Abstract
Saffron (Crocus sativus L.) has been considered as a medicinal plant since ancient times and also widely used as food additive for its color, taste and odor. The pharmacological properties of saffron and its main constituents, crocin and safranal have been evaluated using different in vivo and in vitro models. Additionally, other lines of studies have found toxicological effects of saffron. However, a comprehensive review that covers all aspects of its toxicity has not been published yet. The current study provides classified information about the toxic effects of saffron and its constituents in various exposure conditions including acute, sub-acute, sub-chronic and chronic studies. Therapeutic doses of saffron exhibits no significant toxicity in both clinical and experimental investigations.
Keywords: Crocetin, Crocin, Saffron, Safranal, Toxicity
Introduction
From long time ago, herbal medicines play an important role in cultures and traditions of different nations such as Muslim. Saffron, the vernacular name for Crocus sativus, is a well-known herbal plant. Saffron is a perennial, stemless herb belonging to Iridaceae family. It is cultivated in various countries such as Iran, Greece, Spain, China and Turkey (1, 2).
The chemical constituents of saffron are 63% sugars, 12% protein, 10% moisture, 5% crude fiber, 5% fat, and 5% minerals (% w/w) (2). There are about 150 volatile and non-volatile ingredients in saffron. Safranal is responsible for unique odor of saffron, classified as a volatile agent (3). Non-volatiles constituents include crocin (color agent), crocetin and picrocrocin (bitter flavor) (Figure 1) (4). In regard to distinct smell, color and flavor, saffron is widely used around the world. It is extensively used as a coloring and flavoring factor and as a spice in the manufacturing of cosmetics and preparation of foods (5).
Figure 1.
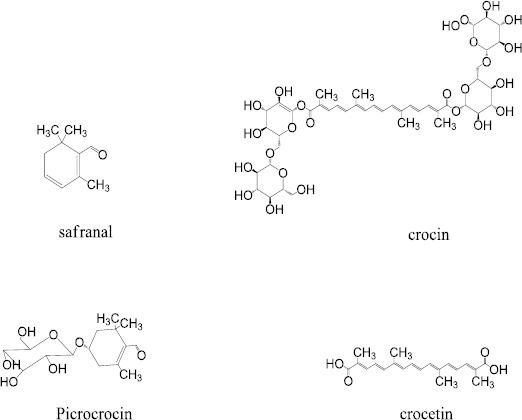
Chemical structures of safranal, crocin, picrocrocin and crocetin
The eminent Iranian medical scientists, Razi and Avicenna, introduced the saffron as a unique herbal plant for treatment of diseases such as depression, delivery difficulties, respiration insufficiency and digestive disorder (2, 6).
Today, many pharmacological aspects of saffron and its components, such as anti-hypertensive (7), anti-tremor (8), neuroprotective (9, 10), anti-depressant (11) anti-tussive (12), and anti-convulsant (13, 14) effects have been investigated.
Regarding to the wide use of saffron and its active components in traditional medicine and current pharmacology, the determination of possible toxic effects is necessary.
In recent years several animal models and clinical trials have been conducted for determination of safety of it.
In double-blind, placebo-controlled study, after one week treatment with saffron tablet (200 and 400 mg/day), no adverse effect on coagulant and anticoagulant system have been reported (15). In another clinical trial, Heidarzadeh et al (2015) evaluated the safety of saffron and crocin in patients with schizophrenia. They found that saffron and crocin (in doses of 15 mg twice daily) didn’t show toxic effects on thyroid, liver, kidney and hematologic systems. (16).
In animal experiments, we found that under acute and sub-acute conditions, the aqueous extract of saffron could decrease toxic effect of safranal. It is showed that after four days co-treatment of safranal (1.2 ml/kg, IP) and saffron (5, 10, 20 and 30 mg/kg, IP), mortality rate diminished significantly in rats. Moreover, in sub-acute model, the concurrent administration of safranal (0.2 ml/kg/day) and saffron (5, 10 and 20 mg/kg/day) resulted in remarkable decrease of mortality in animals (17). Saffron and its major components were safe after oral administration in animal studies (acute exposure) (18), while following sub-acute treatment some toxic effect were mentioned. Administration of ethanolic extract of saffron (0.35, 0.7, 1.05 g/kg) for two weeks decreased hemoglobin (Hb), hematocrit (HCT) and total red blood cells (RBC) counts in rats (19).
Despite the wide use of saffron in many countries as an herbal medicine, there is no well-documented study which categorized the toxic effects of saffron in animal models and human studies. Therefore, in this review paper, different studies in scientific databases including Scopus, MEDLINE and Web of Science databases and local references have been discussed, which evaluate the toxicity of saffron and its main components under the descriptive and developmental toxicity using animal models. Additionally possible toxic effects of saffron and crocin in human clinical trials were mentioned. The keywords for the search were: Crocus sativus, saffron, crocin, safranal, crocetin, toxicity, developmental, animal model and clinical trial.
Toxicological findings on saffron and its major components in animals
Acute toxicity of saffron and its major constituent including crocin and safranal
Generally, the first step into determination of toxicity of chemical substance is acute tests. The aim of the acute test is to acquire LD50 and other toxic effects such as target organs after one or more route of administration. Rat and mice are two usual species which can be used for this test. The animals will be examined 14-day after receiving single dosage to see lethality and other toxic effects (20).
Acute toxicity of saffron
It has been shown that toxicological data on saffron safety is not uniform. Findings exhibited that LD50 values of saffron stigma and petal extracts were 1.6 and 6 g/kg, respectively in mice after IP exposure (18).
The LD50 value of saffron was 4120±556 mg/kg after oral administration in BALB/c mice (18).
Acute toxicity of crocin
It has been found that oral administration of 3 g/kg crocin within 2 days in mice did not cause mortality. A similar result was observed after IP exposure at same dose.
Crocin administration (IP) at 0.5, 1, 1.5, 2 and 3 g/kg did not induce any mortality after 24 and 48 hr. It can be concluded that crocin is a practically low-toxic substance (21) (Table 1).
Table 1.
The LD50 values of saffron, safranal and crocin
| Toxicity | Components | Animals | Route of administration | LD50 | Ref |
|---|---|---|---|---|---|
| Acute | Saffron (aqueous extract) | BALB/c mice | Oral | 4120±556 mg/kg | (18) |
| Saffron (stigma extract) | mice | IP | 1.6 g/kg | (18) | |
| Saffron (petal extract) | mice | IP | 6 g/kg | (18) | |
| Safranal | Male BALB/c mice Female BALB/c mice Male Wistar rats | IP | 1.48 ml/kg 1.88 ml/kg 1.50 ml/kg |
(18) | |
| Male BALB/c mice Female BALB/c mice Male Wistar rats | Oral | 21.42 ml/kg 11.42 ml/kg 5.53 ml/kg |
(18) | ||
| Crocin | Male Razi mice Wistar rats (150-210 g) | IP | 1–5 g/kg | (21) |
Acute toxicity of safranal
In our previous study, the acute toxicity of safranal was evaluated. Our finding showed that LD50 values were 1.48, 1.88 and 1.50 ml/kg in male mice, female mice and male Wistar rats, respectively after IP administration. Using oral administration, these values change to 21.42, 11.42 and 5.53 ml/kg in male mice, female mice and male rats, respectively. We established 0.75 ml/kg as a maximum non-fatal dose in mice (both sexes). After the IP and oral exposure this index was 0.75 ml/kg and 3.5 ml/kg, respectively on male Wistar rats. As clearly shown in acquired data, there is a significant difference in LD50 values after IP administration in comparison to oral exposure. First pass metabolism and lower absorption following oral administration can explain this difference (18) (Table 1).
