Abstract
The nuclear orphan receptor COUP-TFII is widely expressed in multiple tissues and organs throughout embryonic development, suggesting that COUP-TFII is involved in multiple aspects of embryogenesis. Because of the early embryonic lethality of COUP-TFII knockout mice, the role of COUP-TFII during limb development has not been determined. COUP-TFII is expressed in lateral plate mesoderm of the early embryo prior to limb bud formation. In addition, COUP-TFII is also expressed in the somites and skeletal muscle precursors of the limbs. Therefore, in order to study the potential role of COUP-TFII in limb and skeletal muscle development, we bypassed the early embryonic lethality of the COUP-TFII mutant by using two methods. First, embryonic chimera analysis has revealed an obligatory role for COUP-TFII in limb bud outgrowth since mutant cells are unable to contribute to the distally growing limb mesenchyme. Second, we used a conditional-knockout approach to ablate COUP-TFII specifically in the limbs. Loss of COUP-TFII in the limbs leads to hypoplastic skeletal muscle development, as well as shorter limbs. Taken together, our results demonstrate that COUP-TFII plays an early role in limb bud outgrowth but not limb bud initiation. Also, COUP-TFII is required for appropriate development of the skeletal musculature of developing limbs.
The muscles of the limbs and ventral body wall originate from the somites, epithelial spheres of paraxial mesoderm that form in a rostral-to-caudal direction along both sides of the neural tube (10, 12, 13, 15). The somites give rise to two separate muscle-forming cell populations. First of all, cells of the dorsomedial dermomyotome give rise to the epaxial myotome, which eventually develops into the muscles of the back. These epaxial muscle precursors express MyoD and other muscle-specific proteins and are dependent on signals arising from the notochord and the floor plate for their differentiation (8, 21, 40, 45, 49). Second, cells of the ventrolateral dermomyotome generate the hypaxial muscle lineage, whose developmental fate is dependent on the rostrocaudal positioning of the somite. At interlimb levels, the lateral dermomyotome generates the thoracic and abdominal muscles (14). For somites that are located adjacent to the limbs, however, the muscle precursor cells of the lateral dermomyotome undergo an epithelial-mesenchymal transition and migrate to the limbs and diaphragm, where they subsequently proliferate and then undergo differentiation (5, 7, 11, 22). These migrating muscle precursors do not express myogenic determination genes until after they have reached their targets in the limbs. Although the signals regulating hypaxial cell specification are not as well understood, it is believed that signals from the surface ectoderm are required for their formation (9, 20, 53, 55).
Once the migrating muscle precursor cells reach the limbs, they populate the dorsal and ventral mesenchyme as two premuscular masses. These premuscular masses consist of two parts, a surface layer of proliferating muscle precursor cells and a deeper layer of differentiating myoblasts that express myogenic differentiation markers such as myogenin. These two populations strike a balance between proliferation and differentiation (42) such that any increase in proliferation leads to an increase in muscle size. However, if terminal differentiation is induced prematurely, there has not been enough time for adequate proliferation to occur and the result is smaller muscles (28). This balancing act is mediated by the activity of several signals. An increase in the proliferative pool can be induced by FGFs, IGFI, and BMPs, whereas a decrease in muscle mass can be achieved through the use of BMP antagonists such as noggin (1, 4, 26, 31). Furthermore, activation of the Notch pathway has been shown to cause muscle precursor cells to continue to express Pax3 and Myf5, downregulating MyoD and thereby preventing the onset of terminal differentiation (17). Recently, Sonic hedgehog (SHH) has been demonstrated to play a role in limb skeletal muscle size by acting as a survival and proliferation factor. Loss of SHH activity results in hypoplastic limb muscles, whereas ectopic overexpression in the chick is able to cause muscle hypertrophy, possibly by upregulating the BMP pathway (2, 23, 37).
Chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) is a nuclear orphan receptor of the steroid-thyroid hormone receptor superfamily (57). COUP-TFII is most highly expressed during embryonic development, and mutation of this gene results in early embryonic lethality because of defects in angiogenesis and heart development (43, 44, 47). Previous study of cell cultures has indicated a role for COUP-TFII in muscle development by showing that COUP-TFII inhibits the expression and transcriptional function of MyoD, thereby preventing muscle differentiation (3, 41). Furthermore, COUP-TFII lies downstream of SHH signaling and may therefore be a mediator of the SHH pathway in certain developmental situations (35, 36). Although COUP-TFII is well defined biochemically, its physiological functions during embryogenesis remain largely undefined. This is in large part because of the fact that the COUP-TFII mutant is an early embryonic lethal mutant that dies prior to E10 because of angiogenesis and cardiovascular failure, preventing the study of the role of COUP-TFII in the development of tissues and organs at later stages. In this study, we addressed the function of COUP-TFII during embryonic limb development. We used both embryonic chimera and tissue-specific knockout techniques to bypass the early embryonic lethality of the COUP-TFII knockout. By embryonic chimera analysis, we demonstrated that COUP-TFII is required for mesenchymal cells to contribute to the distally growing limb bud in chimeric embryos. In addition, tissue-specific knockout of COUP-TFII in the limb bud results in hypoplastic musculature. Therefore, COUP-TFII is required for limb bud outgrowth and proper limb muscle development.
MATERIALS AND METHODS
Generation of stable ES cell lines.
Stable embryonic stem (ES) cell lines homozygous for the COUP-TFII mutation were generated and marked with the ubiquitously expressed ROSA26-LacZ allele (27), and COUP-TFII+/− mice were naturally mated with COUP-TFII+/− ROSA26+/− mice (mixed 129Sv-C57BL/6 background). Blastocysts were then isolated by flushing the uterine horns of pregnant females at embryonic day 3.5 (E3.5), where the day of vaginal plug detection is designated E0.5. Uteri were flushed with sterile ES medium (high-glucose Dulbecco modified Eagle medium, 15% fetal calf serum, 1% l-glutamine, 0.5% penicillin-streptomycin, 0.18% β-mercaptoethanol) supplemented with 0.02 M HEPES. Blastocysts were then transferred onto a 60-mm-diameter tissue culture dish containing a feeder layer of mitotically inactivated SNL cells in ES medium and allowed to adhere and grow for approximately 4 to 6 days at 37°C in 5% CO2. Once the inner cell mass outgrowth had reached an appropriate size (large colonies but prior to any cell death or differentiation), colonies were picked and disaggregated first by treatment with 0.25% trypsin-EDTA and then pipetting up and down. Disaggregated colonies were plated into individual wells of a feeder layer-coated 24-well dish. Cells were fed with new medium daily and passed 1:1 every 3 days to a new well until ES cells became apparent. ES cell lines were genotyped by PCR for COUP-TFII and by PCR and β-galactosidase (β-gal) staining for LacZ. ES cell lines were also karyotyped for normal chromosome complement as described by Hogan et al. (32). Both wild-type control and mutant ES cell lines were generated in this fashion.
