Abstract
Objective(s):
Studies have confirmed that microgravity, as a mechanical factor, influences both differentiation and function of mesenchymal stem cells. Here we investigated the effects of simulated microgravity on neural differentiation of human adipose-derived stem cells (ADSCs).
Materials and Methods:
We have used a fast rotating clinostat (clinorotation) to simulate microgravity condition. Real-time PCR and flow cytometry analysis were used to evaluate the regulation of neurotrophins, their receptors, and neural markers by simulated microgravity and their impact on neural differentiation of cells.
Results:
Our data revealed that simulation microgravity up-regulated the expression of MAP-2, BDNF, TrkB, NT-3, and TrkC both before and after neural differentiation. Also, the neural cells derived from ADSCs in microgravity condition expressed more MAP-2, GFAP, and synaptophysin protein in comparison to the 1G control.
Conclusion:
We showed that simulated microgravity can enhance the differentiation of mesenchymal stem cells into neurons. Our findings provide a new strategy for differentiation of ADSCs to neural-like cells and probably other cell lineages. Meanwhile, microgravity simulation had no adverse effects on the viability of the cells and could be used as a new environment to successfully manipulate cells.
Keywords: ADSCs, Microgravity, Neural differentiation, Neurotrophin
Introduction
While the peripheral nervous system has an intrinsic capacity for repair and regeneration, the mammalian central nervous system (CNS) has a very limited ability to replenish neuronal loss following an injury, and is unable to regenerate correct axonal and dendritic connections between cells (1). Although an adult human brain has a few neural stem cells (2, 3), these cells have a limited capacity to participate in regenerating processes (4). In recent years, stem cell biology has provided the novel option for the study and cell therapy of neurodegenerative diseases (5-7). Due to ethical, biological, and safety issues, therapeutic use of embryonic stem cells has not been generally accepted. In the past decades, more attention has been paid to the use of adult stem cells in regenerative medicine (8-10).
Mesenchymal stem cells (MSCs) are heterogeneous populations of multipotent stem cells that can be isolated from different adult tissues, including bone
marrow (7-9), adipose tissue (11), umbilical cord blood (12), peripheral blood (13), dermis (14), amniotic fluid (15), and even tumors (16). Since adipose tissues are abundant and easy to access by less invasive methods, they are ideal sources of adult stem cells. They are able to differentiate into mesodermal and non-mesodermal cell lineages, such as osteocytes (17), adipocytes (18), chondrocytes (19), cardiomyocytes(20), fibroblasts (21), myofibroblasts (22), epithelial cells (23), hepatocytes (24), and neurons (25).
Many studies have shown that neural-like cells derived from MSCs have the ability to repair CNS injuries (26-28). However, recovery from injury after transplantation of these cells depends on the secretion of the appropriate amount of growth factors and their ability to survive for a long period. To date, different methods have been employed to induce neural differentiation of MSCs, and to increase the efficiency of differentiation, such as using chemical inducers, growth factors, and co-culture with neural cells (7-9, 27-29). In
recent years, biophysical forces such as flow, shear stress, and microgravity are increasingly being used as a nove culture methodology to manipulate cells along with biochemical techniques (30-33). Microgravity, as a mechanical factor, has been confirmed to affect cell growth and physiology. Numerous studies have demonstrated the effect of microgravity on intracellular signaling mechanisms, cell secretions, and gene expression. It has been shown that microgravity can stimulate the cell proliferation and cell differentiation, and triggers cells to grow within three-dimensional aggregates (34-36). Furthermore, simulated microgravity can accelerate differentiation of MSCs (37).
Briefly, the aim of present study was to investigate the effect of simulated microgravity on neural differentiation of adipose-derived stem cells (ADSCs). We have analyzed the expression of neurotrophins (NGF, BDNF, and NT-3) and their receptors (high-affinity tropomyosin-related kinase receptors; TrkA, TrkB, TrkC) as main regulators of neuronal survival and death before and after transdifferentiation of ADSCs.
Materials and Methods
All experiments were approved by the Clinical Research Ethics Committee of the Medical Laser Research Center, ACECR, Tehran, Iran. Each human subject signed a consent form.
Isolation and culture of ADSC
Human subcutaneous adipose tissue was obtained from 5 healthy women (aged 34–48) undergoing cosmetic liposuction surgery and transferred to the Aerospace Research Institute, Tehran, Iran in sterile conditions. After removing the blood phase, adipose samples were washed with Hank’s balanced salt solution (HBSS; Biowest, France) and used for stem cell isolation.
Cell isolation was done as described by Zuk et al, (2001) (38). Briefly, adipose tissue (suspended in HBSS solution) was digested with 1 mg/ml of collagenase type 1 A (Sigma, USA) for 60 min at 37 °C and continuously shaken to detach the stromal cells from adipocytes. Then, the adipose suspension was diluted with equal volume of DMEM media (Dulbecco’s Modified Eagle Medium; Biowest, France) supplemented with 10% fetal bovine serum (FBS; Biowest, France) to neutralize the collagenase and centrifuged at 400 g for 10 min. After discarding the supernatant (floated adipose cells), the stromal vascular pellet was dispersed in DMEM media containing 10% FBS, 1% antibiotic-antimycotic solution (Biowest, France) and seeded on a T25 flask (TPP, Switzerland). Cultures were incubated in humidified incubator at 37 °C with 5% CO2. On the next day, cellular debris, as well as the non-attached cells were eliminated by changing the medium and replaced with the fresh medium. Adherent MSCs were maintained at 80% confluence and sub-cultured with trypsin/EDTA (Biowest, France) when required. Cell count was determined by hemocytometer. Cells at passages 4 or later were used for the analysis of MSC surface markers and differentiation experiments.