It is suggested to determine the acute toxic effects of other constituents which will be helpful to understand whole aspect of saffron toxicity.
Sub-acute toxicity of saffron and its major constituent
In sub-acute toxicity test, information about impact of the chemical substance on living organism after repeated exposure is acquired. Hematological and biochemical parameters, food intake, body weight and other factors are analyzed in this test (20).
Sub-acute toxicity of saffron
The sub-acute toxicity of saffron (stigma) ethanolic extract has been carried out in rats by Mohajeri et al. Results showed that intraperitoneal administration of ethanolic extract (0.35, 0.7, 1.05 g/kg) for two weeks decreased body weight of rats in the dose dependent manner. Additionally, decreased Hb, HCT and RBC counts were reported. Vice versa, total white blood cells (WBC) counts were increased dose dependently. Increased alanine aminotransferase (ALT), aspartate amino-transferase (AST) enzymes, which are involved in liver injury, were observed. Also, the level of serum urea, uric acid and creatinine were significantly increased. The pathological findings showed that ethanolic extract induced mild to severe hepatic and renal injuries (19).
In a sub-acute experiment conducted by Karimi et al (2004), the aqueous extract of petal (1.2, 2.4, and 3.6 g/kg) and stigma (0.16, 0.32, and 0.48 g/kg) of saffron could lead to significant decrease of body weight in rats. Also, reduced levels of Hb, HCT and RBC counts as well as anemia were observed. Liver and lung injuries were reported in animals which received petal extract (22).
In another study, the effect of saffron on spermatogenesis index in rats was carried out. The finding of this study exhibited oral administration of 200 mg/kg of saffron for 28 days significantly decreased spermatogenesis index including: repopula-tion index (RI), spermatogenesis index (SI) and tubular differentiation index (TDI) (23) (Figure 2).
Figure 2.
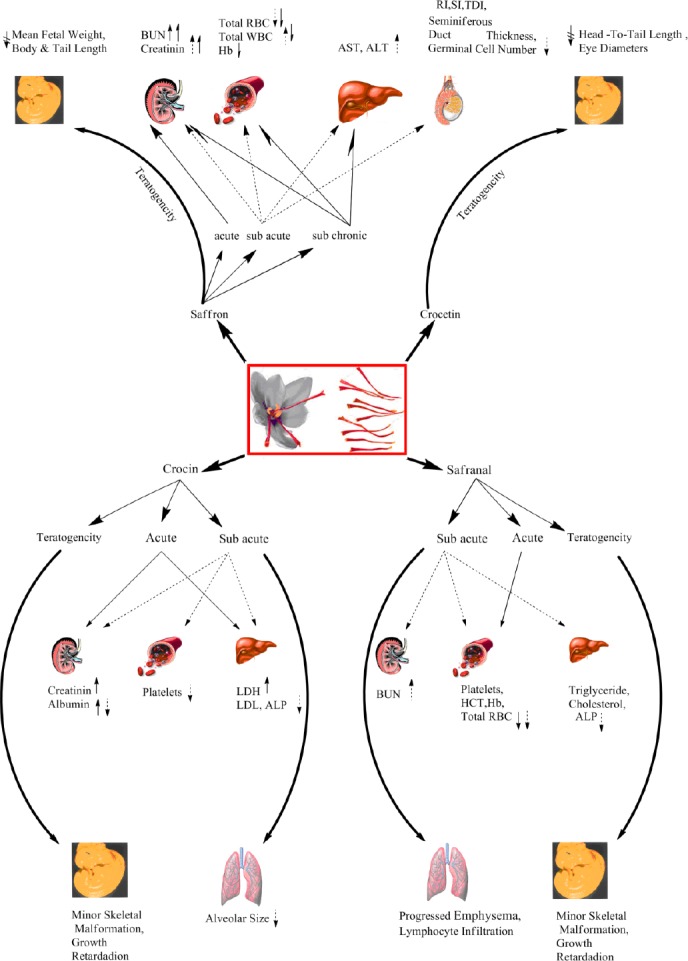
Schematic of toxic effects of saffron, crocin, safranal and crocetin in different animal toxicity test
Sub-acute toxicity of crocin
In a sub-acute study on crocin in 2010, we examined various factors including weight changes, amount of food intake, biochemical, hematological and pathological parameters in rats. Following IP administration of crocin (180 mg/kg) once a day for 21 days, increased platelets and creatinin levels were observed. At the same dose, a reduction in weight, food intake, alveolar size (in lung) and also minor myosin light chain atrophy were detected. Administration of crocin (90 mg/kg) decreased levels of albumin and alkaline phosphatase (ALP) while increased the level of LDL. Significant pathological lesions weren’t observed in main organs (heart, liver, spleen kidney and lung) after exposure to 15, 45, 90 and 180 mg/kg doses of crocin.
Finally, according to this report, the entire lesions were negligible to show serious toxicity and crocin was found to be safe substance at the pharmacological doses (21).
In another study, the liver toxicity of crocin was examined. The findings showed that IP administration of 50, 100 and 200 mg/kg of crocin once a week for four weeks in rats didn’t alter serum parameters including ALT, AST, ALP, urea, uric acid and creatinine, malondialdehyde (MDA) and gluthatione (GSH) content in liver. Also, using histo-pathological examination, no significant toxicity was observed (24) (Figure 2).
Sub-acute toxicity of safranal
In our previous study, the sub-acute toxicity of safranal (0.1, 0.25 and 0.5 ml/kg) on rats within 21-day exposure was evaluated. The results showed that oral administration of safranal markedly decreased important hematological factors including RBC counts, HCT, Hb and platelets. Reduced levels of cholesterol, triglyceride, ALP with parallel increase of serum urea nitrogen exhibited remarkable effect of safranal on biochemical parameters. In Pathological examinations noticeable lesion in different tissues (heart, liver and spleen) was not observed, while safranal could induce histopathological changes in lung and kidney (3, 18). Also, the evaluation of immunotoxic effect of safranal didn’t show any significant toxicity on humoral and cellular immune system of mice after IP exposure to 0.1, 0.5 and 1 ml/kg doses for 21 days (25) (Figure 2).
Sub-chronic toxicity of saffron
In sub-chronic test the duration of study is 30-90 days. This type of study conducted on dogs, rats and mice. At the end of the test, biochemical and hematological analysis are done as well as other factors such as weight of the major organs, food consumption, respiratory and cardiovascular function are assessed (20).
