Abstract
The Fas-activated serine/threonine phosphoprotein (FAST) is tethered to the outer mitochondrial membrane, where it interacts with BCL-XL (17). Here we show that RNA interference-mediated knockdown of endogenous FAST results in apoptosis, whereas overexpressed recombinant FAST inhibits Fas- and UV-induced apoptosis, indicating that FAST is a survival protein. The antiapoptotic effects of FAST are regulated by interactions with the translational silencer TIA-1: a FAST mutant lacking its TIA-1-binding domain does not inhibit apoptosis, and overexpressed recombinant TIA-1 inhibits the antiapoptotic effects of FAST. Because the antiapoptotic effects of FAST require ongoing protein synthesis, we hypothesized that FAST might function by preventing TIA-1-mediated silencing of mRNAs encoding inhibitors of apoptosis. Consistent with this hypothesis, FAST promotes the expression of cotransfected reporter proteins, a process that requires its TIA-1-binding domain and is inhibited by overexpressed recombinant TIA-1. More compellingly, recombinant FAST increases the expression of endogenous cIAP-1 and XIAP, but not GAPDH, in transfected HeLa cells. Because FAST is released from mitochondria in cells undergoing Fas- or UV-induced apoptosis, we propose that FAST serves as a sensor of mitochondrial stress that modulates a TIA-1-regulated posttranscriptional stress response program.
We have identified the RNA-binding proteins TIA-1 and TIAR as downstream effectors of the PKR-eukaryotic translation initiation factor 2 α subunit (eIF2α) translational control pathway (15). In cells subjected to environmental stress, PKR-induced phosphorylation of the translation initiation factor eIF2α reduces the concentration of eIF2/GTP/tRNAiMet, the ternary complex that loads the initiator tRNA onto the small ribosomal subunit. Under these conditions, TIA-1 promotes the assembly of a noncanonical 48S preinitiation complex that inhibits protein translation (2). The PKR/eIF2/TIA-1 pathway controls both protein synthesis and cell survival. Overexpression of PKR (9), a phosphomimetic mutant of eIF2α (29) or TIA-1 (14, 32) inhibits protein translation and promotes apoptotic cell death. In contrast, dominant-negative mutants of PKR or a nonphosphorylatable mutant of eIF2α promote translation and inhibit apoptosis (3, 37).
Just as translational arrest is linked to apoptotic cell death, enhanced translation is linked to malignant transformation. Overexpression of translation initiation factor eIF4E in NIH 3T3 cells is sufficient to induce cellular transformation (16). Moreover, translation initiation factor eIF4E is overexpressed in a variety of human cancers, including breast, lung, and colon (25). The signaling cascades that regulate the ability of eIF4E to initiate translation also play a prominent role in oncogenesis. AKT, a kinase activated in many cancers, indirectly activates eIF4E and enhances protein translation (36). AKT indirectly activates mTOR, another kinase that activates eIF4E, and enhances protein translation. Inhibitors of mTOR (e.g., rapamycin) are emerging as important chemotherapeutic agents (36). Taken together, these results support the concept that regulation of protein translation plays a critical role in regulating cellular proliferation and cell death.
Mitochondria also play a critical role in determining whether cells live or die. BCL-2 family members that promote cell survival (e.g., BCL-2 and BCL-XL) or cell death (e.g., BAK) are concentrated at the outer mitochondrial membrane (7). Interactions between these proteins regulate a permeability transition pore that allows the release of cytochrome c, AIF, Smac/DIABLO, and Omi, proteins that collectively promote apoptotic cell death (26). In Jurkat T cells, apoptosis triggered by ligation of the Fas receptor is dependent upon a mitochondrial activation pathway (4). Fas ligation results in the activation of caspase-8, which cleaves the BH3-only protein BID. Truncated BID (tBID) is translocated from the cytosol to the mitochondrial membrane, where it interacts with BCL-2 and BCL-XL (5). When the neutralizing capacity of BCL-2 and BCL-XL is exceeded, tBID targets the proapoptotic proteins BAX and BAK, allowing the release of apoptotic effectors from mitochondrial stores (26). The release of smac/DIABLO promotes apoptosis by inhibiting IAP proteins, a family of E3 ligases that directly and indirectly inhibit the activation of apoptotic caspases (20). Tian et al. previously described a TIA-1-interacting protein (33) that resides at the outer mitochondrial membrane in association with BCL-XL (17). This Fas-activated serine/threonine phosphoprotein (FAST) is tethered to mitochondria by a lysine/arginine-rich domain at its carboxyl terminus (17). Although FAST is constitutively phosphorylated on serine and threonine residues (33), it is rapidly dephosphorylated in Jurkat cells treated with anti-Fas antibodies. This result led us to propose that FAST may be involved in a signaling cascade that regulates apoptotic cell death (33).
Here we show that FAST is a survival protein that constitutively suppresses apoptotic cell death. The full antiapoptotic effects of FAST require active NF-κB and ongoing protein synthesis, suggesting that FAST can promote the expression of antiapoptotic proteins, some of which are expressed in an NF-κB-dependent manner. FAST increases, and TIA-1 reduces, the expression of cotransfected reporter proteins. TIA-1 also inhibits the ability of FAST to inhibit apoptosis. Most importantly, recombinant FAST increases the expression of endogenous inhibitors of apoptosis (i.e., cIAP-1 and XIAP) in HeLa transfectants, providing a possible mechanism for its antiapoptotic effects. Thus, interactions between FAST and TIA-1 influence both protein expression and susceptibility to apoptosis. Because FAST is displaced from mitochondria in cells undergoing Fas- or UV-induced apoptosis, we propose that FAST serves as a sensor of mitochondrial stress that promotes cell survival by enhancing the expression of antiapoptotic proteins.
MATERIALS AND METHODS
Cells, antibodies, and reagents.
COS-7 cells and HeLa cells were obtained from the American Type Culture Collection. Cells were maintained in 10% fetal bovine serum (FBS) in Dulbecco's modified Eagle medium (DMEM). The preparation of affinity-purified rabbit polyclonal antisera reactive with FASTN was described previously (17). Antibodies obtained from commercial sources include antihemagglutinin (anti-HA) (murine monoclonal antibody clone 16B12, immunoglobulin G1; Berkeley Antibody Co.), anti-FLAG (murine monoclonal antibody clone; Sigma Chemical Co., St. Louis, Mo.), anti-BCL-XL (rabbit polyclonal and mouse monoclonal; Santa Cruz Biotechnology, Inc.), anti-Myc (rabbit polyclonal; Santa Cruz Biotechnology, Inc.), anti-active caspase 3 (rabbit polyclonal; Promega), rabbit polyclonal anti-cIAP-1 (R and D), mouse monoclonal anti-XIAP (Stressgen), mouse monoclonal anti-GAPDH (Research Diagnostics), and anti-β-galactosidase (mouse monoclonal; Promega). Isotype-specific secondary antibodies (multiple labeling grade) were obtained from Jackson ImmunoResearch Labs, West Grove, Pa. Hoechst dye no. 33258 and other chemicals were obtained from Sigma.
Plasmid constructions.
The construction of expression vectors encoding full-length and mutant FAST were described previously (17). pMT2-HA-TIA1, pMT2-HA-TIA1-RRM, and pMT2-HA-TIA-1-PRD (previously designated TIA-1ΔRRM) were described previously (14). The full-length coding regions of FAST and TIA-1 were also subcloned into the pcDNA3-FLAG vector. Briefly, FAST and TIA-1 were amplified by PCR with overhanging ends containing BglII/EcoRI and BamHI/EcoRI sites, respectively, and then were digested with the corresponding restriction enzymes and inserted into pcDNA3-FLAG vector predigested with BamHI/EcoRI. The pcDNA3-FLAG-BCL-XL construct was a gift from Tom Chittenden (ImmunoGen, Inc.). Plasmid pCMV4-IκBαΔN was a kind gift from Mark Boothby (Vanderbilt University). pcDNA3-β-gal was obtained from Invitrogen.
Western blot analysis.
Whole-cell extracts were separated on 4 to 20% gradient gels, transferred to nitrocellulose, and probed with the indicated antibodies by using previously described methods (33).
Transfections and immunoprecipitations.
