Abstract
Objective(s):
Despite treatment with antibiotics and vaccination with BCG, tuberculosis (TB) is still considered as one of the most important public health problems in the world. Therefore, designing and producing a more effective vaccine against TB seems urgently. In this study, immunogenicity of a fusion protein which consisting or comprising CFP-10 from Mycobacterium tuberculosis and the Fc-domain of mouse IgG2a was evaluated as a novel subunit vaccine candidate against TB.
Materials and Methods:
The genetic constructs were cloned in pPICZαA expression vector and recombinant vectors (pPICZαA-CFP-10: Fcγ2a and pPICZαA-CFP-10:His) were transformed into Pichia pastoris. To evaluate the expression of recombinant proteins, SDS-PAGE and immunoblotting were used. The immunogenicity of recombinant proteins, with and without BCG were assessed in BALB/c mice and specific cytokines against recombinant proteins (IFN-γ, IL-12, IL-4, IL-17 and TGF-β) were evaluated.
Results:
The levels of IFN-γ and IL-12 in mice that received recombinant proteins was higher than the control groups (BCG and PBS). Thus, both recombinant proteins (CFP-10:Fcγ2a and CFP-10:His) could excite good response in Th1-cells. The Fc-tagged protein had a stronger Th1 response with low levels of IL-4, as compared to CFP-10:His. However, the highest level of Th1 response was observed in groups that were vaccinated with BCG (prime) and then received recombinant protein CFP-10: Fcγ2a (booster).
Conclusion:
The results demonstrated that binding mice Fc-domain to CFP-10 protein can increase the immunogenicity of the subunit vaccine. Further studies, might be able to design and produce a new generation of subunit vaccines based on the Fc-fused immunogen.
Keywords: CFP-10, Fcγ2a, Immunogenicity, Mycobacterium – tuberculosis, Subunit vaccine
Introduction
Tuberculosis (TB) which is caused by Mycobacterium tuberculosis (Mtb) is one of the most important infectious diseases and one of the most common causes of death in the world, especially in developing countries. According to the World Health Organization (WHO), about 9 million people are infected annually, of which, 2 million lose their lives (1). Today, the attenuated strain of Mycobacterium bovis, called BCG, is used to induce immunity in many countries. The BCG is the only approved vaccine in humans. The effectiveness of this vaccine varies in different parts of the world and its efficacy have been reported to be from 0 to 80% in different studies (2). In addition, BCG vaccine produces immunity in children and has little effect in preventing pulmonary TB in adults. Therefore, there is an urgent need of developing novel vaccines and vaccination strategies to consistently protect adults against pulmonary TB. The vaccines, such as viral vector vaccines, DNA vaccines, subunit vaccines, attenuated Mtb and recombinant BCG are the most important new vaccines studied over the last twenty years (3).
Given that Mtb is a facultative intracellular bacteria, cell mediated immunity (CMI) and Th1-cells are the main parts of protective immunity in TB disease. These cells play an important role in controlling TB infection through the production of TNF-α, IFN-γ and IL-2. In addition, cytotoxic T-cells take part in the protective immunity through the production of IFN-γ and direct lysis of contaminated macrophages (4).
In recent years, subunit vaccines were considered due to the safety and easy production (5). These vaccines are designed mainly on the basis of secretory proteins and immunodominant antigens. Among these immunogenic antigens of Mtb, two secretory proteins: early secretory antigenic target-6 (ESAT-6) and culture filtrate protein-10 (CFP-10) are of paramount importance (6). These antigens, are respectively expressed by Rv3875 (esxA) and Rv3874 (esxB) genes. Both genes are encoded by difference-1 region (RD1) of the bacterial genome and play a key role in virulence. RD1 locus exists in pathogenic strains of Mtb and M. bovis, but has been deleted in the BCG vaccine strain. The results of several studies suggest that secretory proteins, ESAT-6 and CFP-10, play an important role in the pathogenesis of Mtb. Activation of T-cells by these antigens is an evidence of the suitability of this vaccine. Therefore, regarding the role of these proteins in stimulating the immune response and lack of them in BCG vaccine, it can be concluded that, ESAT-6 and CFP-10 antigens, are apropriate candidates for producing subunit vaccines (6-8).
In designing new subunit vaccines, in addition to selection of antigens stimulating the immune system, using a suitable system for vaccine presentation is also important. One of the most important limitations of these vaccines in stimulating a protective response against TB is lack of adequate presentation of vaccine antigens to T-lymphocytes. Thus, to obtain an appropriate immune response, multiple injection of booster doses of vaccine with suitble time interval must be applied (9). One of the most effective methods to overcome this limitation is using proteins binding to immunoglobulin (Ig) Fc domain (10). In this method, one or more antigens forming subunit vaccines bind to the Fc domain of antibodies. Subunit vaccines binding to Fc domain of antibodies increase the half-life, solubility, stability and ease in purification. Also, binding of immunogenic proteins to the Fc domain of antibodies facilitates its selective uptake by antigen-presenting cells (APCs) via FcγR and consequently enhances the efficiency of cross-presentation for inducing a potent Th1 immune response (10, 11). Macrophages and dendritic cells (DCs) have different types of receptors binding to Fc (FcγR), such as: FcγRI, FcγRIIA, FcγRIIB and FcγRIII, which are bind to the Fc-IgG domain (10-13). Several studies show that targeted presentation of antigens to FcγR receptors on the surface of APCs can increase the uptake and presentation of antigens by these cells for 50-500 times. In addition, selective uptake of foreign antigens by Fc receptors can cause presentation of antigen through cytoplasmic route and eventually MHC-I. This can stimulate CD8+ lymphocytes. Stimulation of cytotoxic T-lymphocytes is one of the effective factors in the removal of macrophages infected with Mtb (14). According to these studies, it can be proposed that binding CFP-10 to mouse IgG2a domain, increases the immunogenic potential of subunit vaccines. Therefore, CFP-10:Fcγ2a and CFP-10:His, were expressed in Pichia pastoris system, and then their immunogenicity was evaluated in mice model with spacific adjuvant and different BCG regimens.
