Abstract
Objective(s):
The effects of Curcuma longa (C. longa) and curcumin on total and differential WBC count and oxidant, antioxidant biomarkers, in rat model of asthma were evaluated.
Materials and Methods:
Total and differential WBC count in the blood, NO2, NO3, MDA, SOD, CAT and thiol levels in serum were examined in control, asthma, Asthmatic rats treated with C. longa (0.75, 1.50, and 3.00 mg/ml), curcumin (0.15, 0.30, and 0.60 mg/ml), and dexamethasone (1.25 μg/ml) rats.
Results:
Total and most differential WBC count, NO2, NO3 and MDA were increased but lymphocytes, SOD, CAT and thiol were decreased in asthmatic animals compared to controls (P<0.001). Total WBC, NO2 and NO3 were significantly reduced in treated groups with dexamethasone and all concentrations of C. longa and curcumin compared to asthmatic group (P<0.001 for all cases). MDA was significantly decreased, but SOD, CAT and thiol increased in treated asthma animals with dexamethasone and two higher concentrations of C. longa and curcumin (P<0.01 to P<0.001). There were significant improvement in eosinophil percentage due to treatment of highest concentration of the extract and curcumin, neutrophil and monocyte due to highest concentration of curcumin and lymphocyte due to highest concentration of the extract and two higher concentrations of curcumin compared to asthmatic group (P<0.01 to P<0.001). Dexamethasone treatment improved monocyte (P<0.001) and lymphocyte (P<0.01) percentages.
Conclusion:
Antioxidant and anti-inflammatory effects of C. longa extract and its constituent curcumin in animal model of asthma was observed which suggest a therapeutic potential for the plant and its constituent on asthma.
Keywords: Curcuma longa, Curcumin, Inflammation, Oxidative stress, Rat model of asthma, WBC
Introduction
Asthma is a chronic disease and is one of the major global health problems (1) which is characterized by inflammation of the airways, variable and recurring airflow obstruction, the airway walls swelling, mucus plug formation, and bronchial hyper responsiveness to physical and pharmacological stimuli (2).
Different inflammatory cells, including eosinophils, lymphocytes, mast cells, and neutrophils involved in airway inflammation of asthma (3).
Airway inflammation is the most prime cause of the recurrent episodes of airflow restriction in asthma and is related with incremented oxidative stress (4). Increased oxidative stress (oxidant antioxidant imbalance) of the airways also contribute in chronic airway inflammation of asthma (5) which is accompanied with increased oxidant activity in the lung and blood (6). The lung is the main exposable organ to environmental oxidants such as air pollutants and cigarette smoke as well as endogenous oxidant produced by mitochondrial electron transport during respiration released from phagocytes (7). There are strong evidences that inflammatory cells from peripheral blood and bronchoalveolar lavage (BAL) fluid of asthmatic subjects generate more superoxide anion radicals than those from healthy controls (8). Measurement of exhaled air in asthmatic patients showed enhanced levels of nitro tyrosine and other markers of oxidative stress (9). The normal lung contains high levels of antioxidant resources to prevent oxidant-induced lung injury. This is supported by the studies demonstrating an oxidant–antioxidant imbalance in asthmatic airways, reduced total antioxidant capacity and individual antioxidants in plasma and BAL fluid of asthmatic subjects (10). Studies indicated that the use of antioxidants may be effective in the treatment of asthma by restoring the oxidant-antioxidant balance (11).
In traditional medicines, plant-based drugs as well as natural antioxidant have been used for either treatment or prevention of lung disease. Curcuma longa (C. longa), or turmeric belonging to Zingiberaceae family is a perennial herb with pointed leaves and bears funnel-shaped yellow flowers which grows to a height of three to five feet (12), and is cultivated extensively in Asia mostly in India, China and other countries with a tropical climate. C. longa has also mentioned in Ayurvedic medicine for its anti-asthmatic and anti-dyspnea effects. Different pharmacological effects such as anti-asthmatic (13), antioxidant (14) and anti-inflammatory (15) effects for this plant have been reported.
Vogel (1842), primarily isolated curcumin, (a bright yellow spice, extracted from the rhizome of C. longa), it was structurally characterized by Milobedeska and colleagues (1910) and synthesized and confirmed by Lampe and colleagues in 1913 (16, 17). Several studies have reported considerable biological properties for curcumin including; anti-inflammatory (18), anti-microbial (19), and hepatoprotective (20). Curcumin has been demonstrated to inhibit nitric oxide and reactive oxygen species (ROS) production in macrophage (21) and to enhance the activity of many antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) (22) and heme oxygenase-1 (OH-1) (23).
In the present study, the effect of the hydro-ethanolic extract of C. longa compared to the effect of its constituent, curcumin on total and differential white blood cell (WBC) count as an indicator of inflammation, and the levels of nitric oxide (NO), malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD) and thiol, in serum of rat model of asthma were investigated.
Materials and Methods
Plant, extract and constituent
C. longa rhizomes were purchased from a local herbal shop in Mashhad, Razavi Khorasan Province, Iran and identified by botanists in the herbarium of Ferdowsi University of Mashhad.
The rhizomes (100 g) was cleaned, grounded, weighed, and homogenized in 96% ethanol at a ratio of 1:10 of plant to ethanol and left to soak for 3 days at 37 °C with occasional shaking and stirring. The mixture was then filtered and the resulting liquid was concentrated under reduced pressure at 45 °C in an Eyela (Heidolph, Germany) rotary evaporator to yield a dark gummy-yellow extract (24). The yield extract was 14%.
Experimental animals and induction of animal model of asthma
The study was performed in sixty nine male Wistar rats weighing approximately 200–250 g in animal house, School of Medicine, Mashhad University of Medical Sciences, Iran. The animals were kept in a 22±2 °C temperature with a 12 hr light/dark cycle and fed with standard diet and tap drinking water ad libitum.
For induction of animal model of asthma, the rats were sensitized by three IP injections of 1 mg/kg chicken egg albumin (Ovalbumin=OVA, grade V, 98% pure; Sigma, St. Louis, MO, USA) in 0.9% sterile saline containing 100 mg Al(OH)3 as adjuvant on days 1, 2 and 3 of the experiment. The animals were then exposed to an aerosolized of 1% OVA concentration for 20 min/day, on days 6, 9, 12, 15, 18 and 21 (25). A solution of 1% OVA in normal saline was aerosolized by delivery of compressed air to a jet nebulizer (26). Exposures were carried out in a whole body inhalation exposure chamber dimensioned 30×20×20 cm. The rat in the control group received IP and inhaled normal saline instead of OVA using the same procedure as OVA administration in sensitized animals.
The study was carried out in the following groups:
A) Control or saline treated (group C).
B) Animal model of asthma or OVA sensitized (group A).
C) Asthma animals treated with the extract of C. longa (0.75, 1.5, 3 mg/ml), (groups CL 0.75, CL 1.50, and CL 3.00).
D) Asthma animals treated with curcumin (0.15, 0.3, 0.6 mg/ml), (groups Cu 0.15, Cu 0.30, and Cu 0.60).
E) Asthma animals treated with dexamethasone (1.25 μg/ml), (group D).