Cells were transfected with SuperFect (QIAGEN, Valencia, Calif.) for immunofluorescence microscopy or Lipofectamine 2000 (Invitrogen) for immunoprecipitation and Western blotting analysis according to the manufacturer's instructions. Cells cultured in 6-well plates (2 × 105 to 3 × 105 cells/well plated 20 h before transfection) were exposed to DNA complexes for 5 h, trypsinized, and replated onto parallel plates for both immunofluorescence (24-well plates containing 11-mm-diameter coverslips) and Western blotting (12-well plates). For immunoprecipitations, cells were transfected in 6-well plates as described above without replating. After 28 h, cells were solubilized in NP-40 lysis buffer (1% NP-40, 150 mM NaCl, 1 mM EDTA, 50 mM Tris, pH 7.8). Lysates were precleared with protein A-Sepharose (Amersham Pharmacia Biotech, Piscataway, N.J.) in 150 μl of NP-40 lysis buffer for 1 h. Subsequently, precleared lysates were incubated with the antibody-protein A-Sepharose complex in 150 μl of NP-40 lysis buffer for 1 h, washed three times, and then boiled in sodium dodecyl sulfate (SDS) sample buffer for separation on a 4 to 20% polyacrylamide gradient gel (Invitrogen). Following transfer to nitrocellulose membranes, the resolved proteins were revealed by probing with the indicated antibodies.
Immunofluorescence.
Cells were plated on 11-mm-diameter glass coverslips on 24-well plates. At various time points after transfection the cells were fixed in 2 to 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min, immersed in −20°C methanol for 10 min, rinsed in PBS, and incubated in blocking buffer (5% normal horse serum in PBS) for 1 h before addition of primary antibodies. Cells were incubated with the indicated antibodies (anti-HA was used at 2.5 μg/ml, anti-β-galactosidase was used at 2.3 μg/ml, and anti-active caspase 3 was used at a 1:1,000 dilution) for 1 h, washed several times in PBS, and incubated for 1 h with diluted isotype-specific secondary antibodies (1:200 for Cy2- or Cy5-labeled secondary antibodies and 1:2,000 for Texas red-labeled or Cy3-labeled secondary antibodies) in blocking solution supplemented with Hoechst dye. Cells were viewed using a Nikon Eclipse 800 microscope equipped with epifluorescence and appropriate filter sets optimized to detect the specific fluorochromes used. Images were digitally captured using a CCD-SPOT RT digital camera and then were merged and compiled using Adobe Photoshop software.
TUNEL assay.
Cells were plated on 11-mm-diameter glass coverslips in 24-well plates and were cultured in the absence or presence of anti-Fas antibody (7C11 was used at 1:200 from culture supernatant, which was predetermined to saturate the Fas receptor; a gift from Michael Robertson, University of Illinois). Cells were then fixed in 4% paraformaldehyde in PBS for 10 min, followed by immersion in −20°C methanol for 10 min. After rehydration in PBS, cells were washed with TdT buffer (Roche) for 5 min and then were incubated with terminal transferase-mediated dUTP nick end labeling (TUNEL) reaction mix (TdT buffer, 20 nM dUTP-biotin, 0.15 μl of terminal transferase) (Roche) for 1 h at 37°C. The reaction was stopped by addition of a solution containing 20 mM sodium citrate and 300 mM NaCl. Cells were then incubated in blocking buffer for 20 min prior to the addition of streptavidin-fluorescein isothiocyanate (Fisher) at 1:1,000 in blocking buffer for 1 h. Cells were then further incubated with anti-HA, anti-caspase 3, and Hoechst dye; washed; and mounted for immunofluorescence microscopy.
Apoptosis assays.
Transfected cells were cultured for 24 to 40 h prior to treatment with apoptotic inducers. The following apoptotic inducers were used: anti-Fas antibody 7C11 (1:200 dilution from culture supernatant titrated to give maximal induction of apoptosis) for 20 h, anti-Fas antibody plus cycloheximide (0.025 μg/ml) for 20 h, and UV irradiation (10 mJ/cm2) followed by recovery for 1 to 2 h. After treating cells with apoptotic inducers, cells were processed for immunofluorescence analysis and TUNEL assay as described above. The morphological scoring of individual transfectants was done blindly. At least 200 cells per experiment were scored for the presence or absence of active caspase-3, nuclear condensation, and DNA fragmentation. The data presented represent means of 3 to 10 independent experiments. The Student's paired t test was used to compare the percentage of transfected cells exhibiting these morphological changes to those of vector controls.
Cell fractionation.
COS-7 or HeLa cells in log phase were harvested by scraping, centrifuged, and resuspended in detergent-free buffer (200 mM mannitol, 70 mM sucrose, 1 mM EGTA, 10 mM HEPES, pH 7.5) containing protease inhibitors (phenylmethylsulfonyl fluoride, leupeptin, aprotinin, and benzamidine) and 14.0 μM 2-mercaptoethanol. Cells were disrupted by shearing (30 times with a 26-guage needle) until cell breakage was about 90% as assessed by phase-contrast microscopy. Nuclei were pelleted by centrifugation at 1,300 × g in a refrigerated microcentrifuge for 5 min. Heavy membrane fractions containing mitochondria (referred to as the P20 fraction) were prepared by centrifuging the resulting supernatants at 14,000 rpm (20,000 × g) for 20 min. The corresponding supernatant is referred to as the S20 fraction. The relative purity of these fractions was previously reported (17).
RNA interference (RNAi).
Coding sequences for small interfering RNAs (siRNAs) with a G+C ratio of 45 to 55% were analyzed by BLAST search to ensure that they did not have significant sequence homology with other genes. To generate mammalian expression vectors expressing human FAST-specific siRNA, we cloned double-stranded DNA oligonucleotides into pSuppressor-Neo vector exactly as described by the manufacturer (Imgenex). The sequences of the sense strands are as follows: ACGCAACTCAGCAGCAAGGTGgagtactgCACCTTGCTGCTGAGTTGCGT (hFASTsiRNA-1) and GGGTTGGAAGCTGCTCTAAGCgagtactgGCTTAGAGCAGCTTCCAACCC (hFAST siRNA-2) (gene-specific sequences are in capitals and hairpin sequences are underlined). pSuperessor2 vector expressing a scramble sequence (sense strand, GCGCGCTTTGTAGGATTCGgagtactgCGAATCCTACAAAGCGCGC) was used as a negative control.
COS-7 cells were transfected with recombinant HA-FAST together with the individual pSuper-siFAST constructs by using Lipofectamine 2000. After 48 h, total cell lysates were analyzed by Western blotting with anti-HA antibody. These same vectors were transfected into HeLa cells by using Lipofectamine 2000. After 48 h, cells were solubilized in 1% NP-40 lysis buffer and were centrifuged at 10,600 × g in a desktop centrifuged for 5 min before analyzing supernatants by Western blotting using anti-FASTN antibody to quantify the expression of endogenous FAST.
RESULTS
FAST is a constitutively active survival factor.
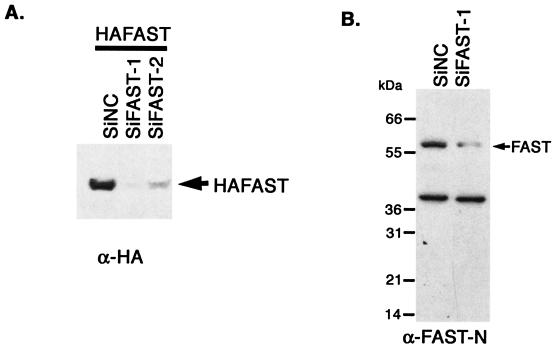
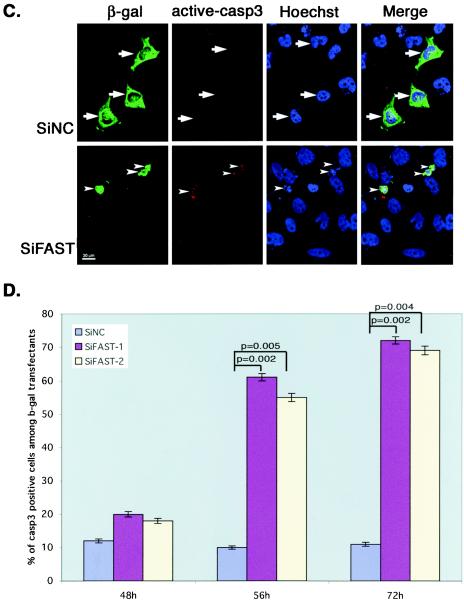
FAST is a mitochondria-associated, BCL-XL-interacting protein that has been proposed to regulate apoptotic cell death (17, 33). To determine whether FAST can regulate apoptosis, we constructed vector-based siRNAs designed to reduce the expression of endogenous FAST. Sequences conforming to published guidelines for targeted knockdown of selected mRNAs (34) were inserted into the pSuppressor2 vector and were cotransfected with HA-FAST into COS-7 cells. After 48 h, cells were processed for Western blotting analysis to quantify the expression of HA-FAST. We identified two different vector-based siRNAs (siFAST-1 and siFAST-2) that significantly and reproducibly lower the expression of HA-FAST (Fig. 1A). We then transfected HeLa cells with siFAST-1 or a negative control siRNA and quantified the expression of endogenous FAST by Western blotting analysis. As shown in Fig. 1B, siFAST-1 reduces the expression of endogenous FAST in HeLa cells. The reduction is less than that in COS-7 cells, because not all cells receive the siRNA in this transient transfection experiment. To selectively visualize transfected cells, we cotransfected HeLa cells with the siRNA vectors together with a vector encoding β-galactosidase. After 48, 56, or 72 h, cells were processed for two-color immunofluorescence microscopy to visualize β-galactosidase and active caspase-3 (Fig. 1C). HeLa cells transfected with si-FAST-1 (Fig. 1C) or si-FAST-2 (data not shown) exhibit cytoplasmic and nuclear condensation and activation of caspase 3. These results are quantified in Fig. 1D. These results reveal that FAST is a survival protein that prevents the spontaneous (or transfection-induced) activation of caspase 3 and apoptosis.