Materials and Methods
Design and gene construction
In order to increase the expression of fusion protein CFP10:His and CFP10:Fcγ2, sequences encoding constructs were optimized based on P. pastoris. Then, optimized constructs were synthesized into pUC57 cloning vector. For the cloning process, restriction sites of enzymes (NotI and XhoI), and the sequence coding restriction site of KEX2 were located upstream of the gene constructs. The gene constructs were rescued from the pUC57 cloning vector by restriction digestion using XhoI and NotI (Thermo Scientific, USA) and transfered into the pPICZαA expression vector. Finally, recombinant vectors of pPICZα A-CFP10:Fcγ2a and pPICZα A-CFP10:His were obtained.
Recombinant vector of pPICZαA-CFP10:His contain c-myc in C-terminal. Moreover, a stop codon was placed at the end of Fc domain in the recombinant vector pPICZα A-CFP10:Fcγ2a to prevent the expression of His-tag. Recombinant vectors were transformed to Escherichia coli Top10F’, and then the transformed E. coli were cultured in Laurie Bertani agar containing 25 μg Zeocin Zeocin™. The recombinant vectors were purified using plasmid extraction kit. Finally, to ensure the accuracy of cloning, both recombinant vectors were fully sequenced over the sites at which the fused DNA fragments were cloned.
Transformation to P. pastoris and selection of transforms
Recombinant vectors were digested and linearized by SacI enzyme; and were then transformed into P. pastoris GS115 cells by the electroporation method. The transformed P. pastoris GS115 cells were selected from non-transformed cells by growth on YPD agar containing 100 µg Zeocin™ (InvivoGen, USA) (after 3 days of incubation at 28 °C). The colonies grown on YPD agar containing Zeocin™ were selected from the largest colonies for protein expression.
PCR colony and colony selection
To approve molecular transformation, right colonies with an integrated recombinant DNA of CFP10:Fc and CFP10:His were selected by PCR with α-factor and AOX1 primers (according to Easy select P. pastoris expression kit).
Expression at low levels and detection of recombinant protein
To identify the best colony expressing recombinant proteins, transformed yeast cells (confirmed by PCR) were cultured in a baffled flask containing 5 ml BMGY (28°C in shaker incubator) to reach the OD600 = 2 (about 16 hr). After reaching the desired OD, to stimulate the expression of recombinant proteins, yeast cells were isolated with centrifugation (5000 rpm for 5 min at 4 °C) and were cultured in BMMY to reach the OD600=1. Then, the flask containing BMMY were kept at 28 °C for 3 days (within shaker incubator with 300 rpm). In this period, 100% methanol was added to the each flask for maximal stimulation of the expression of recombinant proteins. The amount of methanol added in the final environment was 0.5%. Finally, after 3 days, the supernatant was separated by centrifu-gation and the expression amount of recombinant proteins were determined by enzyme-linked immunosorbent assay (ELISA). According to ELISA data, the best colonies expressing protein were determined.
Medium-scale expression of recombinant proteins
The best colonies selected by ELISA were cultured in 1l of BMGY (28 °C and shaker incubation at 300 rpm) to reach the OD600= 2. Then, yeast cells were obtained with centrifuge (5 min at 4 °C and 5000 rpm) and were re-cultured in 2 l BMMY until the OD600=1. Thereafter, baffled flasks were incubated at 300 rpm and 28 °C for 3 days. To increase the expression of recombinant proteins, pure methanol was added to the flasks at a final concentration of 0.5%, each 24 hr.
Purification of recombinant proteins
In order to purify of recombinant protein, the supernatant of the culture was collected with centri-fugation (10 min at 10,000 rpm) and recombinant proteins CFP10:Fcγ2 were purified by HiTrap rProtein A Sepharose Fast Flow column (GE Healthcare, USA). Briefly, the pH of supernatant was adjusted to 7 using 1 M sodium phosphate buffer and was filtered by a filter of 0.45 μm. After washing the column by binding buffer (20 mM sodium phosphate and pH=7), filtered supernatant was passed through HiTrap column at a flow rate of 3 ml/min. A new binding buffer was then utilized to wash the column again before eluting the column strip of the target proteins with an elution buffer (0.1 M sodium citrate, pH 4.6). The mentioned proteins were then collected in microtubes containing neutralization buffer (1M Tris-HCl, pH 9).
Labeled His-tag recombinant protein (CFP10:His) was purified by Ni-NTA garose column (QIAGEN, USA). After washing the column with distilled water, lysis buffer (50 mM potassium phosphate, pH=7.8, 400 mM NaCl, 100 mM KCL, 10% glycerol and 0.5% of Triton X100) was passed through Ni-NTA agarose (with the same volume of the column) to equilibrate the column. The filtered supernatant was passed through the column at a flow rate of 1 ml/min and the column was then washed with lysis buffer (containing 10 and 30 mM imidazole). Finally, for the isolation of recombinant proteins bound to the column, lysis buffer containing 500 mM imidazole was used. To concentrate and desalt the eluted fractions, a Vivaspin 20 ultrafiltration spin column (Sartorius Stedim, Germany) was used. Finally, evaluation of the eluted fractions (containing recombinant fusion proteins) was done by western blotting and using SDS-PAGE.