Recently, the sub-chronic effect of saffron on BALB/c mice following five weeks exposure was reported. Results revealed that oral administration of saffron (4000 and 5000 mg/kg) markedly decreased counts of RBC and WBC as well as, hemoglobin level. Increased BUN and creatinine levels, which are indicative of kidney dysfunction, were detected in animals. This result was confirmed by histopathological examination. Additionally, the activity of liver enzymes including ALT and AST, increased (26) (Figure 2). However, it should be noted that the administrated doses are high. Saffron extract in lower doses exhibited protective effects in different models. For example, administration of saffron aqueous extract (25, 50 and 100 mg/kg/day,
IP for 30 days) protected against ethylene glycol induced calcium oxalate (CaOx) nephrolithiasis in rats. (27). Additionally, the aqueous extract of saffron stigmas (20 and 80 mg/kg, IP) markedly decreased methyl methanesulfonate–induced DNA damage in mice organs (28).
Unfortunately, the sub-chronic toxicity of constituents of saffron is not evaluated. Therefore it is suggested to study the toxic effects of important constituent of saffron especially crocin and safranal in sub-chronic models.
Developmental toxicity of saffron and its main constituents in animals
Teratogenicity tests have been applied on more than four thousand chemical substances. Results showed that about 65 percent of substances found to be nonteratogen. Also, the results showed that about 7 percent in more than one species and 18 percent in most species were teratogen (29).
Herbal plants are used in many countries for different purposes. In pregnant females these products applied for control of symptoms such as nausea, constipation and vomiting. Additionally, some of the plant can promote lactation, stimulating the appetite, reduce menstrual discomfort etc. On the other hands, adverse effects of herbal plants on the fetus have reported in different studies. Regarding to these fact, it is important to know the possible toxic effects of saffron and its components in fetus and breast feeding child (30, 31).
Developmental toxicity of saffron
In 2009, Zeynali et al evaluated the teratogenicity of different doses of aqueous extracts of saffron (0.8, 0.4 and 0.2 %) in BALB/c mice. Their finding revealed that administration of aqueous extract of saffron caused reduction in tail length, biparietal diameter, placental diameter and weight of fetal during gestational period. Also, results showed the saffron extracts elevated mortality rate and the mean number of resorbed fetus in the test group in comparison to control one in the dose dependent manner (32).
In another study, prenatal developmental toxicity of saffron was investigated in male Wistar rats. The result of this study exhibited oral administration of saffron (at the doses of 1000, 250 and 50 mg/kg) didn’t show any effects on food intake, gravid uterine weight, corpora lutea and implantation counts, early and late resorptions, pre and post implantation loss. Skeletal examination did not show any malformations. No biochemical parameters were affected following exposure to saffron extracts (33) (Figure 2).
Developmental toxicity of safranal and crocin
Administration of crocin (200 mg/kg and 600 mg/kg, IP) and safranal (0.075 ml/kg and 0.225 ml/kg, IP) disrupted skeleton formation in mice. Also, the evaluation of maternal and fetal factors indicated that these ingredients adversely affected weight, length, growth, mandible and calvaria of fetuses (34) (Figure 2).
Developmental toxicity of crocetin
The teratogenic effect of crocetin was demonstrated in frog (Xenopus) embryos by American researchers. Crocetin (10, 25, 50, 100 and 200 µM) decreased head-to-tail length and eye diameters in animals but not cement gland length (35) (Figure 2).
Clinical trials on saffron and crocin dosage forms and toxicity
Saffron tablets
Previously, we examined the safety of saffron tablet (200 and 400 mg) in healthy volunteers who received tablets for 7 days. In this study, the electrocardiographic factors (ECG), lipid profile (TG), ionogram (Na+ and K+), kidney biochemical markers and hematological factors were evaluated.
Our clinical findings revealed that mean arterial pressures and standing systolic blood pressure were decreased in persons who received 400 mg tablets (but not 200 mg saffron tablet) (36). We didn’t observe significant toxicity on hematological parameters. Similarly, Ayatollahi and his colleague confirmed that saffron tablets (200 and 400 mg) are safe drug on coagulation system (15).
In Table 2, different side effects of saffron under different duration of exposure have been summarized.
Table 2.
Clinical complications and side effects related to saffron
| Duration of exposure | Dose | Adverse effects | P | N | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Saffron | Drug | Saffron (N) | Drug (N) | Placebo (N) | |||||
| 6 weeks | capsule /30 mg/day (BD) | - | Anxiety | 3 | - | 1 | 0.60 | 40 | (41) |
| Decreased appetite | 2 | 2 | 1.39 | ||||||
| Increased appetite | 5 | 1 | 0.18 | ||||||
| Sedation | 1 | 2 | 1.00 | ||||||
| Nausea | 2 | 1 | 1.00 | ||||||
| Headache | 3 | 2 | 1.00 | ||||||
| Hypomania | 2 | 1 | 1.00 | ||||||
| 22 weeks | capsule/30 mg/day | Donepezil capsule 10 mg/day | Vomiting | 1 | 7 | - | 0.05 | 44 | (42) |
| Dizziness | 2 | 5 | 0.42 | ||||||
| Dry mouth | 5 | 3 | 0.70 | ||||||
| Fatigue | 1 | 4 | 0.35 | ||||||
| Hypomania | 1 | 0 | 1.00 | ||||||
| Nausea | 2 | 6 | 0.25 | ||||||
| 16 weeks | capsule /30 mg/ day | - | Dizziness | 2 | - | 3 | 1.00 | 46 | (43) |
| Dry mouth | 3 | 1 | 0.60 | ||||||
| Fatigue | 1 | 2 | 1.00 | ||||||
| Hypomania | 2 | 0 | 0.48 | ||||||
| Nausea | 2 | 1 | 0.25 | ||||||
| 4 weeks | capsule /15 mg (twice daily) | - | Dry mouth | 3 | - | 2 | NS | 20 | (44) |