Flow cytometry analysis of ADSC
ADSCs at passage 4 underwent FACS analysis for CD34, CD45, CD73, CD90, and CD105 to characterize stem cell surface antigen and the effectiveness of the selection method. These surface antigens are known markers associated with ADSCs (39, 40). Cells were harvested with 25% trypsin/EDTA and suspended in phosphate-buffered saline (PBS) (3 × 105/100 µl for each reaction) and then incubated for 30 min at 4 °C with the phycoerythrin (PE) and fluorescein isothiocyanate (FITC) conjugated antibodies (BD Biosciences PharMingen, USA) against CD90-PE, CD105-FITC, CD73-PE as positive marker and CD34-PE, CD45-FITC as negative marker. Samples were analyzed using a Cyflow Space (Partec, Germany) flow cytometer. Data were then analyzed by the FloMax software (version 2.70).
ADSCs multilineage differentiation potential
Under the appropriate conditions, ADSCs have the ability to differentiate into osteoblasts, adipocytes, and chondrocytes. In this study, to demonstrate multipotent potential of the isolated cells, adipogenic and osteogenic differentiation were induced using human mesenchymal stem cell functional identification kit (R&D systems, USA) according to the manufacturer’s recommendations.
For adipogenic differentiation, ADSCs were plated into a 6-well culture plate at an initial density of 2.1×104 cells per cm2 in Alpha-MEM media (Biowest, France) supplemented with antibiotics and 10% FBS and allowed cells to reach 90-100% confluence. After the cells reached confluence, the medium was replaced by the adipogenic induction medium (R&D systems, USA): α-MEM, hydrocortisone, 3-isobutyl-1-methyl-xanthine, and indomethacin. The medium was renewed every 3 days. After 9 days, adipogenic induction was confirmed by Oil Red O staining of cytoplasmic lipid droplets. Observation of Oil Red droplets with light microscopy indicated the differentiation of ADSCs into adipocytes.
For osteogenic differentiation of ADSCs, ADSCs were plated into a 6-well culture plate at an initial density of 2.1×104 cells per cm2 in Alpha-MEM media supplement-
ed with antibiotic and 10% FBS and allowed cells to reach 50–70% confluence. After the cells reached confluence, the medium was replaced by the osteogenic induction medium (R&D systems, USA): α-MEM, dexamethasone, ascorbic acid-2-phosphate, and β-glycerophosphate. The medium was renewed every 3 days. After 14–21 days, cells started to detach and osteocytes were prepared. Osteogenic induction was confirmed by Alizarin Red S staining of extracellular matrix calcium deposition. Observation of bright orange-red calcium deposits with light microscopy indicated the successful in vitro bone formation. Briefly, cells were washed with PBS and fixed with 10% formalin for 30 min at room temperature and then, washed with distilled water. Cells were stained with 2% Alizarin Red S and incubated at room temperature for 1 hr in the dark. After careful aspiration of the Alizarin Red S staining solution, cells were washed four times with distilled water and examined under light microscopy.
Neural differentiation
ADSCs were plated into a 96-well culture plate. Neural differentiation was induced as described previously (41). Briefly, pre-induction was performed by discarding the medium and adding new DMEM medium containing 20% FBS and 10 ng/ml bFGF (Roche) for 24 hr. On the next day, the medium was removed; then neural induction medium (NIM) was added to the culture and was renewed every day by discarding half of the medium and adding new NIM. The composition of NIM was: DMEM supplemented with 2% DMSO, 10 ng/ml bFGF, 100 μM butylate hydroxyanisole (Sigma, USA), 10 μM Forskolin (Sigma, USA), 25 mM KCl, 2 mM valproic acid, and 5 μg/ml insulin. Samples were divided into 4 groups: 1- control group without rotation (samples in normal gravity; 1G= one gravity) in growth medium, 2- control group without rotation in neural differentiation medium, 3- simulated microgravity group with clinorotation (samples in simulated microgravity: 0.001G) in growth medium, and 4- simulated microgravity group with clinorotation in neural differentiation medium. The cells were monitored continually after neuronal induction and were used for RNA extraction or subjected to assays at specific time points.
Microgravity simulation
2D clinostat was used for simulating microgravity. Through rotation, this device prevents gravity from affecting cells. Clinostat was sterilized by ultraviolet light and 70% ethanol and put in a 37 °C CO2 incubator. ADSCs were seeded at a density of 2 × 106 cells on tissue culture tube (TPP, Switzerland) or at a density of 5 × 104 cells on 96-well plates. After cell adhesion, tubes
or plates were completely filled by medium supplemented with antibiotics and 10% FBS to prevent the presence of air bubbles. To maintain the pH balance, the medium was supplemented with 15 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). Samples were fixed at the center of the device. The clinostat rotation speed was 20 rpm and the rotation times were 6, 24, and 48 hr.
Flow cytometry analysis of differentiated ADSCc
After differentiation, neural-like cells were analyzed by flow cytometry to detect the expression level of neural markers. Cells were permeabilized using 70% ethanol. Non-specific antibody binding was blocked with the combination of 10% goat serum in primary antibodies. The primary antibodies were against the MAP-2 (Cell signaling company, U.S.A), synaptophysin (eBioscience, U.S.A), and GFAP (ebBioscience, U.S.A).
The primary antibodies were added and incubated for 3 hr at room temperature. Binding of primary antibodies was revealed with specific secondary anti-goat IgG-FITC (Abcam, 1:50) for 1 hr at room temperature. Samples were analyzed using a Cyflow Space flow cytometer. Data were then analyzed by the FloMax software.
Analysis of gene expression by real-time quantitative PCR
The expression of neurotrophin, their receptors, ADSC marker, and neural lineage markers were performed by real-time quantitative PCR. Total RNA was extracted from undifferentiated and differentiated samples using an RNA isolation kit (Cell Amp™ Direct RNA Prep Kit for RT-PCR; Takara, Japan), frozen immediately in liquid nitrogen, and stored at −75 °C until the time of use. One microgram of total RNA was used for cDNA synthesis using Prime Script™ RT reagent Kit (Takara, Japan) in a 20 μl reaction and according to the manufacturer’s recommendations.
