Y., and T.M. were supported, in part, by grants-in-aid for the Promotion of Polio Eradication from the Ministry of Health, Labour and Welfare, Japan. H.S. and T.Y. were supported, in part, by grants-in-aid for Development of Expanded Programme on Immunization and Accelerating Measles Control in the Polio-free Era from the Ministry of Health, Labour and Welfare, Japan. A.U. is supported by grants-in-aid from the Japan Society for the Promotion of Sciences. The Polio Regional Reference Laboratory, Australia, is supported by WHO, the Department of Health and Ageing, Canberra, and the Department of Human Services, Melbourne, Australia.
REFERENCES
- 1.Alexander, J. P., Jr., H. E. Gary, Jr., and M. A. Pallansch. 1997. Duration of poliovirus excretion and its implications for acute flaccid paralysis surveillance: a review of the literature. J. Infect. Dis. 175(Suppl. 1):S176-S182. [DOI] [PubMed] [Google Scholar]
- 2.Bellmunt, A., G. May, R. Zell, P. Pring-Akerblom, W. Verhagen, and A. Heim. 1999. Evolution of poliovirus type I during 5.5 years of prolonged internal replication in an immunodeficient patient. Virology 265:178-184. [DOI] [PubMed] [Google Scholar]
- 3.Benyesh-Melnick, M., J. L. Melnick, W. E. Rawls, I. Wimberley, J. Barrera-Oro, E. Ben-Porath, and V. Rennick. 1967. Studies on the immunogenicity, communicability, and genetic stability of oral poliovaccine administered during the winter. Am. J. Epidemiol. 86:112-136. [DOI] [PubMed] [Google Scholar]
- 4.Blomqvist, S., A. L. Bruu, M. Stenvik, and T. Hovi. 2003. Characterization of a recombinant type 3/type 2 poliovirus isolated from a healthy vaccinee and containing a chimeric capsid protein VP1. J. Gen. Virol. 84:573-580. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard, M. J., D. H. Lam, and V. R. Racaniello. 1995. Determinants of attenuation and temperature sensitivity in the type 1 poliovirus Sabin strain. J. Virol. 69:4972-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, B. A., M. S. Oberste, K. Maher, and M. Pallansch. 2003. Complete genomic sequencing shows that polioviruses and members of human enterovirus species C are closely related in the non-capsid-coding region. J. Virol. 77:8973-8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cammack, N., A. Phillips, G. Dunn, V. Patel, and P. D. Minor. 1988. Intertypic genomic rearrangements of poliovirus strains in vaccinees. Virology 167:507-514. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2003. Laboratory surveillance for wild and vaccine-derived polioviruses, January 2002-June 2003. Morb. Mortal. Wkly. Rep. 52:913-916. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2002. Laboratory surveillance for wild poliovirus and vaccine-derived poliovirus, 2000-2001. Morb. Mortal. Wkly. Rep. 51:369-371. [PubMed] [Google Scholar]
- 10.Cherkasova, E. A., E. A. Korotkova, M. L. Yakovenko, O. E. Ivanova, T. P. Eremeeva, K. M. Chumakov, and V. I. Agol. 2002. Long-term circulation of vaccine-derived poliovirus that causes paralytic disease. J. Virol. 76:6791-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiba, Y., H. Murakami, M. Kobayashi, H. Shimizu, H. Yoshida, T. Yoneyama, T. Miyamura, J. Yu, and L.-B. Zhang. 2000. A case of poliomyelitis associated with infection of wild poliovirus in Qinghai Province, China, in October 1999. Jpn. J. Infect. Dis. 53:135-136. [PubMed] [Google Scholar]
