Abstract
Epstein-Barr virus (EBV)-induced lymphoproliferative disease is an important complication in the context of immune deficiency. Impaired T-cell immunity allows the outgrowth of transformed cells with the subsequent production of predominantly B-cell lymphomas. Currently there is no in vivo model that can adequately recapitulate EBV infection and its association with B-cell lymphomas. NOD/SCID mice engrafted with human CD34+ cells and reconstituted mainly with human B lymphocytes may serve as a useful xenograft model to study EBV infection and pathogenesis. We therefore infected reconstituted mice with EBV. High levels of viral DNA were detected in the peripheral blood of all infected mice. All infected mice lost weight and showed decreased activity levels. Infected mice presented large visible tumors in multiple organs, most prominently in the spleen. These tumors stained positive for human CD79a, CD20, CD30, and EBV-encoded RNAs and were light chain restricted. Their characterization is consistent with that of large cell immunoblastic lymphoma. In addition, tumor cells expressed EBNA1, LMP1, and LMP2a mRNAs, which is consistent with a type II latency program. EBV+ lymphoblastoid cell lines expressing human CD45, CD19, CD21, CD23, CD5, and CD30 were readily established from the bone marrow and spleens of infected animals. Finally, we also demonstrate that infection with an enhanced green fluorescent protein (EGFP)-tagged virus can be monitored by the detection of infected EGFP+ cells and EGFP+ tumors. These data demonstrate that NOD/SCID mice that are reconstituted with human CD34+ cells are susceptible to infection by EBV and accurately recapitulate important aspects of EBV pathogenesis.
Epstein-Barr virus (EBV) is a human gammaherpesvirus that predominantly infects B cells and epithelial cells (17, 18, 45, 56). EBV represents an important health concern since 90% of the world's population is infected (14, 55, 70). Although in immunocompetent hosts infection is generally subclinical (14), infection in immunodeficient patients can produce significant morbidity and/or mortality (40). Once a person is exposed to the virus, EBV persists in vivo within infected B cells for the entire life span of the individual. After infection, EBV is maintained in a latent form in which only a few genes are expressed. In contrast, >100 genes are expressed during lytic replication (7). The highly restricted expression of the vast majority of its genes enables EBV to avoid immune detection by cytotoxic T lymphocytes (CTLs) (7). The 10 genes that are generally expressed during latency encode six nuclear proteins, two types of nontranslated RNA, and two membrane proteins. EBV nuclear antigen 1 (EBNA1) is required for replication and for maintenance of the viral genome (70). EBNA2 has multiple functions, including the regulation of expression of latent membrane proteins 1 and 2 (LMP1 and -2), which in turn contribute to the growth and transformation of B cells (7). The EBNA3 family of proteins (3A, 3B, and 3C) encode transcription factors that upregulate viral and cellular genes and play a role in the transformation of human primary B cells (7, 70). The EBNA leader protein (EBNA-LP) stimulates EBNA2 transcriptional activation (27). LMP1 is critically involved in the effective immortalization and proliferation of B cells that are latently infected by EBV, whereas LMP2 (a and b) proteins serve to block reactivation from latency and increase B-cell survival (33, 37, 41). EBV-encoded RNAs (EBERs) are nontranslated viral RNAs of unknown function that might be involved in preventing the programmed cell death of infected cells (35). BamHI rightward-transcript RNAs are present in all latency types but are not essential for transformation, and their role remains to be fully elucidated (70). Interestingly, not all 10 genes are expressed simultaneously during latency in all cases. Different patterns of gene expression have been described, and each one of them has been shown to correlate to a specific disease(s) (1, 36). For example, type 1 latency is defined by the expression of EBNA1 and EBERs and is associated with Burkitt's lymphoma (1, 7, 36). Type 2 latency is defined by EBNA1, LMP1, LMP2, and EBER expression and is associated with nasopharyngeal carcinoma, Hodgkin's disease, and peripheral T-cell lymphoma (1, 7, 36). The type 3 latency pattern is characterized by the expression of EBNA1, EBNA2, EBNA3A, EBNA3B, EBNA3C, EBNA-LP, LMP1, LMP2, and EBERs and is associated with lymphoproliferative disease, X-linked lymphoproliferative disease, and infectious mononucleosis (1, 36). There is no definite latency type designated for healthy carriers, but they do express LMP2 and EBERs and sometimes have EBNA1 expression (64).
The relationship between latent EBV infection and the neoplasias that develop in immunosuppressed individuals has been documented (3, 39, 49). The group of immunodeficiencies that are associated with tumors is broad and extremely heterogeneous (39). Regardless of the origin of the immune deficiency, whether primary or secondary, the most important neoplasias are those of the lymphoid system. They can range anywhere from a benign reactive hyperplasia to B-cell lymphomas. All of these entities share certain characteristics, including their cell type of origin (B cell), extranodal involvement, diffuse and at times aggressive behavior, and as stated earlier and most relevant to this report, association with EBV (3).
Because of EBV's restricted tropism, in vivo studies of EBV pathogenesis have been very limited (42). Whereas Old World primates have been found to be refractory to EBV infection, New World species, including the common marmoset (Callithrix jacchus) and the cottontop tamarin (Sanguinus oedipus oedipus) (54), have been found to be susceptible to infection by EBV. Common marmosets have been infected by direct intramuscular or intraperitoneal (i.p.) injection. Persistent EBV infections can be detected in this model, but EBV-associated lymphomas do not develop (68). Cottontop tamarins that are injected (intramuscularly or i.p.) with EBV develop multifocal large-cell lymphomas. Despite their usefulness as in vivo models of EBV infection, New World primates represent endangered species and can only be used in small numbers because they are rare and costly.
An alternative model that has been used to study EBV-associated B-cell lymphoproliferative disease is the SCID mouse (20, 40, 48, 67). SCID mice lack functional B and T cells due to a deficiency in double-stranded DNA break repair activity involved in the proper joining of V(D)J coding sequences of B- and T-cell antigen receptors during lymphocyte maturation (5). Because of their lack of T and B cells, SCID mice are not capable of rejecting human cells. In fact, SCID mice can sustain human peripheral blood mononuclear cells (PBMC) in the abdomen after i.p. injection for various lengths of time (43, 44). Moreover, when the injected cells are derived from EBV-seropositive donors, they give rise to EBV+ B-cell lymphomas (40, 44, 67). However, there is significant donor to donor variation in the ability to establish tumors in this model. When lymphoblastoid cell lines (LCLs) are injected into SCID mice, they almost invariably produce tumors (46). With very few exceptions (48), the vast majority of studies performed with SCID mice have not addressed in vivo infections with EBV but rather have focused on the ability of infected or transformed cells to produce tumors.
An alternative way by which SCID mice can be reconstituted with human cells is the transplantation of human hematopoietic stem cells (HSC) into preconditioned mice (23). The fact that the majority of human cells in recipient SCID mice are B cells makes them ideal targets for infection with EBV. Unfortunately, the transplantation of human HSC into SCID mice results in relatively low levels of reconstitution with human cells. In contrast, nonobese diabetic SCID (NOD/SCID) mice also support the growth and differentiation of human HSC and result in significantly higher levels of engraftment and reconstitution with human cells. The fact that the majority of the human cells in recipient NOD/SCID mice are B cells makes them ideal targets for infection with EBV (23). For this study, we evaluated the susceptibility of NOD/SCID mice engrafted with human hematopoietic cells to infection with EBV. Our results indicate that mice receiving transplanted human HSC are readily infected with EBV, resulting in high viral loads and the subsequent development of malignant lymphomas.
