Abstract
Alongside the rapid growth in aging populations worldwide, prevention and therapy for age-related memory decline and dementia are in great demand to maintain a long, healthy life. Here we found that iso-α-acids, hop-derived bitter compounds in beer, enhance microglial phagocytosis and suppress inflammation via activation of the peroxisome proliferator-activated receptor γ. In normal mice, oral administration of iso-α-acids led to a significant increase both in CD11b and CD206 double-positive anti-inflammatory type microglia (p < 0.05) and in microglial phagocytosis in the brain. In Alzheimer's model 5xFAD mice, oral administration of iso-α-acids resulted in a 21% reduction in amyloid β in the cerebral cortex as observed by immunohistochemical analysis, a significant reduction in inflammatory cytokines such as IL-1β and chemokines including macrophage inflammatory protein-1α in the cerebral cortex (p < 0.05) and a significant improvement in a novel object recognition test (p < 0.05), as compared with control-fed 5xFAD mice. The differences in iso-α-acid-fed mice were due to the induction of microglia to an anti-inflammatory phenotype. The present study is the first to report that amyloid β deposition and inflammation are suppressed in a mouse model of Alzheimer's disease by a single component, iso-α-acids, via the regulation of microglial activation. The suppression of neuroinflammation and improvement in cognitive function suggests that iso-α-acids contained in beer may be useful for the prevention of dementia.
Keywords: Alzheimer disease, inflammation, microglia, neuroinflammation, phagocytosis
Introduction
With the rapid growth in aged populations, cognitive decline and dementia are becoming an increasing burden not only on patients and their families, but also on national healthcare systems worldwide. Because of the lack of a disease-modifying therapy for dementia, preventive approaches such as diet, exercise, and learning are being explored. In etiological studies of lifestyle, for example, low to moderate consumption of alcohol, such as wine and beer, might reduce the risk of developing dementia (1–3). Individuals who consumed low to moderate levels of alcoholic beverages on a daily basis were shown to have significantly lower risk of the development of cardiovascular and neurodegenerative disease, as compared with individuals who abstained from alcohol beverages or drank heavily. Apart from the effects of alcohol itself, resveratrol, a polyphenolic compound in red wine, has neuroprotective (4–6) and cardioprotective (7, 8) activities. On the other hand, beer has remained the most consumed alcoholic beverage in the world for more than a thousand years, and hops, the female inflorescences of the hop plant (Humulus lupulus L.), have been used in beer-brewing since 822; to date, however, no constituents of beer have been reported to be beneficial for preventing dementia.
Hops are used as both a preservative and a flavoring agent in the beer-brewing process. The bitter taste of beer originates from α-acids in hops. Because iso-α-acids activate the peroxisome proliferator-activated receptor-γ (PPAR-γ)2 (9–12), iso-α-acids in beer have antioxidant and anti-metabolic syndrome activities and are also reported to prevent diet-induced obesity in rodents and to improve hyperglycemia, which has also been confirmed in humans (13). PPAR-γ is known as a therapeutic target in Alzheimer disease (14), which prompted us to study the effects of iso-α-acids, as beer components, on the pathogenesis of Alzheimer's disease.
In the present study, we examined whether, as agonists of PPAR-γ, the iso-α-acids present in beer might increase microglial phagocytosis of amyloid β (Aβ) and suppress inflammation in neuronal tissue. We also evaluated the ability of iso-α-acids to prevent Alzheimer's disease-like pathology and cognitive decline in a mouse model of Alzheimer's disease.
Results
Iso-α-acids Enhanced Aβ Phagocytosis and Promoted Anti-inflammatory Activity of Primary Cultured Microglia
Primary microglia were treated with iso-α-acids, and phagocytic activity was determined by measuring the fluorescence of fluorescein amidite (FAM)-labeled Aβ. Aβ could be seen engulfed by CD11b-positive microglia (Fig. 1A), and 0.3 and 1 μm iso-α-acids (IAAs), trans-isohumulone (tIH), and cis-isohumulone (cIH) significantly enhanced microglial Aβ phagocytosis (Fig. 1B). Phagocytosis was increased in a concentration-dependent manner (Fig. 1C). Next, we examined effects of six iso-α-acids contained in hop extracts on Aβ phagocytosis activity. tIH, cIH, trans-isocohumulone, and cis-isocohumulone significantly enhanced phagocytosis, but trans-isoadhumulone and cis-isoadhumulone did not; trans-isohumulone showed the greatest effect (Fig. 1E). Flow cytometry analysis showed that iso-α-acids treatment led to significantly higher expression of the class B scavenger receptor CD36 in microglia (Fig. 1D). To examine the mechanism of the iso-α-acid-activated microglial phagocytosis, microglia were treated with the selective PPAR-γ antagonist T0070907. Neither enhanced phagocytosis nor increased surface expression of CD36 was observed in the presence of T0070907 (Fig. 1, F and G), suggesting that the Aβ phagocytosis and surface expression of CD36 induced by iso-α-acids occurred through PPAR-γ.
FIGURE 1.