Real-time PCR was performed using StepOnePlus real-time PCR (Applied Biosystems, USA) and SYBR Green real-time Master Mix kit (Takara, Japan). Cycling conditions were: 94 °C for 2 min; followed by 40 cycles of 95 °C for 5 sec and 60 °C for 30 sec. To ensure specificity of PCR products, PCR melt curves were performed for each gene after real-time PCR. Changes in the fold
number were calculated by using the 2ΔΔCt method, which was normalized by glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH) as the housekeeping gene. Relative expression was evaluated using the REST 2009 software (version 2.0.13). Gene-specific primers were designed and ordered as described in Table 1. All primers were synthesized by Macrogen (South Korea).
Table 1.
Sequence of PCR primers
| Gene | Ref Seq | Primer sequence (5´ to 3´) | PCR product length (bp) |
|---|---|---|---|
| NGF | NM_002506.2 | Forward : GGTCATCATCCCATCCCATCTT Reverse: GTTCACCTCTCCCAACACCATC |
131 |
| BDNF | NM_001709.4 | Forward: GAAGGCTGCCCCCATGAAAG Reverse: GACTACTGAGCATCACCCTGG |
248 |
| NTF3 (NT-3) | NM_002527.4 | Forward : CACCGAACTGCTGCGACAAC Reverse: CGTTTCCGCCGTGATGTTCT |
147 |
| TrkA | NM_002529.3 | Forward : CTTCCTCCGATCCCATGGAC Reverse: CCCACTAGACAGTTGCGTGT |
180 |
| TrkB | NM_006180.4 | Forward: ACACCACGAACAGAAGTAATGAA Reverse: CCCACCACAGACGCAATCAC |
106 |
| TrkC | NM_002530.3 | Forward: CGCAGTCTTCACACGCTCAAC Reverse: AGAGTGTGGTGAGCCGGTTAC |
163 |
| MAP-2 | NM_002374.3 | Forward: TGGCGGACGTGTGAAAATTG Reverse: ATGGTCCACACGGGCTTTAG |
166 |
| GNL3 | NM_206826.1 | Forward: TCCGAGCTTCATCGTATCTCC Reverse: ACTTCAATACTTGCTGGACTTCG |
72 |
| GAPDH | NM_002046.5 | Forward: GGAAGGTGAAGGTCGGAGTCA Reverse: TCATTGATGGCAACAATATCCACT |
101 |
MTT assay
Cell viability was done as described previously (41) by using the colorimetric 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Briefly, 20 μl of MTT reagent (Sigma, USA; 10 mg/ml) was added to 5 × 104 cells cultured in 96 well plates in a final volume of 200 μl. The cells were incubated for 3 hr at 37 °C. Then 200 μl of dimethyl sulfoxide (DMSO) was introduced, as the solvent reagent. The amount of formazan produced from MTT cleavage was quantitated with an ELISA plate reader (BioTech Company, USA), at 570 nm wavelength.
Statistical analysis
All data are presented as the mean±SEM. One-Way ANOVA with Tukey’s post hoc test, as well as non-parametric Mann–Whitney tests, were used for comparison of the expression of selected genes in all samples using SPSS 15. P<0.05 was considered significant. All experiments were replicated at least twice.
Results
Isolation of ADSC and culture
We extracted mesenchymal cells from human adipose tissue and cultured the cells as mentioned above. We selected them positively based on their ability to adhere to the plastic surface of the cell culture flask. ADSCs exhibited the fibroblast-like spindle shape after 48 hr and reached 70-80% of cell confluence after 4 days (Figure 1). Morphology of ADSCs has been changed after exposure to simulated microgravity. We observed that cells became rounded after 3 days of microgravity treatment (Figure 2).
Figure 1.
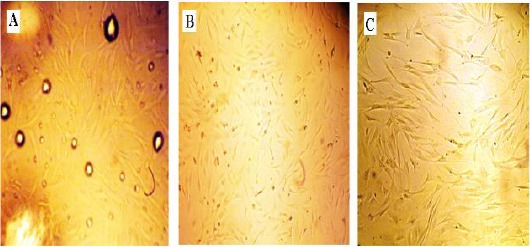
Isolation of ADSCs with the enzymatic method from lipoaspirate tissues. Fibroblast-like spindle shape cells were observed after 48 hr culture of the stromal vascular pellet in cell culture flask. (A) 3 hr after extraction; note the presence of oil spots (remains of digested fat). (B and C) After changing medium ADSCs at 70% of confluence at passage 2 (B) and at passage 4 (C) were observed
Figure 2.
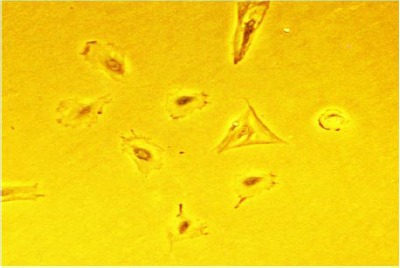
Morphology of ADSCs after 3 day exposure to microgravity. Round cells were observed after 3 day exposure to simulated microgravity
Cell surface markers
Flow cytometry analysis of the cells at passage 2 demonstrated that more than 95% of the cells were positive for MSCs associated antigens, CD90, CD73, and CD105 and negative for hematopoietic markers such as CD34 and CD45 antigens (Figure 3E). These results confirmed that the isolated cells were MSCs. The presence of surface markers was examined by RT-PCR, too. Extracted cells showed the mRNA expression of CD90, CD73, and CD105 (Figure 3E). We cannot detect the expression of CD34 and CD45 markers by RT-PCR (data not shown).
Figure 3.