- 12.Christodoulou, C., F. Colbere-Garapin, A. Macadam, L. F. Taffs, S. Marsden, P. Minor, and F. Horaud. 1990. Mapping of mutations associated with neurovirulence in monkeys infected with Sabin 1 poliovirus revertants selected at high temperature. J. Virol. 64:4922-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuervo, N. S., S. Guillot, N. Romanenkova, M. Combiescu, A. Aubert-Combiescu, M. Seghier, V. Caro, R. Crainic, and F. Delpeyroux. 2001. Genomic features of intertypic recombinant Sabin poliovirus strains excreted by primary vaccinees. J. Virol. 75:5740-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De, L., B. Nottay, C. F. Yang, B. P. Holloway, M. Pallansch, and O. Kew. 1995. Identification of vaccine-related polioviruses by hybridization with specific RNA probes. J. Clin. Microbiol. 33:562-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowdle, W. R., E. de Gourville, O. M. Kew, M. A. Pallansch, and D. J. Wood. 2003. Polio eradication: the OPV paradox. Rev. Med. Virol. 13:277-291. [DOI] [PubMed] [Google Scholar]
- 16.Furione, M., S. Guillot, D. Otelea, J. Balanant, A. Candrea, and R. Crainic. 1993. Polioviruses with natural recombinant genomes isolated from vaccine-associated paralytic poliomyelitis. Virology 196:199-208. [DOI] [PubMed] [Google Scholar]
- 17.Gavrilin, G. V., E. A. Cherkasova, G. Y. Lipskaya, O. M. Kew, and V. I. Agol. 2000. Evolution of circulating wild poliovirus and of vaccine-derived poliovirus in an immunodeficient patient: a unifying model. J. Virol. 74:7381-7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgescu, M. M., J. Balanant, A. Macadam, D. Otelea, M. Combiescu, A. A. Combiescu, R. Crainic, and F. Delpeyroux. 1997. Evolution of the Sabin type 1 poliovirus in humans: characterization of strains isolated from patients with vaccine-associated paralytic poliomyelitis. J. Virol. 71:7758-7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgescu, M. M., F. Delpeyroux, M. Tardy-Panit, J. Balanant, M. Combiescu, A. A. Combiescu, S. Guillot, and R. Crainic. 1994. High diversity of poliovirus strains isolated from the central nervous system from patients with vaccine-associated paralytic poliomyelitis. J. Virol. 68:8089-8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georgescu, M. M., M. Tardy-Panit, S. Guillot, R. Crainic, and F. Delpeyroux. 1995. Mapping of mutations contributing to the temperature sensitivity of the Sabin 1 vaccine strain of poliovirus. J. Virol. 69:5278-5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgopoulou, A., and P. Markoulatos. 2001. Sabin type 2 polioviruses with intertypic vaccine/vaccine recombinant genomes. Eur. J. Clin. Microbiol. Infect. Dis. 20:792-799. [DOI] [PubMed] [Google Scholar]
- 22.Guillot, S., V. Caro, N. Cuervo, E. Korotkova, M. Combiescu, A. Persu, A. Aubert-Combiescu, F. Delpeyroux, and R. Crainic. 2000. Natural genetic exchanges between vaccine and wild poliovirus strains in humans. J. Virol. 74:8434-8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagiwara, A., T. Yoneyama, K. Yoshii, H. Yoshida, H. Shimizu, J. Wada, N. T. H. Thanh, P. V. Tu, and T. Miyamura. 1999. Genetic analysis of wild polioviruses towards the eradication of poliomyelitis from the Western Pacific Region. Jpn. J. Infect. Dis. 52:146-149. [PubMed] [Google Scholar]