SDS-PAGE and Western blot
Expression of recombinant fusion proteins was analyzed using SDS-PAGE and confirmed by Western blotting. A 12% gel was used to visualize the recombinant protein via SDS-PAGE performance (15). Staining of the gels was performed with Coomassie Brilliant Blue G-250 following Bio-Rad Mini PROTEAN electrophoresis (Bio Rad, USA). For Western blotting, the proteins were separated by 12% gel and then the proteins were transferred onto polyvinylidene fluoride (PVDF) membrane. After electrotransfring, PVDF membranes were blocked by BSA 2% (overnight at 4 °C). For identification of Fc-tag recombinant protein (CFP10:Fcγ2a), incubation of membranes was done for probing with goat anti-mouse IgG-HRP antibody (Santa Cruz, USA) at a dilution of 1:10,000 for 1 hr at room temperature. Also, in order to identify CFP10:His fusion protein, PVDF membranes were incubated for an hr at room temperature with His-probe (H3) HRP antibody (Santa Cruz, USA) at a dilution of 1:5000. Finally, the specific recombinant proteins were detected by enhanced chemiluminescence detection system (ECL).
APC-targeting of CFP10:Fcγ2a recombinant protein
Using a direct immunofluorescence assay, confir-mation of Fc-fusion protein binding to Fcγ receptor (FcγRI) on APCs was done. Briefly, CFP10:Fcγ2a protein was added to the immunofluorescence slides, on which mouse macrophages had been fixed and then incubated at 37 °C for 80 min. After washing the cells with phosphate-buffered saline (PBS), they were incubated with fluorescent antibodies consisting of PE anti-mouse CD64 (FcγRI) (BioLegend, USA) and goat anti-mouse IgG2a-FITC (Santa Cruz, USA) in 3% (w/v) BSA in a humidified chamber for 2 hr. Ultimately, a fluorescence microscope (Nikon Eclipse E200, Japan) was employed to view the slides and obtain the images (16).
Preparation of adjuvant compound and subunit vaccine
To prepare appropriate concentrations of fusion proteins (50 µg of each protein), these proteins were diluted in sterile PBS. To prepare adjuvant compounds, DDA (Sigma-Aldrich, UK) and TDM (VacciGrade; IvivoGen, USA) were prepared according to the lipid films method. Then, one hr before injecting the vaccine, the recombinant proteins and DDA/TDB adjuvant were mixed together (DDA/TDB; 250/50 µg) (17).
Immunization
To evaluate the immunogenicity of recombinant proteins, female C57BL/6 mice 6 to 8 weeks of age were used. These mice were obtained from Pasteur Institute (Tehran, Iran) and kept under standard conditions in Mashahad Bu-Ali Research Institue. The mice were randomly divided into 6 groups (each group contained 5 mice). Group A: negative control mice that received 200 μl PBS; Group B: mice that received 5×105 CFU BCG; Group C: mice that received 50 µg of protein CFP10-Fcγ2a in 100 µl PBS plus 100 μl TDB-DDA adjuvant; Group D: mice that received 50 µg of CFP10-His protein in 100 µl PBS plus 100 μl TDB-DDA; Group E: mice that primed with BCG and stimulated by CFP10:Fcγ2a subunit vaccine (50 µg of Fc-tag fusion protein in 100 μl of PBS plus 100 μl of DDA/TDB adjuvant); and Group D: mice that primed with BCG and stimulated by CFP10:His subunit vaccine (50 µg of His-tag fusion protein in 100 μl of PBS plus 100 μl of DDA/TDB adjuvant). The mice were vaccinated subcutaneously 4 times at two weeks intervals. Groups that received BCG and PBS were vaccinated only one-time on the first day. Other groups were vaccinated four times on days 1, 14, 28 and 42. Prime/boost groups were vaccinated on first day with BCG plus each subunit vaccine and then boosted by each recombinant protein mixed with DDA/TDB on days 14, 28 and 42.
Evaluation of the cytokine production
Two weeks after the last vaccination, the mice were sacrificed and their spleen were removed aseptically, and a suspension of the splenocytes were prepared on a plate (each well containing 4×106 cell). These cells were cultured in 1 ml RPMI1640 (Biosera, UK) and then seeded for 72 hr at 37 °C in the presence of 5% CO2 with recombinant proteins CFP10: Fcγ2a (5 μg/ml) and CFP10: His (5 μg/ml). In this process, phyto-hemagglutinin (PHA, Gibco, USA) was used as a positive control (5 μg/ml). After incubation, culture supernatants were collected and the level of cytokines IL-12, IFN-γ, IL-4, TGF-β and IL-17 was determined by commercial ELISA kits (eBioscience, Austria) (according to manufacturer’s company).
Statistical analysis
The data were expressed as mean±standard deviation (SD) of the duplicate samples. Tukey’s multiple comparison tests of One-Way ANOVA were used to determine the statistical significance of the differences at P<0.05.
Results
Design and cloning expression cassettes
Recombinant expression cassettes were designed based on the diagram in Figure 1. After synthesis of expression constructs in plasmid pUC57, these gene constructs were subcloned in pPICZαA expression vector. Plasmid pPICZα A contains α-factor signal sequence (for secretion of recombinant proteins) and promotor gene of alcohol oxidase induced by methanol (AOX1) that promotes high levels of expression and secretion of recombinant proteins into the culture supernatant.
Figure 1.