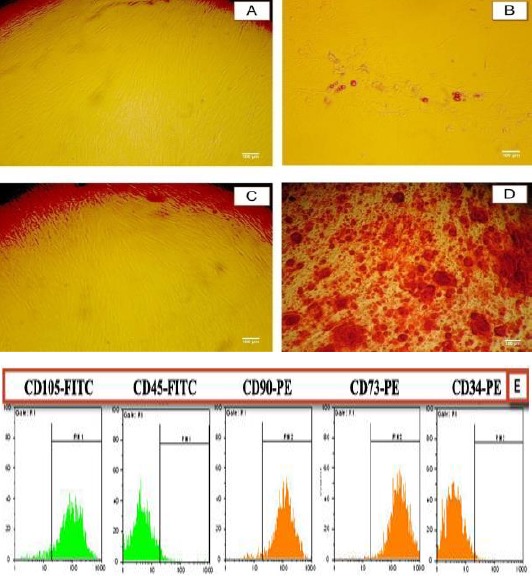
Characterization of ADSCs. Differentiation of human ADSCs to adipocyte and osteoblast lineages. (A and C) ADSCs cultured in control media; (B) ADSCs cultured in adipogenic media for 9 days and stained with alkaline phosphatase; Observation of Oil Red droplets by light microscopy indicated the differentiation of ADSCs into adipocytes. (D) ADSCs cultured in osteogenic media for 15 days and stained with Alizarin Red S. Observation of bright orange-red calcium deposits by light microscopy indicated the successful in vitro bone formation. (E) Cell surface markers analysis of MSC. Third-passage of isolated ADSCs was positive for CD90, CD73, and CD105 (markers of MSC) and negative for CD34 and CD45 markers (hematopoietic markers)
nalysis of ADSCs multipotency
To determine their multipotent capacity, the isolated ADSCs were differentiated into adipocyte and osteocyte. Adipogenic differentiation was demonstrated by accumulation of lipid-rich vacuoles detected with Oil Red O staining (Figure 3B), and osteogenic differentiation was confirmed by calcium deposit production in extracellular matrix observed in Alizarin Red (Figure 3D). These results indicated that the isolated cells exhibit characteristics of mesenchymal stem cells and have adipogenic and osteogenic potentials.
Neural differentiation
Neural induction was done on passage 4 and in 96 well plates. After adding neural induction medium, samples were divided into two groups: simulated microgravity group (samples were placed in clinostat) and control group (cells were differentiated in normal conditions; 1G). Cell morphology starts to change after 3 hr and neural-like cells can be observed (Figure 4).
Figure 4.
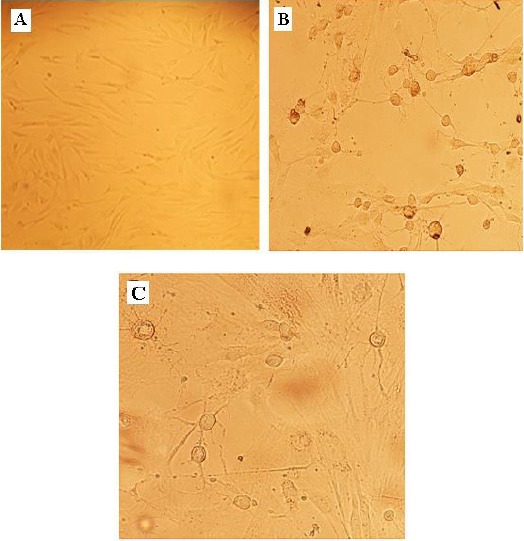
Neuronal differentiation of ADSCs. The phenotype of the cells appeared to change rapidly following the addition of neural-induced medium. (A) Undifferentiated ADSC. (B and C) Cells after 24 hr of neuronal induction, big round cell bodies were formed and neurotic-like processes appeared
Analysis of gene expression by RT-PCR and real-time quantitative PCR
To evaluate the effect of microgravity on neural gene expression, ADSCs were collected after 6, 24, and 72 hr of treatment by clinostat. Neuron-like cells were collected after 6, 24, and 48 hr of treatment by clinostat, too. We employed quantitative reverse-transcription polymerase chain reaction to measure any changes in gene expression of neurotrophins, their receptors, and neuronal markers. Our selected genes were: GNL3 (Nucleostemin), MAP-2, NGF (Ngfb) and its receptor TrkA (Trk1), BDNF and its receptor TrkB (Trk2), NT-3
(Ntf-3) and its receptor TrkC (Ntrk3). Our results showed that microgravity enhanced expression level of these genes before neural induction (Figure 5). The expression of GNL3, MAP-2, BDNF, TrkB, NT-3, and TrkC showed a gradual and significant up-regulation following microgravity treatment. The expression of MAP-2 and BDNF increased up to 2.5 fold over the control level at 72 hr after microgravity simulation, GNL3 and TrkB increased up to 2 fold and NT-3 and its receptor TrkC increased up to 1.7 fold over the control level. The level of NGF expression slightly increased (about 10%) by 72 hr after microgravity simulation and we could not detect any changes in TrkA expression following microgravity treatment in comparison to the control group.
Figure 5.