The extract of C. longa, curcumin and dexame-thasone were prescribed in animals’ drinking water during sensitization period. The number of animals for each curcumin treated group was 7 and for other groups, it was 8. Averagely 40 ml drinking water was used by each rat/day and there wasn’t significant difference in the drinking water used by the animals in different groups.
Total and differential WBC count
At the end of the experiment period, rats were anesthetized by IP injection of ketamine (50 mg/kg). Blood samples (4 ml) were taken by cardiac puncture immediately after anesthetizing and exposing the animals’ chest. Two ml of blood sample was collected into the test tube containing anticoagulant EDTA for counting total and differential WBC. The other part of sample was centrifuged at 3500 rpm for 10 min. Supernatants were collected and stored at -80 °C for measurement of MDA, NO, SOD, CAT levels.
The blood sample was stained with Turk solution (1:10 dilution) and total WBC counted in duplicate in a hemocytometer. Differential cell counts were done on a thin slide prepared with a smearing blood sample, using Wright-Giemsa’s stain. According to staining and morphological criteria, differential cell analysis was carried out under a light microscope by counting 100 cells, and the percentage of each cell type was calculated.
Measurement of oxidant biomarkers
Using a Griess reagent, total stable oxidation products of NO metabolism (NO2-/NO3-) were measured. The Griess reagent contained of sulfanilamide (SULF) and N-(1-Naphthyl) ethylene diamine dihydrochloride (NEDD). The frozen serum was allowed to thaw and to reach a temperature of 25°C followed by being deproteinized by zinc sulfate solution (Sigma, America). Serum was then centrifuged at 10000 g for 10 min. One hundred twenty microlitre of the clear supernatant was mixed with Griess reagents including 50 μl SULF (2% w/v, Sigma, America) in 5% HCl and 50 μl NEDD (0.1% w/v, Sigma, America) in H2O in a test tube and incubated at controlled room temperature for 10 min and the absorbance of samples was then measured at 540 nm for measuring NO2. For the reduction of nitrate to nitrite, 50 μl saturated solutions of vanadium (III) chloride (VCl3; Sigma, America) in 1 M HCl was added and incubated for 2 hr at 30°C in the dark. The absorbance of samples was then measured at 540 nm against a blank containing the same concentrations of ingredients without biological sample. To determine NO2 and NO3 concentrations linear regression were used from standard curve of NaNO2 and the results were expressed as μM (27).
Malondialdehyde (MDA) levels, as an index of lipid peroxidation reacts with thiobarbituric acid (TBA) as a thiobarbituric acid reactive substance (TBARS) which produce a red coloured complex with peak absorbance at 535 nm. Two ml reagent of TBA/trichloroacetic acid (TCA)/HCl was added to 1 ml of serum and the solution was heated in a water bath for 40 min and then cooled. The whole solutions were centrifuged at 1,000×g for 10 min and the absorbance was measured at 535 nm (28). The MDA concentration (C) in nM was calculated as; C= Absorbance/(1.56×105),
Measurement of antioxidant biomarkers
Superoxide dismutase (SOD) activity was measured as previously described (29) by a colorimetric assay. This assay involved generation of superoxide by pyrogallol auto-oxidation and the inhibition of superoxide-dependent reduction of the tetrazolium dye, MTT (3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazolium bromide) to its formazan by SOD and was measured at 570 nm. The level of SOD was expressed as unit (U)/ml. The amount of enzyme causing 50% inhibition in the MTT reduction rate was defined as one unit of SOD activity.
Catalase (CAT) activity was also estimated using the previously described method (30) based on determination of the rate constant, k, (dimension: s-1, k) of hydrogen peroxide decomposition. The constant rate of the enzyme was determined using measurement of the decrease in absorbance at 240 nm per min. Activities were expressed as k (constant rate) per litter and expressed as unit (U)/ml (31).
Total thiol concentration was measured using DTNB as the reagent which reacts with the thiols to produce a yellow coloured complex with a peak absorbance at 412 nm. One ml Trisethylene diamine tetraacetic acid (EDTA) buffer (pH 8.6) was added to 50 μl serum in 1 ml cuvettes and sample absorbance was read at 412 nm against Tris-EDTA buffer alone (A1). Twenty microliter DTNB reagents (10 mmol in methanol) were then added to the mixture and keep it in laboratory temperature for 15 min and the sample absorbance was read again (A2). The absorbance of DTNB reagent was also read as a blank (B). Total thiol concentration (mmol) was calculated using the following equation (32); Total thiol concentration (mmol)= (A2–A1–B)×1.07/0.05×13.6.
Statistical analysis
The statistical analysis was performed using InStat (GraphPad Software, Inc, La Jolla, USA). All data were presented as mean±SEM. The comparisons between the results of treated groups, un-treated asthma group and control group were performed using one way analysis of variance (ANOVA) with Tukey-Kramer’s post-test. The data of three concentrations of extract and curcumin were also compared using ANOVA with Tukey-Kramer’s post-test. Significance was considered at P<0.05.
Results
The effect of the extract of C. longa and its constituent, curcumin on total and differential WBC count
Total WBC count, in the blood of asthma group was significantly higher than those of the control group (P<0.001, Figure 1a). Significant and concentration-dependent decrease in total WBC count was seen in asthmatic animals treated with all concentrations of the extract and curcumin compared to untreated asthma group (P<0.001 for all cases), (Figure 1a). Dexamethasone treatment also significantly reduced total WBC count compared to asthma group (P<0.001, Figure 1a). Reduction of total WBC count in treated groups with all concentrations of curcumin were significantly greater compared to dexamethasone treated group (P<0.05 to P<0.001), (Figure 1a).
Figure 1.
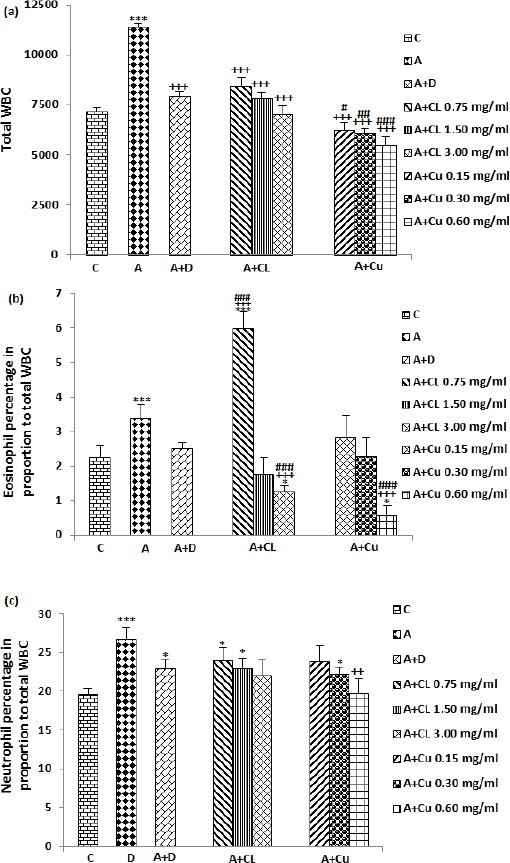
The effect of C. longa and its constituent, curcumin on total WBC number (count/ml of blood), (a), percentage of eosinophil (b) and neutrophil (c) in control animals (C), asthma (A) group, A treated with dexamethasone (D), C. longa (CL) (for each group, n=8) and curcumin (Cu, n=7). Data are presented as mean±SEM values. *P<0.05, ***P<0.001; compared to group C. ++ P<0.01, +++ P<0.001 compared to group A. # P<0.05, ## P<0.01, ### P<0.001 compared to group D. Statistical analyses were performed using one way analysis of variance (ANOVA) with Tukey-Kramer’s post-test
The percentages of eosinophil, neutrophil and monocyte in the blood of asthma group were significantly greater but the percentage of lymphocyte was less than those of the control group (P<0.001 for all cases), (Figures 1 and 2). The percentage of eosinophil was significantly reduced in treated asthma groups with highest concentration of the extract (3 mg/ml) and curcumin (0.6 mg/ml), (P<0.001 for both cases) in comparison with untreated asthma group (Figure 1b). Dexamethasone treatment did not significantly reduced eosinophil percentage compared to untreated asthma group. The effects of highest concentrations of the extract and curcumin on reduction of eosinophil percentage were significantly higher than dexamethasone treated group (P<0.001 for both cases), (Figure 1b). The percentage of eosinophil in asthma group treated with low concentration of the extract was significantly higher than control group (P<0.001), (Figure 1b).