| Restlessness | 2 | 0 | NS | ||||||
| Anxiety | 2 | 0 | NS | ||||||
| Tachycardia | 0 | 1 | NS | ||||||
| Constipation | 1 | 1 | NS | ||||||
| Nausea | 0 | 1 | NS | ||||||
| Reflux | 0 | 1 | NS | ||||||
| Abdominal pain | 0 | 1 | NS | ||||||
| Headache | 0 | 1 | NS | ||||||
| Dizziness | 0 | 1 | NS | ||||||
| Daily drowsiness | 1 | 1 | NS | ||||||
| Morning drowsiness | 1 | 0 | NS | ||||||
| six weeks | capsule /30 mg/ day | Fluoexetine /40mg/day | Daytime drowsiness | 0 | 1 | - | 1.00 | 40 | (45) |
| Morning drowsiness | 1 | 1 | 1.00 | ||||||
| Constipation | 1 | 3 | 0.60 | ||||||
| Nervousness | 0 | 1 | 1.00 | ||||||
| Decreased appetite | 4 | 2 | 0.66 | ||||||
| Dry mouth | 1 | 4 | 0.34 | ||||||
| 8 weeks | capsule / 15 mg bid | capsule fluoxetine /10 mg bid | Anxiety | 4 | 7 | - | 0.48 | 40 | (46) |
| Decreased appetite | 5 | 4 | 1.00 | ||||||
| Increased appetite | 1 | 3 | 0.60 | ||||||
| Sexual dysfunction | 3 | 5 | 0.69 | ||||||
| Tremor | 2 | 5 | 0.40 | ||||||
| Nausea | 3 | 4 | 1.00 | ||||||
| Headache | 2 | 5 | 0.40 | ||||||
| Sweating | 2 | 3 | 1.00 | ||||||
| Heart Pounding | 3 | 2 | 1.00 | ||||||
| Insomnia | 3 | 3 | 1.00 | ||||||
| 6 weeks | capsule /30 mg/day (BD) | - | Anxiety | 4 | - | 2 | 0.66 | 40 | (47) |
| Decreased appetite | 4 | 2 | 0.66 | ||||||
| Stomach pain | 4 | 2 | 0.66 | ||||||
| Tremor | 3 | 1 | 0.60 | ||||||
| Nausea | 5 | 2 | 0.40 | ||||||
| Headache | 3 | 1 | 0.60 | ||||||
| Sweating | 2 | 1 | 1.00 | ||||||
| Heart pounding | 4 | 2 | 0.66 | ||||||
| 6 weeks | Capsule/30 mg/day (BD) | Fluoxetine/20 mg/day (BD) | Anxiety | 3 | 6 | - | 0.45 | 40 | (48) |
| Decreased appetite | 2 | 5 | 0.45 | ||||||
| Increased appetite | 5 | 2 | 0.40 | ||||||
| Sedation | 1 | 0 | 1.00 | ||||||
| Nausea | 2 | 4 | 0.66 | ||||||
| Headache | 3 | 6 | 0.45 | ||||||
| Sexual dysfunction | 0 | 4 | 0.10 | ||||||
| Tremor | 0 | 4 | 0.10 | ||||||
| Sweating | 0 | 3 | 0.23 | ||||||
| 6 weeks | capsule/ 30 mg/day (TDS) | capsule of imipramine 100 mg/day (TDS) | Anxiety | 4 | 1 | 0.32 | 30 | (49) | |
| Decreased Appetite | 2 | 0 | 0.48 | ||||||
| Increased Appetite | 1 | 5 | 0.16 | ||||||
| Sedation | 0 | 6 | 0.01 | ||||||
| Nausea | 2 | 1 | 1.00 | ||||||
| Headache | 3 | 2 | 1.00 | ||||||
| Dry Mouth | 1 | 5 | 0.03 | ||||||
| Hypomania | 2 | 1 | 1.00 | ||||||
| Constipation | 2 | 5 | 0.38 | ||||||
| Urinary Retention | 1 | 5 | 0.16 | ||||||
Crocin tablets
In a randomized, double-blind, placebo-controlled trial, we examined the safety of crocin tablets (20 mg) in healthy volunteers that received tablets for one month. Administrations of crocin tablets decrease partial thromboplastin time, amylase and mixed WBC (monocytes, basophils and eosinophils). According to these results, crocin was found to be relatively safe herbal product (37).
Effect of saffron on miscarriage rate
In the prospective case-control study, miscarriage rate in pregnant females who participated in saffron harvesting was examined. Just the pregnant subject between first and twentieth week of gestation, were studied. The abortion rate is significantly higher among pregnant females whom exposed to high level of saffron in comparison to control (38). The possible mechanism may be through the uterine contraction and/or bleeding which induced by saffron (36, 39, 40).
Effects of saffron on labor
In triple blind clinical study, administration of saffron capsule (250 mg) at the beginning of active stage of labor markedly reduced mean anxiety score from 46.5±18.8 in the placebo group to 26.4±16.9 in saffron group. Also, the mean fatigue score in treated group (57.7±20.9) significantly decreased in com-parison to placebo group (52). In another clinical study, saffron capsule (250 mg) which was used at the active phase of the first stage of labor could diminish pain intensity from 97.4±2.9 in females received placebo to 85.9±8.4 in treated females. Saffron did not show toxicity in infants and mothers (53).
Effect of saffron and its main constituents on normal and cancer cells
Cancer, an important health problem, is a major cause of mortality around the world. Reports have been shown that more than 8 million people are recognized with cancer each year. Findings stated that cancer may be controlled by modifications in life style and environment, including following a healthy diets. Chemoprevention introduced as a promising plan for prevention of cancer. It defined as the use of natural or synthetic agents. In this regard, spiced and herbs opened new horizon for inhibition of tumor growth and progression of cancer. Recently, the large numbers of studies have focused on the anticancer properties of saffron and its main components using in vivo and in vitro models (Table 4) (54, 55).
Table 3.
The effect of saffron and crocin on biochemical parameters in volunteers
| Duration of exposure | Dose | Significant side effect | N | Ref. | |
|---|---|---|---|---|---|
| 1 week Treatment, At 1 month monitoring |
Saffron | Crocin | 60 | (15) | |
| Tablet/ 200 mg/day, 400 mg/day |
- | None | |||
| 12 weeks | Capsule/ 30 mg/day | Capsule/ 30 mg/day | Crocin significantly Decreased the FBS Level | 66 | (50) |
| 12 weeks | Capsule/ 15 mg twice daily | Capsule/ 15 mg twice time a day | WBC count increased significantly in patients receiving saffron, but it was within the normal range | 66 | (51) |
| One month | - | Tablet/ 20 mg | Significant decreased in amylase, mixed white blood cells and PTT | 42 | (37) |
| 1 week | Tablet/ 200 mg/day, 400 mg/day | - | At dose of 400 mg: significant decrease in standing systolic blood pressure, mean arterial pressures, RBC and increase in Na+, BUN and creatinine | 30 | (36) |
| At dose of 200 mg: Decrease in RBC, Hb, HCT, PLT, INR, bleeding time and increase in creatinine | |||||
Table 4.