- 24.Horie, H., H. Yoshida, K. Matsuura, M. Miyazawa, K. Wakabayashi, A. Nomoto, and S. Hashizume. 2002. Isolation of vaccine-derived type 1 polioviruses displaying similar properties to virulent wild strain Mahoney from sewage in Japan. J. Med. Virol. 68:445-451. [DOI] [PubMed] [Google Scholar]
- 25.Kew, O., V. Morris-Glasgow, M. Landaverde, C. Burns, J. Shaw, Z. Garib, J. Andre, E. Blackman, C. J. Freeman, J. Jorba, R. Sutter, G. Tambini, L. Venczel, C. Pedreira, F. Laender, H. Shimizu, T. Yoneyama, T. Miyamura, H. van Der Avoort, M. S. Oberste, D. Kilpatrick, S. Cochi, M. Pallansch, and C. de Quadros. 2002. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 296:356-359. [DOI] [PubMed] [Google Scholar]
- 26.Kew, O. M., M. N. Mulders, G. Y. Lipskaya, E. E. de Silva, and M. A. Pallansch. 1995. Molecular epidemiology of poliovirus. Semin. Virol. 6:401-414. [Google Scholar]
- 27.Kew, O. M., R. W. Sutter, B. K. Nottay, M. J. McDonough, D. R. Prevots, L. Quick, and M. A. Pallansch. 1998. Prolonged replication of a type 1 vaccine-derived poliovirus in an immunodeficient patient. J. Clin. Microbiol. 36:2893-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kew, O. M., P. F. Wright, V. I. Agol, F. Delpeyroux, H. Shimizu, N. Nathanson, and M. A. Pallansch. 2004. Circulating vaccine-derived polioviruses: current state of knowledge. Bull. W.H.O. 82:16-23. [PMC free article] [PubMed] [Google Scholar]
- 29.Kojouharova, M., P. L. F. Zuber, S. Gyurova, L. Fiore, G. Buttinelli, A. Kunchev, N. Vladimirova, N. Korsun, R. Filipova, R. Boneva, E. Gavrilin, J. M. Deshpande, G. Oblapenko, and S. G. Wassilak. 2003. Importation and circulation of poliovirus in Bulgaria in 2001. Bull. W.H.O. 81:476-481. [PMC free article] [PubMed] [Google Scholar]
- 30.Korotkova, E. A., R. Park, E. A. Cherkasova, G. Y. Lipskaya, K. M. Chumakov, E. Feldman, O. M. Kew, and V. I. Agol. 2003. Retrospective analysis of a local cessation of vaccination against poliomyelitis: a possible scenario for the future. J. Virol. 77:12460-12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, J., T. Yoneyama, H. Yoshida, K. Yoshii, H. Shimizu, T. Miyamura, M. Hara, X. H. Hou, H. Zheng, Y. Fang, L.-B. Zhang, and A. Hagiwara. 1995. Genetic analysis of wild-type 1 poliovirus isolates in China, 1985-1993. Res. Virol. 146:415-422. [DOI] [PubMed] [Google Scholar]
- 32.Li, J., L.-B. Zhang, T. Yoneyama, H. Yoshida, H. Shimizu, K. Yoshii, M. Hara, T. Nomura, H. Yoshikura, T. Miyamura, and A. Hagiwara. 1996. Genetic basis of the neurovirulence of type 1 polioviruses isolated from vaccine-associated paralytic patients. Arch. Virol. 141:1047-1054. [DOI] [PubMed] [Google Scholar]
- 33.Lindberg, A. M., P. Andersson, C. Savolainen, M. N. Mulders, and T. Hovi. 2003. Evolution of the genome of human enterovirus B: incongruence between phylogenies of the VP1 and 3CD regions indicates frequent recombination within the species. J. Gen. Virol. 84:1223-1235. [DOI] [PubMed] [Google Scholar]
- 34.Liu, H.-M., D.-P. Zheng, L.-B. Zhang, M. S. Oberste, O. M. Kew, and M. A. Pallansch. 2003. Serial recombination during circulation of type 1 wild-vaccine recombinant polioviruses in China. J. Virol. 77:10994-11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu, H. M., D. P. Zheng, L. B. Zhang, M. S. Oberste, M. A. Pallansch, and O. M. Kew. 2000. Molecular evolution of a type 1 wild-vaccine poliovirus recombinant during widespread circulation in China. J. Virol. 