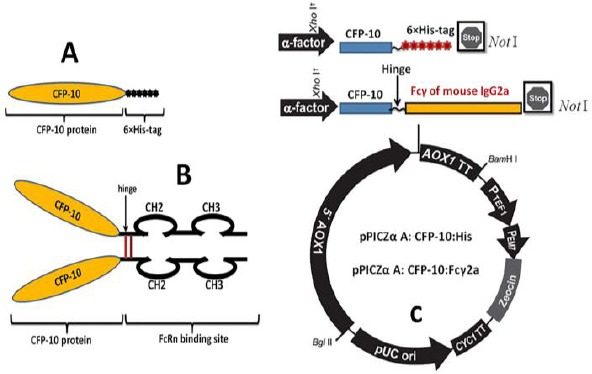
Schematic illustration of the protein constructs. A) Schematic illustration of the genetic fusion of Mycobacterium tuberculosis CFP-10 and 6× His-tag to create a CFP-10:His fusion protein. B) Schematic illustration of the genetic fusion of Mycobacterium tuberculosis CFP-10 and murine Fcγ2a to create a CFP-10:Fcγ2a fusion protein. C) Schematic map of the pPICZα-CFP-10:Fcγ2a and pPICZα-CFP-10:His. The insert was cloned into the XhoI and NotI restriction enzyme sites of pPICZαA vector downstream to the AOX1 promoter.5′ AOX1, alcohol oxidase 1 promoter;AOX1 TT, transcriptional terminator from Pichia pastoris AOX1 gene;TEF1 promoter, transcriptional elongation factor 1 promoter from Saccharomyces cerevisiae; EM7 promoter, synthetic prokaryotic promoter; Zeocin, Zeocin resistance gene; CYC1 TT, transcriptional terminator from Saccharomyces cerevisiae CYC1 gene; pUCori, pUC origin of replication
Transformation and selection of the transforms
After transformation by electroporation, the positive transformed colonies were selected by the YPDS medium containing 100 μg/ml Zeocin™. After isolation of genomic DNA from transformed colonies, entry of recombinant pPICZα A vectors into the genome of P. pastoris was confirmed by PCR with AOX1 and α-factor primers.
Evaluation of the recombinant proteins expression
After expression in low level, clones with the highest absorbance in ELISA were selected for expression at a high level. After high-level expression, recombinant proteins were purified from supernatants collected by columns of HiTrap rProtein A FF (for CFP-10: Fcγ2a) and Ni-NTA Agarose (for CFP-10: His).
For Fc-taged recombinant protein, analysis of purified fractions by SDS-PAGE and Western blot confirmed a 35 kDa protein band (Figures 2A and B). Western blot analysis and SDS-PAGE of fractions collected from the column of Ni-NTA agarose (CFP-10: His) confirmed a 13 kDa protein band (Figurs 2C and D). Based on the molecular weight of the amino acid sequences, CFP10-FCγ2α, CFP10-His proteins were calculated 13 and 35 kDa, respectively (ExPASy, PeptideMass).
Figure 2.
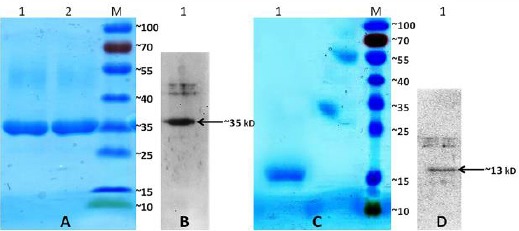
Purification and expression of the recombinant CFP-10:Fcγ2a and CFP-10:His fusion proteins. A) Analysis of the recombinant CFP-10:Fcγ2a protein by 12% SDS-PAGE stained with Coomassie Blue. Lane 1, 2: fractions of the eluted recombinant protein of approximately 35 kDa. Lane M: protein marker. B) The expression of the recombinant CFP-10:Fcγ2a protein was analyzed by western blot using anti mouse IgG-HRP. C) Analysis of the recombinant CFP-10:His protein by 12% SDS-PAGE stained with Coomassie Blue. Lane 1: fractions of the eluted recombinant protein of approximately 13 kDa. Lane M: protein marker. D) The expression of the recombinant CFP-10:His protein was analyzed by Western blot using anti His tag-HRP. Lane M: protein marker
APC-targeting of EF recombinant fusion protein
Direct immunofluorescence assay was employed to demonstrate Fc-taged recombinant protein (CFP10:Fcγ2a) binding to Fcγ receptor (FcγRI) on APCs. Therefore, it shows the binding of CFP10:Fcγ2a to the FcγRI on mouse macrophage cells (Figure 3).
Figure 3.
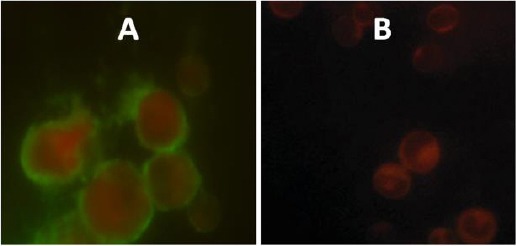
Co-localization of FcγRI (CD64) on macrophages and CFP-10:Fcγ2a recombinant fusion protein. Immunofluorescence staining of macrophages showing CFP-10:Fcγ2a recombinant fusion binds to FcγRI. A and B) Red signal, macrophages stained with PE anti-mouse CD64 (FcγRI) antibody; green signal, CFP-10:Fcγ2a recombinant fusion stained with goat anti-mouse IgG2a-FITC antibody. C) Immunofluorescence staining of macrophages with goat anti-mouse IgG2a-FITC, PE anti-mouse CD64 (FcγRI) without Fc-fusion protein as a negative control (red signal, macrophages stained with PE anti-mouse CD64 (FcγRI) antibody). CFP-10, 10 kDa Culture filtrate antigen; Fcγ2a, Fc fragment of mouse IgG2a; FcγRI, Fcγ receptor I; PE, phycoerythrin; FITC, fluorescein isothiocyanate
Evaluation of the immune response to recombinant proteins
After grouping, BALB/c mice were subcutaneously vaccinated with recombinant proteins CFP-10:Fcγ2a and CFP-10:His (mixed with adjuvant DDB/TDA), BCG and PBS. Prime/boost group received BCG and recombinant proteins at the same time. Then, to evaluate the immune response in the vaccinated mice (Th1, Th2 and Th17 responses), level of cytokines IFN-γ and IL-12 and IL-4 and IL-17 were assessed by ELISA.