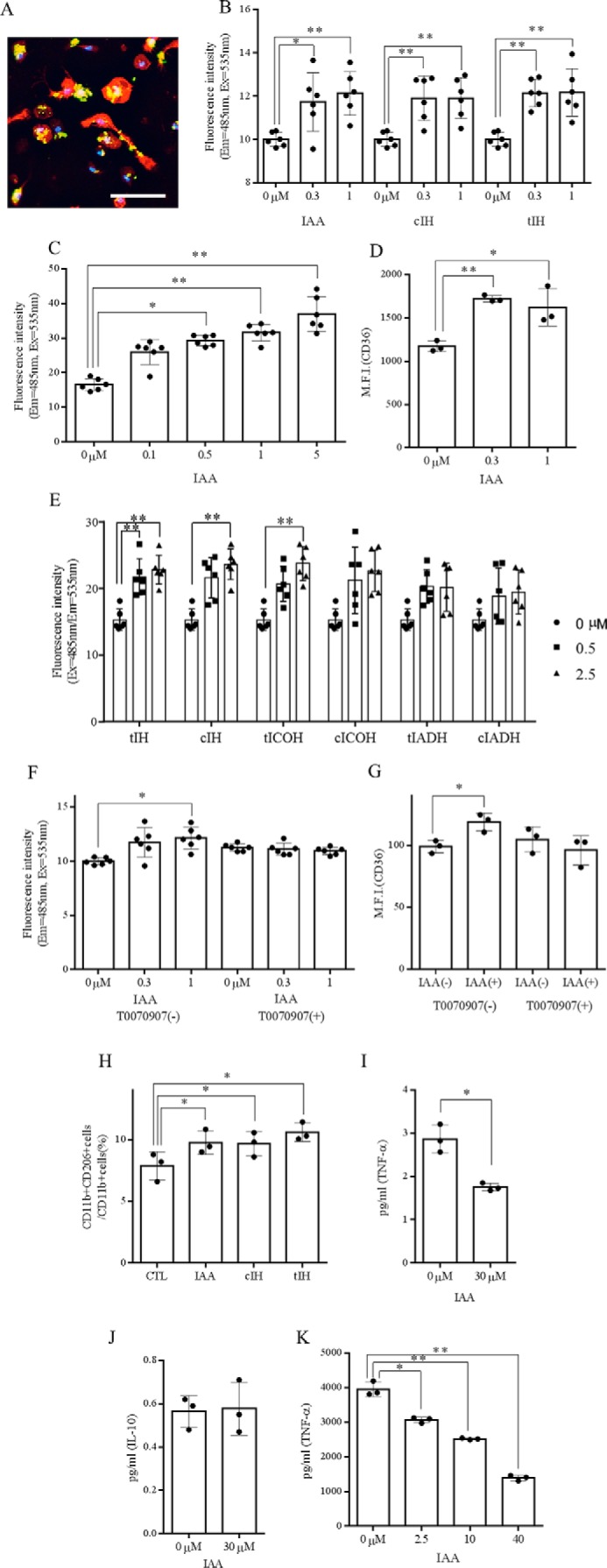
Effect of in vitro treatment with iso-α-acids on microglial phagocytic and anti-inflammatory activity. A, immunofluorescent image of cultured microglia showing CD11b-positive microglia (red) engulfing Aβ-FAM (green). DAPI-stained nuclei are shown in blue. The scale bar indicates 100 μm. B, effects of isomerized hop extract containing 0.3 or 1 μm IAAs, tIH, or cIH on in vitro phagocytosis of Aβ-FAM by CD11b-positive microglia. C, effects of 0.1–5 μm IAAs on in vitro phagocytosis of Aβ-FAM by CD11b-positive microglia. D, expression of CD36 on microglia cells treated with 0, 0.3, or 1.0 μm IAA analyzed by flow cytometry. E, microglial phagocytosis of Aβ-FAM measured after pretreatment with 0, 0.5, and 2.5 μm individual iso-α-acids: tIH, cIH, trans-isocohumulone (tICOH), cis-isocohumulone (cICOH), trans-isoadhumulone (tIADH), and cis-isoadhumulone (cIADH). F and G, phagocytosis of Aβ (F) and CD36 expression (G) in isolated microglia pretreated with 50 nm T0070907, a PPAR-γ antagonist, prior to iso-α-acid treatment. H, ratio of CD11b-positive and CD206-positive cells to CD11b-positive cells in microglia, calculated by flow cytometry after treatment with 3 μm IAA. I and J, ratio of TNF-α-positive (I) and IL-10-positive (J) cells to CD11b-positive microglia. K, concentration of TNF-α in microglial cultural supernatant after pretreatment with 0, 0.5, and 2.5 μm IAA and treatment with 5 ng/ml LPS and 0.5 ng/ml IFN-γ. The data are the means ± S.D. of 3–5 wells/sample. *, p < 0.05; **, p < 0.01.
To investigate the effects of iso-α-acids on anti-inflammatory activity, microglia treated with iso-α-acids were analyzed by flow cytometry, and the production of TNF-α in the cells was investigated after LPS stimulation. Iso-α-acids significantly increased the population of CD11b and CD206 double-positive microglia, a population known to have an M2 anti-inflammatory phenotype (24) (Fig. 1H). Iso-α-acid treatment suppressed the production of TNF-α but not that of IL-10 in CD11b-positive microglia (Fig. 1, I and J). Iso-α-acids also suppressed microglial production of TNF-α after LPS stimulation in a dose-dependent manner (Fig. 1K). Together, these results suggest that iso-α-acids activate PPAR-γ in microglia, leading to Aβ phagocytosis, increased surface expression of CD36, and anti-inflammatory activities.
Microglia from Mice Given Oral Iso-α-acids Showed Enhanced Aβ Phagocytosis
We next investigated the effects of oral administration of iso-α-acids on microglia in mouse brain. After administration of iso-α-acids at various concentrations to wild-type mice for 3 days, microglia were isolated from hippocampus. We then purified the microglial cells and assessed their ability for Aβ phagocytosis and their anti-inflammatory phenotype. Administration of iso-α-acids at 4 and 20 mg/kg significantly enhanced the ability of brain microglial cells to phagocytose Aβ (Fig. 2A). Flow cytometry analysis indicated that the ratio of CD11b and CD206 double-positive microglia to CD11b cells was significantly increased (Fig. 2, B and C) and that the expression of CD36 and CD68 in CD11b-positive cells was increased in mice given oral iso-α-acids (Fig. 2, D and E). After oral administration of iso-α-acids to mouse, various iso-α-acids were detected in brain (Fig. 3 and Table 1), suggesting that iso-α-acids that have been orally administered can penetrate into this organ. Taken together, these data show that iso-α-acids that have been orally administered can penetrate into the brain and that the brain microglia show enhanced anti-inflammation and Aβ phagocytosis abilities.
FIGURE 2.
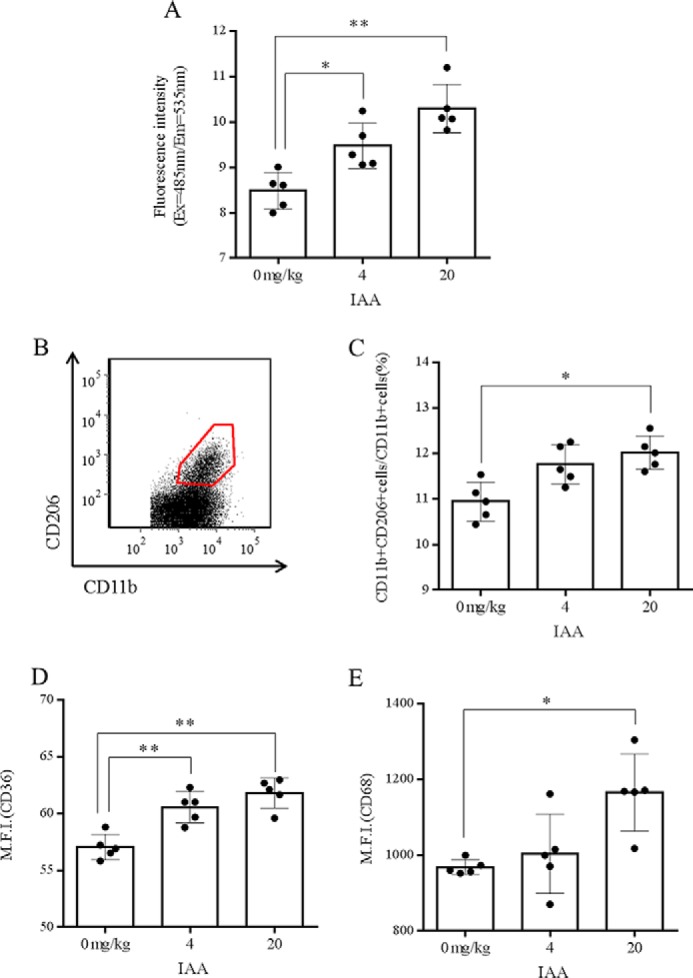
Effect of in vivo treatment with iso-α-acids on microglial phagocytic activity. A, phagocytosis of Aβ by microglia isolated from hippocampi of mice orally administered 0, 4, or 20 mg/kg of IAAs once a day for 3 days. B and C, scatter plots from flow cytometry detection of CD11b-positive cells showing the ratio of CD11b CD206 double-positive to CD11b-positive microglia under the above conditions. CD11b- and CD206-positive cells were gated as shown by the boxed area in B, and the ratio of CD11b-positive cells plus CD206-positive cells to CD11b-positive cells was calculated (C). D and E, expression of CD36 (D), and CD68 (E) in CD11b-positive microglia. The data are the means ± S.D. of 5 mice/group. *, p < 0.05; **, p < 0.01.