MATERIALS AND METHODS
Isolation of human CD34+ cells and transplantation into immunodeficient mice.
Human umbilical cord blood was obtained at Parkland Memorial Hospital (Dallas, Tex.) from uncomplicated births and was processed as previously described (21, 22). Briefly, mononuclear cells were isolated by Ficoll (Amersham Biosciences, Piscataway, N.J.) gradient separation and were enriched for CD34+ cells by positive immunomagnetic isolation according to the manufacturer's instructions (Miltenyi Biotech, Auburn, Calif.). This protocol yielded 90 to 95% pure CD34+ cells. The cells (2 × 105 to 5 × 105 per mouse) were infused intravenously into sublethally irradiated (325 cGy by 137Cs γ-irradiation) 8- to 10-week-old NOD/SCID mice. Mice were maintained in microisolators and were fed sterile food and chlorinated sterile water. Reconstitution with human cells was evaluated by flow cytometry as indicated below.
Virus preparation, in vivo infections, and tissue harvest.
Stocks of the enhanced green fluorescent protein (EGFP)-tagged virus (B95-8)EBfaV-GFP and Akata virus laboratory strains (57, 58) were kindly provided by J. Sixbey and prepared by treating B95-8 cells harboring EBfaV-GFP with 20 ng of tetradecanoyl phorbol acetate/ml for 4 days or the Akata cell line by induction with anti-human immunoglobulin (Sigma, St. Louis, Mo.). Cell-free supernatants were concentrated 10 (EBfaV-GFP) or 20 (Akata) times by tangential flow filtration (Labscale TFF; Millipore Corp., Bedford, Mass.) through a Pellicon XL device (Millipore). To test the infectivity of EBfaV-GFP, we incubated 1 ml of concentrated virus with 106 BL2 cells (an EBV-negative Burkitt's lymphoma cell line) for 90 min. The virus supernatant was removed, and the cells were resuspended in complete medium (RPMI 1640 [Cellgro, Herdon, Va.] supplemented with 10% fetal bovine serum [FBS; HyClone, Logan, Utah], 2 mM glutamine [Cellgro], 10 U of penicillin [Invitrogen, Carlsbad, Calif.]/ml, and 10 μg of streptomycin [Invitrogen]/ml). At 2 days postinfection, approximately 40% of the BL2 cells were EGFP positive. Alternatively, genome equivalents were determined by real-time PCR analysis as indicated below. Mice were infected with approximately 8 × 104 to 40 × 104 EBV genome equivalents by injection (in 30 or 150 μl) directly into the spleen. Mice were sacrificed at 5 to 6 weeks postinfection. Spleens, peripheral blood, and bone marrow were harvested essentially as previously described (21, 22). In addition, tumor samples were collected, fixed in formalin, and processed for histology. Paraffin-embedded hematoxylin-and-eosin-stained sections from eight mice were reviewed by a pathologist who was blinded to prior mice treatments. Immunohistochemical studies were performed on an automated ES system (Ventana Medical Systems Inc., Tucson, Ariz.). All samples were stained with the pan-T-cell markers CD3 (Dako, Carpinteria, Calif.) and CD5 (Novocastra Laboratory, Newcastle, United Kingdom), the pan-B-cell markers CD20 and CD79a (Dako), CD30 (Dako), the myeloid marker CD15 (Biocare Medical, Walnut Creek, Calif.), and CD10 (Novocastra Laboratory). In situ hybridization studies were performed for kappa and lambda light chains as well as EBERs for all eight mouse samples (Dako). Appropriate isotype controls were used for all stains. In addition, tumor samples were dissociated, resuspended as single-cell suspensions, pelleted, and stored frozen in 10% dimethyl sulfoxide (Sigma)-90% FBS (HyClone) in liquid nitrogen. LCLs from peripheral blood, bone marrow, and spleens were established by plating 105 cells in 96-well dishes with 200 μl of RPMI (Invitrogen) supplemented with 20% FBS, 1% penicillin-streptomycin-glutamine (Invitrogen), 1% sodium pyruvate (Invitrogen), and 1 mM mercaptoethanol (Sigma).
PCR analysis.
EBV DNA levels in peripheral blood were determined by real-time PCR analysis essentially as previously described (29). Briefly, total DNAs were isolated from whole mouse blood (50 μl) by use of a blood extraction kit (Qiagen, Valencia, Calif.) according to the manufacturer's protocol. The DNAs were eluted in a 150-μl volume. Ten microliters of DNA was used for real-time PCR. Analyses of EBNA1, EBNA2, LMP1, and LMP2a expression were performed by reverse transcription-PCR (RT-PCR) essentially as described previously (2). RNAs were isolated from splenocytes, LCLs, or tumor cells by use of an RNeasy kit (Qiagen). DNAs were removed by digestion with DNase from a DNA-free kit (Ambion, Austin, Tex.). cDNAs were prepared by use of a Superscript first-strand synthesis system for RT-PCR kit (Invitrogen). The PCR primers and amplification conditions used were previously published (2, 63). PCR products were resolved in 1.5% agarose gels and visualized with ethidium bromide. The identities of PCR amplification products were confirmed by direct DNA sequencing performed at the UT Southwestern Medical Center's sequencing facility.
Flow cytometry analysis.
Mononuclear cells were isolated from bone marrow, peripheral blood, and spleens of engrafted mice. Reconstitution with human cells, EGFP expression in human B cells in infected mice, and LCL phenotypes were determined by flow cytometry on a FACSCalibur flow cytometer running the CellQuest software package (BD Biosciences, San Jose, Calif.). To reduce the nonspecific binding of monoclonal antibodies (MAbs), we incubated the cells with mouse immunoglobulins (Igs) (10 μg/ml; Sigma) on ice for 20 min before the addition of a human-specific MAb or an isotype control MAb. Samples were stained with the following MAbs: anti-CD3-fluorescein isothiocyanate (IgG1, clone UCHT1), anti-CD5-allophycocyanin (APC) (IgG1, clone UCHT2), anti-CD10-phycoerythrin (PE) (IgG1, clone HI10a), anti-CD19-PE (IgG1, clone HIB19), anti-CD21-APC (IgG1, clone B-ly4), anti-CD30-biotin (IgG1, clone BerH8), anti-CD45-PE (IgG1, clone HI30), anti-CD45-APC (IgG1, clone HI30), and anti-CD79a-APC (IgG1, clone HM47) (all from BD Pharmingen, San Diego, Calif.). Anti-CD19-PerCP-Cy5.5 (IgG1, clone SJ2SC1) and anti-CD33-PerCP-Cy5.5 (IgG1, clone P67.6) were obtained from BD Biosciences. Biotinylated antibodies were revealed with streptavidin-PE from BD Biosciences. Appropriate isotype controls were used for all stains.
Fluorescence microscopy analysis of tumors and LCLs obtained from mice infected with EGFP-tagged EBV.
Organs with tumors were removed intact and were analyzed for EGFP expression under a dissecting microscope (SMZ1500; 0.5× WD136) with a UV light source (Nikon, Lewisville, Tex.). Images were recorded with a Nikon digital DXM1200 camera and analyzed with Nikon ACT-1, version 2.11, software. LCLs were analyzed for EGFP expression under a DM IRBE inverted microscope (Leica, Deerfield, Ill.) using a Photometrics Coolsnap HQ camera (Tucson, Ariz.) and were acquired digitally with the Meta Series 5.0 software package.
