Abstract
Background
Worldwide, oral sucrose is standard of care in many neonatal intensive care units to relieve procedural pain in neonates. This study aims to determine if time interval between sucrose administration and heelstick correlates with pain scores.
Methods
Neonates were prospectively studied with variable time intervals and assessed with the Premature Infant Pain Profile-Revised (PIPP-R).
Results
150 neonates were included with a median gestational age of 30+6 (IQR 27+6–33+2) weeks and a median time interval of 72 (IQR 39–115) seconds between sucrose administration and heelstick. In multiple regression analysis, this time interval was not significantly related to the PIPP-R (B=0.004, 95% CI −0.005 to 0.013, p=0.37). Providing non-nutritive sucking combined with sucrose was significantly related to lower PIPP-R scores (B=−3.50, 95% CI −4.7 to −2.3, p<0.001).
Conclusions
Our study suggests that there is no need to wait 2 min after sucrose administration before a painful procedure. Sucrose-induced non-nutritive sucking shows a fast pain-relieving effect in neonates.
Keywords: Pain, Neonatology, premature, Analgesia, Sucrose
What is already known on this topic?
Administration of sucrose to neonates prior to a heelstick procedure significantly reduces pain.
Sucrose is most effective in combination with non-nutritive sucking.
Most studies and guidelines recommend administering sucrose at least 2 min before a painful procedure.
What this study adds?
No correlation was found between pain intensity and time interval between administration of sucrose and the heelstick procedure, suggesting that 2 min waiting is unnecessary.
Sucrose-induced non-nutritive sucking was the only variable significantly correlated with a reduction in pain scores.
Introduction
Oral administration of sucrose significantly reduces procedural pain in newborns.1 It is most effective when combined with non-nutritive sucking (NNS).1 This intervention is therefore recommended in international neonatal pain guidelines.2
In most studies, sucrose is administered 2 min prior to the painful procedure.1 To our knowledge, only one study evaluated the effect of this time interval and found it optimal.3 Because this study included healthy newborns only, we aimed to test whether this conclusion also holds true for premature or critically ill neonates.
We hypothesised that pain scores for premature or critically ill term neonates will be lowest when a time interval of approximately 2 min is used. The aim of the study was to determine if the time interval between sucrose administration and heelstick is correlated with pain scores.
Methods
This prospective study was conducted from September 2014 to February 2015 at a level 3 neonatal intensive care unit. Eligible for inclusion were all preterm and critically ill term neonates with an indication for blood sampling by heelstick. Patients were selected by convenience sampling and could be included only once. This study consisted of two consecutive parts, A and B. In part A, 100 heelstick procedures were performed without any guidance on time interval. In part B, the medical team was instructed to adhere to a 2 min time interval3 during 50 heelstick procedures. This way, we expected a large variation of time intervals and a sufficient population to perform a regression analysis. The institutional ethical review board waived the need for approval (MEC-2014-357).
Data collection
The procedure, starting with the commencement of sucrose administration (T=0), was timed with a stopwatch. The pacifier was gently applied into the mouth and sucrose was administered inside the cheek. Without pressure, the nurse held the pacifier in place until the infant started to suck.
Primary outcome was the pain score obtained with the validated Premature Infant Pain Profile-Revised (PIPP-R),4 applied during the first 30 s after the heelstick. A score <7 suggests no or little pain, 7–12 slight-to-moderate pain and scores >12 are thought to reflect moderate-to-severe pain. All assessments were performed by a research nurse (NM) trained to score the PIPP-R (linearly weighted kappa compared with a trained neonatologist=0.85). If one of the PIPP-R items could not be assessed, a proportional score was calculated by multiplying the total score by 7/6.
Other variables recorded were: gestational age, postnatal age, NNS at time of the prick, number of doses, total volume of sucrose administered, size of the lance (BD Microtainer Quickheel Lancet Preemie or Infant), number of heel squeezes and number of pricks needed. All heelsticks were performed by trained lab personnel, while a nurse or healthcare assistant provided facilitated tucking.
Our clinical treatment protocol prescribed the use of sucrose 24% with a maximum volume of 0.5 or 2.0 cc in patients with bodyweight <1000 or >1000 g, respectively.
Data analysis
The association between PIPP-R and the time interval was estimated with Spearman's rank correlation coefficient. Multiple linear regression analysis with PIPP-R total score as outcome variable and the time interval in seconds as predictor variable was applied. The relevant covariates postnatal age in days, NNS, total volume of sucrose in millilitres and gender were also entered into the regression model. Gestational age was not included because it is included in the PIPP-R score. A sensitivity analysis was performed by replacing time interval in seconds with study phase (part A or part B) in the regression model.