FIGURE 3.
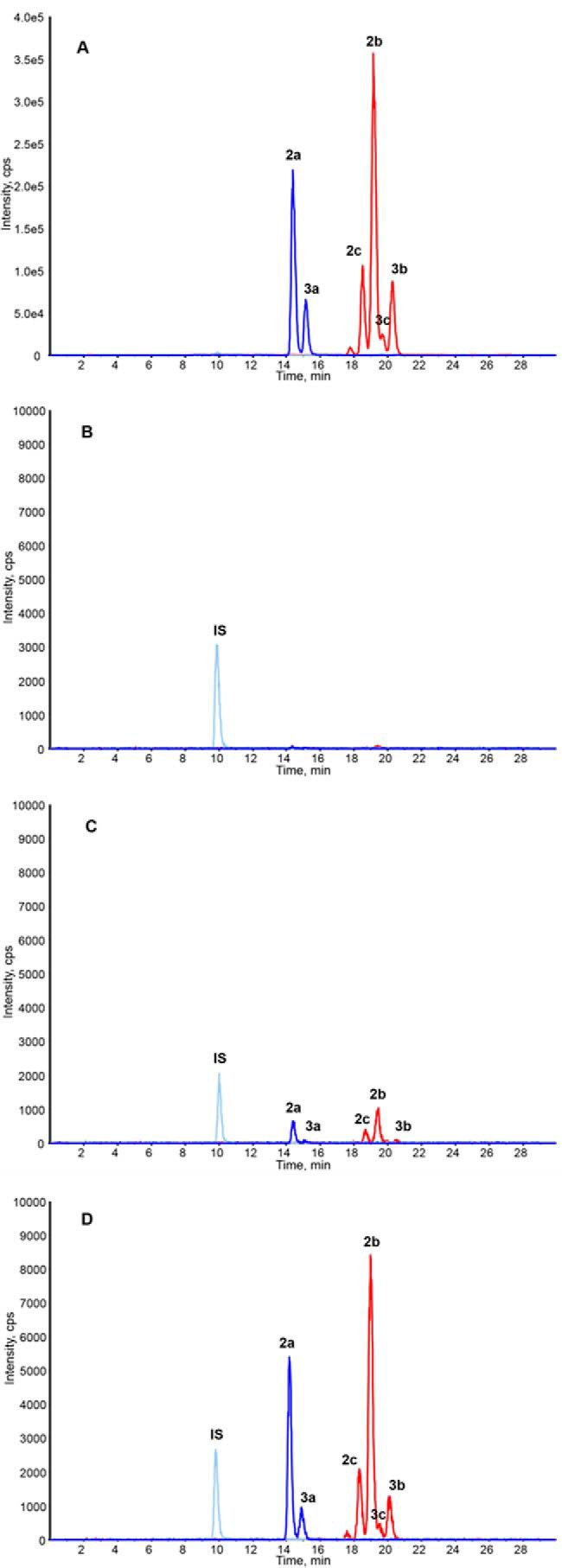
Permeability of iso-α-acids through the blood-brain barrier. A–D, HPLC-MS/MS chromatograms of an IHE (sample for administration) (A), vehicle (B), IHE containing 0.5 g/kg of iso-α-acids (C), and IHE containing 2 g/kg of iso-α-acids (D). The peaks are labeled according to the compounds shown in Fig. 7.
TABLE 1.
Iso-α-acid specific parameters for LC-MS/MS analysis
DP, declustering potential; CXP, cell exit potential; CE, collision energy.
| Compound | Q1 mass | Q3 mass | DP | CE | CXP |
|---|---|---|---|---|---|
| atomic mass units | atomic mass units | V | V | V | |
| cis-Isocohumulone (2a) | 347.00 | 250.90 | −75 | −24 | −13 |
| trans-Isocohumulone (3a) | 347.00 | 250.90 | −75 | −24 | −13 |
| cis-Isohumulone (2b) | 360.97 | 264.90 | −30 | −24 | −13 |
| trans-Isohumulone (3b) | 360.97 | 264.90 | −30 | −24 | −13 |
| cis-Isoadhumulone (2c) | 360.97 | 264.90 | −30 | −24 | −13 |
| trans-Isoadhumulone (3c) | 360.97 | 264.90 | −30 | −24 | −13 |
| trans-Dihydrohumulinic acid (IS) | 267.00 | 154.60 | −50 | −28 | −1 |
Administration of Iso-α-acids Substantially Reduced Aβ Burden and Inflammation in 5xFAD Mice
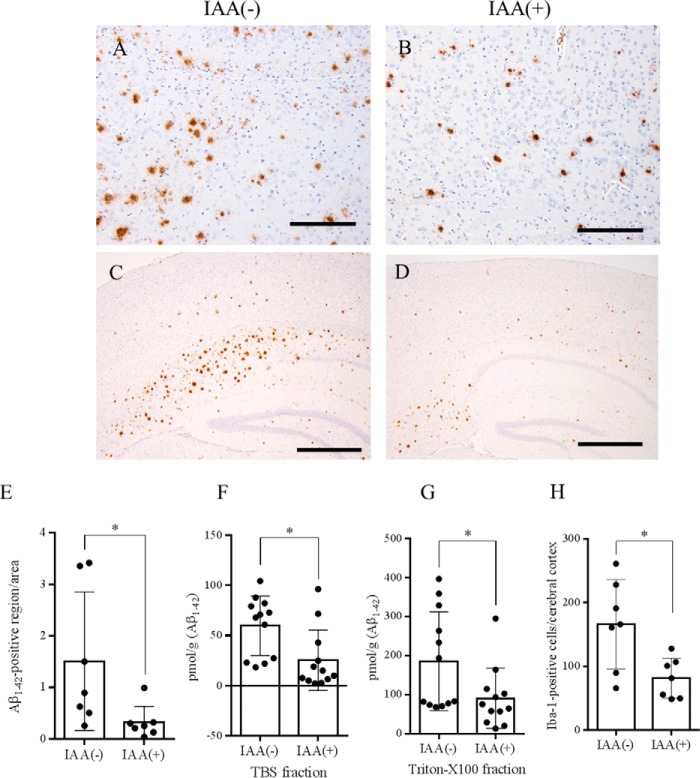
To investigate the effects of iso-α-acids in an animal model of Alzheimer's disease, 5xFAD transgenic mice were fed pellets containing 0.05% (w/w) iso-α-acids or vehicle for 3 months. As shown in Fig. 4, immunohistochemical analysis revealed a significant reduction in Aβ1–42 burden (21% versus the vehicle administration group) in cerebral cortex (Fig. 4, A–E). The immunohistochemical finding was confirmed by quantification of Aβ levels using ELISA. In the TBS-soluble fractions and TBS-insoluble and Triton X-100-soluble fractions of the cerebral cortex, Aβ1–42 levels were significantly reduced in the group given iso-α-acids (Fig. 4, F and G). Immunohistochemical analysis for microglial infiltration revealed a significant reduction in Iba-1-positive microglial infiltration in the cerebral cortex (Fig. 4H).