Figure 2.
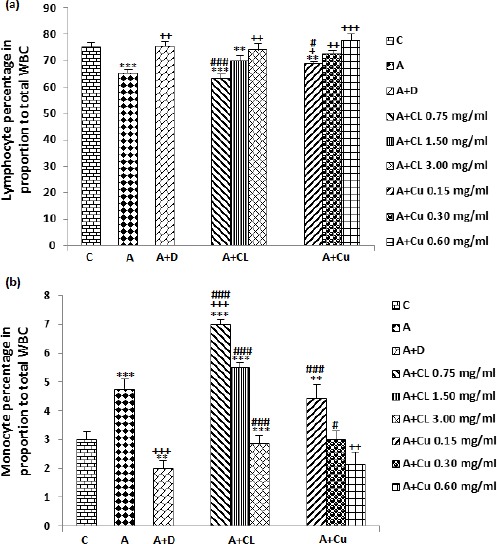
The effect of C. longa and its constituent, curcumin on percentage of lymphocyte (a) and monocyte (b) in control animals (C), asthma (A) group, A treated with dexamethasone (D), C. longa (CL), (for each group, n=8) and curcumin (Cu, n=7). Data are presented as mean±SEM values. *P<0.05, **P<0.01, ***P<0.001; compared to group C. +P<0.05, ++P<0.01, +++P<0.001 compared to group A. # P<0.05, ## P<0.01, ### P<0.001 compared to group D. Statistical analyses were performed using ANOVA with Tukey-Kramer’s posthoc test
The percentage of neutrophil in treated group with only highest concentration of curcumin was significantly reduced compared to untreated asthma group (P<0.01), (Figure 1c). However, the percentage of neutrophil were significantly higher in A treated groups with dexamethasone, two lower concentrations of the extract and medium concentration of curcumin compared to the control group (P<0.05 for all cases), (Figure 1c).
Compared to asthma group, the percentage of lymphocyte in dexamethasone, highest concentration of the extract (P<0.01) and all three concentrations of curcumin (P<0.05 to P<0.001) were significantly increased (Figure 2a). The change in percentage of lymphocyte due to treatment of low concentration of the extract and curcumin was significantly lower compared to the effect of dexamethasone (P<0.001 and P<0.05 receptively), (Figure 2a). The percentage of lymphocyte in asthma groups treated with two lower concentrations of the extract and low concentration of curcumin were higher (P<0.01 to P<0.001) compared to control group (Figure 2a).
Significant decreases were seen in the percentage of monocyte in treated asthma groups with highest concentration of curcumin (P<0.01) and dexamethasone (P<0.001), (Figure 2b). The percentage of monocyte were significantly higher in treated groups with all concentrations of the extract (P<0.001 for all cases) and two lower concentrations of curcumin (P<0.001 for low and P<0.05 for medium concentration) compared to dexamethasone treated group (Figure 2b). In asthma groups treated with two higher concentrations of the extract (P<0.001) and low concentration of curcumin (P<0.01), the percentage of monocyte was significantly higher compared to control group (Figure 2b).
The effects of high concentration of the extract (3.00 mg/ml) on total WBC count, eosinophil, lymphocyte and monocyte as well as the effect of its medium concentration (1.50 mg/ml) on eosinophil and monocyte were significantly higher than its low concentration (0.75 mg/ml, P<0.01 to P<0.001). The effect of highest concentration of curcumin (0.60 mg/ml) on eosinophil, lymphocyte and monocyte percentage were significantly higher than its lower concentration (0.15 mg/ml, P<0.05 to 0.01), (Table 1).
Table 1.
Comparisons of total and differential WBC count in asthma (A) groups treated with three concentrations of Curcuma longa (CL, 0.75, 1.50, and 3.00 mg/ml) and curcumin (Cu, 0.15, 0.30, and 0.60 mg/ml)
| Substances | Total WBC (Cunt/ml) | Eosinophil (Percentage) | Neutrophil (Percentage) | Lymphocyte (Percentage) | Monocyte (Percentage) |
|---|---|---|---|---|---|
| A+CL 0.75 | 8400.00±452.37 | 6.00±0.46 | 24.00±1.61 | 63.00±1.82 | 7.00±0.38 |
| A+CL 1. 50 | 7806.25±303.17 | 1.75±0.49 | 23.00±1.30 | 69.75±2.08 | 5.50±0.19 |
| +++ | +++ | ||||
| A+CL 3.00 | 7025.00±412.64 | 1.26±0.16 | 22.00±2.03 | 73.87±2.47 | 2.87±0.29 |
| + | +++ | ++ | +++ | ||
| A+Cu 0.15 | 6207.14±415.68 | 2.85±0.63 | 23.85±2.08 | 68.87±0.80 | 4.43±0.48 |
| ** | *** | *** | |||
| A+Cu 0.30 | 6042.86±263.32 | 2.28±0.56 | 22.29±0.80 | 72.43±1.46 | 3.00±0.31 |
| * | *** | ||||
| A+Cu 0.60 | 5471.43±426.20 | 0.57±0.30 | 19.71±1.90 | 77.57±2.58 | 2.15±0.40 |
| + | ++ | *** ++ |
Data were presented as mean±SEM
Comparison of CL vs Cu:* P<0.05, **P<0.01, *** P<0.001 using unpaired t test
Comparison of CL 3.00 and CL 1.50 vs CL 0.75:+ P<0.05, ++ P<0.01, +++ P<0.001 using ANOVA test
Comparison of Cu 0.60 and Cu 0.30 vs Cu 0.15:+ P<0.05, ++ P<0.01, +++ P<0.001 using ANOVA test
Comparison of CL 3.00 vs CL 1.50:# P<0.05, ## P<0.01, ### P<0.001 using ANOVA test
Comparison of Cu 0.60 vs Cu 0.30:#P<0.05, ## P<0.01, ### P<0.001 using ANOVA test
The effects of all concentrations of curcumin on monocyte, its two lower concentrations on total WBC and its lowest concentration on eosinophil were significantly higher than those of the extract concentrations (P<0.05 to P<0.001), (Table 1).