Statistics.
Statistical analysis was performed with GraphPad Prism, version 4.0 (GraphPad Software Inc., San Diego, Calif.). Data are presented as means of experimental measurements and standard errors of the means (SEM).
RESULTS
Reconstitution of immunodeficient mice with human CD34+ cells.
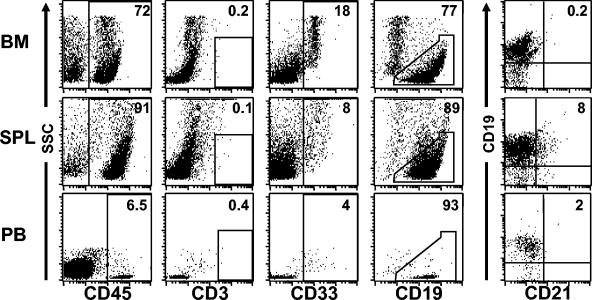
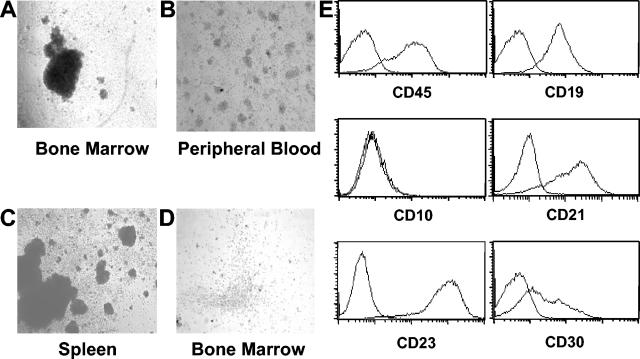
SCID mice are readily reconstituted with human hematopoietic stem cells (21, 23). However, their overall levels of reconstitution are relatively low (23). In this regard, NOD/SCID mice serve as better recipients of human hematopoietic stem cells because they consistently produce significantly higher levels of reconstitution with human cells that are sustained for months after engraftment (23). We chose human cord blood CD34+ cells for transplantation into preconditioned (325 cGy of γ-irradiation) NOD/SCID mice because they have been shown to result in higher levels of reconstitution than CD34+ cells isolated from bone marrow or fetal livers (28). In addition, the use of cord blood greatly reduces the possibility of contamination with EBV-infected B cells. CD34+ cells were isolated by immunomagnetic selection and were >90% pure, as determined by flow cytometry. Cells (2 × 105 to 5 × 105 per mouse) were injected into preconditioned recipient mice via the tail vein. Reconstitution with human cells in mouse peripheral blood was monitored by whole blood staining with human-specific anti-CD45. At 6 to 8 weeks posttransplantation, the average level of human cells in the peripheral blood of recipient mice was 4% ± 1.6% (range, 2 to 7%; n = 10). A flow cytometry analysis of the human cells in recipient mice demonstrated that the majority of the human cells present in the different hematopoietic tissues harvested from engrafted mice were B lymphocytes (Fig. 1). Low levels of myeloid cells were also present, and no T cells were found in any of the mice used in these experiments. These results were consistent with our previous observations and those from other laboratories (21, 22, 32). Interestingly, human B cells expressing detectable levels of human CD21, the major EBV receptor, were only detected in the spleen (Fig. 1). Therefore, EBV infections were performed via intrasplenic injections.
FIG. 1.
Engraftment of preconditioned NOD/SCID mice with human CD34+ cells results predominantly in the production of human B cells. A flow cytometry analysis of human cells (CD45+) that were present in the bone marrow, spleens, and peripheral blood of reconstituted mice showed the presence of abundant CD19+ human B cells in all hematopoietic tissues. Consistent with previous reports, human CD33+ myeloid cells, but no human T cells (CD3+), were detected in these mice in addition to B cells. CD21+ B cells were only present in the spleen. Note that the analysis of CD19, CD33, CD3, and CD21 expression was performed on cells gated through hCD45. Data are from one representative mouse of a group of eight mice.
Infection of NOD/SCID mice reconstituted with human CD34+ cells results in high levels of EBV DNA in peripheral blood.
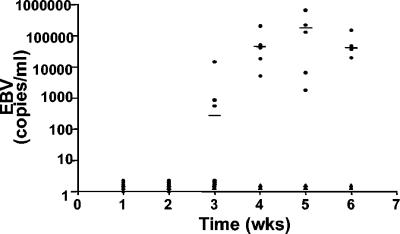
To determine the susceptibility of NOD/SCID mice that were reconstituted with human CD34+ cells to infection with EBV, we injected the virus into mice via the spleen at 8 to 10 weeks posttransplantation. EBV infections were monitored by real-time PCRs of peripheral blood DNAs every 7 days postinfection. Human beta-actin DNA was used as an internal control for all amplifications. No viral DNA was detected in any sample at the first and second week postinfection (Fig. 2). Viral DNA was present in three of six infected mice at 3 weeks postinfection. Four weeks after infection, all samples (six of six) obtained from mice infected with EBV showed high levels of viral DNA that remained high for the rest of the experiment (6 weeks). None of the blood samples obtained from human cell recipient but uninfected mice or from mice that were not human cell recipients but were infected with EBV had detectable levels of EBV DNA (Fig. 2 and data not shown). At the time of harvest, the level of reconstitution with human cells in the bone marrow was 57% ± 3% (range, 47 to 65%; n = 6).
FIG. 2.
Analysis of EBV loads in peripheral blood after infection of NOD/SCID mice that were reconstituted with human CD34+ cells. DNAs were extracted from blood samples collected at weekly intervals from infected and control (transplant recipients that were not infected) mice. Samples were analyzed for the presence of EBV DNA by real-time PCR. Note that no virus was detected in any of the mice during the first 2 weeks postinfection and that by 4 weeks postinfection all animals had high levels of viral DNA in their blood. •, infected mice; ▴, control uninfected mice.
Sequelae of EBV infection in NOD/SCID mice.
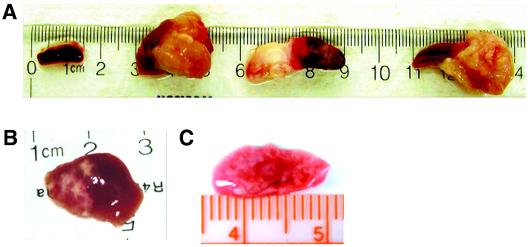
EBV-infected mice began to show signs of illness by 5 weeks postinfection. The first notable changes were weight loss and a reduction in overall physical activity. All mice were harvested for analysis at 5 to 7 weeks postinfection. At the time of harvesting, it was noted that all infected mice developed obvious tumors in the spleen (n = 10) (Fig. 3A). In addition, a gross analysis of other tissues, including livers and lungs, also showed signs of disseminated disease (Fig. 3B). Two control groups of mice (transplant recipients that were not infected or nonrecipients that were infected with EBV) were maintained in parallel (n = 6). Animals in these groups did not develop tumors and did not present gross or histological evidence of neoplasia in any of the tissues examined (Fig. 3A and data not shown). These results demonstrate that NOD/SCID mice that are reconstituted with human CD34+ cells can be experimentally infected with EBV and that their infection results in the development of tumors.
FIG. 3.
Development of tumors after infection with EBV in NOD/SCID mice that were reconstituted with human CD34+ cells. At 5 to 6 weeks postinfection, all infected animals had developed tumors in their spleens. (A) Spleen from a control (not infected) reconstituted mouse and spleens from three different EBV-infected mice (n = 7). Disseminated disease was also seen in other organs, including livers (B) and lungs (C).