FIGURE 4.
Effect of oral iso-α-acids on Aβ deposition in the brain of Alzheimer's disease model mice. A–D, immunohistochemistry of Aβ1–42 distribution in the cerebral cortex of mice fed with control diet (A and C) and IAA-containing diet (B and D). E, quantification of Aβ1–42-positive areas in cerebral cortex calculated by ImageJ. F–H, soluble (F) and insoluble (G) Aβ1–42, the number of Iba-1 positive cells (H) in the cortex of 5xFAD transgenic mice fed with CTL or IAA diet for 2.5 months quantified by ELISA. The data in the ELISA assay are represented as the means ± S.E. of 13 mice (wild-type group with control diet, 5xFAD group with control diet, 5xFAD group with IAA diet), or 5 mice (wild-type group with IAA diet). The data in the immunohistochemistry represent the means ± S.D. of 6–7 mice/group. *, p < 0.05. The scale bars represent 200 μm in A and B and 500 μm in C and D.
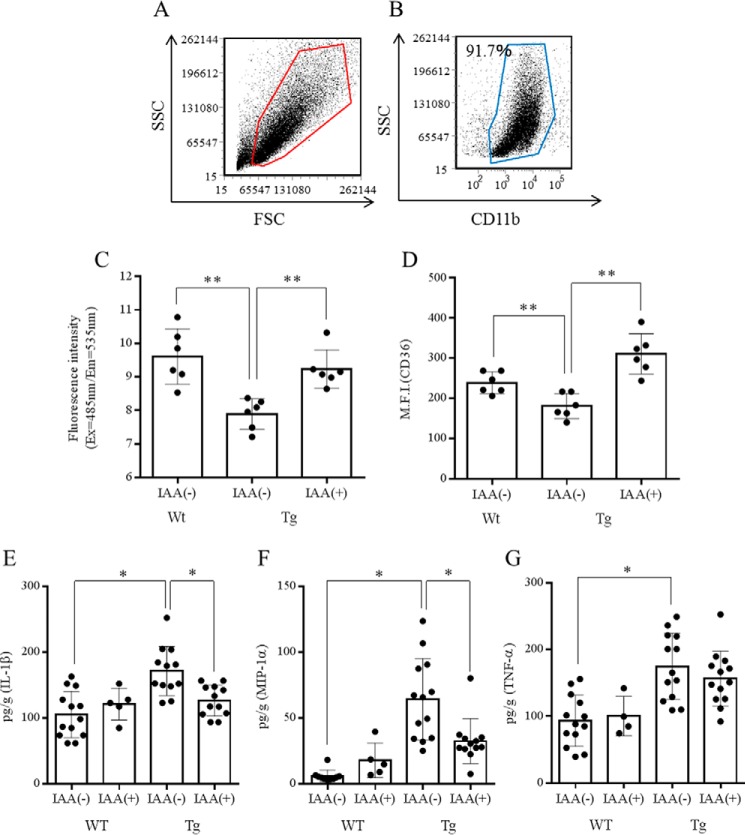
To evaluate the phenotype of microglia, microglia from 5xFAD and wild-type mice were isolated and compared in the phagocytosis assay and flow cytometry analysis (Fig. 5, A and B). Both phagocytic activity toward Aβ and expression of CD36 in microglia were significantly lower in 5xFAD mice than in age-matched control mice. Oral administration of iso-α-acids alleviated both the dysfunction of phagocytosis and the reduced expression of CD36 in 5xFAD mice (Fig. 5, C and D).
FIGURE 5.
Characterization of microglia in the brains of mice fed a diet with or without iso-α-acids for 2.5 months. A and B, purity of isolated microglia after MACS (>90%) in the scatter plot of forward scatter (FSC) and side scatter (SSC). C, Aβ phagocytosis in microglia isolated from the wild-type, control, and IAA groups. D, expression of CD36 on the surface of microglia. E–G, concentration of IL-1β (E), TNF-α (F), and MIP-1α (G) in the cerebral cortex. The data represent the means ± S.D. of 7 or 13 mice/group. *, p < 0.05; **, p < 0.01.
Next, levels of cytokines and chemokines were quantified by a Bio-Plex system. The concentrations of IL-1β, TNF-α, and MIP-1α in cerebral cortex were significantly higher in the 5xFAD mice than in age-matched control mice (Fig. 5, E and G). There are no differences in cytokine levels between wild-type mice with and those without iso-α-acid administration. Oral administration of iso-α-acids alleviated the increase in cytokine and chemokine levels in 5xFAD mice. The levels of IL-1β and MIP-1α were significantly lower in the iso-α-acids diet group than in the vehicle diet group (Fig. 5, E and G). These results suggest that oral administration of iso-α-acids suppresses Aβ deposition and inflammation in an animal model of Alzheimer's disease.
Intake of Iso-α-acids Improved Cognitive Function in 5xFAD Mice
The effects of oral administration of iso-α-acids on cognitive function in 5xFAD mice were examined by a novel object recognition test. The time spent exploring around the novel object was significantly longer for 5xFAD mice given iso-α-acids than for 5xFAD mice given vehicle (Fig. 6A). Regarding the discrimination index (15), 5xFAD mice given iso-α-acids showed a significant improvement as compared with control 5xFAD mice (Fig. 6B), indicating that administration of iso-α-acids can improve cognitive function in a mouse model of Alzheimer's disease.
FIGURE 6.
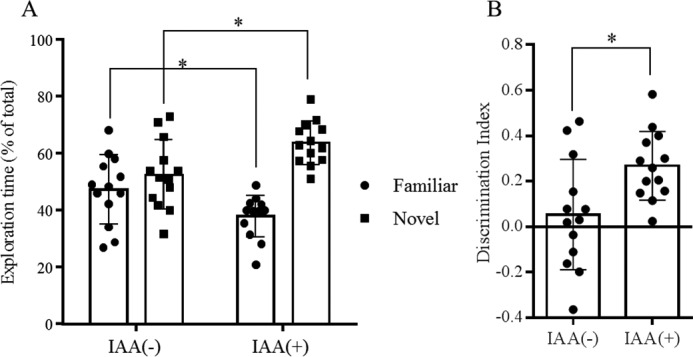
Novel object recognition test of episodic memory. A, time spent by 5xFAD transgenic mice fed a control diet or a diet containing 0.05% (w/w) IAAs for 2.5 months exploring novel and familiar objects during 5 min of re-exploration as a percentage of the total time spent exploring objects. B, discrimination index (time spent with object A − time spent with object B)/(total time exploring both objects). The data represent the means ± S.D. of 13 mice/group. *, p < 0.05.