FIG. 1.
Knocking down FAST results in apoptosis. (A) COS-7 cells were transfected with pMT2-HA-FAST together with a vector-based negative control RNAi (pSuppressor2-SiNC), FAST RNAi-1 (pSuppressor2-SiFAST-1), or FAST RNAi-2 (pSupressor2-SiFAST-2). After 48 h, cells were processed for Western blotting analysis to quantify the expression of recombinant HA-FAST. (B) HeLa cells were transfected with either negative control RNAi (SiNC) or FAST RNAi (SiFAST-1). After 48 h, cells were processed for Western blotting analysis to quantify the expression of endogenous FAST. (C) HeLa cells were transfected with pcDNA3-β-galactosidase together with either negative control RNAi (SiNC) or FAST RNAi (siFAST-1 or siFAST-2) before being processed for immunofluorescence microscopy using anti-β-galactosidase, anti-active caspase 3, or Hoechst dye. Arrows point out healthy transfected cells. Arrowheads point out transfected cells that are undergoing apoptosis. Size bar, 20 μm. (D) The mean percentages (±standard errors; n = 3) of transfected cells (revealed using anti-β-galactosidase) that exhibit active caspase-3 are presented as a bar graph. Calculated P values for selected comparisons are shown. β-gal, β-galactosidase.
FAST inhibits Fas- and UV-induced activation of caspase 3.
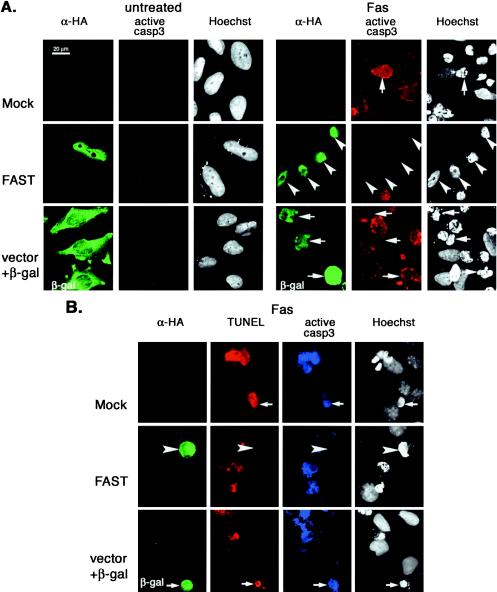
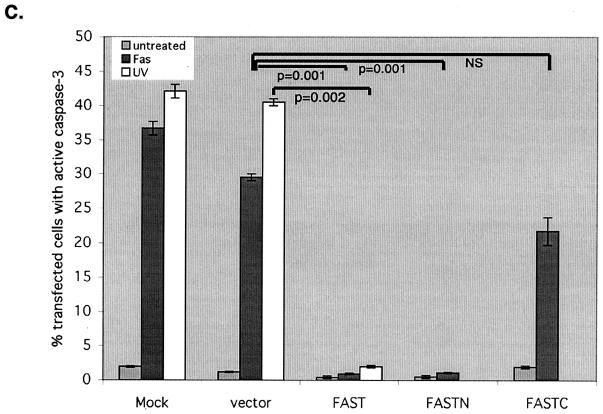
It was previously reported that FAST is dephosphorylated in Jurkat cells subjected to Fas ligation (33), and it has been suggested that FAST may regulate Fas-induced apoptosis (17). We therefore tested whether overexpression of recombinant FAST inhibits Fas-induced apoptosis. HeLa cells were transfected with HA-FAST or a vector control together with β-galactosidase (to identify transfected cells), and then they were cultured in the absence or presence of anti-Fas antibody before processing for three-color immunofluorescence microscopy to quantify the activation of caspase 3 (anti-active caspase 3) and to assess nuclear morphology (Hoechst) (Fig. 2A). Although endogenous FAST is localized to mitochondria and the nucleus (17), overexpressed recombinant HA-FAST has a heterogenous distribution (17). In some cells it is predominantly nuclear (Fig. 2A), whereas in other cells it is found in both the nucleus and the cytoplasm. In untreated cells, nuclear morphology is similar in transfected and untransfected cells and caspase 3 is not activated (Fig. 2A, left panels). In cells treated with anti-Fas, mock-transfected cells (treated with SuperFect in the absence of plasmid DNA; designated Mock in Fig. 2A), untransfected cells, and cells transfected with vector plus β-galactosidase (Fig. 2A) commonly exhibit active caspase 3 as well as nuclear condensation and fragmentation. In contrast, HA-FAST transfectants very rarely contain active caspase 3 (Fig. 2A). Although HA-FAST transfectants exhibit nuclear and cytoplasmic condensation, they do not undergo nuclear fragmentation (i.e., condensed nuclei do not exhibit typical nuclear blebs). The TUNEL assay reveals that FAST transfectants do not exhibit apoptotic DNA cleavage (Fig. 2B). In mock-transfected cells or cells transfected with vector control plus β-galactosidase, all of the cells expressing active caspase 3 (Fig. 2B, blue coloring) are also stained by the TUNEL assay (Fig. 2B, red). In contrast, HA-FAST transfectants are uniformly negative for both active caspase 3 and TUNEL staining (Fig. 2B). Despite the absence of active caspase 3 and the lack of DNA fragmentation, HA-FAST transfectants round up and detach from the plate, indicating that they are not spared from Fas-induced death. This result is consistent with the known ability of Fas ligation to trigger caspase-independent death (12). Thus, in HA-FAST-overexpressing cells, some components of the death program are intact, whereas others are absent. The mean percentage (±standard error; n = 3 independent experiments) of transfected cells showing activation of caspase 3 in response to various stimuli is quantified in Fig. 2C. The antiapoptotic effects of FAST are not restricted to Fas-induced apoptosis: FAST is also a potent inhibitor of UV-induced apoptosis (Fig. 2C). Like full-length FAST, FASTN (a truncation mutant comprising amino acids 1 to 372 [17]) prevents the Fas-induced activation of caspase 3. In contrast, FASTC (a truncation mutant comprising amino acids 372 to 550 that resides at the mitochondrial membrane in association with BCL-XL [17]) does not significantly affect Fas-induced activation of caspase 3.
FIG. 2.
FAST inhibits Fas- and UV-induced apoptosis. (A) HeLa cells were mock transfected or transfected with vectors encoding HA-FAST or β-galactosidase, cultured in the absence (untreated) or presence of anti-Fas antibody (FAS) before being processed for immunofluorescence microscopy using anti-HA, anti-β-galactosidase, anti-active caspase 3, and Hoechst dye. Arrows point out cells undergoing Fas-induced caspase-dependent apoptosis. Arrowheads point out FAST transfectants in which caspase 3 is not activated. Size bar, 20 μm. (B) HeLa cells were mock transfected or transfected with either FAST or β-galactosidase (β-gal), cultured in the presence of anti-Fas antibody, and then processed for TUNEL analysis (red) and immunofluorescence (anti-HA, green; anti-active caspase 3, blue) or Hoechst staining. Arrows point out untransfected cells or β-galactosidase-transfected cells. Arrowheads point out FAST transfectants. (C) The mean percentages (±standard errors; n = 3) of transfected cells (vector control, HA-FAST, HA-FASTN, or HA-FASTC; revealed using anti-HA or anti-β-galactosidase) cultured under the indicated conditions that exhibit active caspase 3 are presented as a bar graph. Calculated P values for selected comparisons are shown.