Generation of chimeric embryos.
ES cells were plated and grown until they reached 90% confluency; they were then passed 1:3 one time until they reached 90% confluency once again prior to trypsinization and resuspension in injection medium (ES medium) before being placed on ice. After 30 min, most SNL feeder cells and debris have settled to the bottom of the tube. E3.5 C57BL/6 blastocysts were flushed out of uteri with injection medium. Malformed or early stage embryos were sorted out, and the remaining mature blastocysts were washed in M2 medium. Twelve to 14 ES cells were microinjected into the blastocoels of the embryos. After all of the embryos had been microinjected, they were transferred into the uteri of day 2.5 pseudopregnant ICR foster mothers at no more than 10 embryos per uterine horn. Subsequently, embryos were dissected at mid-gestational stages for 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining and analysis. Embryonic age was determined by the uterine age of the foster mothers.
β-Galactosidase staining of embryos.
Embryos were dissected into cold 1× phosphate-buffered saline (PBS; Gibco) and fixed in 2% paraformaldehyde (PFA; Sigma) in 1× PBS-0.125 M piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES; pH 6.9)-1 mM MgCl2-5 mM EGTA (pH 8.0) for 15 to 45 min at room temperature, depending on the size of the embryo (E9.5 to E12.5, respectively). Embryos were then washed three times with PBS for 20 min each time. Embryos were stained in 2 mM MgCl2-0.01% deoxycholate-0.02% NP-40-100 mM phosphate buffer (pH 8.0)-5 mM K4Fe(CN)6-5 mM K3Fe(CN)6-1 mg of X-Gal per ml. After staining, embryos were washed in PBS and stored in 4% PFA at 4°C.
Whole-mount in situ hybridization.
Whole-mount in situ hybridization was performed with probes to myogenin (from Eric Olson) and Lbx1 (from Krzysztof Jagla) as templates for the generation of digoxigenin-labeled antisense riboprobes. The protocols used were essentially the same as those published previously (46, 48).
Immunohistochemical staining.
Embryos were fixed in 2% PFA-PBS (pH 7.4) for 1 h, cryoprotected in 30% sucrose-PBS (overnight, 4°C), and frozen in PBS containing 15% sucrose and 7.5% gelatin. Frozen tissues were sectioned at a thickness of 10 μm in a cryostat. The sections were air dried for 1 h at room temperature and stored at −20°C until used.
The air-dried sections were washed in PBS and incubated in blocking buffer (PBS, 1% bovine serum albumin, 5% serum, 10 μg of Fab fragment donkey anti-mouse IgG [heavy and light chains] per ml, 0.02% Triton X-100) for 30 min prior to incubation with the primary antibody (monoclonal anti-Pax3 [1:500; DSHB], anti-COUP-TFII [1:5,000; kindly provided by Toshiya Tanaka, Department of Molecular Biology and Medicine, The University of Tokyo], or monoclonal anti-phospho-histone H3 [1:200; Cell Signaling Technology]) overnight at 4°C. After being washed three times with PBS, the sections were incubated with biotin-SP-conjugated donkey anti-mouse IgG (heavy and light chains; Jackson ImmunoResearch Laboratories) for 60 min at room temperature. Antibody binding was visualized with TSA kit no. 22 (Molecular Probes) in accordance with the manufacturer's recommendation.
For double immunostaining, the sections were first incubated with the diluted first primary antibody, monoclonal anti-COUP-TFII (1:5,000) as described above and staining was amplified with TSA kit no. 22. Subsequently, sections were incubated with the monoclonal anti-Pax3 second primary antibody and visualized by conventional fluorescent staining. Briefly, upon completion of the first immunostaining of COUP-TFII, the sections were treated with the blocking buffer for 30 min and then incubated with anti-Pax3 antibody overnight at 4°C. Antibody binding was visualized with the Cy3-conjugated donkey anti-mouse IgG (heavy and light chains; Jackson ImmunoResearch Laboratories) secondary antibody.
RESULTS
Expression of COUP-TFII indicates a role in limb and muscle development.
To determine the function of COUP-TFII during embryonic limb development, we began by examining the tissue-specific expression pattern of the COUP-TFII gene. This was done by using a LacZ knock-in mouse generated in our laboratory, in which a nuclear LacZ gene was used to replace the COUP-TFII gene, thereby being expressed under the control of the COUP-TFII regulatory region (56). Therefore, the expression of COUP-TFII during limb development was monitored in heterozygotes by measuring β-gal activity by X-Gal staining. When E9.5 embryos were stained with X-Gal, it was found that COUP-TFII was expressed in the lateral plate mesoderm (LPM) from the earliest stages of limb bud initiation and outgrowth (Fig. 1A). Since the embryonic limb is initiated and first appears as an outgrowth of the LPM around early E9 (33, 58), this expression suggests that COUP-TFII has an important function during early limb bud development, either during limb bud initiation itself or in events following induction of the limb bud.
FIG. 1.
