Abstract
Objective(s):
Artemisia is a genus of herbs and small shrubs forms an important part of natural vegetation in Iran. It has been reported that several Artemisia species possess anti-proliferative effects. Considering the value of this genus in anti-cancer researches we have chosen Artemisia biennis for cytotoxic and mechanistic studies.
Materials and Methods:
In this study we have investigated the cytotoxic and apoptotic effects of petroleum ether, dichloromethane, ethyl acetate, ethanol, and ethanol: water (1:1 v/v) extracts of A. biennis Willd. on two cancer human cell lines (K562 and HL-60) and J774 as normal cells.
Results:
CH2Cl2 extract was found to have the highest anti-proliferative effect on cancer cells. IC50 values obtained in AlamarBlue® assay for CH2Cl2 extract were 64.86 and 54.31 µg/ml on K562 and HL-60 cells respectively. In flow cytometry histogram of the cells treated with CH2Cl2 extract, sub-G1 peak was induced. DNA fragmentation, increased in the level of Bax and cleavage of PARP protein all showed the induction of apoptosis with CH2Cl2 extract after 48 hr contact with cells.
Conclusion:
The results can corroborate the cytotoxic and apoptotic effects of the CH2Cl2 extract of A. biennis on the K562 and HL-60 cancer cell lines.
Keywords: Artemisia biennis, Asteraceae, Apoptosis, Leukemic cell lines
Introduction
Artemisia (Asteraceae) is regarded as one of the large and diverse genera of the plants with about 400 species. Some species of the genus have a reputation as anti-hepatotoxic and anti-malarial agents, food additives (1, 2) antimicrobial or antiviral (3-5) and anti-inflammatory agents (6). Among 43 reported species of the genus in Iran, two are endemic to the country (7, 8). Flavonoids, coumarins, sterols, polyacethylenes, monoterpens, sesquit- erpenes and sesquitrpenelactons are the main classes of reported phytochemicals from the genus (1, 9).
Herbal extracts of the same genus have been shown to possess anti-prolifrative and anti-apoptotic effects (10-14). The apoptosis induction of Artemisia lavandulaefolia via the mitochondrial and MAPKs pathways and apoptosis in HeLa cells by Artemisia princeps Pampanini cv. Sajabal through caspase-mediated activation of the mitochondrial death pathway and inhibition of tumor growth of HeLa xenograft mice are two examples in this genus (15).
Artemisia biennis Willd. With the Persian names of “Dermaneye dosaaleh” and “ Dermaneye mortafa”, grows
wildly in Iran (16). Presence of alpha-pinene (10.2%), 1, 8- cineole (10.1%), Artemisia ketone (11.4%) and camphor (24.6%) has been reported as the main components of the essential oil (17). Based on another study, (Z)-β-ocimene (34.7%), (E)-β-farnesene (40.0%), the acetylenes (11.0%) and (Z) - and (E)-en-yn-dicycloethers were found to be the major components of the essential oil. Notable effects against Aspergillus niger, Cryptococcus neoformans and Fonsecaea pedrosoi as well as weak antioxidant and free radical scavenging activities have been reported for the essential oil (18). The hydro-ethanol extract of A. biennis exhibited significant effects on in vitro leishmanicidal activity while dichloromethane extract of A. biennis in comparison with six other Artemisia species possessed the highest cytotoxicity on the cervical cancer cell line (19, 20). The hydro-ethanol extract of A. biennis was found to have higher total phenolic content and antioxidant activity (21). Apoptosis inducing ability in the plant have led to the identification of many bioactive anti-cancer and chemoth- erapeutic agents. Apoptosis may be triggered in cancer cells via multiple signaling path ways, such as the receptor, mitochondrial and mitogen-activated protein kinase (MAPK) pathways (22).