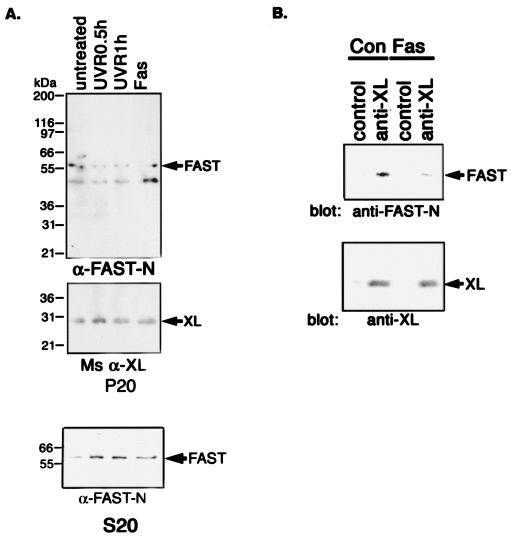
Fas ligation and UV irradiation displace FAST from mitochondria and disrupt FAST-BCL-XL interactions.
The ability of FASTN, but not FASTC, to inhibit the activation of caspase 3 indicates that mitochondrial tethering is not required for the antiapoptotic effects of FAST. We used subcellular fractionation and coimmunoprecipitation analysis to determine whether FAST is displaced from the mitochondrial membrane in response to apoptotic stimuli. HeLa cells were left untreated or were treated with UV irradiation or anti-Fas antibody, and then they were processed to prepare fractions enriched in mitochondria (20,000 × g for the pellets designated P20) as well as supernatants containing soluble material (S20) (17). Western blotting analysis was used to quantify the amounts of FAST and BCL-XL in the pellets and the amount of FAST in the corresponding supernatants (S20). As shown in Fig. 3A, both UV irradiation and anti-Fas antibodies reduce the amount of FAST, but not BCL-XL, found in the P20 fraction. Importantly, the amount of FAST found in the S20 fraction was correspondingly increased, indicating that the expression of FAST is not reduced by these treatments. These results suggest that FAST is selectively displaced from the mitochondria in cells undergoing UV- or anti-Fas antibody-induced apoptosis. In a parallel analysis, HeLa cells cultured in the absence or presence of anti-Fas antibody were solubilized, immunoprecipitated with anti-BCL-XL or control antisera, and separated by SDS-polyacrylamide gel electrophoresis (PAGE). Immunoblotting analysis revealed that FAST coprecipitates with BCL-XL, as previously reported (17). In cells undergoing Fas-induced apoptosis, the amount of coprecipitated FAST was significantly reduced (Fig. 3B). This result indicates that FAST is displaced from BCL-XL in cells undergoing Fas-induced apoptosis.
FIG. 3.
(A) Stress stimuli displace endogenous FAST from mitochondria. HeLa cells were left untreated or were treated with UV irradiation (10 mJ/cm2 followed by 0.5 or 1 h recovery) or anti-Fas antibody (1:200 dilution from culture supernatants) for 6 h before being processed to obtain pellets enriched in mitochondria (P20) and corresponding supernatants (S20). Individual fractions were processed for Western blotting analysis with anti-FASTN antibody or anti-BCL-XL antibody. (B) Fas ligation interrupts FAST/BCL-XL interaction. HeLa cell extracts prepared from cells cultured in the absence or presence of anti-Fas antibody were processed for coimmunoprecipitation with either rabbit anti-BCL-XL antibody or an isotype-matched control antibody (rabbit anti-Myc). Immunoprecipitates were then processed for Western blotting analysis with anti-FAST-N antibody or mouse anti-BCL-XL antibody.
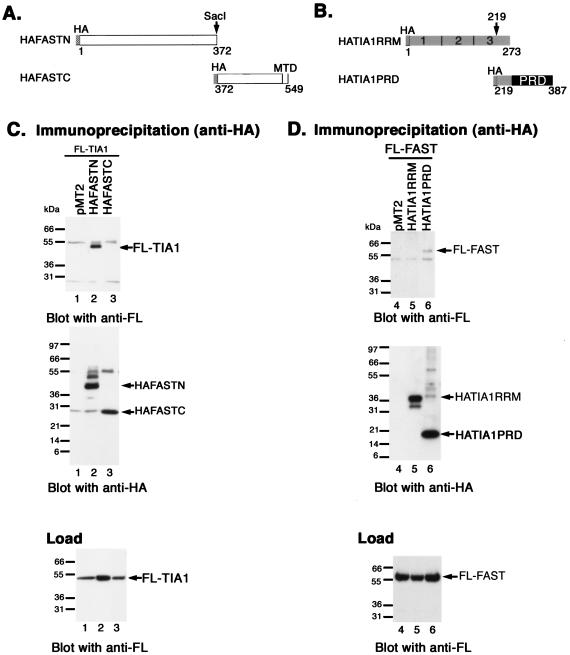
FAST-TIA-1 interactions.
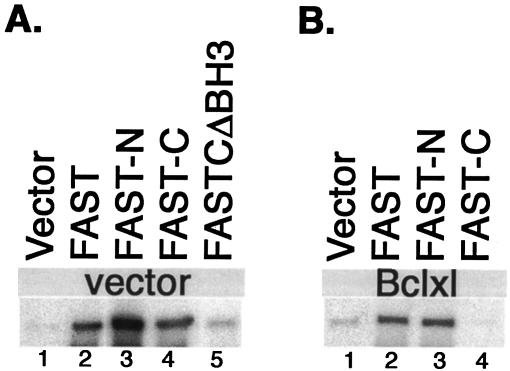
It was previously reported that FAST binds to TIA-1, an RNA-binding protein that promotes stress-induced translational arrest (13-15) and apoptosis (33). We used coimmunoprecipitation analysis of truncation mutants to map the interaction domains within FAST and TIA-1. COS-7 cells were cotransfected with FLAG-TIA-1 together with pMT2 vector pMT2-HA-FASTN or pMT2-HA-FASTC (Fig. 4A). Cell lysates were immunoprecipitated with anti-HA and then were sequentially immunoblotted with anti-FLAG and anti-HA (Fig. 4C). Lysates were also immunoblotted with anti-FLAG to quantify the expression of FLAG-TIA-1 (Fig. 4C, load). The immunoprecipitates revealed that FLAG-TIA-1 interacts with HA-FASTN but not HA-FASTC. Thus, the TIA-1 interaction domain and the caspase 3 inhibitory domain (see Fig. 2) are both encoded by FASTN. We also cotransfected COS-7 cells with FLAG-FAST together with pMT2 vector, pMT2-HA-TIA-1-RRM123 (a truncation mutant comprising amino acids 1 to 273 that encodes the RNA-binding domains of TIA-1) or pMT2-TIA-1-PRD (a truncation mutant comprising amino acids 219 to 387 that encodes the prion-related protein interaction domain of TIA-1) (Fig. 4B). Immunoprecipitation analysis revealed that FAST binds to TIA-1-PRD but not to TIA-1-RRM (Fig. 4D).
FIG. 4.
Identification of FAST/TIA1 interaction sites. (A) Schematic depiction of HA-FASTN and HA-FASTC truncation mutants. MTD, mitochondrial tethering domain; BH3, BCL2 homology 3-related domain. (B) Schematic depiction of HA-TIA-1 truncation mutants. 1, 2, 3, RNA-recognition motifs; PRD, prion-related domain. (C) COS-7 cells were cotransfected with pcDNA3-FLAG-TIA1 and the indicated HA-FAST truncation mutants. After 28 h, cells were processed for coimmunoprecipitation analysis with mouse anti-HA antibody followed by Western blotting analysis with mouse anti-FLAG antibody, followed by mouse anti-HA antibody. Lysates from transfected cells were analyzed by Western blotting with anti-FLAG antibody (load). (D) COS-7 cells were cotransfected with pcDNA3-FLAG-FAST and the indicated HA-TIA1 truncation mutants. Lysates from transfected cells were immunoprecipitated with anti-HA antibody and then were sequentially blotted with anti-FLAG antibody and anti-HA antibody. Lysates from transfected cells were analyzed by Western blotting with anti-FLAG antibody (load). FL, FLAG.
