Abstract
Aims
Metaplastic breast carcinoma (MBC) is a rare subtype of breast carcinoma less responsive to conventional chemotherapy than ductal carcinoma. In molecular terms, MBCs usually cluster with triple-negative breast cancers (TNBCs), but have a worse prognosis than TNBCs. Studies investigating MBCs for specific biomarkers of therapy response are rare and limited by the methodological approaches. The aim of the present study was to characterise MBCs on a molecular level and test programmed death-ligand 1 (PD-L1) biomarker expression in MBCs for future therapeutic interventions.
Methods
We profiled 297 samples (MBC (n=75), TNBC (n=106), human epidermal growth factor receptor 2 (HER2)-positive breast cancers (n=32) and hormone-positive breast cancers (n=84)) by next-generation sequencing. Immunohistochemistry for PD-L1 and programmed cell death 1 (PD-1) expression was performed using automated procedures.
Results
The most commonly mutated genes in MBCs included TP53 (56%) and PIK3CA (23%). Pathogenic mutations in other genes, including HRAS, FBXW7, PTEN, AKT1 and SMAD4, were rare. PD-L1 expression was detected in a significantly higher proportion of MBCs (46%) than in other subtypes (6% each in hormone-positive and HER2-positive breast cancers, and 9% in TNBC, not otherwise specified, p<0.001). PD-1-positive tumour infiltrating lymphocytes (TILs) varied greatly in MBCs.
Conclusions
Comprehensive profiling of a large cohort of this rare subtype of breast carcinoma highlighted the predominance of TP53 mutation and increased PD-L1 expression in carcinoma cells. These results can be exploited in clinical trials using immune checkpoint inhibitors.
Keywords: BREAST CANCER, BREAST PATHOLOGY, TUMOUR BIOLOGY, GENETICS
Introduction
Metaplastic breast carcinomas (MBCs) are rare and aggressive tumours comprising ∼1% of all breast cancers.1 These heterogeneous tumours are composed of biphasic components, including conventional adenocarcinoma and metaplastic cellular and matrix components such as squamous, chondroid, spindle, rhabdoid or osseous. They are commonly triple-negative (oestrogen receptor (ER) negative/progesterone receptor (PR) negative and human epidermal growth factor receptor 2 (HER2) negative), and placed under the triple-negative breast cancer (TNBC) category. Despite shared biological characteristics, these tumour types have different clinical behaviours. MBCs are characterised by larger tumour size at presentation, lower rates of lymph node involvement and higher rates of recurrence.2 More importantly, they respond less frequently to chemotherapy than TNBCs and carry a worse prognosis with 5-year cumulative survival rates of 49%–64%.3 4 In efforts to better comprehend MBCs biologically, Ross et al5 genetically profiled 20 MBCs using hybridisation capture from ∼255 cancer-associated genes and discovered alterations in the TP53, PIK3CA, MYC, KMT2D (MLL2), PTEN, CKDN2A/B, CCND3, CCNE1, EGFR and KDM6A genes.
The tumour immune microenvironment has also come under scrutiny recently for exploration of new therapeutic strategies.6 An immunosuppressive microenvironment is maintained through several tumour-immune cell interactions including programmed cell death 1 (PD-1, CD279) receptor-ligand interaction. Programmed death-ligand 1 (PD-L1 or B7-H1), one of the two ligands of PD-1, is usually expressed on the surface of immune cells, such as antigen-presenting cells, and can be aberrantly expressed on cancer cells. PD-L1 binding to PD-1, a CD8 T-cell receptor, produces co-inhibitory signals that lead to inactivation of tumour infiltrating lymphocytes (TILs) and facilitate tumour progression. The suppression of PD-1/PD-L1 interaction using specific inhibitors has been correlated with significant and durable responses in many malignancies.7–10 No studies have investigated PD-L1 expression in MBCs; a few publications have analysed PD-L1 expression in TNBCs which have shown higher PD-L1 expression than hormone-positive and HER2-positive breast cancers (19%–39%).6 11–14
In this study, we analysed the distribution of PD-L1 expression in MBCs in comparison to hormone-positive, HER2-positive and TNBCs. We also analysed the presence of PD-1 expression in TILs within the MBC cohort and searched for correlations between immune-related biomarkers and genetic alterations.