IFN-γ and IL-12
The results showed significant IFN-γ in mice that received a dose of BCG (as Prime) and then three doses of the protein CFP10: Fcγ2a (as boost) as compared to the other groups (P<0.05) (Figure 4A). In addition, IFN-γ levels in groups that received CFP-10: Fcγ2a was significant as compared to the group that received BCG, CFP10: His and PBS (P<0.05) (Figure 4A). Furthermore, the mice that received a single dose of BCG (as prime) and three doses of the protein CFP10: Fcγ2a (as boost) had the highest levels of IL-12 (P<0.05) (Figure 4B). The level of IL-12 in groups that received recombinant protein CFP10: Fcγ2a was significant as compared to the rats vaccinated with CFP10: His BCG and PBS (P<0.05).
Figure 4.
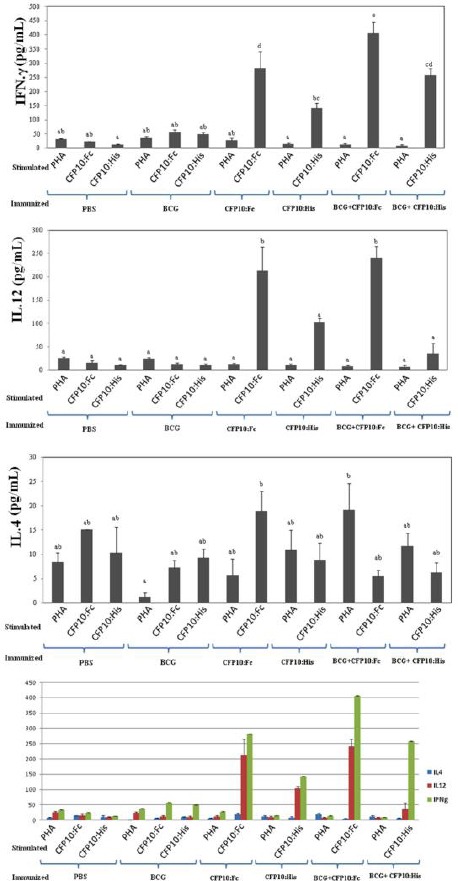
Cytokine releases from splenocytes of immunized mice. IFN-γ, IL-12, and IL-4 secretion in splenocytes was evaluated following stimulation with CFP-10:Fcγ2a, CFP-10:His, and PHA. Mice were immunized with CFP-10:Fcγ2a in DDA/TDM, and CFP-10:His in DDA/TDM, respectively. PBS and BCG were used as controls. Freshly isolated spleen cells were plated in duplicate at 5×106 cell per well in 24-well plate and incubated with CFP-10:Fcγ2a and CFP-10:His (10 μg/ml) and PHA (5μg/mL) for 72 h at 37 °C, 5 % CO2. IFN-γ, IL-12, and IL-4 were detected using mouse IFN-γ, IL-12, and IL-4 ELISA kits. A and B) CFP-10:Fcγ2a stimulated splenic cells from immunized mice with CFP-10:Fcγ2a had the highest numbers of IFN-γ and IL-12 secretion than CFP-10:His, BCG, or PBS immunized groups (P <0.05). C) Both CFP-10:Fcγ2a- and CFP-10:His immunized groups showed increased level of IL-4 production, as a marker of Th2, compare to BCG and PBS control groups (P<0.05). D) Differential IFN-γ, IL-12 and IL-4 production in the vaccinated mice showed all immunizes groups were pushed toward Th1 responses. *There is no significant difference between the groups with at least one shared letter
IL-4
The level of IL-4 production in mice groups showed no significant relationship between the groups. Although, the level of production of IL-4 was higher in mice that received one dose of BCG (as prime) and three doses of the recombinant protein as compared to other groups, it was statistically not significant (P>0.05) (Figure 4C). In addition, the level of IL-4 was higher in mice that received CFP-10: Fcγ2a as compared to the other groups (P>0.05) (Figure 4C). A comparison between IFN-γ and IL-4 productions was demonstrated that immunization with CFP-10: Fcγ2a could stimulate Th1 immune response (Figure 4D).
IL-17
The level of IL-17 had no significant differences between the all groups (P>0.05). The overall level of IL-17 in all the mice groups was less than 0.1 pg, which is lower than the limit of detection level (data not shown).
IFN-γ/IL-4
The ratio of IFN-γ/IL-4, which represent the level of Th1 and Th2 response, showed that the mice that received a single dose of BCG and three doses of recombinant had the highest ratio as compared to the other groups; IFN-γ/IL-4 ratio had a significant difference in this group as compared to the group that received CFP10: Fcγ2a and BCG (P>0.05). In addition, IFN-γ/IL-4 ratio in mice that received a single dose of BCG and three doses of the recombinant protein CFP10: Fcγ2a was higher than that of mice that received one dose of BCG and three doses of recombinant protein CFP10: His and the group that received CFP10: His, although the difference was not significant (P>0.05). The results showed the cytokine response in all groups tended towards Th1, although the severity of Th1 responses in the group that received one dose of BCG and three doses of the recombinant protein CFP10: Fcγ2a was higher than that of other groups (P>0.05).
Discussion
BCG has been used as the only existing vaccine to prevent TB for nearly a century. But due to the lack of efficacy of BCG vaccine in the prevention of pulmonary TB in adults, it seems that designing and producing a new and more effective vaccine is an urgent and important need for controlling TB (2, 3).