Discussion
The present study has identified a novel physiological function of iso-α-acids, constituents of hops used in beer making, in the prevention of Alzheimer's disease-like symptoms by enhancing microglial phagocytosis, suppressing inflammation, and improving cognitive function. All six stereoisomers of the three iso-α-acid congeners enhanced microglial phagocytosis in cultured cells. In particular, trans-isohumulone and cis-isohumulone, both of which are plentiful in beer, had potent activity as compared with the other congeners.
Iso-α-acids enhanced the surface expression of CD36, which mediates the innate host response to Aβ. CD36 is known to be involved in fatty acid metabolism, heart disease, taste, and dietary fat processing in the intestine (16–21). CD36 on the surface of microglia is reported to play an important role in Aβ phagocytosis in the brain; therefore, it is thought to be a potential preventive or therapeutic target for Alzheimer's disease (22, 23). Our findings suggest that iso-α-acids enhance Aβ phagocytosis by increasing CD36 expression. Moreover, in mice given iso-α-acids orally, a transformation of CD11b-positive microglia to CD206-positive M2 anti-inflammatory type microglia was observed. Generally, type 1 microglia are involved in inflammation and M2 type in anti-inflammatory and tissue repairing processes (24–26), suggesting that iso-α-acids stimulate both microglial phagocytosis and an anti-inflammatory phenotype. Microglia treated with iso-α-acids also showed enhanced intake of foreign substances other than Aβ. On the other hand, iso-α-acids had no effects on the production of Aβ by neurons (data not shown).
Our group previously reported that iso-α-acids bind and activate PPAR-γ (9). Activation of PPAR-γ, a nuclear receptor, contributes to the enhancement of microglial phagocytosis (27–30). PPAR-γ is known as a drug discovery target to improve resistance to insulin, and pioglitazone, a potent PPAR-γ agonist (31, 32), is used to treat type 2 diabetes (33). Blockade of PPAR-γ using an antagonist reduces the phagocytosis enhanced by iso-α-acids. Activation of PPAR-γ has anti-diabetes, anti-arterial sclerosis, anti-tumor, and anti-inflammation effects (34–36), and pro-phagocytic effects on Aβ (28). Recently, PPAR-γ has been considered a potential preventive or therapeutic target for Alzheimer's disease (14). Here, iso-α-acids were found to reduce the levels of Aβ and inflammatory cytokines and to improve cognitive function in 5xFAD model mice via PPAR-γ activation.
Iso-α-acids from hops are plentiful in beer, providing bitter flavor at a concentration of about 20–40 mg/liter, and have been consumed for more than a thousand years. The safety of iso-α-acids was confirmed both in tests using rodents and in human studies. Our group previously reported that administration of iso-α-acids led to an improvement of hyperglycemia and a decrease in body fat in humans. Between 16 and 48 mg of iso-α-acids were administered in the experiment, and a dose of 32–48 mg significantly reduced fasting blood glucose in humans (13). The doses in that report were based on a study of anti-obesity effects using C57BL/6 mice (10). The amount of iso-α-acids given to 5xFAD mice in the present report was lower than that in the study of anti-obesity effects in mice. Therefore, it is expected that a dose of less than 32–48 mg of iso-α-acids would have beneficial effects on neuroprotection in humans. In summary, because iso-α-acids suppress neuroinflammation and improve cognitive function, they may be useful for the prevention of dementia.
Experimental Procedures
Animals
Alzheimer's disease model mice, B6SJL-Tg mice (APPSwFlLon,PSEN1*M146L*L286V) (37), hereafter referred to as 5xFAD transgenic mice, were purchased from The Jackson Laboratory (Sacramento, CA) and maintained by crossing hemizygous transgenic mice with B6SJLF1/J mice in the experimental facility at the University of Tokyo. The 5xFAD transgenic mice overexpress mutant human APP(695) with the Swedish (K670N,M671L), Florida (I716V), and London (V717I) familial Alzheimer's disease (FAD) mutations, along with human PS1 harboring two FAD mutations, M146L and L286V. Nontransgenic WT littermates were used in all experiments. All experiments were approved by the Animal Care and Use Committee of the Graduate School of Agricultural and Life Sciences at the University of Tokyo and conducted in strict accordance with their guidelines.
Mice under 3 months of age were fed with a standard purified rodent growth diet (AIN-93G; Oriental Yeast, Tokyo, Japan), and those over 3 months were fed with a maintenance diet (AIN-93M; Oriental Yeast). Pregnant C57BL/6J mice and 8-week-old C57BL/6J mice (Charles River Japan, Tokyo, Japan) were maintained at the Kirin Company Ltd. All experiments were approved by the Animal Experiment Committee of Kirin Company Ltd. and conducted in strict accordance with their guidelines. All efforts were made to minimize suffering.
Preparation of Iso-α-acids
α-Acids consist predominantly of three congeners, cohumulone (1a), humulone (1b), and adhumulone (1c) (Fig. 7). During the brewing process, they are each isomerized into two epimeric isomers, cis-iso-α-acids (2a–c) and trans-iso-α-acids (3a–c) (Fig. 7). A purchased isomerized hop extract (IHE) (Hopsteiner, Mainburg, Germany) with 30.5% (w/v) iso-α-acids, comprising trans-isocohumulone (1.74% w/v), cis-isocohumulone (7.61% w/v), trans-isohumulone (3.05% w/v), cis-isohumulone (14.0% w/v), trans-isoadhumulone (0.737% w/v), and cis-isoadhumulone (3.37% w/v), was used to isolate individual iso-α-acids as previously described (38).
FIGURE 7.
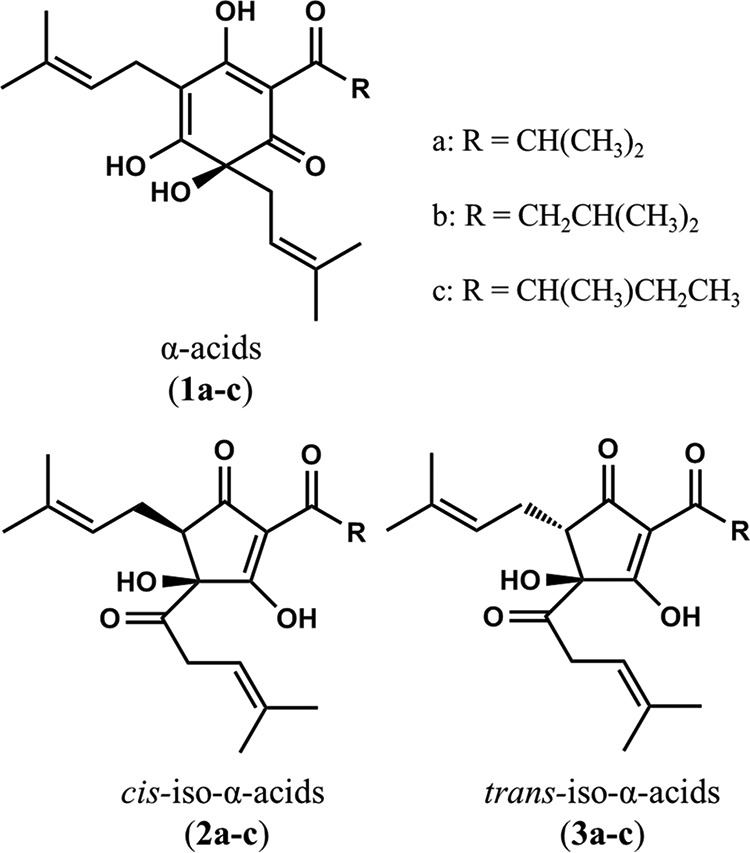
Chemical structures of α-acids and iso-α-acids. Shown are the structures of α-acids cohumulone (1a), humulone (1b), and adhumulone (1c); cis-iso-α-acids cis-isocohumulone (2a), cis-isohumulone (2b), and cis-isoadhumulone (2c); and trans-iso-α-acids trans-isocohumulone (3a), trans-isohumulone (3b), and trans-isoadhumulone (3c).