The use of whole plant extracts in biological studies versus single phytochemicals offers synergistic/antago- nistic effects of the complex mixture of secondary metabolites in the plant (23). Mixtures of compounds present in plant extracts provide the essential combinations of single components that affect multitude targets with much less toxicity of each individually low amount of compounds (24). Based on the anti-proliferation and apoptosis inducing potential of different flavonoids and terpenoids isolated from Artemisia genus (25, 26), this study was designed to examine the ability of petroleum ether (PE), dichloromethane (CH2Cl2), ethyl acetate (EtOAc), ethanol (EtOH) and EtOH/H2O (1:1 v/v) extracts obtained from A. biennis to induce apoptosis in apoptosis-proficient HL-60 and apoptosis-resistant K562 cells, which differ in sensitivity to cytotoxic insults (27). This study also explored the possible mechanism(s) of the apoptosis arbitrated to the plant. The results show for the first time that the A. biennis could induce apoptosis in the human leukemia cells through a mitochondria and caspase-dependent pathway.
Materials and Methods
Reagents and chemicals
AlamarBlue® (resazurin) from Sigma (Saint Louis, MO, USA); RPMI-1640 and FBS from Gibco; Lympholyte®-H from Cedarlane (Canada, CL5020); β-actin (#4970) and PARP (#9542) antibodies, anti-rabbit IgG (#7074) HRP linked antibody from Cell Signaling technology (Boston, USA); ECL Western blotting detection reagent from Bio-RaD (USA); the fluorescent probe propidium iodide (PI), protease inhibitor cocktail, phosphatase inhibitor cocktail, sodium citrate, Triton X-100, phenylmethylsulfonyl fluoride and QuantiPro BCA Assay Kit from Sigma (Steinheim, Germany); all the solvents used for extraction were purchased from Caledon and Scharlau.
Plant material
Aerial parts of A. biennis Willd. were collected from Zoshk (Razavi Khorasan province, Iran) in September 2010. Samples were identified by Dr Valiollah Mozaffarian (Research Institute of Forest and Rangelands, Tehran, Iran). The voucher specimen (No. 12570) has been deposited in the Herbarium, Department of Pharmacognosy, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran.
Preparation of extracts and extracts
The dried powdered aerial parts (120 g) of A. biennis were extracted with petroleum ether (40-60), CH2Cl2, EtOAc, EtOH and EtOH/H2O (1:1 v/v) respectively (Sequential maceration with ca. 3×1.2 L of each solvent). The extracts were filtrated with filter paper and dried using rotary evaporator at a reduced pressure at a temperature below 45 °C to yield 6.13, 8.78, 0.57, 1.84 and 11.99 g of each extract (Figure 1).
Figure 1.
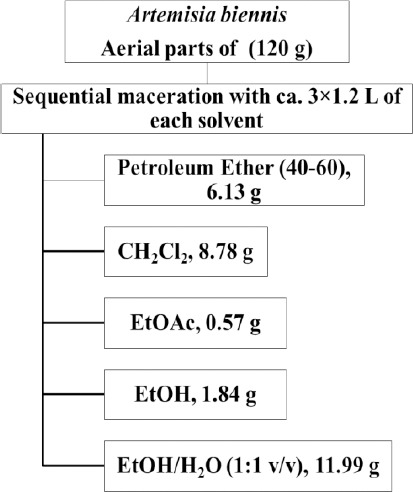
Extraction scheme of PE, CH2Cl2, EtOAc, EtOH and EtOH/H2O (1:1 v/v) extracts of Artemisia biennis
All of the isolated extracts were dissolved in dimethylsulfoxide (DMSO) and then were subjected to cytotoxic and apoptosis assays.
Cell cultures and treatment
The human leukemic cancer cell lines HL-60 (C217) and K562 (C122) and non-malignant cell line, J774 cell (C483), were obtained from Pasture Institute (Tehran, Iran) and maintained in RPMI-1640 medium with 10% v/v fetal bovine serum and 100 u/ml penicillin and 100 μg/ml streptomycin at 37 °C in a humidified atmosphere of 5 % CO2 and 95% of air.
In vitro cell proliferation
The AlamarBlue® cell viability reagent functions as a cell health indicator by using the reducing power of living cells to quantitatively measure the proliferation of various human and animal cell lines, bacteria, plant, and fungi allowing to establish relative cytotoxicity of agents within various chemical classes.
When cells are alive, they maintain a reducing environment within the cytosol of the cell.