One of the most important strategies which used in the design of new vaccines against TB are subunit vaccines, which contains one or more immunodominant antigens of Mtb. Antigens constructing subunit vaccine is based on higher ability of antigens to stimulate protective immune responses (5). Moreover, in most cases, administration of subunit vaccines alone cannot induce a strong and long-lasting immune response. Thus, these vaccines need a suitable adjuvant and delivery system to efficiently get to the immune cells (5). Several studies have demonstrated that targeting of immunogenic protein to Fcγ receptors (on the surface of macrophages and dendritic cells) can potentiate its selectively uptake and increase CMI and antibody responses both in-vitro and in-vivo (11, 16, 18). In fact, previous studies have shown that fusion of Fc domain of antibodies with immunogenic proteins constructing subunit vaccines increased their immunogenicity (11, 16, 18). In this study, to increase the targeted presentation and immunogenicity of CFP-10 protein of Mtb, the protein was expressed as fusion with mice Fcγ2a in P. pastoris system and its immunogenicity was compared with the CFP-10:His fusion protein.
Studies on humans and animal models indicated that Th1-cells producing IFN-γ are the most important cellular immunity in stimulating a protective response against Mtb infection (19).
IFN-γ produced by Th1-lymphocytes, activates macrophages to remove and kill bacteria that are being reproduced (20). Thus, the number of T-cells producing IFN-γ, as well as the amount of this cytokine, determines the effectiveness of the vaccine against TB. In addition, IL-12, produced by macrophages and dendritic cells, play a major role in the differentiation of naive T-cells to Th1-cells (21). On the other hand, recent studies indicated that IL-17 produced by Th17-lymphocytes plays an important role in granuloma maturation and attracts neutrophils to the infection site by stimulating the production of chemokines (22). Contrary to Th1 and Th17 lymphocytes, Th2 lymphocytes suppress Th1 responses through the production of IL-4 and hence can contribute to the spread of TB (23). Thus, in appropriate microenvironment and conditions at the infection site (high levels of IL-12), intact T-cells differentiate into Th1 and cause a protective response against M. tuberculosis by the production of IFN-γ, while in the presence of cytokines IL-1β, the intact T-cells differentiate into Th2 and cause a protective response against extracellular bacteria by the production of IL-4, IL-13 and IL-15. An important factor that determines the type of cytokines present in the infection site or vaccination, is the type of antigen (24, 25).
In designing a subunit vaccine against TB, selection of immunodominant antigens that can induce protective immune response seems important. Among antigens of Mtb, CFP-10 is the most important secretory protein that have been widely used in the design of new vaccines against TB because it has epitopes that stimulate Th1 response (26); as some studies have demonstrated that vaccinations with a DNA vaccine and a recombinant adenovirus expressing CFP-10 can cause a protective immune response in animal models (27).
As a result of the numerous disadvantages of bacterial expression system (such as small production, containing low CG and insolubility of expression proteins), in this study, recombinant proteins (CFP-10: Fcγ2a and CFP10: His) were expressed in the P. pastoris expression system. In addition, the mice Fc should have been glycolized for proper functioning which is only done in eukaryotic expression systems (28-30).
The results of this study showed significantly higher levels of IFN-γ and IL-12 in mice immunized with CFP-10: Fcγ2a as compared to other groups (Figure 4A and B). Moreover, high levels of cytokines IFN-γ and IL-12 were produced in mice immunized with recombinant protein CFP-10: His (Figure 4A and B). However, the level of these cytokines were less when compared with the group that received recombinant Fc-taged fusion protein.
These results confirmed that administration of both forms of recombinant proteins stimulate a protective immune response with involvement of Th1-cells. However, the level of immune response was higher in mice that received Fc fused protein.
Moreover, cellular immune response (with involvement of Th1 lymphocytes) in mice vaccinated with CFP-10: FCγ2a was associated with a positive feedback between IL-12 and INF-γ that shows the higher effect of this protein on dendritic cells for producing IL-12.
In this study, high levels of IL-4 in mice vaccinated with CFP-10: Fcγ2a confirmed the results of other studies (Figure 4C). These studies showed that the protective Th1 response is always associated with a low level of Th2 response (23, 31). It seems that a low level of Th2 response can prevent the immunopathologic effects of a protective cellular immunity and balance immune system of the host after removing the infection (31).
In the present study, levels of IL-17 was low in all vaccinated groups and no significant difference was observed between the groups. The role of Th-17 in response to vaccines studied in animal models has been controvertially reported. One study showed no hypersensitivity effect of low levels of IL-17 against TB, while another study concluded that the level of IL-17 cause granuloma formation or expression of chemokines that ultimately increases the sensitivity to TB (22, 32).
An important strategy to increase the immuno-logic memory of BCG vaccine is the Prime/boost regimen. In the present study, mice were vaccinated with BCG (prime) and then, the subunit vaccines were injected to enhance the effect of BCG (boost). Several studies have shown that this method strengthens the immunologic memory of BCG (33, 34). In this context, the results of this study showed that mice that received the BCG (prime) and were then vaccinated with recombinant protein CFP-10: Fcγ2a (boost) had the highest level of protective Th1 response (IL-12 and IFN- γ) among the mice groups (Figure 4). This indicates that the CFP-10: Fcγ2a recombinant protein can be used to enhance the effect of BCG.
According to the obtained results, CFP-10:FCγ2a recombinant protein, as compared to protein CFP-10:His, stimulate higher levels of cellular immunity response. Previous studies have shown that targeting the antigens to Fc receptors (FCγRs) on the surface of APCs not only increased their uptake and presentation to T-lymphocytes, but also changed the processing and presentation type (11, 35, 36). Therefore, it seems that using Fc fusion protein can simultaneously stimulate the T-lymphocytes CD4+ and CD8+. This issue is very important in creating a protective immune response against intracellular bacteria (10, 11, 16, 18).