Materials and methods
Samples and patients
Two hundred and ninety-seven formalin-fixed paraffin-embedded (FFPE) primary breast tissue samples from Thomas Jefferson University Hospital (Philadelphia, Pennsylvania, USA) and Caris Life Sciences (Phoenix, Arizona, USA) included MBC (n=75), TNBC, not otherwise specified (n=106), HER2-positive breast cancers (n=32) and hormone-positive (ER and PR) breast cancers (n=84). The TNBCs, HER2-positive and hormone-positive breast cancers were limited to histologic subtype: invasive ductal carcinoma, not otherwise specified. The mean age of the MBC group was 63 years. The H&E stained slides were re-reviewed by a board-certified pathologist to confirm the diagnosis of MBC. The diagnostic criteria included the presence of ductal carcinoma coexisting with a metaplastic component as defined by the WHO (2012) classification. Histologically, the MBC group consisted of carcinomas with metaplastic elements including 20 spindle, 18 squamous, 16 chondroid, 12 mixed, 5 myoepithelial, 3 not specified and 1 angiosarcomatoid. The tissue samples included biopsies and/or resection specimens irrespective of therapeutic status.
Within the MBC group, hormone receptor and HER2 status information were available for 71 patients. ER and PR immunohistochemistry (IHC) were considered positive when nuclear staining was identified in >1% of the tumour cells.15 HER2 scoring was based on circumferential membranous staining using the HER2 testing algorithm: Scores 0 and 1+ were considered negative, score 2+ was considered equivocal and reflexively tested with in situ hybridisation (ISH) and score 3+ was called positive on IHC alone.16 The majority of MBCs were triple negative (63/71, 89%). Among the remaining eight cases (11%), three were ER and PR positive only, two were PR positive only, two were ER positive only and one was HER2 positive/ER negative/PR negative. Within the invasive ductal carcinoma groups, the hormone-positive samples were ER and PR positive, and HER2 negative by IHC and HER2/CEP17 ISH. Among the HER2-positive cancers, all cases were HER2 positive by IHC and/or ISH. Of these, 16 were ER positive/PR negative, 11 were ER positive/PR positive and 5 were ER negative/PR positive.
TNBC samples were negative for ER, PR and HER2. The study was approved by the Institutional Review Board at both institutions.
Immunohistochemistry
FFPE tissue sections were stained for PD-L1 (clone: antihuman PD-L1 rabbit monoclonal antibody SP142, Spring Bioscience) and PD-1 (Clone EH12.1, BD Biosciences/Pharmingen) using automated procedures (Ventana BenchMark XT). For PD-L1 IHC, PD-L1 overexpression was estimated as a percentage of total tumour cells and categorised by intensity of staining (0–3+): 0 for no staining, 1+ for weak cytoplasmic staining, 2+ for moderate membranous staining and 3+ for strong membranous staining. Tumour samples with ≥2+ intensity in ≥5% of the tumour cells were considered positive for PD-L1 overexpression.6 17–19 Dendritic cells and macrophages were consistently positive for PD-L1 and lymphocytes in lymphoid follicles were positive for PD-1, serving as internal positive controls for IHC. PD-1 expression (membranous staining at any intensity) in TILs was assessed by counting positively stained lymphocytes in 10 consecutive high power fields (hpf) rich in lymphocytes within the tumour (400× magnification).