Primary Microglia Cell Culture
Primary microglial cells were isolated from mouse brain via magnetic cell sorting (MACS) after conjugation with anti-CD11b antibodies as previously described (39). Isolated CD11b-positive cells (>90% pure evaluated by flow cytometer) were plated in poly-d-lysine (PDL)-coated 96-well plates (BD Biosciences) and cultured in DMEM/F-12 (Gibco) medium supplemented with 10% fetal calf serum (Gibco) and 100 units/ml penicillin/streptomycin (Sigma-Aldrich).
Phagocytosis of 6-Carboxyfluorescein-labeled Aβ1–42 by Microglia
Microglial phagocytosis of 6-carboxyfluorescein-labeled Aβ1–42 (Aβ-FAM; AnaSpec, Fremont, CA) was evaluated using a plate-based assay as previously described (28). Microglial cells isolated from newborn mice were plated at a density of 50,000 cells/well in PDL-coated 96-well plates and incubated with 500 nm Aβ-FAM for 24 h after sample treatment for 12 h. After the medium was removed, extracellular Aβ-FAM was quenched with 0.2% trypan blue, pH 4.4. Cellular fluorescence intensity of the entire areas of 5 wells/sample was measured at 485-nm excitation/535-nm emission using a plate reader (Molecular Devices, Sunnyvale, CA). In some experiments, the cells were treated with 50 nm T0070907 (Tocris, Bristol, UK), a selective antagonist of PPAR-γ, 1 h prior to pretreatment with iso-α-acids.
In Vitro Cytokine Production Assay
Microglia isolated from newborn C57BL/6J mice were plated at a density of 30,000/well in a PDL-coated plate, treated with each sample for 12 h, and then treated with LPS (5 ng/ml; Sigma-Aldrich) and IFN-γ (0.5 ng/ml; R&D Systems, Minneapolis, MN) for 12 h. After stimulation, supernatants were applied to a TNF-α production assay. To quantify TNF-α, the supernatant was measured with an ELISA kit (eBiosciences, San Diego, CA). For measuring intracellular cytokine production, microglia were treated with leukocyte activation mixture with BD GolgiPlug (BD Biosciences) and analyzed with by flow cytometry after staining with anti-mouse CD11b-APC-Cy7 (M1/70; BD Pharmingen, San Diego, CA), anti-mouse TNF-α-FITC (MP6-XT22; BD Pharmingen), and anti-mouse IL-10-APC (JES5-16E3; BD Pharmingen) antibodies and the Cytofix/Cytoperm fixation/permeabilization kit (BD Biosciences). Populations of CD206- and CD11b-positive cells were analyzed with a flow cytometer after staining with anti-mouse CD11b-APC-Cy7 (M1/70; BD Pharmingen) or anti-mouse CD206-PerCP-Cy5.5 (C068C2; BioLegend, San Diego, CA) antibodies.
In Vivo Phagocytosis Assay
To evaluate the effects of iso-α-acids administered orally on microglial phagocytic activity, 8-week-old C57BL/6J male mice (n = 5 mice in each group) were given 0, 4, or 20 mg/kg iso-α-acids orally once a day for 3 days. 3 h after the last administration, brain microglia were isolated via MACS, and the phagocytic activity of the microglia was measured using Aβ-FAM as described above. Populations of CD206- and CD11b-positive cells and the expression of cell markers were analyzed with a flow cytometer after pretreating with a Cytofix/Cytoperm fixation/permeabilization kit (BD Biosciences) and staining with anti-mouse CD36-APC (72-1; BioLegend), anti-mouse CD68-APC (FA-11; BioLegend), anti-mouse CD11b-APC-Cy7 (M1/70; BD Pharmingen), or anti-mouse CD206-PerCP-Cy5.5 (C068C2; BioLegend) antibodies. Flow cytometry data are expressed as the mean median mode of the fluorescence intensity.
Analysis of Aβ Deposition and Inflammation in 5xFAD Transgenic Mice
To evaluate the effects of iso-α-acids on Alzheimer's-like disease, 2.5-month-old transgenic 5xFAD and wild-type male mice were fed a diet with or without 0.05% (w/w) iso-α-acids for 3 months (n = 5 in wild-type mice given iso-α-acids; n = 13 in other groups). The mice were then euthanized, and the brains were removed. The left hippocampus and cerebral cortex were homogenized in TBS buffer (Wako) with a multibead shocker (Yasui Kikai, Osaka, Japan). After centrifugation at 50,000 × g for 20 min, the supernatant was collected. The pellets were homogenized again in TBS containing 1% Triton X-100 (Wako), and the supernatant was collected after centrifugation. The total protein concentration of each supernatant was measured with a BCA protein assay kit (ThermoScientific, Yokohama, Japan). The first supernatant was assayed for quantifying soluble Aβ1–42 (Wako) by ELISA. For quantifying cytokines and chemokines, the supernatant was evaluated by a Bio-Plex assay system (Bio-Rad). The second supernatant was used for quantifying insoluble Aβ1–42 (Wako) by ELISA.
To evaluate Aβ deposition and microglial infiltration immunohistochemically, the right brain hemispheres (n = 6–7 in each group) were fixed in 10% formalin solution (Wako), paraffin-embedded, and cut in 5-μm sections. The hippocampus and cerebral cortex (bregmma 2.30 mm posterior) were analyzed with a monoclonal anti-human Aβx-42 antibody (12F4; Millipore, Billerica, MA) or a polyclonal anti-Iba-1 antibody (Wako). The size of the positive region per area was measured using ImageJ image analysis software (National Institutes of Health). To evaluate microglia activity, the right brain hemispheres (n = 7 in each group) were removed, and isolated CD11b-positive microglia were used for the phagocytosis assay and flow cytometry analysis described above.