The effect of saffron on normal and cancer cell line under in vivo and in vitro conditions
| Condition | type | Dose or Concentration | Toxic responses | Ref. | |
|---|---|---|---|---|---|
| In vivo | Normal | Saffron: 20.7 g/kg | The dose was equivalent to LD50 and was non toxic | (54) | |
| Saffron: 1.2 to 2 g/BW |
Nausea, vomiting, diarrhea, and bleeding were observed | (54) | |||
| Saffron: 4 g/day | Non-toxic | (54) | |||
| Saffron: 200 to 400 mg/day |
Saffron decreased slightly red blood cells and platelets (but, these alterations were in normal ranges) | (36) | |||
| Cancerous | Saffron: 100 mg/kg | Saffron extract inhibited onset and progression of induced skin tumors and delay papilloma onset in rats | (56) | ||
| Saffron: 100, 150, and 175 mg/kg |
Saffron inhibited gastric cancer progression dose dependently | (57) | |||
| Saffron extract + cystein 50 mg/kg | Saffron extract along with cysteine significantly reduced cisplatin toxicity | (58) | |||
| Saffron: 20, 40, and 80 mg/kg | Saffron significantly reduced genotoxicity of anti-cancer drugs | (59) | |||
| In vitro | Normal | Fetal lung fibrobl-ast |
Saffron: 0, 0.25,0.5, 1.0, 2.0 and 4 mg/ml |
No changes in cell viability (IC50 :19.99) | (60) |
| L929 | Saffron: 500, 1000, 1500 and 2000 μg/ml | Cell viability didn’t decrease significantly | (61) | ||
| L929 | Saffron: 200–2000 μg/ml | Cell viability did not reduce significantly | (62) | ||
| Cancerous | MCF-7, SKNM and HeLa | Saffron: 0, 0.25,0.5, 1.0, 2.0 and 4 mg/mL | Concentration-dependent inhibitory effect (IC50 : 0.78, 1.66 and 1.92 on MCF-7, SKNM and HeLa, respectively) | (60) | |
| A549 | Saffron: (500, 1000, 1500 and 2000 μg/ml) | Ethanolic extract of saffron decreased cell viability in malignant cells as a concentration and time-dependent manner (IC50 : 1500 and 565 μg/ml after 24 and 48 hr, respectively) | (61) | ||
| MCF-7 | 200–2000 μg/ml | Decreased cell viability in MCF-7 cells as a concentration and time -dependent manner with an IC50 of 400 ± 18.5 lg/ml after 48 h. apoptotic cell death, increased Bax protein expression | (62) | ||
| MIA-PaCa-2 | Crocetin: 50-200 μmol/l | Crocetin has a significant antitumorigenic effect through the stimulation of apoptotic pathways. | (63) | ||
Anti-tumor activity of different doses of safranal (0.1, 15, 20, 50 µg/ml) have investigated in cultured neuroblastoma cells. Safranal with IC50 11.1 µg/ml and 23.3 µg/ml inhibited cell proliferation and induced cell apoptosis after 24 and 48 hr, respectively (54). The anti-proliferative effects of crocin and saffron extract on different colorectal cancer cell lines (HCT-116, SW-480, and HT-29) were evaluated. Saffron extract at 1 mg/ml and 3 mg/ml reduced cell proliferation in HCT-116 cells, to 45.5% and 6.8% respectively. Saffron extract (1 mg/ml) did not change the growth of non-cancer
The proliferation was significantly decreased in HCT-116, SW-480, and HT-29 to 2.8%, 52%, and 16.8%, respectively following exposure to crocin (1 mM) for 48 hr (64). Saffron extract significantly inhibited proliferation of cancerous cell line SKNSH (malignant cells derived from bone metastases of a neuroblastoma), HeLa (malignant cells from an adenocarcinoma from the uterine cervix) and MCF-7 (malignant cells from a breast tumor) with an IC50 of 1.66 mg/ml, 1.92 mg/ml and 0.78 mg/l respectively. Also, the toxic effect of saffron on normal human lung fibroblasts was evaluated. The IC50 was 19.9 mg/ml which exhibited saffron has selective inhibitory effects in cancerous cells (60).
Saffron extract was incubated on the MCF-7 cells in different concentrations (200–2000 μg/ml) for 24, 48 and 72 hr. The IC50 value was 400±8.5 µg/ml after 48 hr. While saffron significantly induced apoptosis cell death in cancerous cell line did not show remarkable toxicity in L929 cells (non-malignant cells) (62).
In another study, the ethanolic extract of saffron reduced cell viability in A549 cells (lung cancer cells) in the concentration and time-dependent manner with the IC50 1500 and 565 μg/ml after 24 and 48 h exposure, respectively. The cell viability in L929 cells was not changed following exposure to different concentrations of saffron (61).
It has been shown that oral administration of 200 mg/kg of saffron extract in mice markedly inhibit the growth of ascites tumors originated from Ehrlich ascites carcinoma (EAC), sarcoma-180 (S-180) and Dalton’s lymphoma ascites (DLA). In addition, the life span of tumor-bearing mice increased 2-3 times (55). Using an experimental model in rats, the effect of long-term (13 weeks) treatment of crocin on colon adenocarcinoma was investigated. In this study, crocin (400 mg/kg) was administrated once a week from 1 to 13 weeks. Adenocarcinoma induced by SC injection of DHD/K12-PROb cells into the chest of animals. Results showed long-term exposure to crocin increased life span and decreased tumor growth in female rats without major toxic effects (but not in male rats) (65).
Additionally saffron extract (100 mg/kg, oral) significantly inhibited two-stage initiation/promotion [dimethylbenz[a]anthracene (DMBA)/croton oil] skin carcinogenesis in mice (56). Administration of saffron (100, 150 mg/kg, IP) reduced gastric cancer progression in the Wistar albino rat. In vivo studies revealed anti-cancer effects of saffron in doses (100-150 mg/kg), while other studies showed saffron in doses (20 g/kg, oral) had no toxic effects on normal cells (54).
Interestingly, it has been demonstrated that saffron and its constituents selectively inhibit cancer cell proliferation in both in vitro and in vivo models, while these compounds don’t have toxic effect on normal cells in the therapeutic doses. (54).
Mutagenic and antimutagenic effect of saffron and its main constituents
Abduullaev et al assessed the antimutagenic and co-mutagenic effect of saffron and its ingredients. They used Ames/Salmonella test system, two well-known mutagenic agents BP (benzo[a]-pyrene) and 2AA (2-amino-antracene). The concentration of the saffron extract in the cultures ranged from 50 to 1500 mg/plate for Salmonella typhimurium. 100-400 µg/plate of saffron ingredients (crocin, kaemferol, picrocrocin and safranal) were used for co-mutagencity study. It has been shown the non-mutagenic, as well as non-antimutagenic effect of saffron against BP-induced mutagenicity. In addition, the co-mutagenic effect of saffron on 2-AA-induced mutagencity was reported. Safranal is responsible for co-mutagenic effect of it (66). The genotoxicity of crocetin was evaluated using Ames test, rec-assay, and sister chromatid exchange (SCE) in V79 cells. Results of all tests showed crocetin didn’t induce significant genotoxicity (67).
Administration of saffron aqueous extract (20 and 80 mg/kg, IP), crocin (50, 200 and 400 mg/kg, IP) or safranal (72.75 mg/kg and 363.75 mg/kg, IP) markedly reduced methanesulfonate (MMS)-induced DNA damage in liver, lung, spleen and kidney organs in NMRI mice (28, 68). Pretreatment of Swiss albino mice with saffron aqueous extract (20, 40 and 80 mg/kg) could significantly inhibit the genotoxicity of cisplatin, cyclophosphamide, mitomycin C and urethane which evaluated using bone marrow micronucleus test (69). Additionally pretreatment with saffron extract prevented cellular DNA damage (strand breaks) which induced by these anti-tumor drugs (70). Saffron reduced comet tail length, tail moment and percent DNA in the tail. In another study administration of crocin (50, 100 and 200 mg/kg, IP) or safranal (0.025, 0.05 and 0.1 ml/kg, IP) three times per week alone or with DZN (20 mg/kg/day, orally) for 4 weeks did not arrest the genotoxicity induced by diazinon in rats (71). Treatment of C3H10T1/2 cells (mouse mesenchymal) with crocetin (0.01, 0.05 and 0.10 mM) prevented benzo(a)pyrene-induced genotoxicity and neoplastic transformation through increasing the activity of GST and decreasing the formation of a B(a)P-DNA adduct (72).