Cytological characterization of tumors found in NOD/SCID mice infected with EBV.
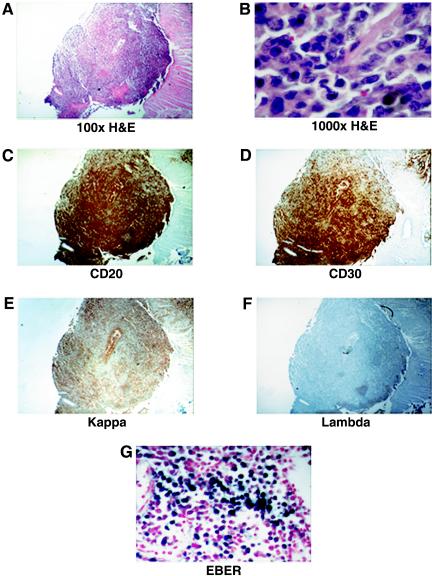
A histological analysis of the tumors found in EBV-infected mice showed diffuse displacement of the spleen by atypical large lymphocytes, some of which had features consistent with immunoblasts (Fig. 4 and Table 1). All tumors analyzed stained positive for human CD20, CD30, and CD79a and were negative for CD3, CD10, and CD15. In addition, two of the tumors were positive for CD5. This phenotype is consistent with that of large-B-cell lymphomas. All tumors were light chain restricted: most tumors were lambda chain positive, but one was kappa chain positive. In addition, all tumors analyzed were positive for EBERs (by in situ hybridization), confirming their association with EBV (Fig. 4). These results demonstrate that the tumors arising in NOD/SCID mice that are reconstituted with human cells and experimentally infected with EBV are of human origin and that their phenotypic characterization is consistent with their classification as large-B-cell immunoblastic lymphomas.
FIG. 4.
Histological characterization of EBV-induced tumors in infected NOD/SCID mice. (A and B) Hematoxylin and eosin (H&E) staining of a representative tumor originating from the spleen of an infected mouse (magnification, ×100 and ×1,000, respectively). (C to F) Immunohistochemical staining with antibodies to human CD20 and CD30 and with antibodies to human immunoglobulin kappa or lambda chains. (G) In situ staining for EBERs in a section from the same tumor.
TABLE 1.
Histological characterization of tumors from EBV-infected mice
| Mouse no. | Presence of staining for marker
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD3 | CD5 | CD10 | CD15 | CD20 | CD30 | CD79a | κ chain | λ chain | EBER | |
| M2 | − | + | − | − | + | + | + | + | − | + |
| M5 | − | − | − | − | + | + | + | + | − | + |
| M7 | − | + | − | − | + | + | + | − | + | + |
| M11 | − | − | − | − | + | + | + | + | − | + |
| M13 | − | − | − | − | + | + | + | + | − | + |
| M14 | − | − | − | − | + | + | + | + | − | + |
Molecular analysis of EBV latency genes in tumor samples and LCLs from infected mice.
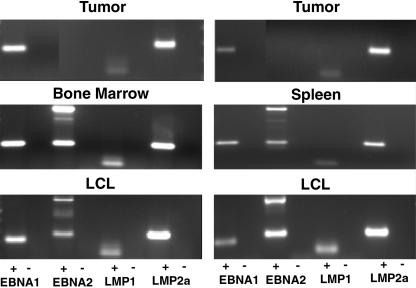
RT-PCR analysis was used to analyze the latency patterns of tumors in EBV-infected mice. The expression of EBNA1, EBNA2, LMP1, and LMP2a was analyzed essentially as previously described (2, 12, 63). Samples from three different spleen tumors obtained from three different mice were used for this analysis. EBNA1, LMP1, and LMP2a were expressed in all three samples (Fig. 5 and data not shown). No EBNA2 expression was detected in these samples despite repeated attempts and the use of two different sets of primers. These results are consistent with a type II latency pattern of gene expression for the EBV-induced tumors in these mice.
FIG. 5.
Latency analysis of EBV in tumor, bone marrow, and spleen cells and in vitro-expanded LCLs. RT-PCR analyses of EBV gene expression were performed with RNAs obtained from tumor cells isolated from two different infected mice (top), from spleen and bone marrow cells (middle), and from LCLs expanded in vitro from bone marrow and spleen cultures (bottom). The PCR products shown correspond to EBNA1, EBNA2, LMP1, and LMP2a. The plus and minus signs indicate whether or not the RNA samples were reverse transcribed prior to PCR amplification. Note the absence of the PCR amplification product for EBNA2 in the two tumor samples and its presence in the bone marrow, spleen, and LCL samples (all were amplified with the same sets of primers and under identical conditions).
An analysis of the expression of EBNA1, EBNA2, LMP1, and LMP2a in RNA samples isolated from bone marrow and spleen cells (not tumor cells) from infected animals demonstrated the expression of all four genes, consistent with a type III latency pattern of gene expression (Fig. 5, middle panels). These results show differential patterns of latency in tumors versus cells that were present in the bone marrow and spleen.
Ex vivo culturing of peripheral blood cells, splenocytes, and bone marrow cells from multiple mice infected with EBV resulted in the establishment of LCLs (Fig. 6). The human origin of these cells and their identity as B lymphocytes were confirmed by their expression of human CD45 and human CD19 (Fig. 6). In vitro-cultured LCLs were characterized by their expression of human CD21, CD23, and CD30 and by a lack of expression of human CD10. A molecular analysis of their latency pattern by RT-PCR showed that all LCLs obtained after in vitro culturing expressed EBNA1, EBNA2, LMP1, and LMP2a (Fig. 5, bottom panels). This pattern of gene expression is characteristic of the type III latency program.
FIG. 6.
Flow cytometric analysis of LCLs derived from EBV-infected mice. (A to C) In vitro-cultured cells obtained from the bone marrow, peripheral blood, and spleens of infected mice resulted in the outgrowth of EBV+ LCLs. (D) In contrast, note the absence of cell clumps from a bone marrow sample obtained from a mouse that was a transplant recipient of human CD34+ cells but was not infected with EBV. (E) Flow cytometric analysis of in vitro-expanded LCLs for cell surface expression of human CD45, CD19, CD10, CD21, CD23, and CD30.
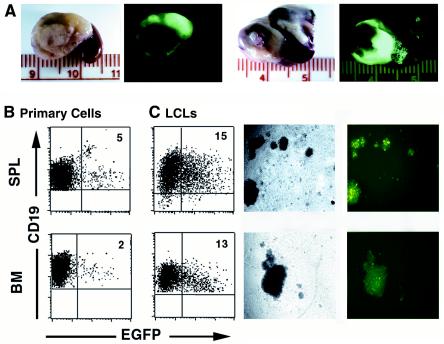
In situ imaging of tumors after infection of NOD/SCID mice that were reconstituted with human CD34+ cells by EGFP-tagged EBV.