Molecular methods
The Illumina TruSeq Amplicon cancer hotspot panel and Illumina MiSeq next-generation sequencing (NGS) were used for analysis of genomic DNA extracted from FFPE tumour tissues of MBCs following microdissection. The panel tests mutation hotspots of 45 genes that can be found at: http://www.carismolecularintelligence.com/next-generation-sequencing-profile. The extended NGS gene panel used for analysis of the control groups consisted of 592 cancer-related genes sequenced using Agilent SureSelect XT and the Illumina NextSeq instrument. A list of the 592 gene panels can be found at: http://www.carismolecularintelligence.com/pdf/MI%20ProfileX%20Menu%20v10.pdf All reported variants were detected with >99% confidence based on the frequency of the mutation present and amplicon coverage. A full sequence BRCA1/2 gene analysis was performed using TruSeq Custom Amplicon BRCA1 and BRCA2 panel. Mutations were classified into categories (pathogenic, presumed pathogenic, variant of unknown significance, likely benign and benign) by board-certified clinical molecular geneticists using available database sources and scientific literature.
Statistical methods
Correlations between variables were identified using χ2 test and two-tailed Fisher's exact test (p≤0.05).
Results
PD-1 and PD-L1 expression in MBCs and invasive ductal carcinoma groups
The differences in PD-L1 expression in MBCs and the invasive ductal carcinoma groups are summarised in table 1.
Table 1.
PD-L1 status in tumour cells of MBCs and invasive ductal carcinoma cases (46% in metaplastic vs 6%–9% in other subtypes combined, p<0.001)
| Breast cancer subtype | PD-L1 status Cut-off ≥2+ intensity/≥5% tumour cells |
Total | |
|---|---|---|---|
| Negative | Positive | ||
| Metaplastic carcinoma | 39 (54%) | 33 (46%) | 72 |
| TNBC-NOS | 93 (91%) | 9 (9%) | 102 |
| HER2-positive breast cancer | 30 (94%) | 2 (6%) | 32 |
| Hormone-positive breast carcinoma | 79 (94%) | 5 (6%) | 84 |
| Total | 241 (83%) | 49 (17%) | 290 |
HER2, human epidermal growth factor receptor 2; MBC, metaplastic breast carcinoma; PD-L1; programmed death-ligand 1; TNBC, triple-negative breast cancer.
PD-L1 expression was positive in 33 of 72 (46%) interpretable MBC cases. Within the invasive ductal carcinoma groups, nine TNBCs (9%), two HER2-positive cancers (6%) and five hormone-positive breast carcinomas (6%) showed PD-L1 expression. Overall, there was a statistically significant difference in expression of PD-L1 in MBCs versus the TNBCs, HER2-positive and hormone-positive breast cancers (p<0.001). Total TILs were not enumerated; however, they were noted to vary greatly within the MBC cohort by histologic examination. The mean number of PD-1-positive TILs in 10 hpf was 68.3 in 70 interpretable cases (median: 22.5, range: 0–400). The data were dichotomised around the median PD-1 expression into two groups, high versus low PD-1. We then categorised PD-L1 expression in tumour cells and PD-1 expression in TILs into four categories (type 1–4) based on the presence or absence of PD-L1 and high or low PD-1 expression similar to the melanoma study by Teng et al.20 These results are summarised in table 2.
Table 2.
Categorisation of MBCs based on PD-L1 expression in tumour cells and low or high PD-1 expression in TILs
| Type | Tumour microenvironment (PD-L1/PD-1 TILs) |
Number of cases (n=71) |
|---|---|---|
| 1 | PD-L1 positive, high PD-1* | 16 (23%) |
| 2 | PD-L1 negative, low PD-1* | 22 (31%) |
| 3 | PD-L1 positive, low PD-1* | 14 (20%) |
| 4 | PD-L1 negative, high PD-1* | 19 (26%) |
*PD-1 categorisation as high or low is done around the median of 22.5.
MBC, metaplastic breast carcinoma; PD-1, programmed cell death 1; PD-L1; programmed death-ligand 1; TIL, tumour infiltrating lymphocyte.