Conclusion
In the present study, we reviewed a variety of articles that examined the toxicity of saffron and its constituents. By considering the LD50 values, it’s clear that safranal is more toxic than crocin and saffron in acute models. Results have revealed that in animal studies, crocin at the pharmacological doses didn’t show important damage on main body organs.
Nevertheless, clinical trial on crocin tablet showed that this component will be safe herbal product in therapeutic doses. Similar studies on saffron tablets didn’t report important clinically toxicity in healthy volunteers. In comparison with saffron and crocin, safranal has more toxic effect on hematological and biochemical indices. Saffron, crocin, safranal and crocetin showed some embryonic malformation in animal’s models at high doses but not in pharmacological doses. Similarly, it has been shown that exposure to very high levels of saffron may increase miscarriage rate in pregnant females. Regarding insufficient clinical trials on safety of saffron in pregnancy, it is suggested that pregnant women should avoid using high dose of saffron. Finally, many in vitro studies showed that saffron and its constituents selectively inhibited cancer cell proliferation, while didn’t exert toxic effect on normal cells.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Razavi BM, Hosseinzadeh H. Saffron as an antidote or a protective agent against natural or chemical toxicities. Daru. 2015;23:31. doi: 10.1186/s40199-015-0112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mollazadeh H, Emami SA, Hosseinzadeh H. Razi’s Al-Hawi and saffron (Crocus sativus): a review. Iran J Basic Med Sci. 2015;18:1153–1166. [PMC free article] [PubMed] [Google Scholar]
- 3.Rezaee R, Hosseinzadeh H. Safranal: from an aromatic natural product to a rewarding pharmacological agent. Iran J Basic Med Sci. 2013;16:12. [PMC free article] [PubMed] [Google Scholar]
- 4.Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Hosseinzadeh H. Saffron: a herbal medicine of third millennium. Jundishapur J Nat Pharm Prod. 2014;9:1–2. doi: 10.17795/jjnpp-16700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosseinzadeh H, Nassiri-Asl M. Avicenna’s (Ibn Sina) the Canon of Medicine and saffron (Crocus sativus): a review. Phytother Res. 2013;27:475–483. doi: 10.1002/ptr.4784. [DOI] [PubMed] [Google Scholar]
- 7.Imenshahidi M, Razavi BM, Faal A, Gholampoor A, Mousavi SM, Hosseinzadeh H. Effects of chronic crocin treatment on desoxycorticosterone acetate (doca)-salt hypertensive rats. Iran J Basic Med Sci. 2014;17:9–13. [PMC free article] [PubMed] [Google Scholar]
- 8.Amin B, Malekzadeh M, Heidari MR, Hosseinzadeh H. Effect of Crocus sativus extracts and its active constituent safranal on the harmaline-induced tremor in mice. Iran J Basic Med Sci. 2015;18:449–458. [PMC free article] [PubMed] [Google Scholar]
- 9.Mehri S, Abnous K, Khooei A, Mousavi SH, Shariaty VM, Hosseinzadeh H. Crocin reduced acrylamide-induced neurotoxicity in Wistar rat through inhibition of oxidative stress. Iran J Basic Med Sci. 2015;18:902–908. [PMC free article] [PubMed] [Google Scholar]
- 10.Dorri SA, Hosseinzadeh H, Abnous K, Hasani FV, Robati RY, Razavi BM. Involvement of brain-derived neurotrophic factor (BDNF) on malathion induced depressive-like behavior in subacute exposure and protective effects of crocin. Iran J Basic Med Sci. 2015;18:958–966. [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseinzadeh H, Motamedshariaty V, Hadizadeh F. Antidepressant effect of kaempferol, a constituent of saffron (Crocus sativus) petal, in mice and rats. Pharmacologyonline. 2007;2:367–370. [Google Scholar]
- 12.Hosseinzadeh H, Ghenaati J. Evaluation of the antitussive effect of stigma and petals of saffron (Crocus sativus) and its components, safranal and crocin in guinea pigs. Fitoterapia. 2006;77:446–448. doi: 10.1016/j.fitote.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76:722–724. doi: 10.1016/j.fitote.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Hosseinzadeh H, Sadeghnia H. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: involvement of GABAergic and opioids systems. Phytomedicine. 2007;14:256–262. doi: 10.1016/j.phymed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Ayatollahi H, Javan AO, Khajedaluee M, Shahroodian M, Hosseinzadeh H. Effect of Crocus sativus L. (saffron) on coagulation and anticoagulation systems in healthy volunteers. Phytother Res. 2014;28:539–543. doi: 10.1002/ptr.5021. [DOI] [PubMed] [Google Scholar]
- 16.Mousavi B, Bathaie SZ, Fadai F, Ashtari Z. Safety evaluation of saffron stigma (Crocus sativus L.). aqueous extract and crocin in patients with schizophrenia. Avicenna J Phytomed. 2015;5:413–419. [PMC free article] [PubMed] [Google Scholar]
- 17.Ziaee T, Razavi BM, Hosseinzadeh H. Saffron reduced toxic effects of its constituent, safranal, in acute and subacute toxicities in rats. Jundishapur J Nat Pharm Prod. 2014;9:3–8. doi: 10.17795/jjnpp-13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.HosseinZadeh H, Shakib SS, Sameni AK, Taghiabadi E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran J Pharm Res. 2013;12:93–99. [PMC free article] [PubMed] [Google Scholar]
- 19.Mohajeri D, Mousavi G, Mesgari M, Doustar Y, Khayat Nouri M. Subacute toxicity of Crocus sativus L. (saffron) stigma ethanolic extract in rats. Am J Pharmacol Toxicol. 2007;2:189–193. [Google Scholar]
- 20.Eaton DL, Gilbert SG. Principles of Toxicology. In: Kilassen CD, Watkins Jb III, editors. Casarett & Doull, s Essentials of Toxicology. 3rd ed. New York: McGraw-Hill; 2015. pp. 16–18. [Google Scholar]
- 21.Hosseinzadeh H, Shariaty VM, Sameni AK, Vahabzadeh M. Acute and sub-acute toxicity of crocin, a constituent of Crocus sativus L (saffron), in mice and rats. Pharmacologyonline. 2010;2:943–951. [Google Scholar]
- 22.Karimi G, Taiebi N, Hosseinzadeh H, Shirzad F. Evaluation of subacute toxicity of aqueous extract of Crocus sativus L. stigma and petal in rats. J Medicina Plants. 2004;4:29–35. [Google Scholar]