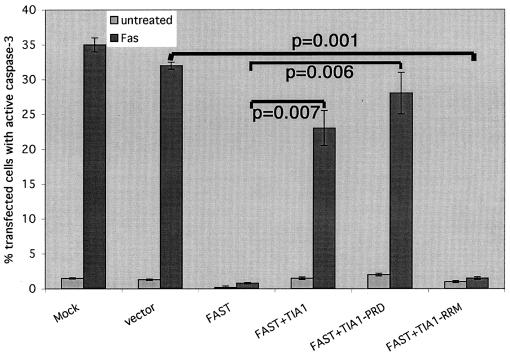
Our results reveal that FASTN binds to TIA-1 and also inhibits Fas-induced apoptosis. To determine whether the antiapoptotic effects of FAST are modulated by TIA-1, we transfected HeLa cells with FAST in the absence or presence of TIA-1, TIA-1-RRM, or TIA-1-PRD. TIA-1 and TIA-1-PRD, but not TIA-1-RRM, prevent FAST-mediated inhibition of caspase 3 activation (Fig. 5). This result suggests that interactions between FAST and TIA-1 modulate the antiapoptotic effects of FAST.
FIG. 5.
TIA-1 inhibits the antiapoptotic effects of FAST. HeLa cells were transfected with the indicated constructs, cultured in the absence (untreated) or presence (Fas) of anti-Fas antibody, and then processed for immunofluorescence microscopy using anti-HA (or anti-β-galactosidase), anti-active caspase 3, or Hoechst dye. The mean percentages (±standard errors; n = 3) of transfected cells (revealed using anti-HA or anti-β-galactosidase) cultured under the indicated conditions that exhibit active caspase 3 are presented as bar graphs. Calculated P values for selected comparisons are shown.
Interactions between FAST, TIA-1, and BCL-XL regulate protein expression.
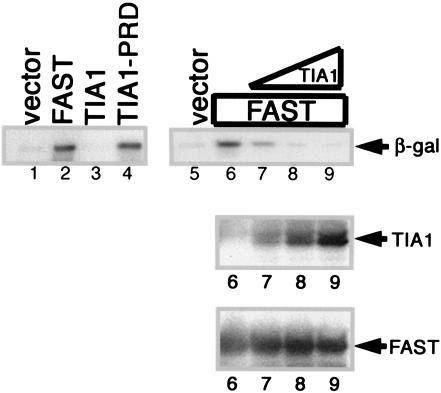
Our data reveal that interactions between FAST and TIA-1 can influence the activation of caspase 3. It was previously reported that TIA-1 is a translational silencer that inhibits the expression of tumor necrosis factor alpha (TNF-α) in macrophages (23) as well as cotransfected reporter proteins in COS-7 cells (14). In contrast, TIA-1-PRD functions as a trans-dominant inhibitor of endogenous TIA-1 and enhances the expression of cotransfected reporter proteins (14). In COS-7 cells transfected with pcDNA3-β-gal, TIA-1 inhibits, and TIA-1-PRD promotes, the expression of β-galactosidase (Fig. 6, left panel). Like TIA-1-PRD, FAST strongly enhances the expression of β-galactosidase in this assay. This effect is reversed in a dose-dependent manner by cotransfection with TIA-1. These results reveal that FAST and TIA-1 antagonistically regulate the expression of β-galactosidase in this assay.
FIG. 6.
FAST enhances the expression of cotransfected β-galactosidase. The left panel shows COS-7 cells cotransfected with a β-galactosidase reporter together with pMT2 alone, pMT2-HA-FAST, pMT2-HA-TIA-1, or pMT2-HA-TIA-1-PRD. After 48 h, cells were processed for Western blotting analysis to quantify the expression of β-galactosidase (β-gal). The right panel shows COS-7 cells cotransfected with a β-galactosidase reporter together with pMT2-HA-FAST and increasing amounts of pMT2-HA-TIA-1 (5:1, 2:1, 1:1). After 48 h, cells were processed for Western blotting analysis to quantify the expression of β-galactosidase and the expression of recombinant TIA-1 and FAST.
If enhanced protein expression is required for the antiapoptotic effects of FAST, then FASTN, but not FASTC, should promote the expression of cotransfected β-galactosidase. Surprisingly, FAST, FASTN, and FASTC all enhance the expression of β-galactosidase in this assay (Fig. 7A). Because FASTC possesses the mitochondrial tethering domain and the BCL-XL interaction domain, we suspected that its effects on β-galactosidase expression might result from displacement of endogenous FAST from the mitochondrial membrane. Consistent with this hypothesis, FASTCΔBH3 (a truncation mutant lacking its BCL-XL-binding domain [17]) only slightly enhances the expression of cotransfected β-galactosidase (Fig. 7A, lane 5). Moreover, cotransfection with BCL-XL eliminates the ability of FASTC (but not FAST or FASTN) to enhance the expression of β-galactosidase (Fig. 7B). Taken together, these results demonstrate that the ability of FAST to enhance protein expression correlates with its ability to inhibit apoptosis.
FIG. 7.
FASTC increases reporter gene expression and is regulated by BCL-XL. (A) COS-7 cells were cotransfected with a β-galactosidase reporter together with pcDNA3 vector and pMT2 alone, pMT2-HA-FAST, pMT2-HA-FASTN, pMT2-HA-FASTC, or pMT2-HA-FASTCΔBH3. After 48 h, cells were processed for Western blotting analysis to quantify the expression of β-galactosidase. (B) COS-7 cells were cotransfected with a β-galactosidase reporter together with pcDNA3-BCL-XL and pMT2 alone, pMT2-HA-FAST, pMT2-HA-FASTN, or pMT2-HA-FASTC.
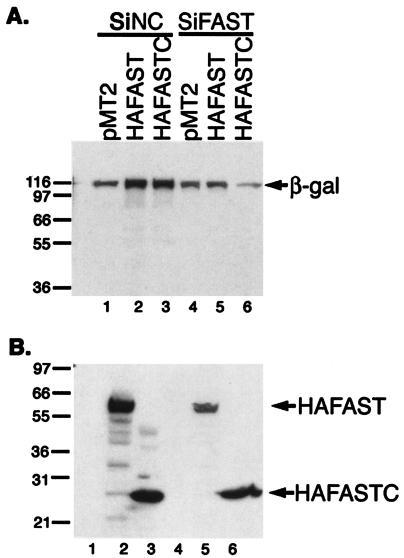
To confirm that FASTC requires endogenous FAST to enhance the expression of β-galactosidase, we used siFAST-1 to selectively reduce the expression of endogenous FAST in FASTC transfectants. COS-7 cells were transfected with either a control siRNA or siFAST-1, together with β-galactosidase and either pMT2 vector, pMT2-HA-FAST or pMT2-HA-FASTC. Cell lysates were subjected to immunoblotting analysis to quantify the expression of β-galactosidase (Fig. 8A) as well as HA-FAST and HA-FASTC (Fig. 8B). In cells treated with control siRNA, HA-FAST and HA-FASTC were expressed at similar levels (Fig. 8B, lanes 2 and 3), and both enhanced the expression of β-galactosidase (Fig. 8A, lanes 2 and 3). In cells treated with siFAST-1, the expression of HA-FAST, but not HA-FASTC, was significantly reduced (Fig. 8B, lanes 5 and 6), consistent with the fact that siFAST-1 targets a sequence that is not present in FASTC. Despite the high expression of HA-FASTC, the expression of β-galactosidase was significantly less than that in the vector control. This suggests that the FASTC-mediated increase in β-galactosidase expression is dependent upon endogenous FAST.
FIG. 8.
Effects of siFAST-1 on β-galactosidase expression. COS-7 cells were cotransfected with a β-galactosidase (β-gal) reporter together with pMT2-HA-FAST, pMT2-HA-FASTN, or pMT2-HA-FASTC and either pSupressor2-siNC (lanes 1 to 3) or pSupressor-2-siFAST-1 (lanes 4 to 6). After 48 h, cells were processed for Western blotting analysis to quantify the expression of β-galactosidase (A) or individual HA-FAST constructs (B). Molecular size markers are shown at the left. Bands corresponding to β-galactosidase, HA-FAST, and HA-FASTC are indicated by arrows.