Expression of COUP-TFII. (A and B) Whole-mount X-Gal staining of E9.5 embryos. COUP-TFII is expressed in the LPM (arrow and box in panel A) and somites (arrowheads in panel B). (C) Cross section of X-Gal-stained E9.5 embryos. At limb levels, COUP-TFII is expressed in the myotome and lateral dermomyotome, which gives rise to migrating muscle precursors (arrow). (D and E) Whole-mount X-Gal staining of E10.5 embryos. COUP-TFII is still expressed in somites (arrowheads in panel D) and becomes expressed in the dorsal and ventral muscular masses in the limb, as well as in the AER. (F) Cross section of X-Gal-stained E10.5 embryo. COUP-TFII is expressed in the myotomes and dorsal and ventral muscle masses, as well as the AER (arrowhead). (G and H) Whole-mount X-Gal staining of E11.5 embryos. COUP-TFII is expressed in somites (arrowheads in panel G), in premuscular masses (arrows in panel G), and in the AER (arrow in panel H). (I) Cross section of an X-Gal-stained E11.5 embryo. COUP-TFII is expressed in premuscle masses, as well as in other mesenchymal tissues of the limb. (J) Whole-mount X-Gal staining of an E12.5 embryo. COUP-TFII is expressed in the developing muscle bundles (arrow). (K and L) Frontal sections of fore and hind limbs of X-Gal-stained embryos at E12.5. Expression of COUP-TFII can be clearly seen in individual muscle bundles (arrows) and nonmuscle mesenchyme. Abbreviations: M, myotome; DM, dermomyotome; DMM, dorsal myogenic mass; VMM, ventral myogenic mass.
Interestingly, COUP-TFII is also expressed in the embryonic somites. COUP-TFII expression in the somites begins at E8.5 (data not shown) and can be clearly seen by E9.5 (Fig. 1B). This expression in the somites suggests that COUP-TFII plays a role in muscle development. At the limb levels, COUP-TFII is highly expressed throughout both the myotome and the dermomyotome of the developing somites (Fig. 1C). Therefore, it is possible that COUP-TFII functions in both epaxial and hypaxial lineages of somite-derived muscle cells (11, 22). First, its expression in the myotome suggests that COUP-TFII is important for development of the muscles of the body proper, including the intercostal muscles, abdominals, and thoracic muscles. Second, its expression in the ventrolateral dermomyotome at limb levels suggests a role in the migrating hypaxial muscle lineage, which consists of cells that migrate from the somites to form the muscles of the limbs and diaphragm (7, 11, 20, 22, 52).
This expression pattern in muscle precursor cells persists in older embryos. At E10.5, COUP-TFII is still highly expressed in the somites (Fig. 1D). However, COUP-TFII is no longer expressed throughout the mesenchyme as it is at E9.5, but rather it is expressed in the fore and hind limb buds in a centralized core of the mesenchyme (Fig. 1E). Cross sections of these limbs demonstrate that COUP-TFII is expressed in two distinct centralized mesenchymal masses (Fig. 1F). One of these masses is located in the dorsal half of the limb, and the other is located in the ventral half. This expression pattern is highly reminiscent of the location of the dorsal and ventral premuscle masses, which are groups of mesenchymal tissue that eventually differentiate into the skeletal muscle elements of the limbs (11). Additionally, COUP-TFII is expressed in the apical ectodermal ridge (AER) at this age (Fig. 1F; also E11.5, in Fig. 1H). By E11.5, COUP-TFII is still expressed in the myogenic mesenchyme (Fig. 1G), and by E12.5, COUP-TFII is expressed in the individual differentiating muscle bundles of both the fore and hind limbs (Fig. 1J, K, and L). COUP-TFII also appears to be expressed in other mesenchymal tissues, which are not associated with developing muscle at both E11.5 and E12.5.
COUP-TFII is required for somitic myogenesis.
On the basis of the somitic expression of COUP-TFII in the myotomes and limb level dermomyotomes, we hypothesized that COUP-TFII may play a role in embryonic muscle development. To determine if COUP-TFII plays a role in hypaxial muscle precursor cell migration, we analyzed COUP-TFII null mutants for the expression of Lbx1, a hypaxial lineage marker (30). Whole-mount in situ hybridization for Lbx1 in both wild-type and COUP-TFII null mutant embryos shows positively expressing cells in both the somites and the limbs, but at a slightly reduced level in COUP-TFII mutant embryos (Fig. 2A and B). This result suggests that muscle precursor cells are able to migrate to the limb but may be at a reduced level.
FIG. 2.
Expression of marker genes for muscle migration and muscle differentiation. (A and B) Whole-mount in situ hybridization of E9.5 wild-type and mutant embryos for Lbx1. Lbx1 expression is detected in both somites and limbs of COUP-TFII embryos, indicating that muscle precursor cells are able to migrate into the limbs. (C and D) Whole-mount in situ hybridization of wild-type and COUP-TFII mutant embryos for myogenin. Expression of myogenin is significantly decreased in caudal somites of COUP-TFII mutant embryos compared to that in wild-type embryos (arrowheads).
Next, we examined the expression of the myogenic determination factor myogenin in wild-type and COUP-TFII mutant embryos in order to determine if COUP-TFII plays a role in myogenic differentiation. Since COUP-TFII mutants are embryonic lethal prior to E10 (44), we analyzed E9.5 embryos at the 22-somite stage of development by whole-mount in situ hybridization. myogenin expression in COUP-TFII mutant embryos is significantly lower than in wild-type embryos of the same age (Fig. 2C and D). Although myogenin is still expressed in the mutant embryos, it does not extend as far in the caudal axis as in wild-type embryos and the level of myogenin expression is much reduced at the caudal end. This indicates that COUP-TFII is important for muscle development and suggests that either COUP-TFII is directly involved in the myogenic differentiation pathway or that, in the absence of COUP-TFII, there are fewer differentiating muscle cells.
Chimera analysis reveals an early cell-autonomous role for COUP-TFII in limb bud outgrowth.
On the basis of the COUP-TFII expression pattern in limbs, we examined the role of COUP-TFII during limb development. To circumvent the early lethality of our mutant, we used an embryonic chimera approach (50). This approach consists of microinjecting wild-type or COUP-TFII mutant ES cells into wild-type blastocysts in order to generate chimeric embryos that are composed of a mixture of ES cell-derived and blastocyst-derived cells (32, 34). Since the ES cells were derived from mice containing the ubiquitously expressed ROSA26-LacZ transgene (27), they can be differentiated from the wild-type blastocyst-derived cells by detecting β-gal enzyme activity with X-Gal.