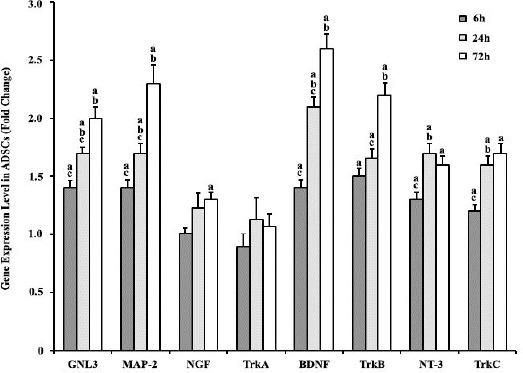
Real-time PCR analysis of GNL3, MAP-2, NGF, TrkA, BDNF, TrkB, NT-3, and TrkC in ADSCs; 6 hr, 24 hr, and 72 hr after simulated microgravity treatment compared with the 1G group. Gene’s expressions were normalized to endogenous GAPDH in the same samples. The expression of GNL3, MAP-2, BDNF, TrkB, NT-3, and TrkC showed a gradual and significant up-regulation following microgravity treatment. Results represent three independent experiments. P<0.05 considered as significant. (a) Statistically different from the control group, (b) statistically different from the previous time-scale group, and (c) statistically different from the next time-scale group
After neural induction, compared to the control group (1G experiment), the expression of GNL3 slightly decreased 48 hr after microgravity treatment (Figure 6). In contrast to GNL3, simulated microgravity increased the expression of MAP-2, BDNF, and TrkB. Following the microgravity treatment, the expression of MAP-2 increased gradually (50%) by 48 hr. The expression of BDNF and its receptor TrkB increased 50% following microgravity induction (after 6 hr) and remained constant at this level throughout the rest of the experiments, for 48 hr. Expression changes of NGF and TrkA were similar before and after neural induction. The level of NGF and NT-3 expression slightly increased (about 10%) by 48 hr after microgravity simulation. We could not detect any changes in TrkA expression in comparison to the control group. Interestingly, in comparison to the alteration of NT-3 expression, the expression of Trk-C came back to the control levels by 48 hr after microgravity simulation.
Figure 6.
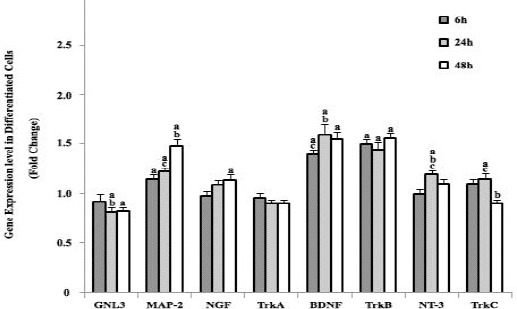
Real-time PCR analysis of GNL3, MAP-2, NGF, TrkA, BDNF, TrkB, NT-3, and TrkC at 6 hr, 24 hr, and 48 hr after induction in simulated microgravity groups vs. 1G group. Genes’ expressions were normalized to endogenous GAPDH in the same samples. Simulated microgravity increased the expression of GNL3, MAP-2, BDNF, and TrkB. Results represent three independent experiments. P<0.05 considered as significant. (a) statistically different from the control group, (b) statistically different from the previous time-scale group, and (c) statistically different from the next time-scale group
MTT assay
The viability and metabolic activity of the cells before and after differentiation to neural-like cells in simulated microgravity and normal conditions were analyzed using the MTT assay. MTT detects cell death at later stages of apoptosis, in which metabolization of tetrazolium salts is reduced. As it is evident in Figure 7, simulated microgravity had no significant effects on the viability of the cells before or after differentiation to neural-like cells (P>0.05). However, after neural differentiation, cell viability is decreased (38.3%) in both groups (1G condition and simulated microgravity condition).
Figure 7.
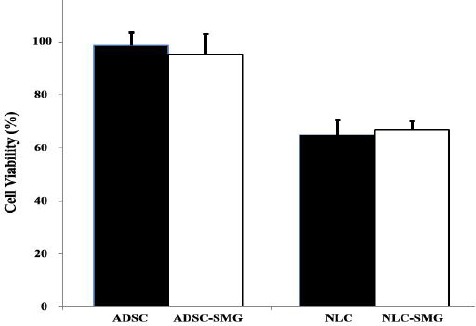
Cell viability of ADSCs cultured in simulated microgravity (SMG), before and after induction of neural differentiation. As it is evident, simulated microgravity had no effects on the viability of the cells in undifferentiated or differentiated cells. The error bars represent standard error of the mean for triplicate experiments. ADSC: ADSCs cultured in normal condition (1G), NLC: neural-like cells 24 hr after differentiation
Flow cytometry analysis of ADSCs and differentiated ADSCs
Quantitative analysis of neural markers through flow cytometry indicated that GFAP was expressed in
55.95%, 59.50%, and 59.69% of ADSCs, neural-like cells cultured in 1G and neural-like cells cultured in simulated microgravity conditions, respectively. The protein expression of MAP-2 and synaptophysin (SYN) was 30.74% and 70.05% in ADSCs, 36.18% and 75.30% in neural-like cells cultured in 1G, 44.69% and 83.97% in neural-like cells cultured in simulated microgravity conditions (Figure 8).
Figure 8.
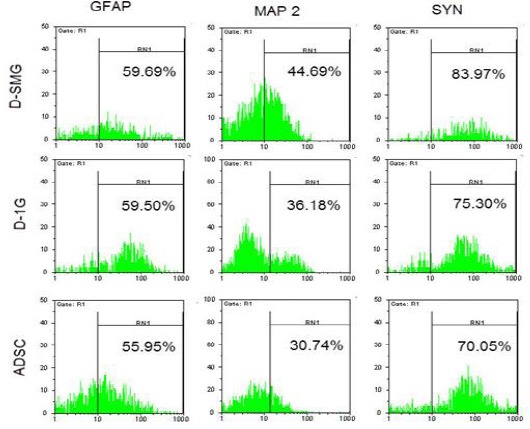
After ADSCs were cultured for 24 hr in differentiation medium, flow cytometry analysis of neural markers (GFAP, MAP-2, and synaptophysin) were done from ADSCs (control), D- SMG(differentiation in SMG) groups, and D-1G (differentiation in 1G) group
Discussion
In recent years, different studies have demonstrated that MSCs from adult tissues are multipotent cells, capable of differentiating into neural-like cells both in vitro and in vivo. Most researchers used chemical reagents or growth factors to induce neural differentiation of MSCs (3, 5-9, 25-29). These neural-like cells, derived biochemical manipulation of MSCs, have been shown to express some characteristics of neuronal cells. However, stability and efficiency of generated cells vary from one study to another, which is most likely due to differences in inducing reagents and the source of stem cells population. Recently, much more attention has been paid to biophysical forces, including microgravity to manipulate stem cells especially with the aim of cell therapy and transplantation (30-33). Microgravity, as a mechanical factor, has been confirmed to affect cell growth and physiology. It has been shown that microgravity can stimulate the cell proliferation and differentiation, and triggers cells to grow within three-dimensional aggregates that are an ideal condition for tissue engineering. Furthermore, simulated microgravity can accelerate differentiation of MSCs (34-37). In this study, we showed that microgravity increased the differentiation of MSCs to neural-like cells with biochemical factors.