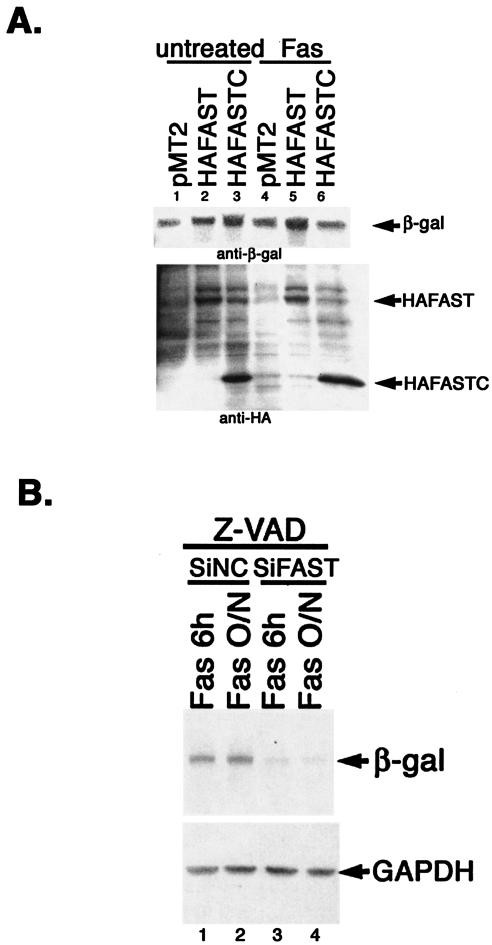
In HeLa cells, HA-FAST inhibits Fas-induced apoptosis, whereas HA-FASTC does not (Fig. 2). We therefore compared the ability of HA-FAST and HA-FASTC to promote the expression of β-galactosidase in HeLa cells cultured in the absence or presence of anti-Fas antibody. As shown in Fig. 9A, both HA-FAST and HA-FASTC enhance the expression of β-galactosidase in untreated HeLa cells, consistent with results obtained with COS-7 cells. In HeLa transfectants treated with anti-Fas antibody for 6 h (a time at which there is no morphological evidence of apoptotic cell death), the expression of β-galactosidase is increased compared to that of untreated cells. In cells treated with anti-Fas, HA-FAST, but not HA-FASTC, further enhances the expression of β-galactosidase (Fig. 9A, lanes 5 and 6). The expression of HA-FASTC is not reduced under these conditions (Fig. 9A, lower panel), indicating that the reduced expression of β-galactosidase is selective and not due to cell loss from apoptosis. Taken together, these results suggest that general protein expression is increased in cells subjected to Fas ligation. Under these conditions, HA-FAST, but not HA-FASTC, further enhances protein expression. The difference may indicate that HA-FASTC enhances protein expression indirectly by displacing endogenous FAST from the outer mitochondrial membrane. Thus, the inability of HA-FASTC to enhance protein synthesis correlates with its inability to inhibit Fas-induced apoptosis.
FIG. 9.
Fas ligation modulates the expression of β-galactosidase. (A) HeLa cells were cotransfected with a β-galactosidase reporter together with pMT2 vector, pMT2-HA-FAST, or pMT2-HA-FASTC, and then they were cultured in the absence or presence of anti-Fas antibody before being processed for Western blotting analysis to quantify the expression of β-galactosidase (β-gal) (upper panel), HA-FAST, and HA-FASTC (lower panel). (B) HeLa cells were transfected with negative control siRNA or siFAST-1 and cultured for 28 or 36 h in the presence of z-VAD (100 nM; Enzyme Systems Products), and then they were treated with anti-Fas antibody for 6 h or overnight, as indicated, before being processed for immunoblotting analysis to quantify the expression of β-galactosidase and endogenous GAPDH.
If the increased expression of β-galactosidase observed in cells treated with anti-Fas antibodies is due to the liberation and/or activation of endogenous FAST, the expression of β-galactosidase should be reduced in cells treated with siFAST-1. To test this hypothesis, HeLa cells were transfected with siRNA control or siFAST-1 and then were treated with anti-Fas antibody in the presence of z-VAD (added to inhibit siFAST-induced apoptosis). Under these conditions, siFAST-1 significantly reduced the expression of β-galactosidase (Fig. 9B), suggesting that the increased protein expression observed in cells treated with anti-Fas antibody is dependent upon endogenous FAST.
Evidence that FAST modulates the NF-κB-dependent survival pathway.
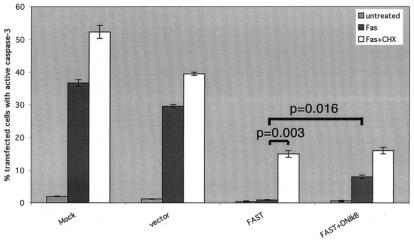
Although FASTN is not tethered to mitochondria, it binds to TIA-1, enhances reporter protein expression, and inhibits apoptosis. The antiapoptotic effects of FAST are unlikely to target mitochondrion-associated regulators of apoptosis. The correlation between TIA-1 binding, reporter protein expression, and inhibition of apoptosis suggests that FAST might inhibit TIA-1-induced translational silencing directly and that the antiapoptotic effects of FAST may result from increased expression of one or more antiapoptotic proteins. In HeLa cells, Fas ligation leads to the NF-κB-dependent transcription of several caspase inhibitors (e.g., c-IAP-1, c-IAP-2, and c-FLIP) (19). Pharmacologic inhibitors of translation (e.g., cycloheximide) potentiate Fas-induced apoptosis by blocking the expression of these caspase inhibitors (19). We therefore examined the effects of cycloheximide and mutant IκBαΔN (an IκBα truncation mutant that functions as a dominant inhibitor of NK-κB [6]) on Fas-induced apoptosis in FAST transfectants. As shown in Fig. 10, both cycloheximide and IκBαΔN partially reverse the FAST-mediated inhibition of caspase activation. These results support the hypothesis that the antiapoptotic effects of FAST are mediated in part by enhanced expression of NF-κB-induced antiapoptotic proteins.
FIG. 10.
The antiapoptotic effects of FAST require active NF-κB and ongoing protein synthesis. HeLa cells were transfected with the indicated constructs and then were cultured in the absence (untreated) or presence of anti-Fas antibody (Fas), with or without cycloheximide (CHX; 0.025 μg/ml), before being processed for immunofluorescence microscopy using anti-HA (or anti-β-galactosidase), anti-caspase 3, or Hoechst dye. The mean percentages (±standard errors; n = 3) of transfected cells (revealed using anti-HA or anti-β-galactosidase) that exhibit active caspase-3 are presented as bar graphs. Calculated P values for selected comparisons are shown.
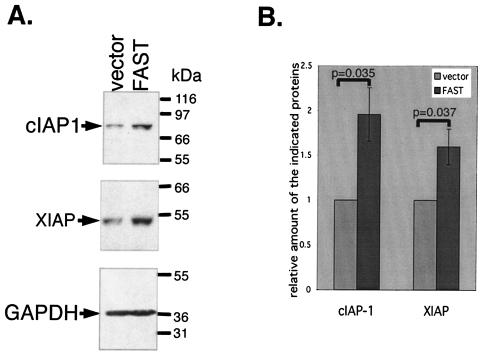
FAST enhances expression of endogenous cIAP-1 and XIAP.
To determine whether FAST increases the expression of endogenous inhibitors of apoptosis, we transfected HeLa cells with recombinant FAST or a vector control. After 48 h, cells were processed for Western blotting analysis to quantify the expression of cIAP-1, XIAP, and GAPDH. Figure 11A shows that expression of endogenous cIAP-1 and XIAP, but not GAPDH, is increased in FAST transfectants compared to that in controls. The increased expression of cIAP-1 and XIAP is impressive considering that only transfected cells (20 to 30%) are subject to the regulatory effects of FAST. The significance of this result was verified by quantifying the expression of cIAP-1 and XIAP by using densitometric scanning in three independent experiments and subjecting the results to statistical analysis. Thus, recombinant FAST selectively induces the expression of inhibitors of apoptosis in HeLa transfectants.
FIG. 11.
FAST promotes the expression of cIAP-1 and XIAP in HeLa transfectants. (A) HeLa cells were transfected with cDNAs encoding a vector control or recombinant FAST. After 48 h, cells were processed for Western blotting to quantify the expression of endogenous cIAP-1, XIAP, and GAPDH. These blots are representative of three independent experiments. (B) The expression of cIAP-1 and XIAP was quantified by densitometry. Protein expression in vector transfectants was assigned a value of 1. The relative increase in expression observed in FAST transfectants is indicated as means ± standard errors (n = 3). P values were calculated using the Student's t test.
DISCUSSION
In previous work, FAST was identified as a TIA-1-interacting phosphoprotein that is rapidly dephosphorylated in Jurkat cells undergoing Fas-induced apoptosis (33). It was subsequently shown that FAST is found at the outer mitochondrial membrane, where it associates with the antiapoptotic protein BCL-XL (17). In gene array studies, FAST was found to be overexpressed in peripheral blood lymphocytes derived from patients with immune-mediated inflammatory diseases, conditions in which aberrant apoptosis contributes to disease pathogenesis (18, 21). Taken together, these results suggested that FAST might be involved in the regulation of apoptosis.