Results
One hundred and fifty patients were included with a gestation age between 24+1 and 42+1 weeks. One patient in part B received a second dose of sucrose during the time interval and was excluded from the analysis. Table 1 shows background characteristics and main study parameters.
Table 1.
Patients' background characteristics and main study parameters (N=149)
| Variables | Part A (N=100) | Part B (N=49) | p Value |
|---|---|---|---|
| Boy/girl; N (%) | 61 (61)/39 (39) | 24 (49)/25 (51) | 0.16 |
| Gestational age in weeks+days | 30+6 (28+5–33+2) | 30+6 (26+6–33+2) | 0.26 |
| Birth weight in grams | 1483 (1158–2015) | 1280 (888–1950) | 0.18 |
| Postnatal age in days | 4 (2–8) | 5 (2–10) | 0.11 |
| Time interval in seconds | 48 (31–79) | 127 (107–153) | <0.001 |
| Non-nutritive sucking; N (%) | 48 (48) | 23 (47) | 0.90 |
| Patients with one dose; N (%)* | 98 (98%) | 44 (90%) | 0.27 |
| Total volume of sucrose in mL | 0.5 (0.5–0.75) | 0.5 (0.5–0.75) | 0.78 |
| Size of the lance: preemie; N (%)/infant; N (%) | 12 (12)/88 (88) | 7 (14)/42 (86) | 0.69 |
| Unable to score nasolabial furrow; N (%) | 44 (44) | 30 (61) | 0.05 |
| Duration of procedure in seconds | 112 (59–191) | 154 (97–257) | 0.014 |
| Number of squeezes | 17 (8–28) | 22 (13–36) | 0.04 |
| Analgesics/sedatives; N (%)† | 5 (5) | 5 (10) | 0.23 |
| PIPP-R | 6 (0–9) | 7 (2–9) | 0.22 |
Data are presented as median (IQR) for all continuous variables. Part A and part B data were compared using the χ2 test for categorical variables and the Mann-Whitney U test for continuous variables.
*Patients who received one dose during the total procedure (from sucrose administration until the end of the blood collection).
†Patients who received continuous or intermittent morphine/fentanyl and/or midazolam during or <3 hours before the heelstick procedure.
PIPP-R, Premature Infant Pain Profile-Revised.
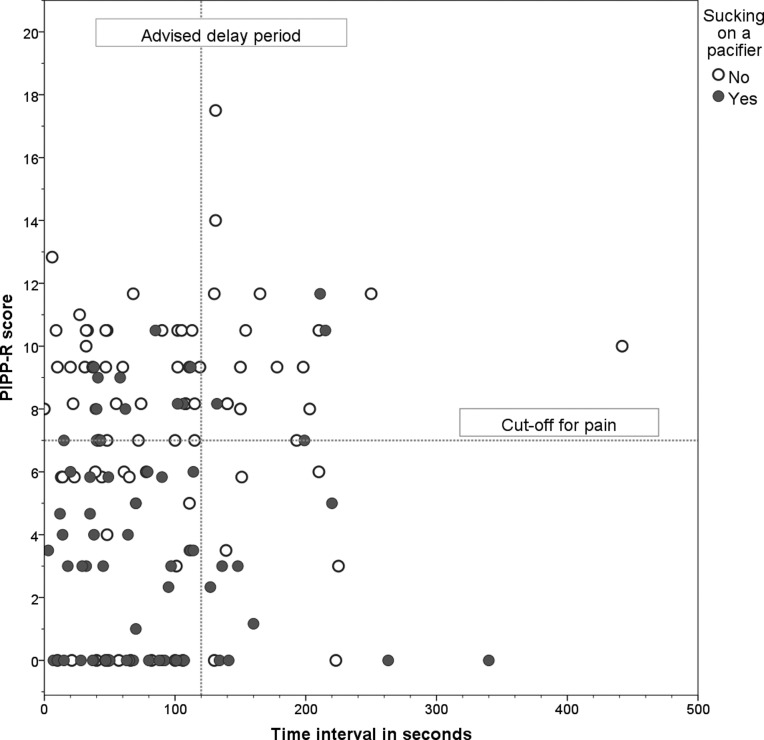
The correlation between PIPP-R and time interval was 0.11 (95% CI −0.05 to 0.27, p=0.17). Large variations in both time interval and PIPP-R scores were found (figure 1).
Figure 1.
Correlation between time interval after sucrose administration and Premature Infant Pain Profile-Revised (PIPP-R) score (N=149). Reference lines are at the time interval of 120 s and the cut-off score for PIPP-R of 7 or higher. Markers differentiate between yes/no non-nutritive sucking.