Effect of the extract of C. longa and its constituent, curcumin on oxidant biomarkers
NO2, NO3 and MDA levels were significantly increased in asthma animals compared to control group (P<0.001 for all cases), (Figure 3). NO2 level was significant and concentration-dependently decreased in treated asthma groups with dexamethasone, three concentrations of the extract and curcumin compared to asthma group (P<0.001 for all cases), (Figure 3a). The effect of treatment with highest concentration of curcumin on NO2 level was significantly higher compared to dexamethasone treatment (P<0.01, Figure 3a). In treated groups with dexamethasone and two lower concentrations of extract NO2 level was significantly higher compare to control group (P<0.05 to P<0.001), (Figure 3a).
Figure 3.
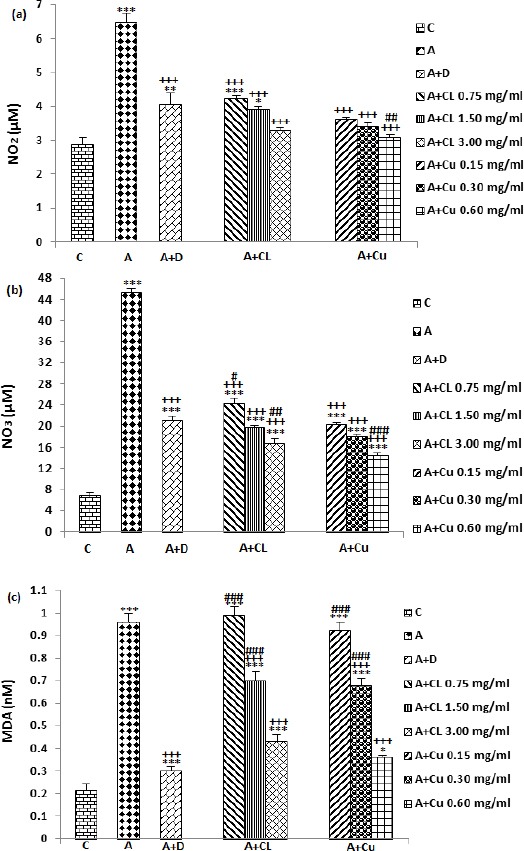
The effect of Curcuma longa and its constituent, curcumin on NO2 (a), NO3 (b) and MDA (C) concentration in control (C), and asthma (A) groups, A treated with dexamethasone (D), C. longa (CL) (for each group, n=8) and curcumin (Cu, n=7). Data are mean±SEM values. *P<0.05, ** P<0.01*** P<0.001; compared to group C. +++ P<0.001 compared to group A. # P<0.05, ## P<0.01, ### P<0.001 compared to group D. Statistical analyses were performed using ANOVA with Tukey-Kramer’s post-hoc test
The treatment of asthma animals with dexamethasone and all concentrations of the extract and curcumin caused significant reduction in NO3 level compared to untreated asthma group (P<0.001 for all cases). Compared with dexamethasone treated group, NO3 level was significantly lower in treated group with low concentration of the extract but was higher in treated groups with two higher concentrations of the extract and all concentrations of curcumin (P<0.05 to P<0.001), (Figure 3b).
MDA level in treated groups with dexamethasone, two higher concentrations of the extract and curcumin was significantly decreased compared to untreated asthma group (P<0.001 for all cases). The effect of two lower concentrations of extract and curcumin on MDA level was significantly lower compared to dexamethasone treated group (P<0.001 for all cases). MDA level in treated groups with dexamethasone, all concentrations of the extract and curcumin were significantly higher compared with control group (P<0.001 for all cases), (Figure 3c).
The effect two higher concentrations of extract (1.50, 3.00 mg/ml) on NO2, NO3 and MDA level were significantly higher than its low concentration (0.75 mg/ml), (P<0.05 to P<0.001). In addition, the effect of high concentration of extract (3.00 mg/ml) on NO2, NO3 and MDA level was higher than its medium concentration (1.50 mg/ml), (P<0.001). The effect of two higher concentrations of curcumin (0.30. 0.60 mg/ml) on NO3 and MDA level and its high concentration on NO2 level were significantly higher than its low concentration (0.15 mg/ml), (P<0.05 to P<0.001). A significant difference was seen between high and medium concentrations of curcumin (0.30, 0.60 mg/ml) on NO3 and MDA levels (P<0.01). In addition, The effect of low concentration of the extract on NO2 and NO3 levels and its medium concentration on NO2 level was statistically lower compared to corresponding concentrations of extract (P<0.01 to P<0.001), (Table 2).
Table 2.
Comparisons of various oxidant and antioxidant variables in in asthma (A) groups treated with three concentrations of Curcuma longa (CL, 0.75, 1.50, and 3.00 mg/ml) and curcumin (Cu, 0.15, 0.30, and 0.60 mg/ml)
| Substances | NO2 (µM) | NO3 (µM) | MDA (nM) | SOD (U/ml) | CAT (U/ml) | Thiol (µM) |
|---|---|---|---|---|---|---|
| A+CL 0.75 | 4.22±0.09 | 24.24±0.99 | 0.99±0.04 | 0.01±0.002 | 0.01±0.002 | 0.05±0.01 |
| A+CL 1. 50 | 3.90±0.09 | 19.77±0.42 | 0.70±0.04 | 0.03±0.002 | 0.03±0.003 | 0.09±0.00 |
| + | ++ | +++ | +++ | +++ | + | |
| A+CL 3.00 | 3.30±0.08 | 16.73±0.88 | 0.43±0.03 | 0.06±0.001 | 0.08±0.001 | 0.20±0.01 |
| +++ ### | +++ # | +++ ### | +++ ### | +++ ### | +++ ### | |
| A+Cu 0.15 | 3.60±0.08 | 20.25±0.42 | 0.92±0.04 | 0.03±0.003 | 0.02±0.002 | 0.07±0.00 |
| *** | ** | ** | ||||
| A+Cu 0.30 | 3.41±0.10 | 17.99±0.50 | 0.68±0.03 | 0.04±0.002 | 0.04±0.002 | 0.12±0.01 |
| ** | + | +++ | ++ | +++ | + | |
| A+Cu 0.60 | 3.07±0.10 | 14.34±0.49 | 0.36±0.01 | 0.07±0.002 | 0.09±0.002 | 0.25±0.01 |
| ++ | +++ ### | +++ ### | *** +++ ### | +++ ### | * +++ ### |
Data were presented as mean±SEM
Comparison of CL vs Cu: *P<0.05, ** P<0.01, *** P<0.001 using unpaired t test
Comparison of CL 3.00 and CL 1.50 vs CL 0.75:+ P<0.05, ++ P<0.01, +++ P<0.001 using ANOVA test
Comparison of Cu 0.60 and Cu 0.30 vs Cu 0.15: + P<0.05, ++P<0.01, +++ P<0.001 using ANOVA test
Comparison of CL 3.00 vs CL 1.50: # P<0.05, ##P<0.01, ### P<0.001 using ANOVA test
Comparison of Cu 0.60 vs Cu 0.30: # P<0.05, ## P<0.01, ### P<0.001 using ANOVA test
Effect of the extract of C. longa and its constituent, curcumin on antioxidant biomarkers
Compared with control group, SOD, CAT and thiol levels were significantly decreased in asthma group (P<0.001 for all cases), (Figure 4). SOD level significantly and concentration-dependently increased in asthma groups treated with all concentrations of curcumin and the extract compared to untreated asthma group (P<0.001 for all cases). Dexamethasone treatment also significantly enhanced SOD level compared to asthma group (P<0.05 to P<0.001), (Figure 4a). Although the effects of treatment with two lower concentrations of curcumin and dexamethasone on SOD level were lower, the effect of treatment with highest concentration of the extract and curcumin on these markers was significantly higher compared to the effect of dexamethasone treatment (P<0.001 for all cases), (Figure 4a). In addition, SOD level was significantly lower in treated groups with dexamethasone, all concentrations of the extract and curcumin compared to control group (P<0.001 for all cases), (Figure 4a).