With two independently generated mutant ES cell lines, chimeric embryos were stained for β-gal enzyme activity. The percent chimerism of each embryo was visually estimated by examining sections of each embryo for the extent of X-Gal staining. Five mutant chimeras composed of greater than 50% mutant cells analyzed at E10.5 show a distinct white patch at the distal end of the limb bud (Fig. 3A), while chimeric embryos generated with wild-type ES cells show an even distribution of X-Gal-positive tissue (Fig. 3D). This result indicates that the mutant cells are not able to maintain contribution to the limb bud and demonstrates that COUP-TFII has a cell-autonomous function during limb development. By E11.5 and E12.5, it is very clear that COUP-TFII mutant cells are unable to contribute to the limb bud, since virtually the entire limb is devoid of blue-staining mutant cells by these ages (Fig. 3B and C). In contrast, wild-type cells have no difficulty populating the entire limb bud at E11.5 and E12.5 (Fig. 3E and F). This suggests that COUP-TFII has a critical cell-autonomous function during limb bud outgrowth.
FIG. 3.
COUP-TFII is required cell autonomously in limb mesenchyme. Whole-mount X-Gal staining (A to F) and cross sections of X-Gal-stained chimeric embryos (G to L). (A to C) Chimeric embryos restrict mutant cells progressively starting at E10.5, as shown by the white patches visible in chimeric E10.5 limbs (arrow in panel A) and the almost completely white limbs at E11.5 and E12.5 (arrows in panels B and C). (D to F) Wild-type chimeras do not have restriction of ES cell-derived cells from the limbs (arrows), which are uniformly blue. (G to I) Cross sections of X-Gal-stained mutant chimeras show that exclusion of mutant cells begins at E9.5 (arrow in panel G) and is largely complete by E12.5. Mutant cells are able to contribute to the AER (arrowhead in panel H). (J to L) Wild-type ES cell-derived cells are able to contribute to the limbs at all stages.
To analyze this phenotype further, we examined cross sections of chimeric limbs and found that while COUP-TFII mutant cells are initially able to contribute to the early stages of limb bud initiation and outgrowth at E9.5 (Fig. 3G), COUP-TFII mutant cells do begin to restrict from the very distal tip of the limb bud. By E10.5, the mutant cells are absent from approximately half of the limb bud, and by E11.5 only scattered mutant cells are present in the limb (Fig. 3H and I). In contrast, wild-type ES cell-derived cells can contribute to the whole limb at all of the stages examined without any obvious deficiencies (Fig. 3J to L). These results suggest that COUP-TFII is not required for limb bud initiation, since the early limb bud still contains COUP-TFII mutant cells. Rather, it is more likely that COUP-TFII plays an important, cell-autonomous role in later stages of limb bud outgrowth. This observation is consistent with the fact that the conventional COUP-TFII knockout, which is embryonic lethal prior to E10, still displays an early limb bud. Interestingly, COUP-TFII also appears only to be required cell autonomously for contribution to the limb mesenchyme, since both the surface ectoderm of the limb and the AER still contain COUP-TFII mutant cells (arrowhead in Fig. 3H).
Conditional ablation of COUP-TFII in limbs reveals a hypoplastic-muscle phenotype.
To further assess the function of COUP-TFII in limb outgrowth, the Cre/loxP system was used to ablate COUP-TFII in the limbs. We have generated an allele of COUP-TFII in which the COUP-TFII locus is flanked by loxP sites (COUP-TFIIflox) (56). Upon exposure to the Cre recombinase enzyme, the COUP-TFII coding region is excised and replaced with a LacZ reporter gene, which is then expressed under the control of the COUP-TFII regulatory region. Consequently, tissue-specific deletion of the COUP-TFII gene in cells that normally express COUP-TFII can be identified by X-Gal staining. To generate a limb-specific knockout of COUP-TFII, we used the Prx1-Cre transgenic mouse. This mouse contains a transgenic Cre recombinase gene under the control of the Prx1 enhancer, which directs Cre expression specifically throughout the limb bud mesenchyme, body wall tissue, and some craniofacial mesenchyme (38). When this strain was crossed with our COUP-TFIIflox mice (Fig. 4A and B) or with ROSA reporter mice (54; data not shown), staining for β-gal activity demonstrated that recombination occurs specifically in the limb bud (Fig. 4A and B). The recombination induced by the Prx1-Cre transgene is initiated around E9.5 in the forelimb and is not completed until E10.5. In the hind limb at E9.5, however, Cre recombinase activity was only detected in a few scattered cells (Fig. 4A).
FIG. 4.
Conditional mutation of COUP-TFII in the limb. (A) Whole-mount X-Gal staining of embryos derived by crossing Prx1-Cre mice with COUP-TFIIflox mice. Significant recombination has occurred in the forelimb at E9.5, but few cells are recombined in the hind limb. (B) Histological sections of an embryo identical to that in Fig. 5A show a high percentage of recombination in the forelimb at this age. (C) Conditional-mutant forelimbs at E15.5 are shorter than those of a wild-type littermate, although individual limb elements appear to be appropriately specified. The bar approximately brackets the limb between the shoulder and the tip of the digits. Abbreviations: fl, forelimb; hl, hind limb; Cond. KO, conditional knockout.
When embryos containing the Prx1-Cre transgene and COUP-TFIIflox alleles were analyzed, we observed that while patterning and other general developmental features appeared to be unaffected, the mutant limbs were almost always noticeably shorter than those of their controlled littermates (Prx1-Cre or COUP-TFIIflox). This first appeared distinctively at E12.5 and was particularly obvious at E15.5 (Fig. 4C), when the mutant limb was approximately 85% of the length of that of the wild-type littermate. When these embryos were stained for skeletal preps, however, it appeared that all of the skeletal elements were intact (data not shown). Consequently, we believe that COUP-TFII may play a role in limb outgrowth, and this may reflect a function of the early expression of COUP-TFII in the LPM at E9. Since recombination of COUP-TFIIflox did not take place until E9.5, at a time when COUP-TFII is already expressed, the incomplete excision of COUP-TFII during this critical time period may contribute to the partial shortening of the limbs seen at later ages. Although there was shortening of the forelimbs, there was no defect in the hind limbs of the conditional knockouts at any of the embryonic development stages; this may have been due to the low Cre recombinase activity in the hind limbs of the Prxl-Cre mice used (Fig. 4A).