We could isolate ADSCs from 15 ml of adipose tissues. The morphology and characteristics of isolated cells were similar to that reported by others. Cells were isolated based on their ability to adhere to the plastic flask and confirmed through flow cytometry and differentiation to adipocytes and osteocytes. Flow cytometry analysis approved that extracted cells were positive for MSC surface antigens (CD73, CD90, and CD105), but negative for hematopoietic antigens (CD34 and CD45) (42). Also our results showed that the isolated cells had multi-lineage differentiation potential (17, 18). They were successfully differentiated into adipocytes and osteocytes. Accumulation of lipid droplets and calcium was detected by Oil Red O staining and Alizarin Red S staining at days 9 and 21 of culture in the induced medium. These results indicate that the isolated cells were ADSCs.
Since the first attempt for differentiating MSCs to neural cells by Woodbury et al in 2000, different methods have been suggested. Woodbury used β-mercaptoethanol (BME), dimethylsulfoxide (DMSO) and butylated hydroxyanisole (BHA) to differentiate human MSCs and showed the expression of neuron-specific enolase (NSE) and
neurofilament-M in differentiated cells (25). In another study, neural differentiation of these cells was done using EGF and BDNF. They could detect the expression of neuron biomarkers such as nestin, glial fibrillary acidic protein (GFAP), and neuron-specific nuclear protein (NeuN) (43). Some other factors that have been used for neural differentiation of MSCs include: fibroblast growth factor 8 (FGF-8), nerve growth factor (NGF), platelet-derived growth factor (PDGF), forskolin, 3-isobutyl-1-methylxanthine (IBMX), dibutyryl cAMP, NT-3, NT-5, glial cell line-derived neurotrophic factor (GDNF), and retinoic acid (RA) (44-50). We used Woodbury’s protocol for neural differentiation of human ADSCs, and compared the effectiveness of microgravity condition in this induction method. The morphology of cells began to change rapidly, and neural-like cells appeared in 3 hr after induction, which is why we chose this protocol. It is the appropriate protocol for simulating microgravity conditions by the clinostat instrument.
In the current study, we observed that microgravity had a positive effect on the neural differentiation potential of MSCs. We found out that that the expression of neural marker MAP-2 increased both before and after neural differentiation in ADSCs under simulated microgravity conditions. The flow cytometry analysis confirmed the real-time PCR results as the number of MAP-2 positive cells were increased before and after differentiation in a simulated microgravity environment, too. These results confirmed data reported by Chen et al (2011) and Yuge et al (2010) suggesting that simulated microgravity conditions enhanced neural differentiation potential of MSCs in vivo and in vitro, respectively (51, 52). Chen et al showed up-regulation of OCT-4 (pluripotent surface marker for stem cells) in rat bone marrow MSCs treated with simulated microgravity, which confirmes our results. The similar result was obtained for the neural marker, synaptophysin. Our data revealed that microgravity increased the number of synaptophysin positive cells with differentiation. However, in contrast to MAP-2 and synaptophysin, the numbers of GFAP positive cells were identical in both control (neural-like cells in 1G condition) and simulated microgravity groups after neural induction. GFAP is a neural marker for astrocytes in the CNS. It seems that microgravity had no any effects on ADSCs differentiation into astrocytes.
Furthermore, the present study revealed that microgravity environment increased neurotrophin’s expression and their specific Trk receptors especially BDNF and TrkB. The expression of neurotrophins and their receptors are crucial for cell transplantation of neurodegenerative diseases. NTs are target-derived factors, which induce the survival, differentiation, development and function of different neurons both in peripheral and central nervous system (CNS). They act through two classes of receptors: the high-affinity Trk family of tyrosine kinase receptors and the low-affinity pan-NT receptor p75 (p75NTR). Reports suggest that binding of NTs to their common p75 receptor in the absence of Trk receptors causes neuronal death (53). It is clear from our study that microgravity increased the expression of BDNF, NT-3, TrkB, and TrkC both before and after neural induction. Also, the expression of NGF was increased after microgravity simulation but it was negligible. Based on the expression levels of NGF obtained in control groups (before and after differentiation) before microgravity treatment, weightlessness had no impact on the expression of NGF before and after differentiation. Chen et al (51) reported up-regulation of NGF and BDNF in MSCs after microgravity induction, too. In contrast to the up-regulation of NGF and BDNF, their experiment detected the expression of neurotrophins decreased significantly either in microgravity environment or in normal gravity conditions after neural induction. The present observation stands in contrast to our study, which may be due to differences in neural induction protocol and time of gene expression analysis. They used b-FGF, human epidermal growth factor (hEGF), dibutyryl cyclic AMP (dbcAMP) and isobutylmethylxanthine (IBMX) to induce neural induction and evaluated gene expression of neurotrophins after 7 day induction. Also, we found that the morphology of ADSCs was changed from spindle to round under microgravity conditions compared to the 1G control, similar to that reported by others (51, 52). Chen et al demonstrated that the morphology changes of MSCs in a microgravity environment is due to microtubules and cytoskeleton reorganization that could affect cell fate. Researchers in 2004 have shown that cell shape, cytoskeletal tension, and RhoA could regulate stem cell lineage commitment (54).
Conclusion
Our findings provide a new strategy for differentiation of ADSCs to neural-like cells and probably other cell lineages. Based on our results, microgravity could be used as a new method besides traditional techniques to increase the efficiency of cell transplantation in medicine. Meanwhile, microgravity simulation had no adverse effects on the viability of the cells and could be used as a new environment to successfully manipulate cells.