Figure 4.
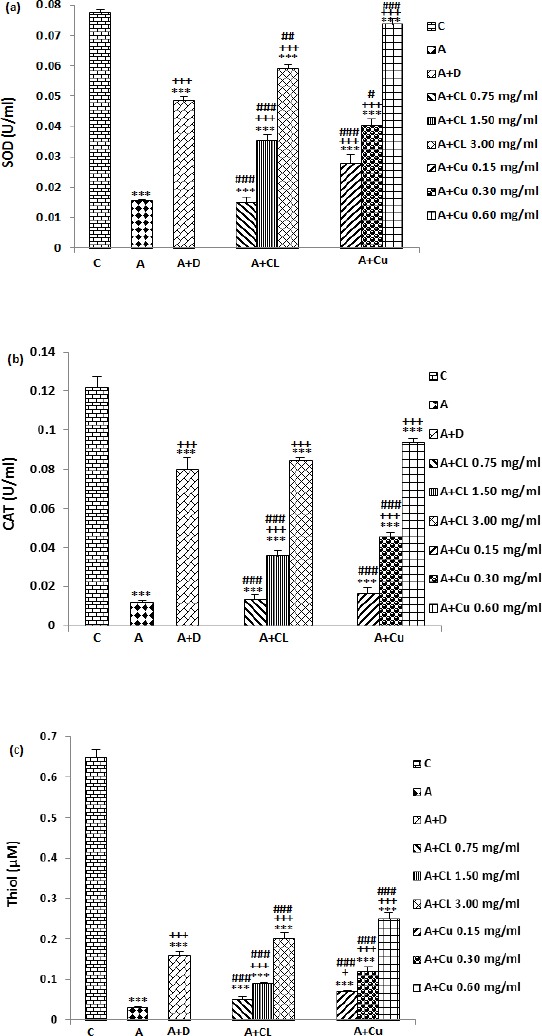
The effect of Curcuma longa and its constituent, curcumin on serum levels of SOD (a), CAT (b) and Thiol (c) in control (C) and asthma groups (A), A treated with dexamethasone (D), C. longa (CL), (for each group, n=8), and curcumin (Cu, n=7). Data are mean ± SEM values. ***P<0.001; compared to group C. + P<0.05, +++P<0.001 compared to group A, #P<0.05, ## P<0.01, ### P<0.001 compared to group D. Statistical analyses were performed using ANOVA with Tukey-Kramer’s post-test
Treatment with dexamethasone, two higher concentrations of the extract and curcumin caused significant increase in CAT level compared to untreated asthma group (P<0.001 for all cases), (Figure 3b). The effect of treatment with two lower concentrations of the extract and curcumin on CAT level was significantly less than dexamethasone treatment (P<0.001 for all cases), (Figure 4b). However, CAT level in treated groups with dexamethasone, all concentrations of the extract and curcumin was significantly lower than control group (P<0.001 for all cases), (Figure 4b).
A concentration-dependent and significant increase of thiol level was observed in treated groups with two higher concentrations of the extract, all concentrations of curcumin and dexamethasone compared to untreated asthma group (P<0.05 for low concentration of curcumin and P<0.001 for other cases), (Figure 4c). Thiol level in treated groups with two lower concentrations of extract and curcumin was significantly lower (P<0.001 for all cases), but, in treated groups with highest concentration of the extract and curcumin was higher (P<0.001 for both cases) compared to dexamethasone treated group (Figure 4c). The concentration of total thiol in asthma groups, treated with dexamethasone, all concentrations of the extract and curcumin was significantly lower compared to control group (P<0.001 for all cases), (Figure 4c).
The effect of two higher concentrations of extract and curcumin on SOD, CAT and thiol levels were significantly higher than their low concentration (P<0.05 to P<0.001). In addition, the effect of high concentration of the extract and curcumin on SOD, CAT and thiol levels were higher than its medium concentration (P<0.001 for all cases). The effects of high and low concentrations of curcumin on thiol and SOD levels were significantly higher than corresponding concentrations of the extract (P<0.01 for low and P<0.001 for high concentration), (Table 2).
Discussion
C. longa a medicinal plant native to the Indian subcontinent is known to possess anti-inflammatory and antioxidant properties. In the present study, the effect of the hydro-ethanolic extract of C. longa and its constituent, curcumin on total and differential WBC count, and levels of oxidant (NO, MDA) and antioxidant (CAT, SOD and thiol) biomarkers in serum of a rat model of asthma were investigated.
Oxidative stress occurs as a result of the production of ROS in consequence of aerobic metabolism. Inflammatory cells such as activated eosinophils, neutrophils, monocytes, and macrophages can generate ROS in response to various stimuli (33). Previous evidences indicating increased oxidative stress is a major feature of many airway diseases, such as ischemia-reperfusion, cystic fibrosis, chronic obstructive pulmonary disease, and asthma (34). Studies indicated that oxidative stress is an important consequence of lung inflammation in asthma which contributes in induction of airway hyper responsiveness (9), and correlate to antioxidant capacity in the lung and blood (7).
The results of the present study showed increased total WBC, eosinophil, neutrophil and monocyte, but reduction of lymphocyte count in asthma compared to control animal. The findings of the present study indicate the induction of an asthma model (sensitization) in rat. Increased total WBC and eosinophil count was shown in asthmatics, both in animal and human studies compared to healthy controls (35, 36). The reduction of lymphocyte percentage in sensitized rat is in fact due to increased total WBC number. In fact, increased absolute number of the lymphocyte was shown in sensitized animals using similar method of sensitization (37). Increased lymphocyte percentage in treated groups is also due to reduction of total WBC count. Treatment with the extract, curcumin and dexamethasone reduced total WBC, eosinophil, neutrophil and monocyte but increased lymphocyte count. In fact, a previous study showed the effect of the extract of C. longa on total and differential WBC count in asthmatic rat, which support the results of the present study (13). Reduction of eosinophil, neutrophil and monocyte in asthma group treated with C. longa extract and curcumin observed in the present study, suggested the anti-inflammatory effect of the plant. Therefore, this plant may have preventive effect on asthma by reduction of inflammatory cells and airway inflammation. The absent of the effect of the treatment of low concentration of the extract on eosinophil may indicate that this concentration of the extract is not effective on this cell type. However, treated with two higher concentrations resulted in reduction of eosinophil count which showed the effect of the plant on eosinophilic inflammation in asthma.