Novel Object Recognition Test
Cognitive function was assessed in 5.5-month-old mice fed with or without 0.05% iso-α-acids. An object recognition test was performed during the light period in a translucent open box (25 × 40 × 20 cm) with an acrylic roof, placed in a rectangular room. A video camera was mounted above the field to record the trials for off-line analysis. Pairs of two different objects were used: some were 3.0 × 3.0 × 6.0 cm3, rectangular-shaped, transparent, and made of acrylic; the others were 4.5-cm diameter, 5.5-cm high, circular truncated cone-shaped, transparent, and made of grass. Two identical objects were used for habituation and two different pairs for memory testing. In all trials, the objects were placed 20-cm apart in the center of the box. After each trial, the objects were thoroughly cleaned with 70% ethanol to remove odor cues. Prior to the test, all mice were moved to the experiment room and habituated for 1 h. Then one mouse at a time was placed in the box and allowed to explore two identically shaped objects for 10 min. 1 h later, each mouse was reintroduced into the box for 5 min with two objects: a copy of a familiar object and a new object. Mice have a natural tendency to explore novel objects more than familiar objects. A discrimination index was calculated by dividing the difference in time for exploring the novel object and the familiar object by the total time spent exploring both objects: that is, (novel object exploration time − familiar object exploration time)/(total exploration time); thus, a discrimination index of 0 indicated equal exploration of both objects.
Blood-Brain Barrier Permeability
To evaluate the permeability of iso-α-acids into the brain, CD-1 male mice were orally administered 0, 0.5, or 2.0 g/kg iso-α-acids (n = 3/group). 1 h later they were euthanized with CO2 and perfused with saline. The brain was then removed and frozen in liquid nitrogen. Brains were homogenized in PBS buffer (1:1, w/v) and centrifuged at 50,000 × g for 10 min. An aliquot (100 μl) of the supernatant was collected, and a 5-μl ethanol solution of trans-dihydrohumulinic acid (2.0 μg/ml) was added as an internal standard. The solution was partitioned with dichloromethane (200 μl) after acidification with 4.5 n perchloric acid (10 μl). A portion (100 μl) of the dichloromethane layer was collected, dried under a N2 stream, and dissolved in ethanol (50 μl). After filtration, this solution was analyzed by LC-MS/MS to identify iso-α-acids in the brain samples. The LC conditions were as follows: column: 100 × 2.1 mm inner diameter, 1.8 μm, ZORBAX Extend-C18 (Agilent, Santa Clara, CA); solvent: 5 mm CH3COONH4 (pH 9.95)/ethanol, 80:20 (v/v, solvent A) and acetonitrile/ethanol, 60:40 (v/v, solvent B), a linear gradient from 0% B for 0 → 3 min, 0 to 20% B in 3 → 27 min, 20 to 90% B in 27 → 27.1 min, and 90% B for 27.1 → 29 min, and an additional 5 min at 0% B for re-equilibration prior to the next injection; flow rate: 0.15 ml/min; and column temperature: 40 °C. The injection volume was 3.0 μl. A 4000 Q-Trap mass spectrometer (AB Sciex, Tokyo, Japan) was equipped with an ESI source operating in the negative ion mode. Nitrogen was used as the turbo gas at 600 °C. Ion source gases 1 and 2 were set to 60 and 50 p.s.i., respectively, the N2 curtain gas was set to 30 p.s.i., and the collision cell gas was set to 7 p.s.i. The compound-specific mass transition (Q1 → Q3), declustering potential, cell exit potential, and collision energy were optimized for each substance prior to analysis by the infusion of pure reference solutions and are summarized in Table 1. The dwell time for each mass transition was 150 ms. The ion spray voltage was set to −4500 V. Data acquisition and processing were performed using the Analyst software version 1.4.2 (AB Sciex).
Statistical Analysis
All values are expressed as means ± S.D. Data from the production assays for cytokines, chemokines, neurotrophic factors, and synaptophysin and from in vitro antagonist assays were analyzed by two-way ANOVA, followed by the Tukey-Kramer test. Data from the in vitro assays were analyzed by one-way ANOVA, followed by Tukey-Kramer's test or Student's t test. All statistical analyses were performed using the Ekuseru-Toukei 2012 software program (Social Survey Research Information, Tokyo, Japan).
Author Contributions
Y. A. conducted most of the experiments, analyzed the results, and wrote most of the paper. H. N., K. U., and A. D. conducted experiments on 5xFAD mice. Y. T. and A. H. conducted the experiment for the LC/MS/MS analysis of iso-α-acids. A. T. conducted experiments on anti-inflammation and wrote the manuscript.
Acknowledgment
We acknowledge Dr. M. Okamura for assistance in the preparation of the manuscript.
This work was supported by a Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grant-in-aid Project, Scientific Research on Innovation Area (Brain Protein Aging and Dementia control (to A. T.)) and by Japan Agency for Medical Research and Development (AMED) DEMENTIA Research and Development Project 16dk0207026h0301 (to A. T.). The authors declare that they have no conflicts of interest with the contents of this article.
- PPAR-γ
- peroxisome proliferator-activated receptor-γ
- IAA
- iso-α-acids
- Aβ
- amyloid β
- MIP-1α
- macrophage inflammatory protein-1α
- tIH
- trans-isohumulone
- cIH
- cis-isohumulone
- FAD
- familial Alzheimer's disease
- FAM
- fluorescein amidite
- IHE
- isomerized hop extract
- MACS
- magnetic cell sorting
- PDL
- poly-d-lysine
- ANOVA
- analysis of variance.