Resazurin, the active ingredient of AlamarBlue®, is a non-toxic, cell permeable compound that is blue in color and virtually non-fluorescent. Upon entering cells, resazurin is reduced to resorufin, a compound that is red in color and highly fluorescent. Viable cells continuously convert resazurin to resorufin, increasing the overall fluorescence and color of the media surrounding cells (28). About 5×104 K562 and 105 HL-60 cells were seeded in each well of 96-microwell plate and treated with various concentrations of each extract of A. biennis (6.25, 12.5, 25, 50, 100, 200 µg/ml). J774 cell line was used as non-malignant cell line. After 48 hr incubation, AlamarBlue® was added to each well according to the manufacturer’s instructions. After 4 hr in culture, the cell viability was determined by measuring the absorbance at 570 nm and 600 nm using an ELISA microplate reader (Awareness, Palm City, FL, USA). The cytotoxicity of A. biennis extracts was expressed as IC50, which was calculated using Graph Pad Software (Graph Pad prism 5 software) and presented as mean±SEM of three independent experiments with three replicates for each concentration of A. biennis extract.
PI Staining
Apoptotic cells were detected by PI staining of small fragments of DNA in treated cells followed by flow cytometry. It has been reported that following DNA fragmentation the so-called sub-G1 peak can be noticed following incubation of cells in a hypotonic phosphate-citrate buffer containing quantitative DNA-binding dye such as PI. Apoptotic cells that have lost DNA will take up less stain and will show up in the left side of the G1 peak in the histogram (29). Briefly, 106 K562 and HL-60 cells were seeded in each well of a 24-well plate and treated with CH2Cl2 extract of A. biennis in different concentrations (25, 50 and 100 µg/ml) for 48 hr. Floating and adherent cells were then harvested and incubated at 4°C overnight in the dark with 750 μl of a hypotonic buffer (50 μg/ml PI in 0.1% sodium citrate plus 0.1% Triton X-100) before flow cytometric analysis of 104 events using a flow cytometer (Becton Dickinson).
Western blotting analysis
About 107 HL-60 cells were treated with CH2Cl2 extract of A. biennis (25, 50 and 100 μg/ml) for 48 hr. The cells rinsed and harvested with cool PBS for 3 times, the cell pellet was resuspended in a lysis buffer containing 50 mM Tris-HCl (pH=7.4), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 0.2% SDS, 1% Protease inhibitor cocktail, 1% phosphatase inhibitor cocktail and 1 mM phenylmethylsulfonyl fluoride and left on ice for 30 min. After centrifugation at 10000 rpm for 20 min at 4 °C, the cell lysate was collected and protein concentration was determined according to the Bio-Rad Protein Assay kit. Equal amount of proteins were subjected to 8% and 12.5% SDS-page (W/V). The proteins were transferred to a polyvinylidene fluoride (PVDF) membrane and subjected to immune-blotting using Bax, β-actin and, PARP antibody as primary antibodies and anti-rabbit IgG HRP-linked antibody as secondary antibodies, Bcl-2. Bax protein band and PARP cleavage in HL-60 cells were detected by enhanced chemiluminescence using the ECL Western blotting detecting reagent. Images were quantified using Gel-pro Analyser V.6.0 Gel Analysis software (Media Cybernetics, InC, Bethesda, MD).
Statistical analysis
One way analysis of variance (ANOVA) and Bonferroni post hoc test were used for data analysis. All the results were expressed as mean±SEM and P-values below 0.05 were considered statistically significant.
Results
Cytotoxicity of various extracts
Different extracts of A. biennis were examined for cytotoxic potential on K562, HL-60 and normal cell lines (J774). These cells incubated in 37 °C and 5% CO2 with various concentrations of different extracts of A. biennis (0-200 µg/ml) for 48 hr.
Results demonstrated that some extract decreased cell viability in a concentration-dependent manner. In K562 cells n-Hexane, CH2Cl2 and EtOAc extracts and in HL-60 cell n-Hexane and CH2Cl2 extracts at the concentration below 25 µg/ml were significantly cytotoxic (P<0.05). Among different extracts of A. biennis, CH2Cl2 extract demonstrated the most cytotoxic effects on cancer cells (Figure 2), but minimal effect on normal cells (Data not shown). IC50 values (µg/ml) for different extracts of A. biennis in HL-60 and K562 cells are presented in Table 1.
Figure 2.