To analyze whether COUP-TFII plays a role in the development of the skeletal muscle elements of the limbs, we began by looking at the expression of the differentiation marker myogenin by in situ hybridization. At E10.5, myogenin is expressed in the somitic myotomes, as well as in the mesenchyme of the limb buds. In the limb-specific knockout embryos, myogenin was clearly expressed in the limb buds at this age and there appeared to be no detectable difference in expression between the limb-specific COUP-TFII knockout mutant (Fig. 5B) and control (Fig. 5A) embryos. By E11.5, however, the myogenin expression pattern was distinctively altered in the mutants (Fig. 5D) in comparison with that in the control (Fig. 5C). At this embryonic age, what was initially a solid mesenchymal core of differentiating muscle has begun to segregate into separate muscle bundles expressing myogenin. When mutant embryos were examined and compared to control littermates, it was clear that the mutant limb bud contained muscle bundles that did not extend distally to the same extent as in the control (Fig. 5C and D). This result was observed consistently in multiple experiments and strongly suggests that COUP-TFII is required for appropriate development of the limb musculature.
FIG. 5.
Loss of COUP-TFII leads to hypoplastic muscles. (A and B) Whole-mount in situ hybridization for myogenin of control and conditional-knockout (Cond. KO) embryos at E10.5. There is no significant difference in expression (arrows) between control and conditional-knockout embryos. (C and D) Whole-mount in situ hybridization for myogenin of control and conditional-knockout embryos at E11.5. The region of myogenin expression does not extend as far distally in the conditional-knockout embryo as in the control embryo (arrows). (E to H) Trichrome staining of frontal sections of an E17.5 forelimb. Compared to that of control embryos at E17.5 (E and G), conditional-knockout limb bud musculature is hypoplastic and reduced in size (F and H). Although most individual muscle bundles appear to be appropriately specified, they are significantly smaller (arrows).
When the limb-specific mutant embryos were examined histologically, it was found that the mutant limb buds had underdeveloped, hypoplastic musculature. This first became evident as early as E12.5 but became particularly pronounced by E17.5 (Fig. 5E to H). Both wild-type and mutant E17.5 fore limbs were sectioned frontally and compared to each other, and the individual muscle bundles of the mutant embryos were drastically smaller and less well developed. The diameters of the developing bones, however, appeared relatively unchanged. Interestingly, it appeared that all of the individual muscle bundles were present, indicating that COUP-TFII does not play a role in the patterning or specification of muscle tissue. Rather, all of the muscle bundles were hypoplastic in the mutant, suggesting that COUP-TFII is required for the establishment of muscle bundles of the appropriate size.
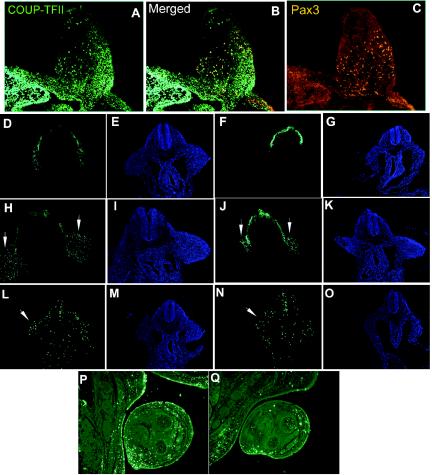
In order to understand the mechanism underlying the role that COUP-TFII plays in limb development, we carried out marker analysis to determine whether COUP-TFII plays a role in muscle precursor cell proliferation and migration. Previous RNA in situ analyses of Lbx1 expression showed that Lbx1 is expressed in the ventral dermomyotome at the limb levels in presumptive migratory muscle precursors, and there is a reduction of Lbx1 expression in COUP-TFII mutant embryos (Fig. 2A and B). This result raises the possibility that COUP-TFII is involved in muscle precursor cell migration. To support this hypothesis, it is important to demonstrate that COUP-TFII is coexpressed in the same precursor cells as the myogenic precursor cell marker Pax3. Therefore, the expression of COUP-TFII and Pax3 was examined in wild-type embryos at E10.5 by immunohistochemistry. The COUP-TFII antibody detected high COUP-TFII expression in the limb and the adjacent mesenchymal cells (Fig. 6A). Specific Pax3 antibody detected Pax3 expression in the dorsal neural tube, in dorsal root ganglia, in dermomyotomes (data not shown), and in scattered cells in the forelimb (Fig. 6C). Most importantly, all positively Pax3-stained cells in the forelimb coexpressed COUP-TFII (Fig. 6B). Therefore, COUP-TFII is expressed in the myogenic precursor cells and may play a role in muscle precursor cell migration.
FIG. 6.
Cell migration and proliferation. (A to C) Transverse sections through the forelimbs of E10.5 wild-type embryos stained with antibodies to detect COUP-TFII (green) and Pax3 (red). Pax3-expressing cells are seen delaminating from the dermomyotome, and migrating muscle precursors coexpress Pax3 and COUP-TFII. (D to K) Transverse sections through the forelimbs of E9.5 (D to G) and E10.5 (H to K) control embryos (D, E, H, and I) and conditional-knockout littermates (F, G, J, and K) stained with antibody Pax3 (green) and 4′,6′-diamidino-2-phenylindole (DAPI) (blue). (L to O) Transverse section through the forelimbs of an E9.5 control embryo (L and M) and a conditional-knockout littermate (N and O) stained with antibody for phospho-histone H3 (green) and DAPI (blue). (P and Q) Frontal sections of the forelimbs of an E14.5 control embryo (P) and a conditional-knockout littermate (Q) stained with antibody for phospho-histone H3 (green).
Next, we analyzed the muscle precursor cell migration in the conditional knockouts and the control littermate embryos by Pax3 immunostaining. We observed a migration defect in the conditional knockouts at E9.5 and E10.5 (Fig. 6F and J) in which the Pax3-positive cells did not extend as far into the limb axis as in the control embryos (Fig. 6D and H). However, the observed migration defect in the conditional knockouts was no longer detected at later stages (i.e., E11.5 or later; data not shown). This suggested that ablation of COUP-TFII delays early muscle precursor cell migration and contributes partly to the defects observed.
To delineate the other factor contributing to the limb defect of the COUP-TFII mutant, we analyzed the proliferation activity of control and conditional-knockout embryos at different developmental stages by phospho-histone H3 immunostaining (a mitotic index marker). We found no significant difference in the proliferation index between control (Fig. 6L) and conditional-knockout (Fig. 6N) embryos at E9.5, while a reduction in the mutant (Fig. 6Q) compared to the controls (Fig. 6P) was observed later, at E14.5. Thus, a reduction in cell proliferation in conditional knockouts is likely also responsible for the observed shorter-limb phenotype in addition to delayed migration.