74:11153-11161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukashev, A. N., V. A. Lashkevich, O. E. Ivanova, G. A. Koroleva, A. E. Hinkkanen, and J. Ilonen. 2003. Recombination in circulating enteroviruses. J. Virol. 77:10423-10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin, J., G. Dunn, R. Hull, V. Patel, and P. D. Minor. 2000. Evolution of the Sabin strain of type 3 poliovirus in an immunodeficient patient during the entire 637-day period of virus excretion. J. Virol. 74:3001-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin, J., E. Samoilovich, G. Dunn, A. Lackenby, E. Feldman, A. Heath, E. Svirchevskaya, G. Cooper, M. Yermalovich, and P. D. Minor. 2002. Isolation of an intertypic poliovirus capsid recombinant from a child with vaccine-associated paralytic poliomyelitis. J. Virol. 76:10921-10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuura, K., M. Ishikura, H. Yoshida, T. Nakayama, S. Hasegawa, S. Ando, H. Horie, T. Miyamura, and T. Kitamura. 2000. Assessment of poliovirus eradication in Japan: genomic analysis of polioviruses isolated from river water and sewage in toyama prefecture. Appl. Environ. Microbiol. 66:5087-5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minor, P. D. 1993. Attenuation and reversion of the Sabin vaccine strains of poliovirus. Dev. Biol. Stand. 78:17-26. [PubMed] [Google Scholar]
- 41.Minor, P. D., and J. W. Almond. 2002. Poliovirus vaccines: molecular biology and immune response, p. 381-390. In B. L. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. ASM Press, Washington, D.C.
- 42.Minor, P. D., and G. Dunn. 1988. The effect of sequences in the 5′ non-coding region on the replication of polioviruses in the human gut. J. Gen. Virol. 69:1091-1096. [DOI] [PubMed] [Google Scholar]
- 43.Mulders, M. N., J. H. Reimerink, M. Stenvik, I. Alaeddinoglu, H. G. van der Avoort, T. Hovi, and M. P. Koopmans. 1999. A Sabin vaccine-derived field isolate of poliovirus type 1 displaying aberrant phenotypic and genetic features, including a deletion in antigenic site 1. J. Gen. Virol. 80:907-916. [DOI] [PubMed] [Google Scholar]
- 44.Nomoto, A., and I. Arita. 2002. Eradication of poliomyelitis. Nat. Immunol. 3:205-208. [DOI] [PubMed] [Google Scholar]
- 45.Nomoto, A., T. Omata, H. Toyoda, S. Kuge, H. Horie, Y. Kataoka, Y. Genba, Y. Nakano, and N. Imura. 1982. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc. Natl. Acad. Sci. USA 79:5793-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oberste, M. S., K. Maher, and M. A. Pallansch. 2004. Evidence for frequent recombination within species human enterovirus B based on complete genomic sequences of all thirty-seven serotypes. J. Virol. 78:855-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oprisan, G., M. Combiescu, S. Guillot, V. Caro, A. Combiescu, F. Delpeyroux, and R. Crainic. 2002. Natural genetic recombination between cocirculating heterotypic enteroviruses. J. Gen. Virol. 83:2193-2200. [DOI] [PubMed] [Google Scholar]
- 48.Otelea, D., S. Guillot, M. Furione, A. A. Combiescu, J. Balanant, A. Candrea, and R. Crainic. 1993. Genomic modifications in naturally occurring neurovirulent revertants of Sabin 1 polioviruses. Dev. Biol. Stand. 78:33-38. [PubMed] [Google Scholar]
- 49.Page, R. D. M. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 50.Philippines National Certification Committee. 2002. Polio eradication in the Philippines. Philippines National Certification Committee, Manila, The Philippines.