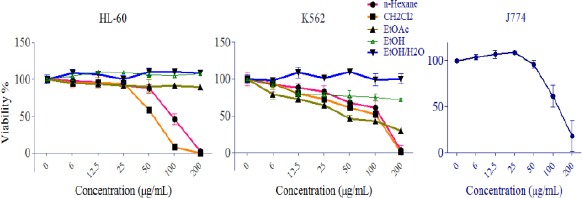
The dose-dependent effects of extracts on the growth of K562 and HL-60 cells and normal J774 cells. PE and CH2Cl2 extracts exhibited cytotoxic activity against apoptosis-proficient HL-60 and apoptosis-resistant K562 cells with minimal cytotoxic effects on normal J774 cells. Values were mean±SEM of at least three independent experiments, each in triplicates
Table 1.
IC50 values (µg/ml) for different extracts of Artemisia biennis in HL-60 and K562 cell lines
| Extracts | |||||
|---|---|---|---|---|---|
| Cell line | PE | CH2Cl2 | EtOAc | EtOH | EtOH/H2O(1:1/v) |
| K562 | 86.18 | 64.86 | 80.91 | >200 | >200 |
| HL-60 | 92.87 | 54.31 | >200 | >200 | >200 |
Apoptosis induction by CH2Cl2 extract
Apoptosis in K562 and HL-60 cell lines was detected with flow cytometry using PI staining test. Cells incubated with various concentrations of CH2Cl2 extract of A. biennis (0, 50, 100 µg/ml) for 48 hr. Sub-G1 peak of treated cells in flow cytometry histograms compared to untreated control cells revealed the induction of apoptosis in treated cells (Figure 3).
Figure 3.
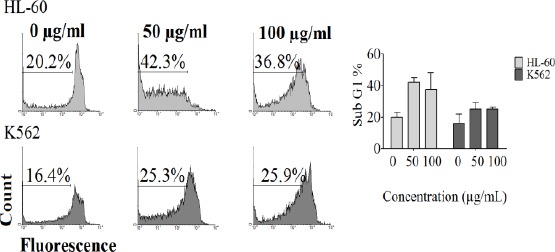
PI staining and flow cytometry analysis of CH2Cl2 extract (0, 25, 50, 100 µg/ml) induced apoptosis in K562 and HL-60 cells
Western blotting
The cleavage of 116 kDa PARP-1 to 89 and 24 kDa fragments was used as an indicator of apoptosis. In HL-60 cells, PARP-1 was cleaved clearly to the 89 kDa and 24 kDa fragments after treatment with CH2Cl2 extract (25, 50 and 100 µg/ml) for 48 hr (Figure 4). Bax proteins play a pivotal role in controlling cytochrome c release and apoptosis initiation via the mitochondrial pathway. CH2Cl2 extract (25, 50 and 100 µg/ml) enhanced the level of Bax protein in HL-60 cells in a concentration-dependent manner (Figure 4).
Figure 4.
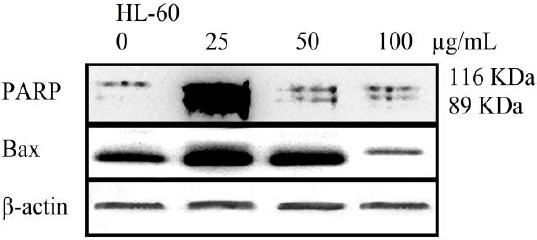
Proteolytic cleavage of poly (ADP-ribose) polymerase (PARP) and increasing in the level of Bax protein in HL-60 cells after 48 hr exposure to CH2Cl2 extracts (0, 25, 50, 100 µg/ml). Β-Actin was used as a loading control. All Western blots were representative of 3 independent experiments
Discussion
Medicinal plants as important natural sources of therapeutic agents are well-known for their less toxic effects than synthetic compounds. Thus, development of novel drugs relies on the progression of plant based therapeutics (30). In this regard, plants are screened for their bioactivity to identify the most potent ones for the desired effects. Different species of the Artemisia have been selected for further analytical and mechanistic study due to potent cytotoxic and anti-tumor properties. In particular, the flavonoids, eupatilin (25), eupafolin (15) and jaceosidin (26) introduced as active principles in Artemisia species which have cytotoxic and apoptosis inducing effects.