Recently, Speck et al. characterized an EBV recombinant virus that incorporates EGFP under the control of the immediate early cytomegalovirus promoter (57, 58). This recombinant virus is capable of infecting and immortalizing human primary B cells in vitro, and infections can be readily monitored as a function of EGFP expression (57, 58). Since EGFP expression could be used as a surrogate marker for EBV infection and to facilitate the in vivo visualization of the generated tumors, we infected transplant recipient mice with the recombinant virus EBfaV-GFP. EBfaV-GFP was generated by homologous recombination with B95-8 (57, 58). Thus, the virus preparations harbored a mixture of EGFP+ and wild-type virus (58). Animals infected with the EGFP-tagged virus (n = 6) were sacrificed at 5 to 6 weeks postinfection, and tumor-bearing organs were analyzed under UV light. Fluorescence signals were observed from all tumors from the mice (Fig. 7A). In addition, the presence of EGFP+ hCD19+ B cells isolated from both peripheral blood and bone marrow was easily determined by flow cytometry (Fig. 7B). The in vitro expansion of bone marrow and spleen cells from mice infected with the EGFP-tagged virus resulted in the establishment of mixed populations of EGFP-positive and -negative LCLs. The presence of both EGFP-positive and -negative cells was consistent with the fact that both the recombinant and the wild-type viruses are present in the stock that was used to infect the mice (57, 58). These results highlight the transformation potential of this EGFP-tagged virus and suggest that EBfaV-EGFP will be useful for in vivo analyses of EBV infections.
FIG. 7.
In situ analysis of EGFP expression in tumors resulting from infection with EBV tagged with EGFP. (A) Infected mice were sacrificed at 5 to 7 weeks postinfection, and internal organs from two different mice with visible tumors were removed for analysis under UV light with a dissecting microscope. (B) EGFP-expressing human B cells were also detected in the spleens (SPL) (not tumor tissue) and bone marrow (BM) of these mice (left dot plots and pictures). (C) LCLs obtained after in vitro culturing of cells from spleens or peripheral blood also expressed EGFP (right dot plots and images).
DISCUSSION
In situations in which the immune system is compromised, herpesviruses are a common cause of morbidity and mortality (39, 49). For example, the incidence of posttransplant lymphoproliferative disorders in solid organ transplant recipients is variable, but it can be as high as 10% for heart-lung transplant recipients (10). In the setting of allogeneic bone marrow transplantation with T-cell depletion, the incidence of posttransplant lymphoproliferative disorders can reach 20% (10). Encouraging results have been obtained with patients receiving heart and/or lung transplants who received prophylactic antiviral treatment (i.e., ganciclovir and acyclovir) (11). However, currently there are no preventative strategies or treatments that can adequately address EBV-related posttransplant disorders. Several possible treatment options are being considered, including the use of antibodies to interleukin-6 (IL-6) or CD20 and the infusion of EBV-specific CTLs (4, 13, 19, 24, 38, 52, 53). These and other possible treatment options would benefit greatly from the availability of preclinical in vivo models in which they could be readily evaluated and optimized.
Experimental in vivo models of herpesvirus infections have provided definitive insights into the molecular basis of disease and have served as comparative models for EBV infection of humans. Two models with significant potential are the infection of rhesus monkeys with lymphocryptovirus and the infection of mice with murine herpesvirus 68 (MHV68) (60). The infection of naïve rhesus monkeys with lymphocryptovirus results in an acute response that resembles infectious mononucleosis, with a subsequent persistent infection and oropharyngeal virus shedding (42). However, further work is needed to clearly establish the utility of this model system. MHV68 infection is characterized by an acute phase that leads to a persistent infection for which B cells are the major site of viral latency (60). Interestingly, lymphoproliferative disease is observed in about 10% of infected mice (59). The incidence of lymphoproliferative disease is dramatically increased if mice are treated with the immunosuppressive drug cyclosporine. However, the direct role of MHV68 in tumorigenesis needs further clarification. There is great potential for MHV68 as a model system of experimental infection with a herpesvirus.
The common marmoset and cottontop tamarin models of experimental infection with EBV have a significant advantage over the above system in that both permit the replication of EBV. Therefore, analyses of EBV-induced pathogenesis and evaluations of therapeutic or vaccine approaches with specific inhibitors of EBV replication and/or latency are possible in these models (6, 9, 15, 16, 69). In addition to the already mentioned difficulties with both experimental systems, one other drawback is the fact that information obtained with either of these two systems would then need to be tested with human cells.
In this study, we evaluated the susceptibility of NOD/SCID mice that were reconstituted with human CD34+ cells to infection by EBV. Our results show that after reconstitution with human CD34+ cells, the predominant type of human cells in these mice are B cells and that reconstituted mice are clearly susceptible to experimental infection with EBV. We showed that infection with EBV results in high levels of EBV DNA in peripheral blood that can be readily quantified by real-time PCR. Furthermore, we showed that mice infected with EBV develop tumors of human origin and that these tumors are large-cell lymphomas of an immunoblastic type that resemble posttransplant lymphoproliferative disease-like lymphomas. Traggiai et al. recently described the use of a different strain of immunodeficient mice in which transplanted human CD34+ cells gave rise to human T cells in addition to human B cells (65). Interestingly, unlike our system of reconstituted NOD/SCID mice, in which all infected animals developed tumors, Traggiai et al. did not find tumors in their EBV-infected mice. The fact that EBV-infected B cells in our system developed into type II rather than type III tumors in vivo was somewhat surprising. Type II tumors such as those seen in patients with Hodgkin's lymphoma are believed to be a consequence of selective pressure against EBNA3 proteins. Since there were no detectable levels of human T cells in these mice, the reasons that we observed type II tumors are unclear at this time. Future work with this system will be needed to clarify this interesting observation. It is clear that the direct injection of EBV into the spleens of transplant recipient mice cannot be considered a natural route of infection, but it should be noted that this route of infection resulted in the development of tumors in 100% of infected mice. We also illustrated the potential utility of an EGFP-tagged virus for the visualization of tumors in situ with the potential future application of imaging technologies for noninvasive real-time monitoring of tumor development and regression.
One interesting observation made with this system was the fact that after infection with EBV, tumors developed in the absence of human T cells. It has been postulated that human T cells play an essential role in tumor development after the inoculation of human PBMC from EBV-seropositive patients into SCID mice (30, 31, 66). The removal of either CD4+ or CD8+ T cells from PBMC isolated from EBV-seropositive individuals was reported to reduce the incidence of tumor formation in SCID mice. These results were interpreted by the authors as suggesting that T cell-B cell contact and/or a soluble factor(s) produced by T cells serves to potentiate the growth of EBV-infected B cells in vivo. Our results suggest that tumor development in immunodeficient mice is not dependent on T cell-B cell interactions or the presence of a soluble factor(s) derived from human T cells. However, it is possible that human T cells were present in reconstituted mice below the level of detection by flow cytometry. Such low levels of human T cells may be sufficient to potentiate the growth of EBV-infected cells in vivo. However, our results are also consistent with the fact that LCLs established in vitro can develop into aggressive tumors in immunodeficient mice in the absence of human T cells (46).
The use of CD34+ cells to reconstitute immunodeficient mice for the study of EBV infection will complement and expand on information obtained with PBMC-SCID-hu mice. One important difference between mice receiving transplanted PBMC and those receiving transplanted CD34+ cells is the lack of xenoreactive T cells in the latter (61, 62). It has been established that the vast majority of T cells that persist in mice inoculated with PBMC represent the expansion of subsets of cells that display reactivity to major histocompatibility complex II antigens. The level and severity of graft-versus-host disease in this system are variable and are dependent to some extent on the size of the inoculum and the overall health of the mouse colony. Since there were no T cells in the transplant recipient mice used for the experiments described in this paper, this issue was completely avoided in our studies. The lack of T cells in these mice also represents an opportunity for the evaluation of adoptive immunotherapy approaches to the treatment of EBV-induced lymphoproliferative disorders. For example, the infusion of EBV-specific CTLs has resulted in posttransplant lymphoproliferative disease regression in both solid organ and hematopoietic stem cell transplant patients (8, 25, 26, 34, 47, 50, 51). Human clinical trials of PBMC infusion after T-cell-depleted bone marrow transplantation have shown tumor regression, but this treatment also caused severe graft-versus-host disease in all patients (47). The generation of EBV-specific T cells for infusion has been shown to be successful and to have greatly diminished the incidence of graft-versus-host disease (50). The substitution of cord-blood-derived CD34+ cells for CD34+ cells from mobilized peripheral blood will greatly facilitate the use of this system to investigate CTL immunotherapy. Thus, the animal model described here may serve as an excellent platform for the evaluation of new, more effective, and safer ways of deriving CTLs in vitro for the treatment of EBV lymphoproliferative disease.