Findings showed that NO2 and NO3, the oxidative products of NO significantly increased in asthma group which also suggest the induction of animal model of asthma in rat. Recent studies has demonstrated that NO is present in exhaled air and that its level is increased in asthma and chronic obstructive pulmonary disease (38). NO is produced via oxidation of L-arginine by nitric oxide synthases enzyme. In the lung, NO acts as a vasodilator, bronchodilator and non-adrenergic non-cholinergic (NANC) neurotransmitter and is an important mediator which may reinforce the inflammatory response in asthma (39). NO is a by-product of airway inflammation and tissue damage and a probable index of oxidative stress in the airway diseases (40). NO has an inhibitory effect on the Th1 and interferon gamma (IFN-γ). This would lead to the increase in the number of Th2 cells and its cytokines, interleukin 4 (IL-4) and IL-5, which augment the inflammatory response (41). NO in air and in fluids of the respiratory tract could cause to a variety of more reactive and thus more toxic nitrogen oxides (42).
Treatment with dexamethasone as positive control, all concentrations of the extract and curcumin significantly decreased NO level. Previous study showed that curcumin treatment normalized the elevated nitrate in asthma rat (13). These results indicated the antioxidant and anti-inflammatory property of the plant and the mechanism of this effect may be mediated through inhibition of nitric oxide synthases enzyme.
The levels of MDA as an oxidant marker of lipid peroxidation significantly increased in asthmatic group which also support the sensitization of animals. A positive correlation was reported between ROS and MDA level (43). Oxidative stress is associated with an increase MDA and protein carbonyls in both BAL fluid and peripheral blood in asthma (4). In patients with different airway diseases including asthma, COPD, and bronchiectasis increased MDA levels in biological fluids was observed (44). MDA was significantly higher in asthmatic patients, and its level was higher during the acute attack of asthma (45).
MDA level was significantly reduced in treated groups with two higher concentrations of extract and curcumin. Therefore, treatment with the extract and curcumin can improve bronchial asthma during an acute asthma attack by reduction of oxidative biomarkers. Decreased MDA level was found in asthmatic animals after treatment with curcumin previously, which supports the findings of the present study (13).
The serum SOD level also decreased in asthma group in the present study. The main antioxidants enzymes in the lungs are SOD, CAT, and glutathione peroxidases as well as heme oxygenase-1, thio-redoxins, peroxiredoxins, and glutaredoxins (46). Since superoxide radical is the primary oxidant generated from a variety of sources, its dismutation by SOD. H2O2 that results from the action of SOD is reduced to water by catalase and the GSH-Pxs. SOD activity has been reported to be decreased (10), increased (47) or unchanged (48) in asthmatics as compared to controls.
In the present study, the SOD activity increased in asthma groups treated with two higher concentrations of the extract and all concentrations of curcumin in a dose-dependent manner. Increased SOD activity was seen in human hepatocyte L02 cell line after treatment with curcumin (49), which confirms the findings of the present study. The loss of SOD activity probably reflects the increased oxidative stress in asthma. Thus, this plant which enhanced SOD activity could be effective in improvement of asthma symptom.
In the present study, the CAT activity significantly reduced in untreated asthma group. CAT is a metalloprotein enzyme and the main scavenger of H2O2. Both animal and human studies have indicated that CAT activity in BAL fluid of asthmatics reduced compared to healthy controls (50). Decrease in CAT activity probably amplifies oxidative stress, contributing to the chronic inflammatory state of the asthmatic airway (51). Two higher concentrations of extract and curcumin reversed CAT activity in treated groups. Other study have shown that curcumin increased CAT activity and the hepatic total antioxidant capacity in liver from rats with hepatic damage and high level of oxidative stress induced by thallium acetate (52). These results showed that C. longa and its constituent, curcumin with antioxidant property are able to inhibit inflammatory responses in asthma.
Results of this study showed that thiol concentration significantly decreased in asthmatic rat which also confirm the induction of animal model of asthma. Thiols are potent antioxidants which protect cells against the oxidative stress. The most thiol antioxidant in the epithelial lining fluid is the tripeptide glutathione (GSH), which is a multifunc-tional intracellular antioxidant and is noticed to be the main thiol-disulphide redox buffer of the cell (53). The antioxidant capacity of thiol compounds is related to the sulphur atom, which can easily accommodate the loss of a single electron (54). GSH is also vital for airway innate immune defense and regulation alveolar macrophage apoptosis and phagocytosis of infectious particles (55). GSH depletion further leads to activation of NF-κB and enhanced pro-inflammatory gene transcription (56).
Treatment with various concentrations of curcumin and extract significantly increased thiol level. The reduced level of glutathione (GSH) in a lung carcinogenesis model induced by benzo (a) pyrene (a major carcinogenic pollutant) in mice was increased by curcumin (57). These findings also indicated antioxidant property of the plant and its constituent curcumin in asthma.
The effects of the extract and its constituent curcumin were concentration dependent. Eosinophil, lymphocyte and monocyte improvement due to high concentration of the extract and curcumin, total WBC due to high curcumin concentration and eosinophil and monocyte due to medium curcumin concentration were significantly higher than their low concentration. The effects of two higher concentrations of the extract and curcumin in almost all oxidant and antioxidant markers were significantly higher than their low concentration. The effects of the highest concentration of the extract and curcumin on most oxidant and antioxidant markers were also higher than the effects of their medium concentration. The concentration dependency effect of the extract and its constituent also support the anti-inflammatory and antioxidant effect of the extract of C. longa and its constituent, curcumin on rat animal model of asthma.
The results of current study also showed that the effects of all concentrations of curcumin on monocyte percentage, the effects of its two lower concentrations on total WBC and the effect of its low concentration on eosinophil percentage were significantly higher than the effects of corresponding concentrations of the extract. In addition, the effect of low concentration of curcumin on NO2, NO3 and SOD, the effect of its medium concentration on NO2 and the effect of its high concentration on SOD and thiol values were higher than the effects corresponding extract concentrations. Therefore the effects of curcumin on total and differential WBC as well as oxidant and antioxidant markers were higher than the extract of the plant at studied concentrations. The curcumin concentrations used in the present study were one fifth of those of the extract of C. longa. However, the curcumin concentration in the pure powder is 31.9% (58), which is higher than the concentrations used in the present study. Therefore, the effect of the extract of the plant on total and differential WBC as well as on oxidant and antioxidant markers on animal model of asthma is suggested to be due to its constituent, curcumin.
The effect of the two lower concentrations of the extract and curcumin on most measured variables were lower than the effect of dexamethasone treatment or there was no significant difference between the effect of dexamethasone and the extract and curcumin treatment except for the effect of the first concentration of curcumin on total WBC count which was significantly higher than the effect of dexamethasone. However, the effects of highest concentration of the extract and curcumin were not significantly different or even higher (on most variables) than the effect of dexamethasone treatment except for the effect of highest concentration of the extract on monocyte percentage. These results also show comparable or even higher anti-inflammatory and antioxidant effect of the highest concentration of the extract of C. longa and its constituent, curcumin on rat animal model of asthma compared to the effect of dexamethasone.