In this study, the anti-proliferative activities of PE, CH2Cl2, EtOAc, ethanol and EtOH/H2O (1:1 v/v) extracts obtained from A. biennis were explored for the first time. According to the results of the present study PE and CH2Cl2 extracts were active against HL-60 cells and PE, CH2Cl2 and EtOAc extracts were active against K562 cell lines. Extract used for extraction of A. biennis have different polarity and draw out phytochemical of various polarity from the plant. Thus, the presence of none/semi polar nature of the active components in A. biennis is assumable. The potent cytotoxic effect of CH2Cl2 extract of A. biennis encourages further study on the pro-apoptotic effects of the extract in HL-60 and K562 cells. In particular, we found that CH2Cl2 extract of A. biennis induced sub G1 peak in flow cytometry histogram of treated HL-60 and K562 cells compared to control untreated cells. The apoptosis induction was confirmed in HL-60 cells, as evidenced by increase in level of Bax protein and cleavage of PARP.
Alteration in the apoptosis pathway is supposed to play a key role in cancer progression (31) and is known as being the particular causes of non-invasive cell death in the body. Apoptosis signaling pathway is mainly regulated by many genes. Caspase families of enzymes are key effectors in cell death-inducing signals from cell surface receptors, mitochondria, or endoplasmic reticulum stress (32). Generally, caspases are classified as initiator and effector caspases and participate in both extrinsic and intrinsic pathways of apoptosis. Effector caspases, such as, caspase-3, -6 and -7, cleave cellular substrates, such as, PARP and lamin A/C, which promote apoptosis. Bax is one of the pro-apoptotic members of the BCL-2 superfamily that forms an opening pore by inserting into the mitochondria outer membrane. When overcome the anti-apoptotic members, pro-apoptotic proteins promote apoptosis via the intrinsic way (29, 33).
Different species of the Artemisia have been selected for further analytical and mechanistic study due to potent cytotoxic and anti-tumor properties. In particular, the flavonoids, eupatilin (25), eupafolin (15) and jaceosidin (26) have been introduced as active principles in Artemisia species, which have cytotoxic and apoptosis inducing effects. The essential oil of A. indica exhibited concentration dependent cytotoxicity against four human cancer cell lines THP-1, A-549, HEP-2 and Caco-2 (34). It has been suggested that A. afra has potential anti-cancer properties because of observed cytoxicity of the ethanol extract against U937 and HeLa cancer cells (35). The CH2Cl2 fraction from A. sacrorum exhibited the highest cytotoxicity in comparison with eight other fractions against HepG2, HT-29 and MCF-7 cells (36). In vitro cytotoxic properties of different extracts from A. absinthium against J-45.01 human acute T leukemia, MDA-MB-231, and MCF-7 cell lines have been proven (37-39). Based on another study, the most potent essential oils of A. absinthium in the brine shrimp (Artemia sp.) test were found to be those containing notable amounts of trans-sabinyl acetate and cis/trans-thujones (40). Ethanol extract of A. argyi exhibited activity against the P388 murine leukemia cell line (41). Dichloromethane extracts of A. annua were more cytotoxic than methanol extracts towards HeLa cancer cells (42). The essential oil of A. herba-alba with the main volatile constituent, verbenol, exhibited significant anti-proliferative activity against the acute lymphoblastic leukemia (CEM) cell line (43). Assessment of the cytotoxicity of ethanolic leaf extracts of A. annua from Chinese and Brazilian origins to molt-4 human leukemia cells revealed the potential application of these extracts in cancer treatment (44).
In this study, the anti-proliferative activities of different extracts obtained from A. biennis were explored for the first time. According to the results of the present study PE and CH2Cl2 extracts were active against HL-60 cells and PE, CH2Cl2 and EtOAc extracts were active against K562 cell lines. Solvents used for extraction of A. biennis have different polarity and drew out phytochemicals of various polarities from the plant. Thus, the presence of the none/semi polar nature of the cytotoxic components in A. biennis is assumable. The potent cytotoxic effect of CH2Cl2 extract of A. biennis encourages further study on the pro-apoptotic effects of the extract in HL-60 and K562 cells. In particular, we found that CH2Cl2 extract of A. biennis induced sub G1 peak in flow cytometry histogram of treated HL-60 and K562 cells compared to control untreated cells. The apoptosis induction was confirmed in HL-60 cells, as evidenced by increase in the level of Bax protein and cleavage of PARP. Taken together, these findings suggest that CH2Cl2 extract obtained from A. biennis induces apoptosis verified by the presence of apoptotic cell populations (sub G1 peak) in flow cytometry histogram of treated cells, increase in level of Bax and cleavage of PARP. In summary, the finding of this study confirmed the potential value of Artemisia genus and in particular A. biennis in the treatment of cancer and malignancies.