DISCUSSION
The nuclear orphan receptor COUP-TFII is highly expressed in multiple tissues throughout embryonic development. In this study, we have investigated the role of COUP-TFII in limb bud outgrowth and muscle development. COUP-TFII is expressed throughout the LPM of the limb field prior to limb bud initiation and then becomes highly expressed in the early limb mesenchyme. In embryonic chimera analyses, COUP-TFII mutant cells are initially able to contribute to the early stages of limb bud development, but at later ages they become progressively restricted from the distally growing limb bud mesenchyme. This shows that COUP-TFII mutant cells are unable to successfully compete against wild-type cells in a chimeric environment during limb bud outgrowth. Furthermore, tissue-specific ablation of COUP-TFII in the developing limb bud mesenchyme also results in shorter limbs, providing additional evidence to support a role for COUP-TFII in limb bud outgrowth. Therefore, COUP-TFII is important for limb bud outgrowth but not limb bud initiation.
To determine whether the shorter limb is caused by more cell death in conditional knockouts, we did terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling staining (29); however, we did not detect any significant difference between the conditional knockouts and the controls by this assay at any stage of development (data not shown). This suggests that the shorter limbs in the conditional-knockout mutant are not due to cell death. Similarly, analysis of COUP-TFII conditional-knockout embryos at E9.5 has failed to show any significant difference in proliferation compared to that in the littermate control embryos. However, at later ages it becomes obvious that the mesenchyme of limbs lacking COUP-TFII proliferates at a lower rate than in wild-type embryos. This further suggests that COUP-TFII is not involved in limb bud initiation but rather is required for the maintenance of limb bud outgrowth. Similar findings have been reported for the FGF signaling pathway. Loss of FGFR1 or its downstream effector Shp2 also leads to defects in limb bud outgrowth (18, 51). Interestingly, loss of FGFR1 also leads to hypoplastic muscles (25), establishing a link between the two developmental processes. FgfR1 expression, however, does not appear to be significantly altered in our mutant, nor does the AER-specific growth factor Fgf8 (data not shown).
In the absence of COUP-TFII, myogenesis does not proceed normally. Expression of myogenin is reduced in the somites at the caudal end of COUP-TFII mutant embryos. It appears that COUP-TFII is involved in hypaxial muscle precursor cell migration since there is a decrease in the expression of Lbx1 in the COUP-TFII null mutant at E9.5. The muscle precursor cell migration defects are also observed in limb buds of the conditional knockout at E9.5 and E10.5 by immunostaining with Pax3 antibody. Both Lbx1 and Pax3 are markers of hypaxial muscle precursor cell migration (6, 16, 19, 24, 39). In addition, the muscles of the limbs fail to develop to the appropriate size when COUP-TFII is specifically ablated in the limb mesenchyme. This defect in muscle development is reflected by the altered expression of the myogenic differentiation gene myogenin in both somitic and limb muscle lineages. This phenotype is manifested as a reduction in muscle size, although it appears that all of the individual muscle bundles of the limb are appropriately specified and develop in their proper positions. Consequently, it appears that COUP-TFII is not involved in the patterning or specification of the limb muscle but may be involved in the maintenance or expansion of the muscle lineage. In addition, COUP-TFII is required for the appropriate migration of limb muscle precursor cells from the somites to the limb buds. Since other genes known to be involved in muscle cell migration are still expressed in the COUP-TFII mutant, this suggests that COUP-TFII may be involved in a novel pathway of muscle cell migration that has not previously been described. It is important to analyze this defect in the future.
A current model of myogenesis proposes that a balance between muscle growth and differentiation must be maintained in order for proper muscle development to occur (28, 42). COUP-TFII has been shown in cell culture to inhibit both the expression and the posttranscriptional regulation of MyoD (3, 41), which is required to initiate the myogenic program, which inevitably causes muscle precursor cells to exit the cell cycle and differentiate. It is therefore conceivable that COUP-TFII is required to inhibit muscle differentiation, thereby maintaining the pool of undifferentiated cells. Furthermore, a recent study suggests that the mitogen SHH is required for repression of the differentiation of myoblasts in order to stimulate their proliferation. Without SHH, the myoblasts differentiate precociously, which eliminates the pool of proliferating myoblasts, leading to less muscle (37). Our laboratory has previously demonstrated that COUP-TFII lies downstream of SHH signaling in vitro (35, 36). Furthermore, we have recently shown that COUP-TFII may act as an effector of SHH signaling in vivo in the development of the stomach (56). Consequently, it is conceivable that COUP-TFII lies downstream of SHH to function in the maintenance and expansion of muscles by inhibiting terminal myogenesis.
In conclusion, we have established critical roles for COUP-TFII in limb and muscle development. COUP-TFII is required for the distal outgrowth of embryonic limbs and also for appropriate embryonic muscle development. COUP-TFII is required for the maintenance of proliferation in the growing limb bud, as well as the migration of muscle precursor cells to the limbs. COUP-TFII represents a novel player in this complex field, and it will be exciting to further define the exact molecular pathways in which COUP-TFII functions.
Acknowledgments
This work was supported by National Institutes of Health grants to S.Y.T. (DK55636, DK57743-project4) and M.-J.T. (DK45641).
We are thankful to Eric Olson for the myogenin probe, Krzysztof Jagla for Lbx1, and Toshiya Tanaka for the COUP-TFII antibody. The Pax3 antibody developed by Charles P. Ordahl was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the Department of Biological Sciences, University of Iowa, Iowa City. We are thankful to Louise Stanley, the Transgenic Microinjection Core at the Baylor College of Medicine, Wei Qian, and Chen Liu for technical assistance.