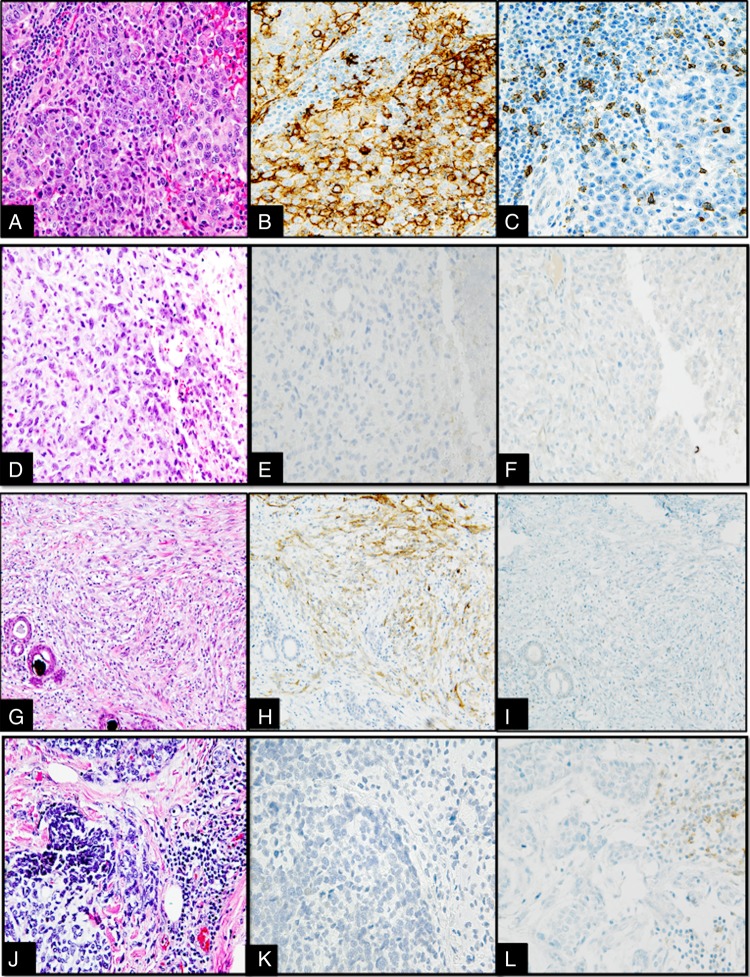
PD-1 and PD-L1 expression in representative cases of the four categories are depicted in figure 1. TILs and PD-1 status of the other breast cancer groups were not analysed for this study.
Figure 1.
(A–L) Interface between tumour and tumour infiltrating lymphocytes (TILs) in different metaplastic breast carcinomas (MBCs) categorised into four categories based on programmed death-ligand 1 (PD-L1) and programmed cell death 1 (PD-1) expression, 400× magnification. (A–C) Type 1 (PD-L1 positive, high PD-1): MBC with squamous metaplastic component (A) showing 3+ intensity PD-L1 staining in 50% of the tumour (B) and high PD-1 expression in the peritumoral lymphocytes (210/10 high power fields) (C). (D–F) Type 2 (PD-L1 negative, low PD-1): MBC with spindle cell metaplastic component (D) with tumour cells showing no increase in expression of PD-L1 by tumour cells (E) and no expression of PD-1 by interstitial lymphocytes/plasma cells (F). (G–I) Type 3 (PD-L1 positive, low PD-1): MBC with spindle cell metaplastic component (G) with moderate overexpression of PD-L1 in the tumour cells (H) and no expression of PD-1 in the TILs (I). (J–L) Type 4 (PD-L1 negative, high PD-1): MBC with areas of chondroid metaplastic component (J) with no PD-L1 overexpression in tumour cells (K) and moderate expression of PD-1 positive in TILs (190/10 high power fields) (L).