Conclusion
The species of Artemisia genus have been demonstrated to possess anti-proliferative effects on cancer cells. In this regard, considering the value of this genus in anti-cancer investigations we have chosen A. biennis for cytotoxic and mechanistic studies. In this study, among the different extracts of A. biennis, CH2Cl2 extract was found to have the highest anti-proliferative effect on cancer cells (K562 and HL-60) and the lowest cytotoxicity on J774 as normal cells. The results show for the first time that the A. biennis could induce apoptosis in the human leukemia cells through a mitochondria and caspase-dependent pathway. Due to their valuable effects, future research will undoubtedly provide more insights and shed further light on the Artemisia genus as promising medicinal plant in anti-cancer therapy.
Acknowledgment
The authors would like to thank Mr. M. Malaekeh for his assistance in flow cytometry. This work was supported by grants from Research Affairs of Mashhad University of Medical Sciences, Mashhad, Iran, and the Specialized Research Fund (No. 910238) for the Pharmacy Doctoral Program.
Conflict of Interest
The authors have no conflict of interest to declare in connection with the content of this manuscript.
References
- 1.Bora KS, Sharma A. The genus Artemisia: a comprehensive review. Pharm Biol. 2011;49:101–109. doi: 10.3109/13880209.2010.497815. [DOI] [PubMed] [Google Scholar]
- 2.Gilani AH, Yaeesh S, Jamal Q, Ghayur MN. Hepatoprotective activity of aqueous-methanol extract of Artemisia vulgaris. Phytother Res. 2005;19:170–172. doi: 10.1002/ptr.1632. [DOI] [PubMed] [Google Scholar]
- 3.Aniya Y, Shimabukuro M, Shimoji M, et al. Antioxidant and hepatoprotective actions of the medicinal herb Artemisia campestris from the Okinawa Islands. Biol Pharm Bull. 2000;23:309–312. doi: 10.1248/bpb.23.309. [DOI] [PubMed] [Google Scholar]
- 4.Kordali S, Cakir A, Mavi A, Kilic H, Yildirim A. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J Agric Food Chem. 2005a;53:1408–1416. doi: 10.1021/jf048429n. [DOI] [PubMed] [Google Scholar]
- 5.Kordali S, Kotan R, Mavi A, Cakir A, Ala A, Yildirim A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J Agric Food Chem. 2005b;53:9452–9458. doi: 10.1021/jf0516538. [DOI] [PubMed] [Google Scholar]
- 6.Abad MJ, Bedoya LM, Apaza L, Bermejo P. The Artemisia L. genus: a review of bioactive essential oils. Molecules. 2012;17:2542–2566. doi: 10.3390/molecules17032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mozaffarian V. Flora of Iran, No 59: Compositae (Anthemideae & Ehinopeae); Research Institute of Forest and Rangelands, Tehran (in Persian) 2008:199–261. [Google Scholar]
- 8.Emami SA, Aghazari F. Les Phanerogames Endemiques de la Flore d’ Iran. Téhéran: Publications de I’Universitéde Téhéran; 2011. pp. 451–452. [Google Scholar]
- 9.Tan RX, Tang HQ, Hu J, Shuai B. Lignans and sesquiterpene lactones from Artemisia sieversiana and Inula racemosa. Phytochemistry. 1998;49:157–161. doi: 10.1016/s0031-9422(97)00889-3. [DOI] [PubMed] [Google Scholar]
- 10.Choi E, Park H, Lee J, Kim G. Anti-cancer, antiobesity, and anti-inflammatory activity of Artemisia species in vitro. J Tradit Chin Med. 2013;33(1):92–97. doi: 10.1016/s0254-6272(13)60107-7. [DOI] [PubMed] [Google Scholar]
- 11.Taghizadeh Rabe SZ, Mahmoudi M, Ahi A, Emami SA. Antiproliferative effects of extracts from Iranian Artemisia species on cancer cell lines. Pharm Biol. 2011;49:962–969. doi: 10.3109/13880209.2011.559251. [DOI] [PubMed] [Google Scholar]