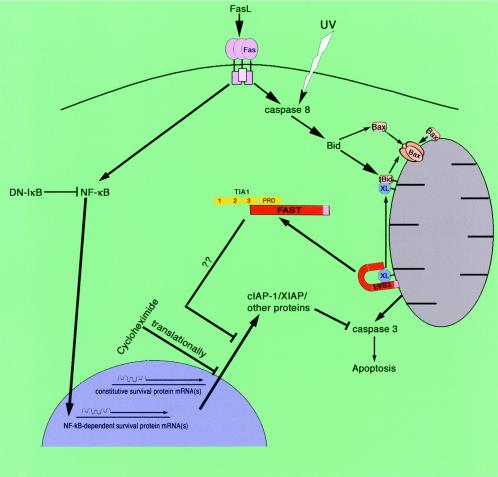
Here we report that reduced expression of endogenous FAST protein by siRNA is sufficient to induce apoptosis in HeLa cells. Conversely, overexpressed recombinant FAST is a potent inhibitor of Fas- and UV-induced apoptosis. Both Fas ligation (22) and UV irradiation (27, 28) induce the activation of caspase-8 and the cleavage of BID. We propose that FAST acts downstream of these events by serving as a sensor of BCL-2 family member dynamics at the mitochondrial membrane. In the model shown in Fig. 12, FAST resides at the outer mitochondrial membrane in association with BCL-XL (17). In response to Fas ligation or UV irradiation, tBID and BAX move to the outer mitochondrial membrane, where they sequester BCL-XL and release FAST from its mitochondrial tether. Consistent with this premise, FAST is displaced from mitochondria in HeLa cells subjected to Fas ligation or UV irradiation (Fig. 3A). FAST is also displaced from BCL-XL in response to Fas ligation (Fig. 3B).
FIG. 12.
Proposed mechanism by which FAST inhibits Fas-induced apoptosis.
Our model predicts that liberated FAST binds to TIA-1, an RNA-binding protein that acts as a translational silencer in both stressed and unstressed cells (1). TIA-1 preferentially targets mRNAs bearing adenine/uridine-rich elements (ARE) in their 3′-untranslated regions (23), although non-ARE transcripts are also regulated by TIA-1 (14). We propose that TIA-1 represses the expression of constitutive or Fas- and UV-induced survival proteins, a process that is disrupted by FAST. Thus, the antiapoptotic effects of FAST are proposed to result from increased expression of survival proteins.
Consistent with this model, FAST and TIA-1 are functional antagonists that interact to determine levels of protein expression. When transfected into COS-7 cells, recombinant FAST increases, and recombinant TIA-1 decreases, the expression of cotransfected β-galactosidase (Fig. 6). In cotransfection experiments, recombinant TIA-1 reverses the ability of recombinant FAST to enhance β-galactosidase expression (Fig. 6). Mutational analysis reveals that the functional effects of FAST require its TIA-1-binding domain: FASTN binds to TIA-1 and enhances the expression of cotransfected β-galactosidase, whereas FASTC does not bind to TIA-1 and does not directly enhance the expression of β-galactosidase. The functional effects of FASTC are complicated by its ability to interact with mitochondria and influence endogenous FAST (Fig. 7 and 8). These results suggest that FAST increases protein expression by binding to, and inhibiting the function of, TIA-1.
Our model further posits that FAST/TIA-1-regulated expression of survival proteins determines susceptibility to apoptosis. Once again, mutational analysis supports this contention: FASTN binds to TIA-1 and inhibits apoptosis, whereas FASTC does not bind to TIA-1 and does not inhibit apoptosis (Fig. 3). Moreover, overexpressed recombinant TIA-1 reverses the ability of recombinant FAST to inhibit apoptosis (Fig. 5).
The importance of the FAST-TIA-1 interactions is further illustrated by analysis of TIA-1 truncation mutants. Coimmunoprecipitation analysis reveals that FAST binds to the prion-related domain (TIA-1-PRD) but not the RNA-binding domain (TIA-1-RRM) of TIA-1. Both TIA-1-PRD (Fig. 6) and TIA-1-RRM enhance the expression of cotransfected β-galactosidase (unpublished data), suggesting that either protein can act as a dominant inhibitor of endogenous TIA-1. Despite its ability to enhance protein expression, TIA-1-PRD inhibits the antiapoptotic effects of FAST (Fig. 5). These results indicate that enhanced protein expression is not sufficient for the antiapoptotic effects of FAST. Similarly, inhibition of protein synthesis by the Drosophila death effector protein, reaper, is necessary, but not sufficient, to induce apoptotic cell death in HeLa cells (31). It is possible that TIA-1-PRD inhibits a FAST-mediated antiapoptotic function that is unrelated to protein expression. Further experiments will be required to resolve this issue.
Several observations suggest that BCL-XL regulates the function of FAST: (i) FASTC truncation mutants lacking the BCL-XL-binding domain no longer increase the expression of cotransfected β-galactosidase; (ii) cotransfection with BCL-XL reduces the ability of FASTC to increase the expression of cotransfected β-galactosidase; and (iii) Fas ligation disrupts FAST:BCL-XL interactions. The release of FAST from the mitochondrial membrane may be analogous to the release of proapoptotic factors (e.g., cytochrome c, AIF, smac/DIABLO, and Omi) from the mitochondrial interstices (7, 26). By antagonizing the effects of these proapoptotic factors, FAST may favor survival by upregulating the expression of antiapoptotic factors.
Protein translation plays an important role in regulating apoptosis. In many cell types, Fas- and TNF-induced apoptosis is potentiated by cycloheximide-induced inhibition of protein synthesis. This appears to result from reduced translation of NF-κB-induced transcripts that carry antiapoptotic genes (19). In addition, transfection of HeLa cells with the proapoptotic Drosophila proteins reaper and grim induces a general translational arrest that is required for apoptotic cell death (11, 31, 38). Thus, reaper- and grim-induced apoptosis may be mechanistically similar to stress-induced apoptosis that is mediated by the PKR/eIF2/TIA-1 pathway. Because FAST interacts with TIA-1, we hypothesized that its antiapoptotic effects might be a consequence of enhanced expression of NF-κB-induced antiapoptotic proteins. Consistent with this hypothesis, the antiapoptotic effects of FAST are partially reversed by both cycloheximide and a dominant-negative inhibitor of NF-κB (Fig. 10). The effects of the dominant-negative inhibitor of NF-κB are significantly less than the effects of cycloheximide, suggesting that FAST might also promote the expression of proteins whose expression is independent of NF-κB.
In the final step of our model (Fig. 12), we propose that FAST and TIA-1 regulate the expression of constitutive or induced survival proteins. In support of this mechanism, recombinant FAST increases the expression of endogenous cIAP-1 and XIAP, inhibitors of apoptosis that are regulated by the NF-κB pathway (30) (Fig. 11). The upregulation of these inhibitors of apoptosis is at least partially selective, as FAST does not affect the expression of endogenous GAPDH. The mechanism by which FAST enhances the expression of cIAP-1 and XIAP remains to be determined. By inhibiting the function of TIA-1, FAST might increase the expression of cIAP-1 and XIAP by promoting their translation. The posttranscriptional regulation of XIAP expression is complex. Transcripts encoding XIAP also encode an internal ribosome entry site (IRES) that enhances translation in response to stress (10). Additional experiments will be required to determine whether FAST and/or TIA-1 regulate translational initiation via the IRES element and whether upregulation of cIAP-1 and XIAP are sufficient to bring about the antiapoptotic effects of FAST.
The ability of FAST to modify the Fas-induced death program suggests that it may participate in some forms of immune-mediated inflammatory disease. Inactivating mutations of Fas leads to autoimmune disease in both rodents and humans (8, 24). Interestingly, FAST is overexpressed in patients with a variety of immune-mediated inflammatory diseases, including rheumatoid arthritis, systemic lupus erythematosus, autoimmune diabetes, and multiple sclerosis (18, 21). Overexpression or misregulation of FAST could contribute to these autoimmune syndromes by delaying the onset of apoptosis, a prelude to autoimmune disease in several experimental systems (35). Taken together, the results presented here offer an explanation of how FAST overexpression might promote the onset of immune-mediated inflammatory disease.