Hemodymeric structure of Fc fusion proteins, which are formed by disulfide bonds to hinge region, increases the immunogenicity capacity of antigens attached to it. In fact, immunization of the Fc domain increases the capacity and size of recombinant proteins and decrease renal excretion. This feature increases the half-life of subunit vaccine proteins and thus extend their half-life (37). Some studies have shown that fusion of some immunogenic proteins of some viruses, such as Influenza A, HIV and ebola to Fc domain stimulate a protective immune response against the viral infection (35, 36, 38).
In addition, the results of this study confirmed the effect of Fc fusion proteins in stimulating immune response. Thus, it seems that further research on Fc fusion proteins can design and produce a new generation of subunit vaccines against TB.
Potential ability of Fc fusion protein to Fc inhibitory receptors (FcγRIIB) which causes a tolerogenic response is the limitation of this delivery system although the results of the co-localization process showed that the Fc fusion protein (CFP-10:Fcγ2a) binds to receptor activating FcγRI (CD64) (Figure 3).
Conclusion
Based on the results of this study, recombinant proteins CFP-10: Fcγ2a stimulates higher levels of cellular immune response as compared to protein CFP-10:His. This indicates that the power of the Fc domain can be used as a selective delivery antigen-presenting system in designing new subunit vaccines against TB to induce a Th1 response and cross-presentation. In addition, the Fc fusion protein can be used to enhance the immune response of BCG vaccine. Therefore, it seems that proteins CFP-10:Fcγ2a can be used as a subunit vaccine against tuberculosis. According to the Th1 response in the mouse model of immunization in the present study and the fact that mice are not appropriate hosts for Mtb challenge, a more suitable animal model, such as guinea pigs or rabbit, is needed to assess the protective immune response of this Fc fusion protein against TB which due to the lack of the necessary conditions and facilities for animal challenge studies in our country is a limitation of the present study.
Acknowledgment
The authors are grateful to Reza Nasr for his valuable advice and help for designing the constructs, and Mastoureh Momen Heravi, Maliheh Moghadam, Medical School, Mashhad university of Medical Sciences, for valuable help. The results described in this paper were part of student thesis. This study was a part of a PhD dissertation by Dr Ali Asghar Baghani and has been supported by Vice Chancellor for Research, Mashhad University of Medical Sciences, Mashhad, Iran. This paper was financially supported by grants from the Mashhad University of Medical Sciences (grant number: 910069).
Conflict of interest
The authors declare no conflict of interest associated with the present manuscript.
References
- 1.World Health Organization. Global tuberculosis report 2015 (WHO) 2015. Available at: www.who.int/about/licensing/copyright_form/en/index.html .
- 2.Nuttall JJ, Davies MA, Hussey GD, Eley BS. Bacillus calmette-guerin (BCG) vaccine-induced complications in children treated with highly active antiretroviral therapy. Int J Infect Dis. 2008;12:e99–e105. doi: 10.1016/j.ijid.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Logan KE, Chambers MA, Hewinson RG, Hogarth PJ. Frequency of IFN-gamma producing cells correlates with adjuvant enhancement of Bacille Calmette-Guerin induced protection against Mycobacterium bovis. Vaccine. 2005;23:5526–5532. doi: 10.1016/j.vaccine.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 4.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 5.Liljeqvist S, Ståhl S. Production of recombinant subunit vaccines: protein immunogens, live delivery systems and nucleic acid vaccines. J Biotechnol. 1999;73:1–33. doi: 10.1016/s0168-1656(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 6.Guo S, Xue R, Li Y, Wang SM, Ren L, Xu JJ. The CFP10/ESAT6 complex of Mycobacterium tuberculosis may function as a regulator of macrophage cell death at different stages of tuberculosis infection. Med Hypotheses. 2012;78:389–392. doi: 10.1016/j.mehy.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Grotzke JE, Lewinsohn DM, et al. RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;51:359–370. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganguly N1, Siddiqui I, Sharma P. Role of M. tuberculosis RD-1 region encoded secretory proteins in protective response and virulence. Tuberculosis. 2008;88:510–517. doi: 10.1016/j.tube.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Ottenhoff TH, Kaufmann SH. Vaccines against Tuberculosis: where are we and where do we need to go?PLoS Pathog. 2012;8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soleimanpour S, Hassannia T, Motiee M, Amini AA, Rezaee SA. Fcy1 fragment of IgG1 as a powerful affinity tag in recombinant Fc-fusion proteins: immunological, biochemical and therapeutic properties. Crit Rev Biotechnol. 2016;6:1–22. doi: 10.3109/07388551.2016.1163323. [DOI] [PubMed] [Google Scholar]
- 11.Soleimanpour S, Farsiani F, Mosavat A, Ghazvini K, Eydgahi MR, Sankian M, et al. APC targeting enhances immunigenicity of a novel multistage Fc-fusion tuberculosis vaccine in mice. Appl Microbiol Biotechnol. 2015;99:10467–10480. doi: 10.1007/s00253-015-6952-z. [DOI] [PubMed] [Google Scholar]
- 12.Adamova E, Walsh MC, Gosselin DR, Hale K, Preissler MT, Graziano RF, et al. Enhanced antigen-specific antibody and cytokine responses when targeting antigen to human Fc-gamma receptor type I using an anti-human Fc-gamma receptor type I-streptavidin fusion protein in an adjuvant-free system. Immunol Invest. 2005;34:417–429. doi: 10.1080/08820130500265372. [DOI] [PubMed] [Google Scholar]