- 12.Tin AS, Sundar SN, Tran KQ, Park AH, Poindexter KM, Firestone GL. Antiproliferative effects of artemisinin on human breast cancer cells requires the down regulated expression of the E2F1 transcription factor and loss of E2F1-target cell cycle genes. Anti-cancer Drugs. 2012;23:370–379. doi: 10.1097/CAD.0b013e32834f6ea8. [DOI] [PubMed] [Google Scholar]
- 13.Lee JG, Kim JH, Ahn JH, Lee KT, Baek NI, Choi JH. Jaceosidin, isolated from dietary mugwort (Artemisia princeps), induces G2/M cell cycle arrest by inactivating cdc25C-cdc2 via ATM-Chk1/2 activation. Food Chem Toxicol. 2013;55:214–221. doi: 10.1016/j.fct.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 14.Park EY, Lee KW, Lee HW, Cho YW, Baek NI, Chung HG, et al. The ethanol extract from Artemisia princeps Pampanini induces p53-mediated G1 phase arrest in A172 human neuroblastoma cells. J Med Food. 2008;11(2):237–245. doi: 10.1089/jmf.2007.609. [DOI] [PubMed] [Google Scholar]
- 15.Ju HK, Lee HW, Chung KS. Standardized flavonoid-rich extract of Artemisia princeps Pampanini cv. Sajabal induces apoptosis via mitochondrial pathway in human cervical cancer HeLa cells. J Ethnopharmacol. 2012;141:460–468. doi: 10.1016/j.jep.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Mozaffarian V. A Dictionary of Iranian Plant Names. Tehran: Farhang Moaser Publishers; 1998. p. 56. [Google Scholar]
- 17.Nematollahi F, Rustaiyan A, Larijani K, Nadimi M. Essential oil composition of Artemisia biennis Willd. and Pulicaria undulata (L.) C.A. Mey., two Compositae herbs growing wild in Iran. J. Essent Oil Res. 2006;18:339–341. [Google Scholar]
- 18.Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 2008;69:1732–1738. doi: 10.1016/j.phytochem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Emami A, Zamani Taghizadeh Rabe SH, Ahi A, Mahmoudi M. Inhibitory activity of eleven Artemisia species from Iran against Leishmania major parasites. Iran. J Basic Med Sci. 2012;15:807–811. [PMC free article] [PubMed] [Google Scholar]
- 20.Emami A, Zamani Taghizadeh Rabe SH, Ahi A, Mahmoudi M. Study on toxic effects of Artemisia spp. extracts from Iran on human cancer cell lines. J Zanjan Univ Med Sci. 2010;18:58–67. [Google Scholar]
- 21.Hatami T, Emami SA, Miraghaee SS, Mojarrab M. Total phenolic contents and antioxidant activities of different extracts and extracts from the aerial parts of Artemisia biennis Willd. Iran J Pharm Res. 2014;13:551–559. [PMC free article] [PubMed] [Google Scholar]
- 22.Mousavi SH, Tayarani-Najaran Z, Hersey P. Apoptosis, from signaling pathways to therapeutic tools. Iran J Basic Med Sci. 2008;11:121–142. [Google Scholar]
- 23.Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365–378. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Russo GL. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem Pharmacol. 2007;74:533–544. doi: 10.1016/j.bcp.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Kim MJ, Kim DH, Na HK, Oh TY, Shin CY, Surh YJ. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces apoptosis in human gastric cancer (AGS;cells. J Environ Pathol Toxicol Oncol. 2005;24:261–269. doi: 10.1615/jenvironpatholtoxicoloncol.v24.i4.30. [DOI] [PubMed] [Google Scholar]
- 26.Lv W, Sheng X, Chen T, Xu Q, Xie X. Jaceosidin induces apoptosis in human ovary cancer cells through mitochondrial pathway. J Biomed Biotechnol. 2008;2008:394802. doi: 10.1155/2008/394802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins LM, Mesner PW, Kottke TJ, Basi GS, Sinha S, Tung JS, et al. Comparison of caspase activation and subcellular localization in HL-60 and K562 cells undergoing etoposide-induced apoptosis. Blood. 1997;90:4283–4296. [PubMed] [Google Scholar]