Acknowledgments
We thank members of the Anderson lab for helpful discussions. We thank Natalie Gilks for making the pcDNA3-FLAG-FAST and pcDNA3-FLAG-TIA-1 constructs.
This work was supported by a fellowship from the Arthritis Foundation (W.L.), a Biomedical Science Award from the Arthritis Foundation (P.A.), and by grants from the NIH (AI50167, AI33600, and AR051472).
REFERENCES
- 1.Anderson, P., and N. Kedersha. 2002. Stressful initiations. J. Cell Sci. 115:3227-3234. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, P., and N. Kedersha. 2002. Visibly stressed: the role of eIF2, TIA-1, and stress granules in protein translation. Cell Stress Chaperones 7:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber, G. N. 2001. Host defense, viruses and apoptosis. Cell Death Differ. 8:113-126. [DOI] [PubMed] [Google Scholar]
- 4.Barnhart, B., E. Alappat, and M. E. Peter. 2003. The CD95 Thpe I/type II model. Semin. Immunol. 15:185-193. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, E. H., M. C. Wei, S. Weiler, R. A. Flavell, T. W. Mak, T. Lindsten, and S. J. Korsmeyer. 2001. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell 8:705-711. [DOI] [PubMed] [Google Scholar]
- 6.Corn, R., M. Aronica, F. Zhang, Y. Tong, S. Stanley, S. J. Kim, L. Stephenson, B. Enerson, S. McCarthy, A. Mora, and M. Boothby. 2003. T cell-intrinsic requirement for NF-κB induction in post-differentiation IFN-γ production and clonal expansion in an Th1 response. J. Immunol. 171:1816-1824. [DOI] [PubMed] [Google Scholar]
- 7.Danial, N., and S. J. Korsmeyer. 2004. Cell death: critical control points. Cell 116:205-219. [DOI] [PubMed] [Google Scholar]
- 8.Dianzani, U., A. Chiocchetti, and U. Ramenghi. 2003. Role of inherited defects decreasing Fas function in autoimmunity. Life Sci. 72:2803-2824. [DOI] [PubMed] [Google Scholar]
- 9.Gil, J., J. Alcami, and M. Esteban. 1999. Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NF-κB. Mol. Cell. Biol. 19:4653-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holcik, M. 2003. Translational upregulation of the X-linked inhibitor of apoptosis. Ann. N. Y. Acad. Sci. 1010:249-258. [DOI] [PubMed] [Google Scholar]
- 11.Holley, C., M. Olson, D. Colon-Ramos, and S. Kornbluth. 2002. Reaper eliminates IAP proteins thorugh stimulated IAP degradation and generalized translational inhibition. Nat. Cell Biol. 4:439-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawahara, A., Y. Ohsawa, H. Matsumura, Y. Uchiyama, and S. Nagata. 1998. Caspase-independent cell killing by Fas-associated protein with death domain. J. Cell Biol. 143:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kedersha, N., S. Chen, N. Gilks, W. Li, I. J. Miller, J. Stahl, and P. Anderson. 2002. Evidence that ternary complex (eIF2-GTP-tRNA(i)(Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell 13:195-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kedersha, N., M. R. Cho, W. Li, P. W. Yacono, S. Chen, N. Gilks, D. E. Golan, and P. Anderson. 2000. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 151:1257-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kedersha, N. L., M. Gupta, W. Li, I. Miller, and P. Anderson. 1999. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 147:1431-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazaris-Karatzas, A., K. S. Montine, and N. Sonenberg. 1990. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature 345:544-547. [DOI] [PubMed] [Google Scholar]
- 17.Li, W., N. Kedersha, S. Chen, N. Gilks, G. Lee, and P. Anderson. 2004. FAST is a BCL-X(L)-associated mitochondrial protein. Biochem. Biophys. Res. Commun. 318:95-102. [DOI] [PubMed] [Google Scholar]
- 18.Maas, K., S. Chan, J. Parker, A. Slater, J. Moore, N. Olsen, and T. M. Aune. 2002. Cutting edge: molecular portrait of human autoimmune disease. J. Immunol. 169:5-9. [DOI] [PubMed] [Google Scholar]
- 19.Micheau, O., S. Lens, O. Gaide, K. Alevizopoulos, and J. Tschopp. 2001. NF-κB signals induce the expression of c-FLIP. Mol. Cell. Biol. 21:5299-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nachmias, B., Y. Ashhab, and D. Ben-Yehuda. 2004. The inhibitor of apoptosis protein family (IAPs): an emerging therapeutic target in cancer. Semin. Cancer Biol. 14:231-243. [DOI] [PubMed] [Google Scholar]
- 21.Neumann, E., F. Kullmann, M. Judex, H. Justen, D. Wessinghage, S. Gay, J. Scholmerich, and U. Muller-Ladner. 2002. Identification of differentially expressed genes in rheumatoid arthritis by a combination of cDNA array and RNA arbitrarily primed-PCR. Arthritis Rheum. 46:52-63. [DOI] [PubMed] [Google Scholar]
- 22.Peter, M. E., and P. H. Krammer. 2003. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 10:26-35. [DOI] [PubMed] [Google Scholar]
- 23.Piecyk, M., S. Wax, A. R. Beck, N. Kedersha, M. Gupta, B. Maritim, S. Chen, C. Gueydan, V. Kruys, M. Streuli, and P. Anderson. 2000. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 19:4154-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieux-Laucat, F., F. Le Deist, and A. Fischer. 2003. Autoimmune lymphoproliferative syndromes: genetic defects of apoptosis pathways. Cell Death Differ. 10:124-133. [DOI] [PubMed] [Google Scholar]
- 25.Rosenwald, I. 2004. The role of translation in neoplastic transformation from a pathologist's point of view. Oncogene 23:3230-3247. [DOI] [PubMed] [Google Scholar]
- 26.Saelens, X., N. Festjens, L. Walle, M. vanGurp, G. Van Loo, and P. Vandenabeele. 2004. Toxic proteins released from mitochondria in cell death. Oncogene 23:2861-2874. [DOI] [PubMed] [Google Scholar]
- 27.Scoltock, A. B., and J. A. Cidlowski. 2004. Activation of intrinsic and extrinsic pathways in apoptotic signaling during UV-C-induced death of Jurkat cells: the role of caspase inhibition. Exp. Cell Res. 297:212-223. [DOI] [PubMed] [Google Scholar]
- 28.Slee, E. A., S. A. Keogh, and S. J. Martin. 2000. Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ. 7:556-565. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava, S. P., K. U. Kumar, and R. J. Kaufman. 1998. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J. Biol. Chem. 273:2416-2423. [DOI] [PubMed] [Google Scholar]
- 30.Stehlik, C., R. de Martin, I. Kumabashiri, J. A. Schmid, B. R. Binder, and J. Lipp. 1998. Nuclear factor (NF)- κB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J. Exp. Med. 188:211-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tait, S., A. Werner, E. De Vries, and J. Borst. 2004. Mechanism of action of Drosophila reaper in mammalian cells: reaper globally inhibits protein synthesis and induces apoptosis independent of mitochondrial permeabilization. Cell Death Differ.11:800-811. [DOI] [PubMed]
- 32.Tian, Q., M. Streuli, H. Saito, S. F. Schlossman, and P. Anderson. 1991. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell 67:629-639. [DOI] [PubMed] [Google Scholar]
- 33.Tian, Q., J. Taupin, S. Elledge, M. Robertson, and P. Anderson. 1995. Fas-activated serine/threonine kinase (FAST) phosphorylates TIA-1 during Fas-mediated apoptosis. J. Exp. Med. 182:865-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuschl, T. 2001. RNA interference and small interfering RNAs. Chembiochem 2:239-245. [Online.] [DOI] [PubMed] [Google Scholar]
- 35.Utz, P. J., T. J. Gensler, and P. Anderson. 2000. Death, autoantigen modifications, and tolerance. Arthritis Res. 2:101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wendel, H. G., E. De Stanchina, J. S. Fridman, A. Malina, S. Ray, S. Kogan, C. Cordon-Cardo, J. Pelletier, and S. W. Lowe. 2004. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 428:332-337. [DOI] [PubMed] [Google Scholar]
- 37.Williams, B. R. 1999. PKR; a sentinel kinase for cellular stress. Oncogene 18:6112-6120. [DOI] [PubMed] [Google Scholar]
- 38.Yoo, S., J. Huh, I. Muro, H. Yu, L. Wang, S. Wang, R. Feldman, R. Clem, H. Muller, and B. Hay. 2002. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat. Cell Biol. 4:416-424. [DOI] [PubMed] [Google Scholar]