REFERENCES
- 1.Amthor, H., B. Christ, and K. Patel. 1999. A molecular mechanism enabling continuous embryonic muscle growth—a balance between proliferation and differentiation. Development 126:1041-1053. [DOI] [PubMed] [Google Scholar]
- 2.Amthor, H., B. Christ, M. Weil, and K. Patel. 1998. The importance of timing differentiation during limb muscle development. Curr. Biol. 8:642-652. [DOI] [PubMed] [Google Scholar]
- 3.Bailey, P., V. Sartorelli, Y. Hamamori, and G. E. Muscat. 1998. The orphan nuclear receptor, COUP-TF II, inhibits myogenesis by post-transcriptional regulation of MyoD function: COUP-TF II directly interacts with p300 and myoD. Nucleic Acids Res. 26:5501-5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton-Davis, E. R., D. I. Shoturma, A. Musaro, N. Rosenthal, and H. L. Sweeney. 1998. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc. Natl. Acad. Sci. USA 95:15603-15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand-Saberi, B., and B. Christ. 1999. Genetic and epigenetic control of muscle development in vertebrates. Cell Tissue Res. 296:199-212. [DOI] [PubMed] [Google Scholar]
- 6.Brohmann, H., K. Jagla, and C. Birchmeier. 2000. The role of Lbx1 in migration of muscle precursor cells. Development 127:437-445. [DOI] [PubMed] [Google Scholar]
- 7.Buckingham, M., L. Bajard, T. Chang, P. Daubas, J. Hadchouel, S. Meilhac, D. Montarras, D. Rocancourt, and F. Relaix. 2003. The formation of skeletal muscle: from somite to limb. J. Anat. 202:59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buffinger, N., and F. E. Stockdale. 1994. Myogenic specification in somites: induction by axial structures. Development 120:1443-1452. [DOI] [PubMed] [Google Scholar]
- 9.Cauthen, C. A., E. Berdougo, J. Sandler, and L. W. Burrus. 2001. Comparative analysis of the expression patterns of Wnts and Frizzleds during early myogenesis in chick embryos. Mech. Dev. 104:133-138. [DOI] [PubMed] [Google Scholar]
- 10.Chevallier, A., M. Kieny, and A. Mauger. 1977. Limb-somite relationship: origin of the limb musculature. J. Embryol. Exp. Morphol. 41:245-258. [PubMed] [Google Scholar]
- 11.Christ, B., and B. Brand-Saberi. 2002. Limb muscle development. Int. J. Dev. Biol. 46:905-914. [PubMed] [Google Scholar]
- 12.Christ, B., H. J. Jacob, and M. Jacob. 1977. Experimental analysis of the origin of the wing musculature in avian embryos. Anat. Embryol. 150:171-186. [DOI] [PubMed] [Google Scholar]
- 13.Christ, B., H. J. Jacob, and M. Jacob. 1974. Origin of wing musculature. Experimental studies on quail and chick embryos. Experientia 30:1446-1449. [DOI] [PubMed] [Google Scholar]
- 14.Christ, B., M. Jacob, and H. J. Jacob. 1983. On the origin and development of the ventrolateral abdominal muscles in the avian embryo. An experimental and ultrastructural study. Anat. Embryol. 166:87-101. [DOI] [PubMed] [Google Scholar]
- 15.Christ, B., and C. P. Ordahl. 1995. Early stages of chick somite development. Anat. Embryol. 191:381-396. [DOI] [PubMed] [Google Scholar]
- 16.Daston, G., E. Lamar, M. Olivier, and M. Goulding. 1996. Pax-3 is necessary for migration but not differentiation of limb muscle precursors in the mouse. Development 122:1017-1027. [DOI] [PubMed] [Google Scholar]
- 17.Delfini, M., E. Hirsinger, O. Pourquie, and D. Duprez. 2000. Delta 1-activated notch inhibits muscle differentiation without affecting Myf5 and Pax3 expression in chick limb myogenesis. Development 127:5213-5224. [DOI] [PubMed] [Google Scholar]
- 18.Deng, C., M. Bedford, C. Li, X. Xu, X. Yang, J. Dunmore, and P. Leder. 1997. Fibroblast growth factor receptor-1 (FGFR-1) is essential for normal neural tube and limb development. Dev. Biol. 185:42-54. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich, S., F. Abou-Rebyeh, H. Brohmann, F. Bladt, E. Sonnenberg-Riethmacher, T. Yamaai, A. Lumsden, B. Brand-Saberi, and C. Birchmeier. 1999. The role of SF/HGF and c-Met in the development of skeletal muscle. Development 126:1621-1629. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich, S., F. R. Schubert, C. Healy, P. T. Sharpe, and A. Lumsden. 1998. Specification of the hypaxial musculature. Development 125:2235-2249. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich, S., F. R. Schubert, and A. Lumsden. 1997. Control of dorsoventral pattern in the chick paraxial mesoderm. Development 124:3895-3908. [DOI] [PubMed] [Google Scholar]
- 22.Duprez, D. 2002. Signals regulating muscle formation in the limb during embryonic development. Int. J. Dev Biol. 46:915-925. [PubMed] [Google Scholar]
- 23.Duprez, D., C. Fournier-Thibault, and N. Le Douarin. 1998. Sonic Hedgehog induces proliferation of committed skeletal muscle cells in the chick limb. Development 125:495-505. [DOI] [PubMed] [Google Scholar]
- 24.Epstein, J. A., D. N. Shapiro, J. Cheng, P. Y. Lam, and R. L. Maas. 1996. Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc. Natl. Acad. Sci. USA 93:4213-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flanagan-Steet, H., K. Hannon, M. J. McAvoy, R. Hullinger, and B. B. Olwin. 2000. Loss of FGF receptor 1 signaling reduces skeletal muscle mass and disrupts myofiber organization in the developing limb. Dev. Biol. 218:21-37. [DOI] [PubMed] [Google Scholar]
- 26.Floss, T., H. H. Arnold, and T. Braun. 1997. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 11:2040-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedrich, G., and P. Soriano. 1991. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5:1513-1523. [DOI] [PubMed] [Google Scholar]
- 28.Fuchtbauer, E. M. 2002. Inhibition of skeletal muscle development: less differentiation gives more muscle. Results Probl. Cell Differ. 38:143-161. [DOI] [PubMed] [Google Scholar]
- 29.Gavrieli, Y., Y. Sherman, and S. A. Ben-Sasson. 1992. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119:493-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross, M. K., L. Moran-Rivard, T. Velasquez, M. N. Nakatsu, K. Jagla, and M. Goulding. 2000. Lbx1 is required for muscle precursor migration along a lateral pathway into the limb. Development 127:413-424. [DOI] [PubMed] [Google Scholar]
- 31.Hannon, K., A. J. Kudla, M. J. McAvoy, K. L. Clase, and B. B. Olwin. 1996. Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J. Cell Biol. 132:1151-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogan, B., R. Beddington, F. Constantini, and E. Lacy (ed.). 1994. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Johnson, R. L., and C. J. Tabin. 1997. Molecular models for vertebrate limb development. Cell 90:979-990. [DOI] [PubMed] [Google Scholar]
- 34.Joyner, A. L. (ed.). 2000. Gene targeting: a practical approach, 2nd ed., vol. 212. Oxford University Press, New York, N.Y.