Multivariate analysis
In multiple regression analysis, too, the time interval was not significantly associated with PIPP-R scores, with an estimated association of 0.004 (95% CI −0.005 to 0.013, p=0.37) points on the PIPP-R per second. NNS was correlated with an average decrease in mean PIPP-R score of 3.5 points (95% CI −4.7 to −2.3, p<0.001). Replacing time interval with study phase in the regression model, the PIPP-R score was on average 0.99 points higher in part B (95% CI −0.29 to 2.26, p=0.13). Postnatal age, gender and volume of sucrose were not significantly correlated to PIPP-R scores.
Discussion
We found that in the studied hospitalised newborns, the heelstick-related pain intensity was not correlated with the length of the time interval between the administration of sucrose and the heelstick. This is in contradiction with the only other comparable study, which led to the worldwide clinical implementation of a 2 min time interval.3 In that study, however, the primary outcome was ‘crying’ and only healthy newborns were included. We used the validated PIPP-R,4 which was not yet available at the time of the previous study and included premature and critically ill term neonates.
Our finding does not correspond with the supposition that a certain length of time must elapse to mediate opioid responses and inhibit nociceptive impulses.5 The absence of a time interval versus effect relationship in our study suggests that sucrose induces a change in the patient's behavioural state rather than a pharmacological effect. Thus, our finding underlines the uncertainties concerning the working mechanisms of sucrose.2
The PIPP-R was applied during the first 30 s following the heelstick, according to the instructions of the instrument, while blood sampling lasted for a median of 2 min. This way, we focused on pain related to the insertion of the lance. According to the previous research, the effect of sucrose can persist 5–10 min after a painful stimulus.5 Since time interval after sucrose administration did not affect relief of the acute pain upon insertion of the lance, we expect that likewise it does not affect relief of the pain related to the preceding squeezing either. If faster onset of action is received, fewer sucrose doses may be needed, which would reduce the possible negative effect of sucrose on neurodevelopment.2
Strictly adhering to a 2 min time interval1 was difficult, for example, when an unstable infant needed time to recover before the heelstick procedure could start.
The study design led to several limitations with uncontrollable confounders that could possibly have influenced time interval and pain intensity. Potential confounders were, for example, the intensity and number of times the heel was squeezed. Also, we did not take into account the administration of analgesics and sedatives. In addition, the research nurse observed the total procedure and thus was not blinded to the time interval. Still, this is the first study that questioned if the worldwide implementation of the 2 min time interval based on one study was indeed justified. In future studies, blinded coders should score the PIPP-R from video recordings. A blinded randomised controlled trial comparing different time intervals is underway.
Conclusion
Our study does not justify the need to wait at least 2 min after sucrose administration, but needs re-evaluation in a randomised controlled trial. Shorter time intervals importantly improve efficiency in busy intensive care units. It is best to give sucrose with a pacifier to stimulate sucrose-induced NNS to reduce pain responses.
Acknowledgments
Ko Hagoort is thanked for editorial assistance.
Footnotes
Contributors: NM conceptualized and designed the study and data collection instruments, carried out data collection and initial analyses, drafted the initial manuscript and approved the final manuscript as submitted. SS conceptualized and designed the study, critically reviewed and revised the manuscript and approved the final manuscript as submitted. JvR supervised initial analyses, critically reviewed and revised the manuscript and approved the final manuscript as submitted. IR critically reviewed and revised the manuscript and approved the final manuscript as submitted. JvdA critically reviewed and revised the manuscript and approved the final manuscript as submitted. MvD conceptualized and designed the study, supervised data collection and initial analyses, critically reviewed and revised the manuscript and approved the final manuscript as submitted.
Competing interests: None declared.
Ethics approval: METC Erasmus MC.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data are available upon request.
References
- 1.Stevens B, Yamada J, Lee GY, et al. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev 2013;1:CD001069 10.1002/14651858.CD001069.pub4 [DOI] [PubMed] [Google Scholar]
- 2.Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine. Prevention and management of procedural pain in the neonate: an update. Pediatrics 2016;137:e20154271 10.1542/peds.2015-4271 [DOI] [PubMed] [Google Scholar]
- 3.Blass EM, Shah A. Pain-reducing properties of sucrose in human newborns. Chem Senses 1995;20:29–35. 10.1093/chemse/20.1.29 [DOI] [PubMed] [Google Scholar]
- 4.Gibbins S, Stevens BJ, Yamada J, et al. Validation of the Premature Infant Pain Profile-Revised (PIPP-R). Early Hum Dev 2014;90:189–93. 10.1016/j.earlhumdev.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 5.Gibbins S, Stevens B. Mechanisms of sucrose and non-nutritive sucking in procedural pain management in infants. Pain Res Manag 2001;6:21–8. 10.1155/2001/376819 [DOI] [PubMed] [Google Scholar]