Conclusion
The results of the present study indicated a preventive effect of C. longa and its constituent curcumin on total and differential WBC, serum levels of NO2, NO3, MDA, CAT and thiol group of animal model of asthma which is comparable to the effect of dexamethasone at used concentrations. Therefore, the results suggest a preventive therapeutic potential for C. longa and its constituent, curcumin on inflammatory cells and oxidative stress in asthma.
Acknowledgment
This study was financially supported by a grant from Research Council of Mashhad University of Medical Sciences. The results described in this paper were part of a PhD student thesis of Farzaneh Shakeri.
References
- 1.Control CfD Prevention. Chronic diseases: the leading causes of death and disability in the United States. 2015;1:16. http://www.cdc.gov/chronicdisease/overview/index htm accessed . [Google Scholar]
- 2.Chung K. Role of inflammation in the hyperreactivity of the airways in asthma. Thorax. 1986;41:657–662. doi: 10.1136/thx.41.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacoste JY, Bousquet J, Chanez P, Van Vyve T, Simony-Lafontaine J, Lequeu N, et al. Eosinophilic and neutrophilic inflammation in asthma, chronic bronchitis, and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 1993;92:537–548. doi: 10.1016/0091-6749(93)90078-t. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad A, Shameem M, Husain Q. Relation of oxidant-antioxidant imbalance with disease progression in patients with asthma -Ann Thorac Med. 2012;7:226–232. doi: 10.4103/1817-1737.102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caramori G, Papi A. Oxidants and asthma. Thorax. 2004;59:170–173. doi: 10.1136/thorax.2002.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacNee W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:50–60. doi: 10.1513/pats.200411-056SF. [DOI] [PubMed] [Google Scholar]
- 7.Nadeem A, Raj HG, Chhabra SK. Increased oxidative stress in acute exacerbations of asthma. J Asthma. 2005;42:45–50. doi: 10.1081/jas-200044774. [DOI] [PubMed] [Google Scholar]
- 8.Cluzel M, Damon M, Chanez P, Bousquet J, De Paulet AC, Michel FB, et al. Enhanced alveolar cell luminol-dependent chemiluminescence in asthma. J Allergy Clin Immunol. 1987;80:195–201. doi: 10.1016/0091-6749(87)90129-1. [DOI] [PubMed] [Google Scholar]
- 9.Saleh D, Ernst P, Lim S, Barnes PJ, Giaid A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. FASEB J. 1998;12:929–37. [PubMed] [Google Scholar]
- 10.Comhair SA, Bhathena PR, Dweik RA, Kavuru M, Erzurum SC. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. The Lancet. 2000;355:624. doi: 10.1016/S0140-6736(99)04736-4. [DOI] [PubMed] [Google Scholar]
- 11.Nadeem A, Masood A, Siddiqui N. Review: Oxidant antioxidant imbalance in asthma: scientific evidence, epidemiological data and possible therapeutic options. Ther Adv Respir Dis. 2008;2:215–35. doi: 10.1177/1753465808094971. [DOI] [PubMed] [Google Scholar]
- 12.Dobelis IN. Magic and medicine of plants. Pleasantville, NY: Reader’s Digest Association; 1986. p. 702. [Google Scholar]
- 13.Sarkar S, Zaidi S, Chaturvedi AK, Srivastava R, Dwivedi PK, Shukla R, et al. Search for a Herbal Medicine: Anti-asthmatic Activity of Methanolic Extract of Curcuma longa. J Pharmacogn Phytochem. 2015;3:59–72. [Google Scholar]
- 14.Selvam R, Subramanian L, Gayathri R, Angayarkanni N. The anti-oxidant activity of turmeric (Curcuma longa) J Ethnopharmacol. 1995;47:59–67. doi: 10.1016/0378-8741(95)01250-h. [DOI] [PubMed] [Google Scholar]
- 15.Anna KT, Suhana ME, Das S, Faizah O, Hamzaini A. Anti-inflammatory effect of Curcuma longa (turmeric) on collagen-induced arthritis: an anatomico-radiological study. Clin Ter. 2011;162:201–207. [PubMed] [Google Scholar]
- 16.Lampe V, Milobedzka J. Studien über curcumin. Berichte der deutschen chemischen Gesellschaft. 1913;46:2235–2240. [Google Scholar]
- 17.Milobedzka J, Kostanecki S, Lampe V. Curcumin. Chem Ber. 1910;43:2163–2170. [Google Scholar]
- 18.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) J Altern Complement Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 19.Han S, Yang Y. Antimicrobial activity of wool fabric treated with curcumin. Dyes and pigments. 2005;64:157–161. [Google Scholar]
- 20.Park EJ, Jeon CH, Ko G, Kim J, Sohn DH. Protective effect of curcumin in rat liver injury induced by carbon tetrachloride. J Pharm Pharmacol. 2000;52:437–440. doi: 10.1211/0022357001774048. [DOI] [PubMed] [Google Scholar]
- 21.Rao M. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 22.Reddy ACP, Lokesh B. Effect of dietary turmeric (Curcuma longa) on iron-induced lipid peroxidation in the rat liver. Food Chem Toxicol. 1994;32:279–283. doi: 10.1016/0278-6915(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 23.Jeong GS, Oh GS, Pae HO, Jeong SO, Kim YC, Shin MK, et al. Comparative effects of curcuminoids on endothelial heme oxygenase-1 expression: ortho-methoxy groups are essential to enhance heme oxygenase activity and protection. Exp Mol Med. 2006;38:393–400. doi: 10.1038/emm.2006.46. [DOI] [PubMed] [Google Scholar]
- 24.Salama SM, Abdulla MA, AlRashdi AS, Ismail S, Alkiyumi SS, Golbabapour S. Hepatoprotective effect of ethanolic extract of Curcuma longa on thioacetamide induced liver cirrhosis in rats. BMC Complement Altern Med. 2013;13:1–17. doi: 10.1186/1472-6882-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salmon M, Walsh DA, Huang TJ, Barnes PJ, Leonard TB, Hay DW, et al. Involvement of cysteinyl leukotrienes in airway smooth muscle cell DNA synthesis after repeated allergen exposure in sensitized Brown Norway rats. Br J Pharmacol. 1999;127:1151–1158. doi: 10.1038/sj.bjp.0702669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babayigit A, Olmez D, Karaman O, Ozogul C, Yilmaz O, Kivcak B, et al. Effects of Ginkgo biloba on airway histology in a mouse model of chronic asthma. Allergy Asthma Proc. 2009;30:186–191. doi: 10.2500/aap.2009.30.3187. [DOI] [PubMed] [Google Scholar]