Acknowledgments
We thank K. Hamra and D. Garbers for the use of their fluorescence microscope; R. Longnecker for the GFP-labeled virus; J. Sixbey and R. Scott for the EBV stocks used for the experiments described in this paper; S. O'Reilly, R. Bibi, N. Guaring-Angulo, and E. Keschman for their excellent technical assistance; P. Denton for his extensive assistance with illustrations; N. Karadikar for helpful comments; C. Sample, J. Sixbey, and members of the Garcia laboratory for critical readings of the manuscript; and J. Gatlin for his multiple contributions during the early stages of this work.
J.V.C. was supported in part by NIH grant CA82055.
Cord blood samples were provided by the Department of Obstetrics and Gynecology's Tissue Procurement Facility of the University of Texas Southwestern Medical Center at Dallas (NIH grant HD011149).
REFERENCES
- 1.Aljurf, M. D., T. W. Owaidah, A. Ezzat, E. Ibrahim, and A. Tbakhi. 2003. Antigen- and/or immune-driven lymphoproliferative disorders. Ann. Oncol. 14:1595-1606. [DOI] [PubMed] [Google Scholar]
- 2.Babcock, G. J., and D. A. Thorley-Lawson. 2000. Tonsillar memory B cells, latently infected with Epstein-Barr virus, express the restricted pattern of latent genes previously found only in Epstein-Barr virus-associated tumors. Proc. Natl. Acad. Sci. USA 97:12250-12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumforth, K. R., L. S. Young, K. J. Flavell, C. Constandinou, and P. G. Murray. 1999. The Epstein-Barr virus and its association with human cancers. Mol. Pathol. 52:307-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollard, C. M., B. Savoldo, C. M. Rooney, and H. E. Heslop. 2003. Adoptive T-cell therapy for EBV-associated post-transplant lymphoproliferative disease. Acta Haematologica 110:139-148. [DOI] [PubMed] [Google Scholar]
- 5.Chang, Y., G. C. Bosma, and M. J. Bosma. 1995. Development of B cells in scid mice with immunoglobulin transgenes: implications for the control of V(D)J recombination. Immunity 2:607-616. [DOI] [PubMed] [Google Scholar]
- 6.Cleary, M. L., M. A. Epstein, S. Finerty, R. F. Dorfman, G. W. Bornkamm, J. K. Kirkwood, A. J. Morgan, and J. Sklar. 1985. Individual tumors of multifocal EB virus-induced malignant lymphomas in tamarins arise from different B-cell clones. Science 228:722-724. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, J. I. 2000. Epstein-Barr virus infection. N. Engl. J. Med. 343:481-492. [DOI] [PubMed] [Google Scholar]
- 8.Comoli, P., M. Labirio, S. Basso, F. Baldanti, P. Grossi, M. Furione, M. Vigano, R. Fiocchi, G. Rossi, F. Ginevri, B. Gridelli, A. Moretta, D. Montagna, F. Locatelli, G. Gerna, and R. Maccario. 2002. Infusion of autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for prevention of EBV-related lymphoproliferative disorder in solid organ transplant recipients with evidence of active virus replication. Blood 99:2592-2598. [DOI] [PubMed] [Google Scholar]
- 9.Cox, C., S. Chang, L. Karran, B. Griffin, and N. Wedderburn. 1996. Persistent Epstein-Barr virus infection in the common marmoset (Callithrix jacchus). J. Gen. Virol. 77:1173-1180. [DOI] [PubMed] [Google Scholar]
- 10.Curtis, R. E., L. B. Travis, P. A. Rowlings, G. Socie, D. W. Kingma, P. M. Banks, E. S. Jaffe, G. E. Sale, M. M. Horowitz, R. P. Witherspoon, D. A. Shriner, D. J. Weisdorf, H. J. Kolb, K. M. Sullivan, K. A. Sobocinski, R. P. Gale, R. N. Hoover, J. F. Fraumeni, Jr., and H. J. Deeg. 1999. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood 94:2208-2216. [PubMed] [Google Scholar]
- 11.Darenkov, I. A., M. A. Marcarelli, G. P. Basadonna, A. L. Friedman, K. M. Lorber, J. G. Howe, J. Crouch, M. J. Bia, A. S. Kliger, and M. I. Lorber. 1997. Reduced incidence of Epstein-Barr virus-associated posttransplant lymphoproliferative disorder using preemptive antiviral therapy. Transplantation 64:848-852. [DOI] [PubMed] [Google Scholar]
- 12.Delecluse, H. J., E. Kremmer, J. P. Rouault, C. Cour, G. Bornkamm, and F. Berger. 1995. The expression of Epstein-Barr virus latent proteins is related to the pathological features of post-transplant lymphoproliferative disorders. Am. J. Pathol. 146:1113-1120. [PMC free article] [PubMed] [Google Scholar]
- 13.Durandy, A. 2001. Anti-B cell and anti-cytokine therapy for the treatment of post-transplant lymphoproliferative disorder: past, present, and future. Transplant. Infect. Dis. 3:104-107. [DOI] [PubMed] [Google Scholar]
- 14.Ehlin-Henriksson, B., J. Gordon, and G. Klein. 2003. B-lymphocyte subpopulations are equally susceptible to Epstein-Barr virus infection, irrespective of immunoglobulin isotype expression. Immunology 108:427-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein, M. A., A. J. Morgan, S. Finerty, B. J. Randle, and J. K. Kirkwood. 1985. Protection of cottontop tamarins against Epstein-Barr virus-induced malignant lymphoma by a prototype subunit vaccine. Nature 318:287-289. [DOI] [PubMed] [Google Scholar]
- 16.Falk, L., F. Deinhardt, L. Wolfe, D. Johnson, J. Hilgers, and G. de-The. 1976. Epstein-Barr virus: experimental infection of Callithrix jacchus marmosets. Int. J. Cancer 17:785-788. [DOI] [PubMed] [Google Scholar]
- 17.Flano, E., D. L. Woodland, and M. A. Blackman. 2002. A mouse model for infectious mononucleosis. Immunol. Res. 25:201-217. [DOI] [PubMed] [Google Scholar]
- 18.Gan, Y. J., J. L. Sullivan, and J. W. Sixbey. 1994. Detection of cell-free Epstein-Barr virus DNA in serum during acute infectious mononucleosis. J. Infect. Dis. 170:436-439. [DOI] [PubMed] [Google Scholar]
- 19.Ganne, V., N. Siddiqi, B. Kamaplath, C. C. Chang, E. P. Cohen, B. A. Bresnahan, and S. Hariharan. 2003. Humanized anti-CD20 monoclonal antibody (Rituximab) treatment for post-transplant lymphoproliferative disorder. Clin. Transplant. 17:417-422. [DOI] [PubMed] [Google Scholar]
- 20.Garnier, J. L., N. R. Cooper, and M. J. Cannon. 1993. Low expression of CD20 and CD23 in Epstein-Barr virus-induced B cell tumors in SCID/hu mice. Am. J. Pathol. 142:353-358. [PMC free article] [PubMed] [Google Scholar]