- 23.Khayatnouri M, Safavi S, Safarmashaei S, Babazadeh D, Mikailpourardabili B. The effect of saffron orally administration on spermatogenesis index in rat. Adv Environ Biol. 2011;5:1514–1521. [Google Scholar]
- 24.Taheri F, Bathaie SZ, Ashrafi M, Ghasemi E. Assessment of crocin toxicity on the rat liver. Modares J Med Sci Pathobiol. 2014;17:67–79. [Google Scholar]
- 25.Riahi-Zanjani B, Balali-Mood M, Mohammadi E, Badie-Bostan H, Memar B, Karimi G. Safranal as a safe compound to mice immune system. Avicenna J Phytomed. 2015;5:441–449. [PMC free article] [PubMed] [Google Scholar]
- 26.Muosa F, AL-Rekabi K, Askar SJ, Yousif EH. Evaluation of the toxic effect of ethanolic extract of saffron in male mice after subchronic exposure. Donnish J Pharm Pharmacol. 2015;1:1–7. [Google Scholar]
- 27.Amin B, Feriz HM, Hariri AT, Meybodi NT, Hosseinzadeh H. Protective effects of the aqueous extract of Crocus sativus against ethylene glycol induced nephrolithiasis in rats. EXCLI J. 2015;14:411–422. doi: 10.17179/excli2014-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hosseinzadeh H, Abootorabi A, Sadeghnia HR. Protective effect of Crocus sativus stigma extract and crocin (trans-crocin 4) on methyl methanesulfonate–induced DNA damage in mice organs. DNA Cell Biol. 2008;27:657–664. doi: 10.1089/dna.2008.0767. [DOI] [PubMed] [Google Scholar]
- 29.Rogers JM. Developmental Toxicology. In: Kilassen CD, Watkins III Jb, editors. Casarett & Doull, s Essentials of Toxicology. 3rd ed. New York: McGraw-Hill; 2015. pp. 150–151. [Google Scholar]
- 30.Taloubi L, Rhouda H, Belahcen A, Smires N, Thimou A, Mdaghri AA. An overview of plants causing teratogenicity: Fenugreek (Trigonella foenum graecum) Int J Pharm Sci Res. 2013;514:516. [Google Scholar]
- 31.Wu M, Hu Y, Ali Z, Khan IA, Verlangeiri A, Dasmahapatra AK. Teratogenic effects of blue cohosh (Caulophyllum thalictroides) in Japanese medaka (Oryzias latipes) are probably mediated through GATA2/EDN1 signaling pathway. Chemical Res Toxicol. 2010;23:1405–1416. doi: 10.1021/tx100205a. [DOI] [PubMed] [Google Scholar]
- 32.Zeynali F, Dashti MH, Anvari M, Hosseini SM, Miresmaeili SM. Studying teratogenic an abortificant effects of different doses of saffron (Crocus sativus) decoction in whole gestational period and the 3rd trimester of gestational period in mice. Int J Reprod Biomed. 2009;7 [Google Scholar]
- 33.Edamula R, Deecaraman M, Kumar DS, Krishnamurthy H, Latha M. Prenatal developmental toxicity of crocus sativus (saffron) in wistar rats. Int J Pharmacol Toxicol. 2014;2:46–49. [Google Scholar]
- 34.Moallem SA, Afshar M, Etemad L, Razavi BM, Hosseinzadeh H. Evaluation of teratogenic effects of crocin and safranal, active ingredients of saffron, in mice. Toxicol Ind Health. 2013;32:285–291. doi: 10.1177/0748233713500818. [DOI] [PubMed] [Google Scholar]
- 35.Martin G, Goh E, Neff A. Evaluation of the developmental toxicity of crocetin on Xenopus. Food Chem Toxicol. 2002;40:959–964. doi: 10.1016/s0278-6915(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 36.Modaghegh M-H, Shahabian M, Esmaeili H-A, Rajbai O, Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine. 2008;15:1032–1037. doi: 10.1016/j.phymed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Mohamadpour AH, Ayati Z, Parizadeh MR, Rajbai O, Hosseinzadeh H. Safety evaluation of crocin (a constituent of saffron) tablets in healthy volunteers. Iran J Basic Medical Sci. 2013;16:39–46. [PMC free article] [PubMed] [Google Scholar]
- 38.Ajam M, Reyhani T, Roshanravan V, Zare Z. Increased miscarriage rate in female farmers working in saffron fields: a possible effect of saffron toxicity. Asia Pac J Med Toxicol. 2014;3:73–75. [Google Scholar]
- 39.Sadraei H, Ghannadi A, Takei-bavani M. Effects of Zataria multiflora and Carum carvi essential oils and hydroalcoholic extracts of Passiflora incarnata Berberis integerrima and Crocus sativus on rat isolated uterus contractions. Int J Aromather. 2003;13:121–127. [Google Scholar]
- 40.Inoue E, Shimizu Y, Shoji M, Tsuchida H, Sano Y, Ito C. Pharmacological properties of N-095, a drug containing red ginseng, polygala root, saffron, antelope horn and aloe wood. Am J Chin Med. 2005;33:49–60. doi: 10.1142/S0192415X05002655. [DOI] [PubMed] [Google Scholar]
- 41.Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, Amini H, Fallah-Pour H, Jamshidi AH, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother Res. 2005;19:148–151. doi: 10.1002/ptr.1647. [DOI] [PubMed] [Google Scholar]
- 42.Akhondzadeh S, Sabet MS, Harirchian MH, Togha M, Cheraghmakani H, Razeghi S, et al. A 22-week, multicenter, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology. 2010;207:637–643. doi: 10.1007/s00213-009-1706-1. [DOI] [PubMed] [Google Scholar]
- 43.Akhondzadeh S, Sabet MS, Harirchian M, Togha M, Cheraghmakani H, Razeghi S, et al. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: a 16-week, randomized and placebo-controlled trial. J Clin Pharm Ther. 2010;35:581–588. doi: 10.1111/j.1365-2710.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 44.Mansoori P, Akhondzadeh S, Raisi F, Ghaeli P, Jamshidi A, Nasehi A, et al. A randomized, double-blind, placebo-controlled study of safety of the adjunctive saffron on sexual dysfunction induced by a selective serotonin reuptake inhibitor. J Med Plants. 2011;1:121–130. [Google Scholar]