Acknowledgment
We are grateful to Setareh Madani for critically reviewing and editing the manuscript. The results reported in this paper were part of a student thesis.
References
- 1.Arvidsson A, Collin T, Kirik D, Kokai Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 2.Singec I, Jandial R, Crain A, Nikkhah G, Snyder EY. The leading edge of stem cell therapeutics. Annu Rev Med. 2007;58:313–328. doi: 10.1146/annurev.med.58.070605.115252. [DOI] [PubMed] [Google Scholar]
- 3.Zietlow R, Lane EL, Dunnett SB, Rosser AE. Human stem cells for CNS repair. Cell Tissue Res. 2008;331:301–322. doi: 10.1007/s00441-007-0488-1. [DOI] [PubMed] [Google Scholar]
- 4.Ninomiya M, Yamashita T, Araki N, Okano H, Sawamoto K. Enhanced neurogenesis in the ischemic striatum following EGF-induced expansion of transit-amplifying cells in the subventricular zone. Neurosci Lett. 2006;403:63–67. doi: 10.1016/j.neulet.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 5.Aharonowiz M, Einstein O, Fainstein N, Lassmann H, Reubinoff B, Ben-Hur T. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS One. 2008;3:e3145. doi: 10.1371/journal.pone.0003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Einstein O, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Polyzoidou E, Lavon I. Transplanted neural precursor cells reduce brain inflammation to attenuate chronic experimental autoimmune encephalomyelitis. Exp Neurol. 2006;198:275–284. doi: 10.1016/j.expneurol.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Hellmann M, Panet H, Barhum Y, Melamed E. D Offen. Increased survival and migration of engrafted mesenchymal bone marrow stem cells in 6-hydroxydopamine-lesioned rodents. Neurosci Lett. 2006;395:124–128. doi: 10.1016/j.neulet.2005.10.097. [DOI] [PubMed] [Google Scholar]
- 8.Cuevas P, Carceller F, Dujovny M, Garcia-Gomez I, Cuevas B, Gonzalez-Corrochano R, et al. Peripheral nerve regeneration by bone marrow stromal cells Neurol Res. 2002;24:634–638. doi: 10.1179/016164102101200564. [DOI] [PubMed] [Google Scholar]
- 9.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- 10.Bradamante S, Barenghi L, Maier M. Stem cells toward the future: The space challenge. Life. 2014;4:267–280. doi: 10.3390/life4020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuk P, Zhu M, Mizuno H, Huang J, Futrell J, Katz A, et al. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Engineering. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 12.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 13.Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. Int J Biochem Cell Biol. 2004;36:585–597. doi: 10.1016/j.biocel.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Haniffa M, Wang X, Holtick U, Rae M, Isaacs J, Dickinson A, et al. Adult human Fibroblasts Are Potent Immunoregulatory Cells and Functionally Equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–1604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 15.Sessarego N, Parodi A, Podesta M, Benvenuto F, Mogni M, Raviolo V, et al. Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica. 2008;93:339–346. doi: 10.3324/haematol.11869. [DOI] [PubMed] [Google Scholar]
- 16.Yan X, Fu C, Chen L, Qin J, Zeng Q, Yuan H, et al. Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res Treat. 2011;132:153–164. doi: 10.1007/s10549-011-1577-0. [DOI] [PubMed] [Google Scholar]
- 17.Birmingham E, Niebur GL, McHugh PE, Shaw G, Barry FP, McNamara LM. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater. 2012;23:13–27. doi: 10.22203/ecm.v023a02. [DOI] [PubMed] [Google Scholar]
- 18.Fink T, Zachar V. Adipogenic differentiation of human mesenchymal stem cells. Methods Mol Biol. 2011;698:243–251. doi: 10.1007/978-1-60761-999-4_19. [DOI] [PubMed] [Google Scholar]
- 19.Alves da Silva ML, Martins A, Costa-Pinto AR, Correlo VM, Sol P, Bhattacharya M, et al. Chondrogenic differentiation of human bone marrow mesenchymal stem cells in chitosan-based scaffolds using a flow-perfusion bioreactor. J Tissue Eng Regen Med. 2011;5:722–732. doi: 10.1002/term.372. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho PH, Daibert AP, Monteiro BS, Okano BS, Carvalho JL, Cunha DN, et al. Differentiation of adipose tissue-derived mesenchymal stem cells into cardiomyocytes. Arq Bras Cardiol. 2013;100:82–89. doi: 10.1590/s0066-782x2012005000114. [DOI] [PubMed] [Google Scholar]
- 21.Lee CH, Moioli EK, Mao JJ. Fibroblastic differentiation of human mesenchymal stem cells using connective tissue growth factor. Conf Proc IEEE Eng Med Biol Soc. 2006;1:775–778. doi: 10.1109/IEMBS.2006.259866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhury R, Webber JP, Gurney M, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget. 2015;20:715–731. doi: 10.18632/oncotarget.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Păunescu V, Deak E, Herman D, Siska I R, T˘anasie G, Bunu C, et al. In vitro differentiation of human mesenchymal stem cells to epithelial lineage. J Cell Mol Med. 2007;11:502–508. doi: 10.1111/j.1582-4934.2007.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu XB, Tao R. Hepatocyte differentiation of mesenchymal stem cells. Hepatobiliary Pancreat Dis Int. 2012;11:360–371. doi: 10.1016/s1499-3872(12)60193-3. [DOI] [PubMed] [Google Scholar]
- 25.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;15(61):364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.Hofstetter C, Schwartz E, Hess D, Widenfalk J, El Manira A, Prockop D J, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu P, Jones L, Tuszynski M. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005;191:344–360. doi: 10.1016/j.expneurol.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- 29.Guo L, Yin F, Meng HQ, Ling L, Huhe T, Li P, et al. Differentiation of mesenchymal stem cells into dopaminergic neuron-like cells in vitro. Biomed environ sci. 2005;18:36–42. [PubMed] [Google Scholar]