- 21.Gatlin, J., M. W. Melkus, A. Padgett, P. F. Kelly, and J. V. Garcia. 2001. Engraftment of NOD/SCID mice with human CD34+ cells transduced by concentrated oncoretroviral vector particles pseudotyped with the feline endogenous retrovirus (RD114) envelope protein. J. Virol. 75:9995-9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatlin, J., A. Padgett, M. W. Melkus, P. F. Kelly, and J. V. Garcia. 2001. Long-term engraftment of nonobese diabetic/severe combined immunodeficient mice with human CD34+ cells transduced by a self-inactivating human immunodeficiency virus type 1 vector. Hum. Gene Ther. 12:1079-1089. [DOI] [PubMed] [Google Scholar]
- 23.Greiner, D. L., R. A. Hesselton, and L. D. Shultz. 1998. SCID mouse models of human stem cell engraftment. Stem Cells 16:166-177. [DOI] [PubMed] [Google Scholar]
- 24.Haddad, E., S. Paczesny, V. Leblond, J. M. Seigneurin, M. Stern, A. Achkar, M. Bauwens, V. Delwail, D. Debray, C. Duvoux, P. Hubert, B. Hurault de Ligny, J. Wijdenes, A. Durandy, and A. Fischer. 2001. Treatment of B-lymphoproliferative disorder with a monoclonal anti-interleukin-6 antibody in 12 patients: a multicenter phase 1-2 clinical trial. Blood 97:1590-1597. [DOI] [PubMed] [Google Scholar]
- 25.Haque, T., C. Taylor, G. M. Wilkie, P. Murad, P. L. Amlot, S. Beath, P. J. McKiernan, and D. H. Crawford. 2001. Complete regression of posttransplant lymphoproliferative disease using partially HLA-matched Epstein Barr virus-specific cytotoxic T cells. Transplantation 72:1399-1402. [DOI] [PubMed] [Google Scholar]
- 26.Haque, T., G. M. Wilkie, C. Taylor, P. L. Amlot, P. Murad, A. Iley, D. Dombagoda, K. M. Britton, A. J. Swerdlow, and D. H. Crawford. 2002. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet 360:436-442. [DOI] [PubMed] [Google Scholar]
- 27.Harada, S., and E. Kieff. 1997. Epstein-Barr virus nuclear protein LP stimulates EBNA-2 acidic domain-mediated transcriptional activation. J. Virol. 71:6611-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holyoake, T. L., F. E. Nicolini, and C. J. Eaves. 1999. Functional differences between transplantable human hematopoietic stem cells from fetal liver, cord blood, and adult marrow. Exp. Hematol. 27:1418-1427. [DOI] [PubMed] [Google Scholar]
- 29.Jebbink, J., X. Bai, B. B. Rogers, D. B. Dawson, R. H. Scheuermann, and R. Domiati-Saad. 2003. Development of real-time PCR assays for the quantitative detection of Epstein-Barr virus and cytomegalovirus, comparison of TaqMan probes, and molecular beacons. J. Mol. Diagn. 5:15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johannessen, I., M. Asghar, and D. H. Crawford. 2000. Essential role for T cells in human B-cell lymphoproliferative disease development in severe combined immunodeficient mice. Br. J. Haematol. 109:600-610. [DOI] [PubMed] [Google Scholar]
- 31.Johannessen, I., and D. H. Crawford. 1999. In vivo models for Epstein-Barr virus (EBV)-associated B cell lymphoproliferative disease (BLPD). Rev. Med. Virol. 9:263-277. [DOI] [PubMed] [Google Scholar]
- 32.Kapp, U., M. Bhatia, D. Bonnet, B. Murdoch, and J. E. Dick. 1998. Treatment of non-obese diabetic (NOD)/severe-combined immunodeficient mice (SCID) with flt3 ligand and interleukin-7 impairs the B-lineage commitment of repopulating cells after transplantation of human hematopoietic cells. Blood 92:2024-2031. [PubMed] [Google Scholar]
- 33.Kaye, K. M., K. M. Izumi, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 90:9150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khanna, R., S. Bell, M. Sherritt, A. Galbraith, S. R. Burrows, L. Rafter, B. Clarke, R. Slaughter, M. C. Falk, J. Douglass, T. Williams, S. L. Elliott, and D. J. Moss. 1999. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc. Natl. Acad. Sci. USA 96:10391-10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komano, J., S. Maruo, K. Kurozumi, T. Oda, and K. Takada. 1999. Oncogenic role of Epstein-Barr virus-encoded RNAs in Burkitt's lymphoma cell line Akata. J. Virol. 73:9827-9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurth, J., T. Spieker, J. Wustrow, G. J. Strickler, L. M. Hansmann, K. Rajewsky, and R. Kuppers. 2000. EBV-infected B cells in infectious mononucleosis: viral strategies for spreading in the B cell compartment and establishing latency. Immunity 13:485-495. [DOI] [PubMed] [Google Scholar]
- 37.Lam, N., and B. Sugden. 2003. LMP1, a viral relative of the TNF receptor family, signals principally from intracellular compartments. EMBO J. 22:3027-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, Z., B. Savoldo, H. Huls, T. Lopez, A. Gee, J. Wilson, M. K. Brenner, H. E. Heslop, and C. M. Rooney. 2002. Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the prevention and treatment of EBV-associated post-transplant lymphomas. Recent Results Cancer Res. 159:123-133. [DOI] [PubMed] [Google Scholar]
- 39.Lopes, V., L. S. Young, and P. G. Murray. 2003. Epstein-Barr virus-associated cancers: aetiology and treatment. Herpes 10:78-82. [PubMed] [Google Scholar]
- 40.Menin, C., L. Ometto, A. Veronesi, M. Montagna, V. Coppola, M. L. Veronese, S. Indraccolo, L. Bruni, B. Corneo, A. Amadori, A. De Rossi, L. Chieco-Bianchi, and E. D'Andrea. 1995. Dominance of a single Epstein-Barr virus strain in SCID-mouse tumors induced by injection of peripheral blood mononuclear cells from healthy human donors. Virus Res. 36:215-231. [DOI] [PubMed] [Google Scholar]
- 41.Miller, C. L., A. L. Burkhardt, J. H. Lee, B. Stealey, R. Longnecker, J. B. Bolen, and E. Kieff. 1995. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity 2:155-166. [DOI] [PubMed] [Google Scholar]
- 42.Moghaddam, A., M. Rosenzweig, D. Lee-Parritz, B. Annis, R. P. Johnson, and F. Wang. 1997. An animal model for acute and persistent Epstein-Barr virus infection. Science 276:2030-2033. [DOI] [PubMed] [Google Scholar]
- 43.Mosier, D. E. 1990. Immunodeficient mice xenografted with human lymphoid cells: new models for in vivo studies of human immunobiology and infectious diseases. J. Clin. Immunol. 10:185-191. [DOI] [PubMed] [Google Scholar]
- 44.Mosier, D. E., G. R. Picchio, M. B. Kirven, J. L. Garnier, B. E. Torbett, S. M. Baird, R. Kobayashi, and T. J. Kipps. 1992. EBV-induced human B cell lymphomas in hu-PBL-SCID mice. AIDS Res. Hum. Retrovir. 8:735-740. [PubMed] [Google Scholar]