- 27.Yousefniapasha Y, Jorsaraei G, Gholinezhadchari M, Mahjoub S, Hajiahmadi M, Farsi M. Nitric oxide levels and total antioxidant capacity in the seminal plasma of infertile smoking men. Cell J. 2015;17:129–136. doi: 10.22074/cellj.2015.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 29.Madesh M, Balasubramanian K. Microtiter plate assay for superoxide dismutase using MTT reduction by superoxide. Indian J Biochem Biophys. 1998;35:184–188. [PubMed] [Google Scholar]
- 30.Aebi H. (13) Catalase in vitro. Meth Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 31.Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 32.Hosseinzadeh H, Sadeghnia HR. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharm Sci. 2005;8:394–399. [PubMed] [Google Scholar]
- 33.Barnes PJ. Reactive oxygen species and airway inflammation. Free Radic Biol Med. 1990;9:235–243. doi: 10.1016/0891-5849(90)90034-g. [DOI] [PubMed] [Google Scholar]
- 34.Bowler RP, Crapo JD. Oxidative stress in airways: is there a role for extracellular superoxide dismutase? American journal of respiratory and critical care medicine. Am J Respir Crit Care Med. 2002;166:38–43. doi: 10.1164/rccm.2206014. [DOI] [PubMed] [Google Scholar]
- 35.Boskabady MH, Ghasemzadeh Rahbardar M, Nemati H, Esmaeilzadeh M. Inhibitory effect of Crocus sativus (saffron) on histamine (H1) receptors of guinea pigtracheal chains. Pharmazie. 2010;65:300–305. [PubMed] [Google Scholar]
- 36.Luksza A, Jones D. Comparison of whole-blood eosinophil counts in extrinsic asthmatics with acute and chronic asthma. Br Med J (Clin Res Ed) 1982;285:1229–1231. doi: 10.1136/bmj.285.6350.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keyhanmanesh R, Boskabady MH, Eslamizadeh MJ, Khamneh S, Ebrahimi MA. The effect of thymoquinone, themain constituent of Nigella sativa ontracheal responsiveness and white blood cell count in lung lavage of sensitized guineapigs. Planta Med. 2010;76:218–222. doi: 10.1055/s-0029-1186054. [DOI] [PubMed] [Google Scholar]
- 38.Kanazawa H, Shoji S, Yoshikawa T, Hirata K, Yoshikawa J. Increased production of endogenous nitric oxide in patients with bronchial asthma and chronic obstructive pulmonary. Clin Exp Allergy. 1998;28:1244–1250. doi: 10.1046/j.1365-2222.1998.00342.x. [DOI] [PubMed] [Google Scholar]
- 39.Ricciardolo FL, Nijkamp FP, Folkerts G. Nitric oxide synthase (NOS) as therapeutic target for asthma and chronic obstructive pulmonary disease. Curr Drug Targets. 2006;7:721–735. doi: 10.2174/138945006777435290. [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez HH, Nieves B, Chumley P, Rivera A, Freeman BA. Nitric oxide regulation of superoxide-dependent lung injury: oxidant-protective actions of endogenously produced and exogenously administer-ed nitric oxide. Free Radic Biol Med. 1996;21:43–52. doi: 10.1016/0891-5849(95)02226-0. [DOI] [PubMed] [Google Scholar]
- 41.Barnes PJ, Liew F. Nitric oxide and asthmatic inflammation. J Immunol. 1995;16(3):128–130. doi: 10.1016/0167-5699(95)80128-6. [DOI] [PubMed] [Google Scholar]
- 42.Robbins RA, Grisham MB. Nitric oxide. Int J Biochem Cell Biol. 1997;29:857–860. doi: 10.1016/s1357-2725(96)00167-7. [DOI] [PubMed] [Google Scholar]
- 43.Sharma A, Bansal S, Nagpal R. Lipid peroxidation in bronchial asthma. Indian J Pediatr. 2003;70:715–717. doi: 10.1007/BF02724313. [DOI] [PubMed] [Google Scholar]
- 44.Corradi M, Pignatti P, Manini P, Andreoli R, Goldoni M, Poppa M, et al. Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. Eur Respir J. 2004;24:1011–1017. doi: 10.1183/09031936.04.00002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shokry DM, El-Tarahony SA. Oxidant-antioxidant balance in childhood asthma. Egypt J Pediatr Allergy Immunol. 2013;11:35–40. [Google Scholar]
- 46.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 47.Kurosawa M, Kobayashi H, Nakano M. Cu-Zn superoxide dismutase activities in platelets from stable bronchial asthmatic patients. Int. Arch. Allergy Immunol. 1993;101:61–65. doi: 10.1159/000236499. [DOI] [PubMed] [Google Scholar]
- 48.Powell CV, Nash AA, Powers HJ, Primhak RA. Antioxidant status in asthma. Pediatric Pulmonol. 1994;18:34–38. doi: 10.1002/ppul.1950180109. [DOI] [PubMed] [Google Scholar]
- 49.Dai C, Tang S, Li D, Zhao K, Xiao X. Curcumin attenuates quinocetone-induced oxidative stress and genotoxicity in human hepatocyte L02 cells. Toxicol Mech Methods. 2015;25:340–346. doi: 10.3109/15376516.2015.1045659. [DOI] [PubMed] [Google Scholar]
- 50.Ghosh S, Masri F, Comhair S, Andreadis A, Swaidani S, Aronica M. Nitration of proteins in murine model of asthma. Am J Respir Crit Care Med. 2003;167:889. [Google Scholar]
- 51.Ghosh S, Janocha AJ, Aronica MA, Swaidani S, Comhair SA, Xu W, et al. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J Immunol. 2006;176:5587–5597. doi: 10.4049/jimmunol.176.9.5587. [DOI] [PubMed] [Google Scholar]
- 52.Abdel-Daim MM, Abdou RH. Protective effects of diallyl sulfide and curcumin separately against thallium-induced toxicity in rats. Cell J. 2015;17:379–388. doi: 10.22074/cellj.2016.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masella R, Di Benedetto R, Varì R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16:577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 54.Karoui H, Hogg N, Fréjaville C, Tordo P, Kalyanaraman B. Characterization of sulfur-centered radical intermediates formed during the oxidation of thiols and sulfite by peroxynitrite ESR-spin trapping and oxygen uptake studies. J Biol Chem. 1996;271:6000–6009. doi: 10.1074/jbc.271.11.6000. [DOI] [PubMed] [Google Scholar]
- 55.Brown LAS, Ping X-D, Harris FL, Gauthier TW. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol. 2007;292:824–832. doi: 10.1152/ajplung.00346.2006. [DOI] [PubMed] [Google Scholar]
- 56.Rahman I, Gilmour PS, Jimenez LA, MacNee W. Oxidative stress and TNF-a induce histone Acetylation and NF-1082B/AP-1 activation in Alveolar epithelial cells: Potential mechanism In gene transcription in lung inflammation. Mol Cell Biochem. 2002:239–248. [PubMed] [Google Scholar]
- 57.Liu Y, Wu Y, Zhang P. Protective effects of curcumin and quercetin during benzo (a) pyrene induced lung carcinogenesis in mice. Eur Rev Med Pharmacol Sci. 2015;19:1736–1743. [PubMed] [Google Scholar]
- 58.Tayyem RF, Heath DD, Al-Delaimy WK, Rock CL. Curcumin content of turmeric and curry powders. Nutr Cancer. 2006;55:126–131. doi: 10.1207/s15327914nc5502_2. [DOI] [PubMed] [Google Scholar]