- 13.Keler T, Guyre PM, Vitale LA, Sundarapandiyan K, van De Winkel JG, Deo YM, et al. Targeting weak antigens to CD64 elicits potent humoral responses in human CD64 transgenic mice. J Immunol. 2000;165:6738–6742. doi: 10.4049/jimmunol.165.12.6738. [DOI] [PubMed] [Google Scholar]
- 14.den Haan JM, Lehar SM, Bevan MJ. CD8 (+) but not CD8 (-) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Mosavat A, Soleimanpour S, Farsiani H, Sadeghian H, Ghazvini K, Sankian M, et al. Fused Mycobacterium tuberculosis multi-stage immunogens with an Fc-delivery system as a promising approach for the development of a tuberculosis vaccine. Infect Genet Evol. 2016;39:163–172. doi: 10.1016/j.meegid.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Davidsen J, Rosenkrands I, Christensen D, Vangala A, Kirby D, Perrie Y, et al. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. Biochim Biophys Acta. 2005;1718:22–31. doi: 10.1016/j.bbamem.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Farsiani H, Mosavat A, Soleimanpour S, Sadeghian H, Akbari Eydgahi MR, Ghazvini K, et al. Fc-based delivery system enhances immunogenicity of a tuberculosis subunit vaccine candidate consisting of the ESAT-6: CFP-10 complex. Mol Biosyst. 2016;12:2189–2201. doi: 10.1039/c6mb00174b. [DOI] [PubMed] [Google Scholar]
- 19.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbst S, Schaible UE, Schneider BE. Interferon gamma activated macrophages kill mycobacteria by nitric oxide induced apoptosis. PLoS One. 2011;6:e19105. doi: 10.1371/journal.pone.0019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Méndez-Samperio P. Role of interleukin-12 family cytokines in the cellular response to mycobacterial disease. Int J Infect Dis. 2010;14:e366–371. doi: 10.1016/j.ijid.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21:455–462. doi: 10.1016/j.cytogfr.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, et al. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–517. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Ansel KM, Lee DU, Rao A. An epigenetic view of helper T cell differentiation. Nat Immunol. 2003;4:616–623. doi: 10.1038/ni0703-616. [DOI] [PubMed] [Google Scholar]
- 25.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helpercell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, Woodworth JS, Shin DS, Morris S, Behar SM. Vaccine-elicited 10-kilodalton culture filtrate protein-specific CD8+T cells are sufficient to mediate protection against Mycobacterium tuberculosis infection. Infect Immun. 2008;76:2249–2255. doi: 10.1128/IAI.00024-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Deng G, Li M, Zeng J, Zhao L, Liu X, et al. A recombinant adenovirus expressing CFP10, ESAT6, Ag85A and Ag85B of Mycobacterium tuberculosis elicits strong antigen-specific immune responses in mice. Mol Immunol. 2014;62:86–95. doi: 10.1016/j.molimm.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Braren I, Greunke K, Umland O, Deckers S, Bredehorst R, Spillner E. Comparative expression of different antibody formats in mammalian cells and Pichia pastoris. Biotechnol Appl Biochem. 2007;47:205–214. doi: 10.1042/BA20060170. [DOI] [PubMed] [Google Scholar]
- 29.Guo Y, Kang W, Zhong Y, Li R, Li G, Shen Y, et al. Purification and characterization of human IL-10/Fc fusion protein expressed in Pichia pastoris. Protein Expr Purif. 2012;83:152–156. doi: 10.1016/j.pep.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Li P, Anumanthan A, Gao XG, Ilangovan K, Suzara VV, Duzgunes N, et al. Expression of recombinant proteins in Pichia pastoris. Appl Biochem Biotechnol. 2007;142:105–124. doi: 10.1007/s12010-007-0003-x. [DOI] [PubMed] [Google Scholar]
- 31.Reljic R, Paul MJ, Arias MA. Cytokine therapy of tuberculosis at the crossroads. Expert Rev Respir Med. 2009;3:53–66. doi: 10.1586/17476348.3.1.53. [DOI] [PubMed] [Google Scholar]
- 32.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gamma delta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 33.Dalmia N, Ramsay AJ. Prime–boost approaches to tuberculosis vaccine development. Expert Rev Vaccines. 2012;11:1221–1233. doi: 10.1586/erv.12.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Husain AA, Warke SR, Kalorey DR, Daginawala HF, Taori GM, Kashyap RS. Comparative evaluation of booster efficacies of BCG, Ag85B, and Ag85B peptides based vaccines to boost BCG induced immunity in BALB/c mice: a pilot study. Clin Exp Vaccine Res. 2015;4:83–87. doi: 10.7774/cevr.2015.4.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konduru K, Bradfute SB, Jacques J, Manangeeswaran M, Nakamura S, Morshed S, et al. Ebola virus glycoprotein Fc fusion protein confers protection against lethal challenge in vaccinated mice. Vaccine. 2011;29:2968–2977. doi: 10.1016/j.vaccine.2011.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu L, Palaniyandi S, Zeng R, Bai Y, Liu X, Wang Y, et al. A neonatal Fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J Virol. 2011;85:10542–10553. doi: 10.1128/JVI.05441-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mekhaiel DN, Czajkowsky DM, Andersen JT, Shi J, El-Faham M, Doenhoff M, et al. Polymeric human Fc-fusion proteins with modified effector functions. Sci Rep. 2011;1:124. doi: 10.1038/srep00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loureiro S, Ren J, Phapugrangkul P, Colaco CA, Bailey CR, Shelton H, et al. Adjuvant free immunization with hemagglutinin-Fc fusion proteins as an approach to influenza vaccines. J Virol. 2010;85:3010–3014. doi: 10.1128/JVI.01241-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