- 45.Niedobitek, G., A. Agathanggelou, H. Herbst, L. Whitehead, D. H. Wright, and L. S. Young. 1997. Epstein-Barr virus (EBV) infection in infectious mononucleosis: virus latency, replication and phenotype of EBV-infected cells. J. Pathol. 182:151-159. [DOI] [PubMed] [Google Scholar]
- 46.Paine-Murrieta, G. D., C. W. Taylor, R. A. Curtis, M. H. Lopez, R. T. Dorr, C. S. Johnson, C. Y. Funk, F. Thompson, and E. M. Hersh. 1997. Human tumor models in the severe combined immune deficient (scid) mouse. Cancer Chemother. Pharmacol. 40:209-214. [DOI] [PubMed] [Google Scholar]
- 47.Papadopoulos, E. B., M. Ladanyi, D. Emanuel, S. Mackinnon, F. Boulad, M. H. Carabasi, H. Castro-Malaspina, B. H. Childs, A. P. Gillio, T. N. Small, J. W. Young, N. A. Kernan, and R. J. O'Reilly. 1994. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N. Engl. J. Med. 330:1185-1191. [DOI] [PubMed] [Google Scholar]
- 48.Pisa, P., M. J. Cannon, E. K. Pisa, N. R. Cooper, and R. I. Fox. 1992. Epstein-Barr virus induced lymphoproliferative tumors in severe combined immunodeficient mice are oligoclonal. Blood 79:173-179. [PubMed] [Google Scholar]
- 49.Razonable, R. R., and C. V. Paya. 2003. Herpesvirus infections in transplant recipients: current challenges in the clinical management of cytomegalovirus and Epstein-Barr virus infections. Herpes 10:60-65. [PubMed] [Google Scholar]
- 50.Rooney, C. M., C. A. Smith, C. Y. Ng, S. Loftin, C. Li, R. A. Krance, M. K. Brenner, and H. E. Heslop. 1995. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet 345:9-13. [DOI] [PubMed] [Google Scholar]
- 51.Rooney, C. M., C. A. Smith, C. Y. Ng, S. K. Loftin, J. W. Sixbey, Y. Gan, D. K. Srivastava, L. C. Bowman, R. A. Krance, M. K. Brenner, and H. E. Heslop. 1998. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 92:1549-1555. [PubMed] [Google Scholar]
- 52.Savoldo, B., J. Goss, Z. Liu, M. H. Huls, S. Doster, A. P. Gee, M. K. Brenner, H. E. Heslop, and C. M. Rooney. 2001. Generation of autologous Epstein-Barr virus-specific cytotoxic T cells for adoptive immunotherapy in solid organ transplant recipients. Transplantation 72:1078-1086. [DOI] [PubMed] [Google Scholar]
- 53.Sherritt, M. A., M. Bharadwaj, J. M. Burrows, L. E. Morrison, S. L. Elliott, J. E. Davis, L. M. Kear, R. E. Slaughter, S. C. Bell, A. J. Galbraith, R. Khanna, and D. J. Moss. 2003. Reconstitution of the latent T-lymphocyte response to Epstein-Barr virus is coincident with long-term recovery from posttransplant lymphoma after adoptive immunotherapy. Transplantation 75:1556-1560. [DOI] [PubMed] [Google Scholar]
- 54.Shope, T., D. Dechairo, and G. Miller. 1973. Malignant lymphoma in cottontop marmosets after inoculation with Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 70:2487-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sitki-Green, D., M. Covington, and N. Raab-Traub. 2003. Compartmentalization and transmission of multiple Epstein-Barr virus strains in asymptomatic carriers. J. Virol. 77:1840-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sixbey, J. W., E. H. Vesterinen, J. G. Nedrud, N. Raab-Traub, L. A. Walton, and J. S. Pagano. 1983. Replication of Epstein-Barr virus in human epithelial cells infected in vitro. Nature 306:480-483. [DOI] [PubMed] [Google Scholar]
- 57.Speck, P., K. A. Kline, P. Cheresh, and R. Longnecker. 1999. Epstein-Barr virus lacking latent membrane protein 2 immortalizes B cells with efficiency indistinguishable from that of wild-type virus. J. Gen. Virol. 80:2193-2203. [DOI] [PubMed] [Google Scholar]
- 58.Speck, P., and R. Longnecker. 1999. Epstein-Barr virus (EBV) infection visualized by EGFP expression demonstrates dependence on known mediators of EBV entry. Arch. Virol. 144:1123-1137. [DOI] [PubMed] [Google Scholar]
- 59.Sunil-Chandra, N. P., J. Arno, J. Fazakerley, and A. A. Nash. 1994. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am. J. Pathol. 145:818-826. [PMC free article] [PubMed] [Google Scholar]
- 60.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275-3279. [DOI] [PubMed] [Google Scholar]
- 61.Tary-Lehmann, M., P. V. Lehmann, D. Schols, M. G. Roncarolo, and A. Saxon. 1994. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J. Exp. Med. 180:1817-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tary-Lehmann, M., A. Saxon, and P. V. Lehmann. 1995. The human immune system in hu-PBL-SCID mice. Immunol. Today 16:529-533. [DOI] [PubMed] [Google Scholar]
- 63.Telenti, A., W. F. Marshall, and T. F. Smith. 1990. Detection of Epstein-Barr virus by polymerase chain reaction. J. Clin. Microbiol. 28:2187-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tierney, R. J., N. Steven, L. S. Young, and A. B. Rickinson. 1994. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J. Virol. 68:7374-7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Traggiai, E., L. Chicha, L. Mazzucchelli, L. Bronz, J. C. Piffaretti, A. Lanzavecchia, and M. G. Manz. 2004. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304:104-107. [DOI] [PubMed] [Google Scholar]
- 66.Veronese, M. L., A. Veronesi, E. D'Andrea, A. Del Mistro, S. Indraccolo, M. R. Mazza, M. Mion, R. Zamarchi, C. Menin, and M. Panozzo. 1992. Lymphoproliferative disease in human peripheral blood mononuclear cell-injected SCID mice. I. T lymphocyte requirement for B cell tumor generation. J. Exp. Med. 176:1763-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagar, E. J., M. A. Cromwell, L. D. Shultz, B. A. Woda, J. L. Sullivan, R. M. Hesselton, and D. L. Greiner. 2000. Regulation of human cell engraftment and development of EBV-related lymphoproliferative disorders in Hu-PBL-scid mice. J. Immunol. 165:518-527. [DOI] [PubMed] [Google Scholar]
- 68.Wedderburn, N., J. M. Edwards, C. Desgranges, C. Fontaine, B. Cohen, and G. de The. 1984. Infectious mononucleosis-like response in common marmosets infected with Epstein-Barr virus. J. Infect. Dis. 150:878-882. [DOI] [PubMed] [Google Scholar]
- 69.Young, L. S., S. Finerty, L. Brooks, F. Scullion, A. B. Rickinson, and A. J. Morgan. 1989. Epstein-Barr virus gene expression in malignant lymphomas induced by experimental virus infection of cottontop tamarins. J. Virol. 63:1967-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Young, L. S., and P. G. Murray. 2003. Epstein-Barr virus and oncogenesis: from latent genes to tumours. Oncogene 22:5108-5121. [DOI] [PubMed] [Google Scholar]