- 35.Krishnan, V., G. Elberg, M. J. Tsai, and S. Y. Tsai. 1997. Identification of a novel sonic hedgehog response element in the chicken ovalbumin upstream promoter-transcription factor II promoter. Mol. Endocrinol. 11:1458-1466. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan, V., F. A. Pereira, Y. Qiu, C. H. Chen, P. A. Beachy, S. Y. Tsai, and M. J. Tsai. 1997. Mediation of Sonic hedgehog-induced expression of COUP-TFII by a protein phosphatase. Science 278:1947-1950. [DOI] [PubMed] [Google Scholar]
- 37.Kruger, M., D. Mennerich, S. Fees, R. Schafer, S. Mundlos, and T. Braun. 2001. Sonic hedgehog is a survival factor for hypaxial muscles during mouse development. Development 128:743-752. [DOI] [PubMed] [Google Scholar]
- 38.Logan, M., J. F. Martin, A. Nagy, C. Lobe, E. N. Olson, and C. J. Tabin. 2002. Expression of Cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33:77-80. [DOI] [PubMed] [Google Scholar]
- 39.Mennerich, D., K. Schafer, and T. Braun. 1998. Pax-3 is necessary but not sufficient for lbx1 expression in myogenic precursor cells of the limb. Mech. Dev. 73:147-158. [DOI] [PubMed] [Google Scholar]
- 40.Munsterberg, A. E., and A. B. Lassar. 1995. Combinatorial signals from the neural tube, floor plate and notochord induce myogenic bHLH gene expression in the somite. Development 121:651-660. [DOI] [PubMed] [Google Scholar]
- 41.Muscat, G. E., S. Rea, and M. Downes. 1995. Identification of a regulatory function for an orphan receptor in muscle: COUP-TF II affects the expression of the myoD gene family during myogenesis. Nucleic Acids Res. 23:1311-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel, K., B. Christ, and F. E. Stockdale. 2002. Control of muscle size during embryonic, fetal, and adult life. Results Probl. Cell Differ. 38:163-186. [DOI] [PubMed] [Google Scholar]
- 43.Pereira, F. A., Y. Qiu, M. J. Tsai, and S. Y. Tsai. 1995. Chicken ovalbumin upstream promoter transcription factor (COUP-TF): expression during mouse embryogenesis. J. Steroid Biochem. Mol. Biol. 53:503-508. [DOI] [PubMed] [Google Scholar]
- 44.Pereira, F. A., Y. Qiu, G. Zhou, M. J. Tsai, and S. Y. Tsai. 1999. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 13:1037-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pownall, M. E., K. E. Strunk, and C. P. Emerson, Jr. 1996. Notochord signals control the transcriptional cascade of myogenic bHLH genes in somites of quail embryos. Development 122:1475-1488. [DOI] [PubMed] [Google Scholar]
- 46.Qiu, Y., A. J. Cooney, S. Kuratani, F. J. DeMayo, S. Y. Tsai, and M. J. Tsai. 1994. Spatiotemporal expression patterns of chicken ovalbumin upstream promoter-transcription factors in the developing mouse central nervous system: evidence for a role in segmental patterning of the diencephalon. Proc. Natl. Acad. Sci. USA 91:4451-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu, Y., V. Krishnan, F. A. Pereira, S. Y. Tsai, and M. J. Tsai. 1996. Chicken ovalbumin upstream promoter-transcription factors and their regulation. J. Steroid Biochem. Mol. Biol. 56:81-85. [DOI] [PubMed] [Google Scholar]
- 48.Qiu, Y., F. A. Pereira, F. J. DeMayo, J. P. Lydon, S. Y. Tsai, and M. J. Tsai. 1997. Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev. 11:1925-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rong, P. M., M. A. Teillet, C. Ziller, and N. M. Le Douarin. 1992. The neural tube/notochord complex is necessary for vertebral but not limb and body wall striated muscle differentiation. Development 115:657-672. [DOI] [PubMed] [Google Scholar]
- 50.Rossant, J., and A. Spence. 1998. Chimeras and mosaics in mouse mutant analysis. Trends Genet. 14:358-363. [DOI] [PubMed] [Google Scholar]
- 51.Saxton, T. M., B. G. Ciruna, D. Holmyard, S. Kulkarni, K. Harpal, J. Rossant, and T. Pawson. 2000. The SH2 tyrosine phosphatase shp2 is required for mammalian limb development. Nat. Genet. 24:420-423. [DOI] [PubMed] [Google Scholar]
- 52.Schafer, K., and T. Braun. 1999. Early specification of limb muscle precursor cells by the homeobox gene Lbx1h. Nat. Genet. 23:213-216. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt, C., B. Christ, M. Maden, B. Brand-Saberi, and K. Patel. 2001. Regulation of Epha4 expression in paraxial and lateral plate mesoderm by ectoderm-derived signals. Dev. Dyn. 220:377-386. [DOI] [PubMed] [Google Scholar]
- 54.Soriano, P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21:70-71. [DOI] [PubMed] [Google Scholar]
- 55.Tajbakhsh, S., U. Borello, E. Vivarelli, R. Kelly, J. Papkoff, D. Duprez, M. Buckingham, and G. Cossu. 1998. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development 125:4155-4162. [DOI] [PubMed] [Google Scholar]
- 56.Takamoto, N., K. Moses, C. Chiang, W. E. Zimmer, R. J. Schwartz, F. J. DeMayo, M. J. Tsai, and S. Y. Tsai. Regulatory linkage shared between COUP-TFII and Hedgehog is central for gastric organogenesis. Submitted for publication.
- 57.Tsai, S. Y., and M. J. Tsai. 1997. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocrinol. Rev. 18:229-240. [DOI] [PubMed] [Google Scholar]
- 58.Xu, X., M. Weinstein, C. Li, and C. Deng. 1999. Fibroblast growth factor receptors (FGFRs) and their roles in limb development. Cell Tissue Res. 296:33-43. [DOI] [PubMed] [Google Scholar]