- 30.Shinde V, Brungs S, Henry M, Wegener L, Nemade H, Rotshteyn T, et al. Simulated microgravity modulates differentiation processes of embryonic stem cells. Cell Physiol Biochem. 2016;38:1483–1499. doi: 10.1159/000443090. [DOI] [PubMed] [Google Scholar]
- 31.Blaber E, Sato K, Almeida E. Stem cell health and tissue regeneration in microgravity. Stem Cells Dev. 2014;23:73–78. doi: 10.1089/scd.2014.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silvano M, Miele E, Valerio M, Casadei L, Begalli F, Campese A, et al. Consequences of simulated microgravity in neural stem cells: Biological effects and metabolic response. J Stem Cell Res Ther. 2015;5:289–296. [Google Scholar]
- 33.Hart DA. Is Adipocyte differentiation the default lineage for mesenchymal stem/progenitor cells after loss of mechanical loading?. A perspective from space flight and model systems. J Biomed Sci Eng. 2014;7:799–808. [Google Scholar]
- 34.Grimm D, Bauer J, Kossmehl P, Shakibaei M, Schönberger J, Pickenhahn H, et al. Simulated microgravity alters differentiation ad increases apoptosis in human follicular thyroid carcinoma cells. FASEB J. 2002;16:604–614. doi: 10.1096/fj.01-0673fje. [DOI] [PubMed] [Google Scholar]
- 35.Claassen DE, Spooner BS. Impact of altered gravity on aspects of cell biology. Int Rev Cyt. 1994;156:301–373. doi: 10.1016/s0074-7696(08)62257-3. [DOI] [PubMed] [Google Scholar]
- 36.Hammond TG, Lewis FC, Goodwin TJ, Linnehan RM, Wolf DA, Hire KP, et al. Gene expression in space. Nat Med. 1999;5:359. doi: 10.1038/7331. [DOI] [PubMed] [Google Scholar]
- 37.Unsworth BR, Lelkes PI. Growing tissues in microgravity. Nat Med. 1998;4:901–907. doi: 10.1038/nm0898-901. [DOI] [PubMed] [Google Scholar]
- 38.Zuk PA, Zhu M, Mizuno H, Huang JI, Futrell WJ, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–226. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell JB, McIntosh K, Zvonic S. Immunophenotype of human adipose-derived cells: Temporal changes in stromalassociated and stem cell-associated markers. Stem Cells. 2006;24:376. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 40.Chen JY, Mou XZ, Du XC, Xiang C. Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins. Asian Pac J Trop Med. 2015;8:739–746. doi: 10.1016/j.apjtm.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 41.Edalat H, Hajebrahimi Z, Movahedin M, Tavallaei M, Amiri S, Mowlaa S J. p75NTR suppression in rat bone marrow stromal stem cells significantly reduced their rate of apoptosis during neural differentiation. Neurosci Lett. 2011;498:15–19. doi: 10.1016/j.neulet.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 42.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 44.Tondreau T, Lagneaux L, Dejeneffe M, Massy M, Mortier C, Delforge A. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72:319–326. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- 45.Hermann A, Maisel M, Storch A. Epigenetic conversion of human adult bone mesodermal stromal cells into neuroectodermal cell types for replacement therapy of neurodegenerative disorders. Expert Opin Biol Ther. 2006;6:653–670. doi: 10.1517/14712598.6.7.653. [DOI] [PubMed] [Google Scholar]
- 46.Greco SJ, Zhou C, Ye JH, Rameshwar P. An interdisciplinary approach and characterization of neuronal cells transdifferentiated from human mesenchymal stem cells. Stem Cells Dev. 2007;16:811–826. doi: 10.1089/scd.2007.0011. [DOI] [PubMed] [Google Scholar]
- 47.Greco SJ, Zhou C, Ye JH, Rameshwar P. A method to generate human mesenchymal stem cell derived neurons which express and are excited by multiple neurotransmitters. Biol Proced Online. 2008;10:90–101. doi: 10.1251/bpo147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kan I, Ben-Zur T, Barhum Y, Levy YS, Burstein A, Charlow T, et al. Dopaminergic differentiation of human mesenchymal stem cells—utilization of bioassay for tyrosine hydroxylase expression. Neurosci Lett. 2007;419:28–33. doi: 10.1016/j.neulet.2007.03.070. [DOI] [PubMed] [Google Scholar]
- 49.Barzilay R, Kan I, Ben-Zur T, Bulvik S, Melamed E, Offen D. Induction of human mesenchymal stem cells into dopamine-producing cells with different differentiation protocols. Stem Cells Dev. 2008;17:547–554. doi: 10.1089/scd.2007.0172. [DOI] [PubMed] [Google Scholar]
- 50.Tondreau T, Dejeneffe M, Meuleman N, Stamatopoulos B, Delforge A, Martiat P. Gene expression pattern of functional neuronal cells derived from human bone marrow mesenchymal stromal cells. BMC Genomics. 2008;9:166. doi: 10.1186/1471-2164-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J, Liu R, Yang Y, Li J, Zhang X, Li J, et al. The simulated microgravity enhances the differentiation of mesenchymal stem cells into neurons. Neuroscience Letters. 2011;505:171–175. doi: 10.1016/j.neulet.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 52.Yuge A, Sasaki Y, Kawahara SL, Wu M, Matsumoto T, Manabe T, et al. Simulated microgravity maintains the undifferentiated state and enhances the neural repair potential of bone marrow stromal cells. Stem Cells Dev. 2010;20:893–900. doi: 10.1089/scd.2010.0294. [DOI] [PubMed] [Google Scholar]
- 53.Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler C M, Bredesen D E. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- 54.McBeath R, Pirone DM. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]