- 45.Shahmansouri N, Farokhnia M, Abbasi S-H, Kassaian SE, Tafti A-AN, Gougol A, et al. A randomized, double-blind, clinical trial comparing the efficacy and safety of Crocus sativus L. with fluoxetine for improving mild to moderate depression in post percutaneous coronary intervention patients. J Affec Disord. 2014;155:216–222. doi: 10.1016/j.jad.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Basti AA, Moshiri E, Noorbala A-A, Jamshidi A-H, Abbasi SH, Akhondzadeh S. Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: a pilot double-blind randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:439–442. doi: 10.1016/j.pnpbp.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 47.Moshiri E, Basti AA, Noorbala A-A, Jamshidi A-H, Abbasi SH, Akhondzadeh S. Crocus sativus L.(petal) in the treatment of mild-to-moderate depression: A double-blind, randomized and placebo-controlled trial. Phytomedicine. 2006;13:607–611. doi: 10.1016/j.phymed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Noorbala A, Akhondzadeh S, Tahmacebi-Pour N, Jamshidi A. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97:281–284. doi: 10.1016/j.jep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Akhondzadeh S, Fallah-Pour H, Afkham K, Jamshidi A-H, Khalighi-Cigaroudi F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: a pilot double-blind randomized trial [ISRCTN45683816] BMC Complement Altern Med. 2004;4:12. doi: 10.1186/1472-6882-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fadai F, Mousavi B, Ashtari Z, Ali BN, Farhang S, Hashempour S, et al. Saffron aqueous extract prevents metabolic syndrome in patients with schizophrenia on olanzapine treatment: a randomized triple blind placebo controlled study. Pharmacopsychiatry. 2014;47:156–161. doi: 10.1055/s-0034-1382001. [DOI] [PubMed] [Google Scholar]
- 51.Mousavi B, Fadai F, Ashtari Z, Hashempour S, Shahhamzei N, Heidarzadeh H. Safety evaluation of saffron stigma (Crocus sativus L.) aqueous extract and crocin in patients with schizophrenia. Avicenna J Phytomed. 2015;5:413–419. [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmadi S, Azhari S, Jafarzadeh H, Rakhshandeh J. R. M. The effect of oral capsules of saffron on anxiety and fatigue during the first stage of labor. J Shahid Sadoughi Univ Med Sci. 2015;23:1915–1926. [Google Scholar]
- 53.Ahmadi S, Azhari S, Rakhshandeh J, Jafarzadeh H. R. M. Effect of saffron oral capsule on pain intensity active phase of the first stage of labor. IJOGI. 2014;17:1–10. [Google Scholar]
- 54.Milajerdi A, Djafarian K, Hosseini B. The toxicity of saffron (Crocus satious L.) and its constituents against normal and cancer cells. JNIM. 2016;3:23–32. [Google Scholar]
- 55.Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.) Exp Biol Med. 2002;227:20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 56.Salomi M, Nair SC, Panikkar K. Inhibitory effects of Nigella sativa and saffron (Crocus sativus) on chemical carcinogenesis in mice. Nutr Cancer. 1991;16:67–72. doi: 10.1080/01635589109514142. [DOI] [PubMed] [Google Scholar]
- 57.Bathaie SZ, Miri H, Mohagheghi M-A, Mokhtari-Dizaji M, Shahbazfar A-A, Hasanzadeh H. Saffron aqueous extract inhibits the chemically-induced gastric cancer progression in the Wistar albino rat. Iran J Basic Med Sci. 2013;16:26–38. [PMC free article] [PubMed] [Google Scholar]
- 58.El Daly E. Protective effect of cysteine and vitamin E, Crocus sativus and Nigella sativa extracts on cisplatin-induced toxicity in rats. J pharm Belg. 1997;53:87–93. [PubMed] [Google Scholar]
- 59.Premkumar K, Abraham SK, Santhiya S, Gopinath P, Ramesh A. Inhibition of genotoxicity by saffron (Crocus sativus L.) in mice. Drug Chem Toxicol. 2001;24:421–428. doi: 10.1081/dct-100106266. [DOI] [PubMed] [Google Scholar]
- 60.Trujillo-Jiménez F, García-López P, Garcia-Carranca A, Abdullaev FI. Effect of saffron on the viability of normal and malignant human cells in vitro. Acta Hortic. 2004:463–470. [Google Scholar]
- 61.Samarghandian S, Boskabady MH, Davoodi S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharmacogn Mag. 2010;6:309–314. doi: 10.4103/0973-1296.71799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mousavi SH, Tavakkol-Afshari J, Brook A, Jafari-Anarkooli I. Role of caspases and Bax protein in saffron-induced apoptosis in MCF-7 cells. Food Chemical Toxicol. 2009;47:1909–1913. doi: 10.1016/j.fct.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 63.Dhar A, Mehta S, Dhar G, Dhar K, Banerjee S, Van Veldhuizen P, et al. Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Mol Cancer Ther. 2009;8:315–323. doi: 10.1158/1535-7163.MCT-08-0762. [DOI] [PubMed] [Google Scholar]
- 64.Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT, et al. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp Oncol. 2007;29:175–180. [PMC free article] [PubMed] [Google Scholar]
- 65.Garc-Olmo DC, Riese HH, Escribano J, Ontañón J, Fernandez JA, Atiénzar M, et al. Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): an experimental study in the rat. Nutr Cancer. 1999;35:120–126. doi: 10.1207/S15327914NC352_4. [DOI] [PubMed] [Google Scholar]
- 66.Abdullaev F, Riveron-Negrete L, Caballero-Ortega H, Hernández JM, Perez-Lopez I, Pereda-Miranda R, et al. Use of in vitro assays to assess the potential antigenotoxic and cytotoxic effects of saffron (Crocus sativus L.) Toxicol In Vitro. 2003;17:731–736. doi: 10.1016/s0887-2333(03)00098-5. [DOI] [PubMed] [Google Scholar]
- 67.Ozaki A, Kitano M, Furusawa N, Yamaguchi H, Kuroda K, Endo G. Genotoxicity of gardenia yellow and its components. Food Chem Toxicol. 2002;40:1603–1610. doi: 10.1016/s0278-6915(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 68.Hosseinzadeh H, Sadeghnia HR. Effect of safranal, a constituent of Crocus sativus (saffron), on methyl methanesulfonate (MMS)-induced DNA damage in mouse organs: An alkaline single-cell gel electrophoresis (comet) assay. DNA Cell Biol. 2007;26:841–846. doi: 10.1089/dna.2007.0631. [DOI] [PubMed] [Google Scholar]
- 69.Premkumar K, Abraham SK, Santhiya ST, Gopinath PM, Ramesh A. Inhibition of genotoxicity by saffron (Crocus sativus L.) in mice. Drug Chem Toxicol. 2001;24:421–428. doi: 10.1081/dct-100106266. [DOI] [PubMed] [Google Scholar]
- 70.Premkumar K, Thirunavukkarasu C, Abraham SK, Santhiya ST, Ramesh A. Protective effect of saffron (Crocus sativus L.) aqueous extract against genetic damage induced by anti-tumor agents in mice. Hum Exp Toxicol. 2006;25:79–84. doi: 10.1191/0960327106ht589oa. [DOI] [PubMed] [Google Scholar]
- 71.Hariri AT, Moallem SA, Mahmoudi M, Hosseinzadeh H. The effect of crocin and safranal, constituents of saffron, against subacute effect of diazinon on hematological and genotoxicity indices in rats. Phytomedicine. 2011;18:499–504. doi: 10.1016/j.phymed.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 72.Chang WC, Lin YL, Lee MJ, Shiow SJ, Wang CJ. Inhibitory effect of crocetin on benzo(a)pyrene genotoxicity and neoplastic transformation in C3HIOT1/2 cells. Anticancer Res. 1996;16:3603–3608. [PubMed] [Google Scholar]