Mutational profile of MBCs and invasive ductal carcinoma groups
Mutational profiles were divided into pathogenic, presumed pathogenic, variants of unknown significance and suspected benign variants. For discussion purposes, only pathogenic/presumed pathogenic alterations were considered. Seventy-two MBCs were tested with the 45-gene NGS mutation panel, which covered regions of the genes in which mutations are commonly found (hotspots). Fifty-seven of these had interpretable results. Failure of the remaining 15 samples was attributed to poor DNA quality in the samples. Of note, 5 of these 15 cases were positive for PD-L1 overexpression (33%). A total of 52 pathogenic/presumed pathogenic alterations were detected in 45 tumours; no mutations were detected in 12 tumours that were successfully sequenced. TP53 alterations were identified in most cases (32/57, 56%) with 20 having isolated TP53 mutations, while 12 also had mutations in other genes. PIK3CA was the second most commonly mutated gene (13/57, 23%) and HRAS was third (3/57). Single cases harboured additional pathogenic mutations of FBXW7, PTEN, AKT1 and SMAD4. Of note, full sequencing of the BRCA1 and BRCA2 genes showed two MBCs samples with pathogenic BRCA1 mutations (R1076fs, S766fs) and one case with BRCA2 mutation of unknown significance. Online supplementary figure S1 compares the different mutations and their frequencies in MBCs. Online supplementary tables S1 and S2 list specific mutations that were seen in each case and the clinical significance of the mutations. The 592 gene NGS panel, which covers the complete protein coding regions of the genes, detected pathogenic mutations in 23 genes (those also present in the 45 gene panel) in the TNBC group; results were available for 96 of 106 tumours. A total of 130 pathogenic alterations were detected in 94 TNBCs. Altogether, 83 of 96 cases had a TP53 mutation (86%), 13 had a PIK3CA mutation (14%), 4 had a BRCA1 mutation (4%), 3 had a BRCA2 mutation (3%), 4 had an AKT1 mutation (4%) and 3 had an ERBB2 (Her2) mutation (3%). The remaining 20 mutations were found at a low frequency of ≤2%. Lists of altered genes and frequencies in the different breast cancer groups are presented in online supplementary tables S3–S5. When tumour types were further subcategorised into mutation types, TP53 versus other mutations (see online supplementary table S6), TP53 mutations were more prevalent in TNBCs and HER2-positive cases than MBCs (88%, 90% vs 71%). No statistically significant correlation was identified between number of molecular alterations and PD-L1 expression in MBCs (p=0.52). Similarly, no statistically significant correlation was discovered between types of mutations (TP53 vs other) and PD-L1 expression in MBCs (p=1.00).
jclinpath-2016-203874supp.pdf (328.5KB, pdf)
Discussion
MBCs are an aggressive subtype of breast carcinoma that are comparatively resistant to conventional chemotherapy making them ideal for genomic and immunologic alteration studies in search for novel therapies. An early phase Ib clinical trial using pembrolizumab, a PD-1 inhibitor in recurrent/metastatic TNBCs with PD-L1 expression, has shown promising results (ClinicalTrials.gov identifier: NCT02447003). Comparable data are accruing with the use of MPDL3280A, a PD-L1 inhibitor.21 22 A recently published study has shown that patients with metastatic melanoma who responded to pembrolizumab had higher levels of CD8+ T lymphocytes (TILs), PD-1 and PD-L1 expressing cells in their pretreatment samples.23 These studies suggest that an increased presence of these markers may be associated with improved responses to PD-1/PD-L1 blockade. Our study shows for the first time that PD-L1 expression in MBCs is significantly greater than in HER2-positive breast cancer, hormone-positive breast cancer and TNBCs. Literature shows substantial variability in PD-L1 expression in TNBCs likely due to use of different analysis platforms. Molecular methods of DNA profiling/gene amplification show PD-L1 expression as high as 39% in TNBCs, while IHC studies show PD-L1 expression only as high as 19% in TNBCs.11–14 Our study showed 9% PD-L1 expression in TNBCs and the variation from literature can be attributed to variability in IHC interpretation and cut-off values. Categorisation of PD-L1 and PD-1 status in MBCs into four groups akin to the melanoma study was done to characterise the tumour microenvironment and gain insight the prognostic implications for use of PD-1/PD-L1 inhibitors in MBCs.20 The melanoma study stratification offered prognostic implications, where patients with type 1 expression had the best prognosis and highest probability of responding to PD-1/PD-L1 inhibitors.18 24 We modified the stratification variables to PD-L1 expression in tumour cells and PD-1 expression in TILs from what was used in the melanoma study. Using PD-L1 and PD-1 expression for categorisation, in our opinion, may be more relevant for PD-1/PD-L1 inhibitors. In our study, 23% of the MBCs fell in the type 1 category, implying that nearly a quarter of the MBCs would be amenable to immune checkpoint therapy. Interestingly, the prognostic value of PD-L1 expression in breast cancers is controversial. Muenst et al showed PD-L1 as a negative prognostic marker in breast cancer, while another recently published study has lent support to the hypothesis that PD-L1 expression may serve as a good prognostic marker.25 26 The prognostic value of increased PD-L1 expression in MBCs would be interesting to address in a prospective cohort. In our study, we discovered a vast variation in PD-1 expression in TILs in MBCs. Further studies to better understand the role of TILs and the tumour immune microenvironment in MBCs need to be pursued.