- 28.O’Brien J, Wilson I, Orton T, Pognan F. Investigation of Alamar Blue (resazurin) fluorescent dye for assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 29.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 30.Shu L, Cheung KL, Khor TO, Chen C, Kong AN. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010;29:483–502. doi: 10.1007/s10555-010-9239-y. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Youle RJ. The role of mitochondria in apoptosis. Annual review of genetics. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 33.Letai A. Pharmacological manipulation of Bcl-2 family members to control cell death. J Clin Invest. 2005;115:2648–2655. doi: 10.1172/JCI26250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rashid S, Rather MA, Shah WA, Bhat BA. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013;138:693–700. doi: 10.1016/j.foodchem.2012.10.102. [DOI] [PubMed] [Google Scholar]
- 35.Spies L, Koekemoer TC, Sowemimo AA, Goosen ED, Van De Venter M. Caspase-dependent apoptosis is induced by Artemisia afra Jacq. ex Willd. in a mitochondria-dependent manner after G2/M arrest. S Afr J Bot. 2013;84:104–109. [Google Scholar]
- 36.Piao GC, Li YX, Yuan HD, Jin GZ. Cytotoxic extract from Artemisia sacrorum Ledeb. against three human cancer cell lines and separation and identification of its compounds. Nat Prod Res. 2012;26:1483–1491. doi: 10.1080/14786419.2011.565473. [DOI] [PubMed] [Google Scholar]
- 37.Wegiera M, Smolarz HD, Jedruch M, Korczak M, Koproń K. Cytotoxic effect of some medicinal plants from Asteraceae family on J-45.01 leukemic cell line - Pilot study. Acta Pol Pharm. 2012;69:263–268. [PubMed] [Google Scholar]
- 38.Shafi G, Hasan TN, Syed NA, Al-Hazzani AA, Alshatwi AA, Jyothi A, et al. Artemisia absinthium (AA): A novel potential complementary and alternative medicine for breast cancer. Mol Biol Rep. 2012;39:7373–7379. doi: 10.1007/s11033-012-1569-0. [DOI] [PubMed] [Google Scholar]
- 39.Baykan Erel S, Gökhan Şenol S, Aydin Köse F, Ballar P. In vitro cytotoxic properties of six Artemisia L. species. Turk J Pharm Sci. 2011;8:247–252. [Google Scholar]
- 40.Judzentiene A, Budiene J, Gircyte R, Masotti V, Laffont-Schwob I. Toxic activity and chemical composition of Lithuanian wormwood (Artemisia absinthium L) essential oils. Rec Nat Prod. 2012;6:180–183. [Google Scholar]
- 41.Lee TK, Vairappan CS. Antioxidant, antibacterial and cytotoxic activities of essential oils and ethanol extracts of selected South East Asian herbs. J Med Plants Res. 2011;5:5284–5290. [Google Scholar]
- 42.Efferth T, Herrmann F, Tahrani A, Wink M. Cytotoxic activity of secondary metabolites derived from Artemisia annua L. towards cancer cells in comparison to its designated active constituent artemisinin. Phytomedicine. 2011;18:959–969. doi: 10.1016/j.phymed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Tilaoui M, Mouse HA, Jaafari A, Aboufatima R, Chait A, Zyad A. Chemical composition and antiproliferative activity of essential oil from aerial parts of a medicinal herb Artemisia herba-alba. Rev Bras Farmacogn. 2011;21:781–785. [Google Scholar]
- 44.Singh NP, Ferreira JFS, Park JS, Lai HC. Cytotoxicity of ethanolic extracts of Artemisia annua to molt-4 human leukemia cells. Planta Med. 2011;77:1788–1793. doi: 10.1055/s-0030-1271157. [DOI] [PubMed] [Google Scholar]