- 51.Rousset, D., M. Rakoto-Andrianarivelo, R. Razafindratsimandresy, B. Randriamanalina, S. Guillot, J. Balanant, P. Mauclère, and F. Delpeyroux. 2003. Recombinant vaccine-derived poliovirus in Madagascar. Emerg. Infect. Dis. 9:885-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 53.Santti, J., T. Hyypia, L. Kinnunen, and M. Salminen. 1999. Evidence of recombination among enteroviruses. J. Virol. 73:8741-8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strebel, P. M., R. W. Sutter, S. L. Cochi, R. J. Biellik, E. W. Brink, O. M. Kew, M. A. Pallansch, W. A. Orenstein, and A. R. Hinman. 1992. Epidemiology of poliomyelitis in the United States one decade after the last reported case of indigenous wild virus-associated disease. Clin. Infect. Dis. 14:568-579. [DOI] [PubMed] [Google Scholar]
- 55.Sutter, R. W., O. M. Kew, and S. L. Cochi. 2003. Poliovirus vaccine—live, p. 651-705. In S. A. Plotkin and W. A. Orenstein (ed.), Vaccines, 4th ed. W. B. Saunders Company, Philadelphia, Pa.
- 56.Technical Consulting Group to the World Health Organization on the Global Eradication of Poliomyelitis. 2002. “Endgame” issues for the Global Polio Eradication Initiative. Clin. Infect. Dis. 34:72-77. [DOI] [PubMed] [Google Scholar]
- 57.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic. Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thorley, B., F. Paladin, and H. Shimizu. 2002. Poliomyelitis due to vaccine-derived polioviruses in the Philippines. In Abstracts of the XIIth International Congress of Virology, Paris, 27 July to 1 August 2002. International Union of Microbiological Societies. EDK Medical and Scientific International Publisher, Paris, France.
- 59.Toyoda, H., C.-F. Yang, N. Takeda, A. Nomoto, and E. Wimmer. 1987. Analysis of RNA synthesis of type 1 poliovirus by using an in vitro molecular genetic approach. J. Virol. 61:2816-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Avoort, H. G., B. P. Hull, T. Hovi, M. A. Pallansch, O. M. Kew, R. Crainic, D. J. Wood, M. N. Mulders, and A. M. van Loon. 1995. Comparative study of five methods for intratypic differentiation of polioviruses. J. Clin. Microbiol. 33:2562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wimmer, E., C. U. Hellen, and X. Cao. 1993. Genetics of poliovirus. Annu. Rev. Genet. 27:353-436. [DOI] [PubMed] [Google Scholar]
- 62.World Health Organization. 2001. Acute flaccid paralysis associated with circulating vaccine-derived poliovirus, Philippines, 2001. Wkly. Epidemiol. Rec. 76:319-320. [PubMed] [Google Scholar]
- 63.World Health Organization. 2000. Certification of poliomyelitis eradication: Western Pacific Region. Wkly. Epidemiol. Rec. 75:399-400. [PubMed] [Google Scholar]
- 64.World Health Organization. 1997. Manual for the virologic investigation of poliomyelitis W.H.O./EPI/GEN/97.1. World Health Organization, Geneva, Switzerland.
- 65.World Health Organization. 2003. Progress towards the global eradication of poliomyelitis, 2002. Wkly. Epidemiol. Rec. 78:138-144. [PubMed] [Google Scholar]
- 66.Yang, C.-F., T. Naguib, S.-J. Yang, E. Nasr, J. Jorba, N. Ahmed, R. Campagnoli, H. van der Avoort, H. Shimizu, T. Yoneyama, T. Miyamura, M. A. Pallansch, and O. Kew. 2003. Circulation of endemic type 2 vaccine-derived poliovirus in Egypt, 1983 to 1993. J. Virol. 77:8366-8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshida, H., J. Li, T. Yoneyama, K. Yoshii, H. Shimizu, T. H. Nguyen, K. Toda, N. T. H. Thanh, P. V. Tu, T. Miyamura, and A. Hagiwara. 1997. Two major strains of type 1 wild poliovirus circulating in Indochina. J. Infect. Dis. 175:1233-1237. [DOI] [PubMed] [Google Scholar]