In genetic profiling, TP53 mutation was the most common and biologically relevant alteration discovered in our study, consistent with recently reported results.5 Potentially targetable mutations identified in our study affected the PIK3CA/Akt/mTOR signalling pathway (PIK3CA, AKT-1, PTEN) proposing benefits of using PIK3CA and mTOR inhibitors in MBCs. A study of five metastatic MBCs treated with the mTOR inhibitor, temsirolimus in combination with liposomal doxorubicin and bevacizumab showed promising preliminary results.27
The limitations of our study included PD-L1 expression analysis based on a single, although well-characterised, antibody used in clinical trials.17 The current literature shows vast variability in PD-L1 IHC interpretation and lack of standardised protocols, except for the recently Food and Drug Administration-approved companion diagnostics kit for use in non-small cell lung carcinomas therapy with pembrolizumab (PD-L1 IHC 22C3pharmDx, Dako). However, it appears that no significant IHC performance differences exist between several recently analysed anti-PD-L1 antibodies raised against the intracytoplasmic domain.28 Furthermore, our preliminary study has shown high concordance (88%–100%) between SP142 antibody used in this study and three other antibodies (SP263, 28-8 and 22c3 clones).29 Other limitations include associated biases secondary to missing clinical data, and different platforms used in NGS.
In summary, our study is the first and largest study to demonstrate overexpression of a targetable checkpoint protein, PD-L1 in MBCs. We have also identified the presence of targetable genetic alterations in a large cohort of MBCs providing options for multitargeted combination therapy.
Take home messages.
Metaplastic breast carcinoma (MBC) is an aggressive and uncommon breast cancer subtype that is less susceptible to chemotherapy than triple-negative breast cancer (TNBC).
Programmed death-ligand 1 (PD-L1)/programmed cell death 1 (PD-1) inhibitors have shown promise in multiple carcinomas, and in our study we discovered PD-L1 overexpression in MBCs compared with TNBC, hormone-positive breast cancer and human epidermal growth factor receptor 2-positive breast cancer.
Next-generation sequencing of MBCs showed mutations most frequently in the TP53 and PIK3CA genes.
PD-1/PD-L1 inhibitors and PIK3CA/mTOR pathway inhibitors may be used in the treatment of aggressive, resistant MBCs.
Footnotes
Handling editor: Runjan Chetty
Funding: Caris Life Sciences.
Competing interests: JS, RF, WC, JK, NX, SR and ZG are employees of Caris Life Sciences. SV has received honoraria from Caris Life Sciences.
Ethics approval: IRB.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Oberman HA. Metaplastic carcinoma of the breast. A clinicopathologic study of 29 patients. Am J Surg Path 1987;11:918–29. 10.1097/00000478-198712000-00002 [DOI] [PubMed] [Google Scholar]
- 2.Schwartz TL, Mogal H, Papageorgiou C, et al. Metaplastic breast cancer: histologic characteristics, prognostic factors and systemic treatment strategies. Exp Hematol Oncol 2013;2:31–6. 10.1186/2162-3619-2-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen IC, Lin CH, Huang CS, et al. Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Cancer Res Treat 2011;130:345–51. 10.1007/s10549-011-1686-9 [DOI] [PubMed] [Google Scholar]
- 4.Bae SY, Lee SK, Koo MY, et al. The prognoses of metaplastic breast cancer patients compared to those of triple-negative breast cancer patients. Breast Cancer Res Treat 2011;126:471–8. 10.1007/s10549-011-1359-8 [DOI] [PubMed] [Google Scholar]
- 5.Ross JS, Badve S, Wang K, et al. Genomic profiling of advanced-stage, metaplastic breast carcinoma by next-generation sequencing reveals frequent, targetable genomic abnormalities and potential new treatment options. Arch Pathol Lab Med 2015;139:642–9. 10.5858/arpa.2014-0200-OA [DOI] [PubMed] [Google Scholar]
- 6.Smyth MJ, Ngiow SF, Ribas A, et al. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol 2016;13:143–58. 10.1038/nrclinonc.2015.209 [DOI] [PubMed] [Google Scholar]
- 7.Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev 2014;23:2965–70. 10.1158/1055-9965.EPI-14-0654 [DOI] [PubMed] [Google Scholar]
- 8.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558–62. 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 11.Ali HR, Glont SE, Blows FM, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol 2015;26:1488–93. 10.1093/annonc/mdv192 [DOI] [PubMed] [Google Scholar]
- 12.Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2014;2:361–70. 10.1158/2326-6066.CIR-13-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabatier R, Finetti P, Mamessier E, et al. Prognostic and predictive value of PD-L1 expression in breast cancer. Oncotarget 2015;6:5449–64. 10.18632/oncotarget.3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett MT, Anderson KS, Lenkiewicz E, et al. Genomic amplification of 9p24.1 targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple negative breast cancer. Oncotarget 2015;6:26483–93. 10.18632/oncotarget.4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784–95. 10.1200/JCO.2009.25.6529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 17.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra37 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatalica Z, Bilalovic N, Palazzo JP, et al. Disseminated histiocytoses biomarkers beyond BRAFV600E: frequent expression of PD-L1. Oncotarget 2015;6:19819–25. 10.18632/oncotarget.4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng MW, Ngiow SF, Ribas A, et al. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 2014;75:2139–45. 10.1158/0008-5472.CAN-15-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson J. Anti-PD-L1 for metastatic triple-negative breast cancer. Lancet Oncol 2015;16:e264 10.1016/S1470-2045(15)70208-1 [DOI] [PubMed] [Google Scholar]
- 22.Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer 2015;112:1421–7. 10.1038/bjc.2015.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014;20:5064–74. 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baptista MZ, Sarian LO, Derchain SFM, et al. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Human Pathol 2016;47:78–84. 10.1016/j.humpath.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 26.Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2014;146:15–24. 10.1007/s10549-014-2988-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moulder S, Moroney J, Helgason T, et al. Responses to liposomal doxorubicin, bevacizumab, and temsirolimus in metaplastic carcinoma of the breast: biologic rationale and implications for stem-cell research in breast cancer. J Clin Oncol 2011;29:e572–5. 10.1200/JCO.2010.34.0604 [DOI] [PubMed] [Google Scholar]
- 28.Mahoney KM, Sun H, Liao X, et al. PD-L1 antibodies to its cytoplasmic domain most clearly delineate cell membranes in immunohistochemical staining of tumor cells. Cancer Immunol Res 2015;3:1308–15. 10.1158/2326-6066.CIR-15-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gatalica Z, Vanderwalde AM, Rose I, et al. Distribution of PD-L1 expression in diverse cancer types: experience with over 10,000 cases. J Clin Oncol 2016;34(Suppl; abstr 11548). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jclinpath-2016-203874supp.pdf (328.5KB, pdf)