References
- 1. Matsui T., Yoshimura A., Toyama T., Matsushita S., and Higuchi S. (2011) Preventive effect of moderation in drinking on dementia. Nihon Rinsho 69, (Suppl. 10) 217–222 [PubMed] [Google Scholar]
- 2. Neafsey E. J., and Collins M. A. (2011) Moderate alcohol consumption and cognitive risk. Neuropsychiatr. Dis. Treat. 7, 465–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horvat P., Richards M., Kubinova R., Pajak A., Malyutina S., Shishkin S., Pikhart H., Peasey A., Marmot M. G., Singh-Manoux A., and Bobak M. (2015) Alcohol consumption, drinking patterns, and cognitive function in older Eastern European adults. Neurology 84, 287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arntzen K. A., Schirmer H., Wilsgaard T., and Mathiesen E. B. (2010) Moderate wine consumption is associated with better cognitive test results: a 7 year follow up of 5033 subjects in the Tromsø Study. Acta Neurol. Scand. Suppl. 2010, 23–29 [DOI] [PubMed] [Google Scholar]
- 5. Porquet D., Griñán-Ferré C., Ferrer I., Camins A., Sanfeliu C., Del Valle J., and Pallàs M. (2014) Neuroprotective role of trans-resveratrol in a murine model of familial Alzheimer's disease. J. Alzheimers Dis. 42, 1209–1220 [DOI] [PubMed] [Google Scholar]
- 6. Witte A. V., Kerti L., Margulies D. S., and Flöel A. (2014) Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 34, 7862–7870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vidavalur R., Otani H., Singal P. K., and Maulik N. (2006) Significance of wine and resveratrol in cardiovascular disease: French paradox revisited. Exp. Clin. Cardiol. 11, 217–225 [PMC free article] [PubMed] [Google Scholar]
- 8. Petrovski G., Gurusamy N., and Das D. K. (2011) Resveratrol in cardiovascular health and disease. Ann. N.Y. Acad. Sci. 1215, 22–33 [DOI] [PubMed] [Google Scholar]
- 9. Yajima H., Ikeshima E., Shiraki M., Kanaya T., Fujiwara D., Odai H., Tsuboyama-Kasaoka N., Ezaki O., Oikawa S., and Kondo K. (2004) Isohumulones, bitter acids derived from hops, activate both peroxisome proliferator-activated receptor α and γ and reduce insulin resistance. J. Biol. Chem. 279, 33456–33462 [DOI] [PubMed] [Google Scholar]
- 10. Yajima H., Noguchi T., Ikeshima E., Shiraki M., Kanaya T., Tsuboyama-Kasaoka N., Ezaki O., Oikawa S., and Kondo K. (2005) Prevention of diet-induced obesity by dietary isomerized hop extract containing isohumulones, in rodents. Int. J. Obes. (Lond.) 29, 991–997 [DOI] [PubMed] [Google Scholar]
- 11. Namikoshi T., Tomita N., Fujimoto S., Haruna Y., Ohzeki M., Komai N., Sasaki T., Yoshida A., and Kashihara N. (2007) Isohumulones derived from hops ameliorate renal injury via an anti-oxidative effect in Dahl salt-sensitive rats. Hypertens. Res. 30, 175–184 [DOI] [PubMed] [Google Scholar]
- 12. Cho Y. C., You S. K., Kim H. J., Cho C. W., Lee I. S., and Kang B. Y. (2010) Xanthohumol inhibits IL-12 production and reduces chronic allergic contact dermatitis. Int. Immunopharmacol. 10, 556–561 [DOI] [PubMed] [Google Scholar]
- 13. Obara K., Mizutani M., Hitomi Y., Yajima H., and Kondo K. (2009) Isohumulones, the bitter component of beer, improve hyperglycemia and decrease body fat in Japanese subjects with prediabetes. Clin. Nutr. 28, 278–284 [DOI] [PubMed] [Google Scholar]
- 14. Agarwal S., Yadav A., and Chaturvedi R. (2016) K peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem. Biophys. Res. Commun., in press [DOI] [PubMed] [Google Scholar]
- 15. Modi K. K., Roy A., Brahmachari S., Rangasamy S. B., and Pahan K. (2015) Cinnamon and its metabolite sodium benzoate attenuate the activation of p21rac and protect memory and learning in an animal model of Alzheimer's disease. PLoS One 10, e0130398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonen A., Campbell S. E., Benton C. R., Chabowski A., Coort S. L., Han X. X., Koonen D. P., Glatz J. F., and Luiken J. J. (2004) Regulation of fatty acid transport by fatty acid translocase/CD36. Proc. Nutr. Soc. 63, 245–249 [DOI] [PubMed] [Google Scholar]
- 17. Abumrad N. A. (2005) CD36 may determine our desire for dietary fats. J. Clin. Invest. 115, 2965–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moore K. J., Kunjathoor V. V., Koehn S. L., Manning J. J., Tseng A. A., Silver J. M., McKee M., and Freeman M. W. (2005) Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 115, 2192–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lynes M. D., and Widmaier E. P. (2011) Involvement of CD36 and intestinal alkaline phosphatases in fatty acid transport in enterocytes, and the response to a high-fat diet. Life Sci. 88, 384–391 [DOI] [PubMed] [Google Scholar]
- 20. Samovski D., Sun J., Pietka T., Gross R. W., Eckel R. H., Su X., Stahl P. D., and Abumrad N. A. (2015) Regulation of AMPK activation by CD36 links fatty acid uptake to β-oxidation. Diabetes 64, 353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sundaresan S., and Abumrad N. A. (2015) Dietary lipids inform the gut and brain about meal arrival via CD36-mediated signal transduction. J. Nutr. 145, 2195–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savill J., Hogg N., Ren Y., and Haslett C. (1992) Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 90, 1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu Y., and Ye R. D. (2015) Microglial Aβ receptors in Alzheimer's disease. Cell Mol. Neurobiol. 35, 71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cherry J. D., Olschowka J. A., and O'Banion M. K. (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J. Neuroinflamm. 11, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Orihuela R., McPherson C. A., and Harry G. J. (2016) Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 173, 649–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang Y., and Le W. (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 53, 1181–1194 [DOI] [PubMed] [Google Scholar]
- 27. Mandrekar-Colucci S., Karlo J. C., and Landreth G. E. (2012) Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer's disease. J. Neurosci. 32, 10117–10128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamanaka M., Ishikawa T., Griep A., Axt D., Kummer M. P., and Heneka M. T. (2012) PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J. Neurosci. 32, 17321–17331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ballesteros I., Cuartero M. I., Pradillo J. M., de la Parra J., Pérez-Ruiz A., Corbí A., Ricote M., Hamilton J. A., Sobrado M., Vivancos J., Nombela F., Lizasoain I., and Moro M. A. (2014) Rosiglitazone-induced CD36 up-regulation resolves inflammation by PPARγ and 5-LO-dependent pathways. J. Leukoc. Biol. 95, 587–598 [DOI] [PubMed] [Google Scholar]
- 30. Li X., Melief E., Postupna N., Montine K. S., Keene C. D., and Montine T. J. (2015) Prostaglandin E2 receptor subtype 2 regulation of scavenger receptor CD36 modulates microglial Aβ42 phagocytosis. Am. J. Pathol. 185, 230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olefsky J. M., and Saltiel A. R. (2000) PPARγ and the treatment of insulin resistance. Trends Endocrinol. Metab. 11, 362–368 [DOI] [PubMed] [Google Scholar]
- 32. Olefsky J. M. (2000) Treatment of insulin resistance with peroxisome proliferator-activated receptor γ agonists. J. Clin. Invest. 106, 467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murphy G. J., and Holder J. C. (2000) PPAR-γ agonists: therapeutic role in diabetes, inflammation and cancer. Trends Pharmacol. Sci. 21, 469–474 [DOI] [PubMed] [Google Scholar]
- 34. Jiang C., Ting A. T., and Seed B. (1998) PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 391, 82–86 [DOI] [PubMed] [Google Scholar]
- 35. Panigrahy D., Huang S., Kieran M. W., and Kaipainen A. (2005) PPARγ as a therapeutic target for tumor angiogenesis and metastasis. Cancer Biol. Ther. 4, 687–693 [DOI] [PubMed] [Google Scholar]
- 36. Schmidt M. V., Brüne B., and von Knethen A. (2010) The nuclear hormone receptor PPARγ as a therapeutic target in major diseases. Scientific World Journal 10, 2181–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oakley H., Cole S. L., Logan S., Maus E., Shao P., Craft J., Guillozet-Bongaarts A., Ohno M., Disterhoft J., Van Eldik L., Berry R., and Vassar R. (2006) Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 26, 10129–10140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Taniguchi Y., Matsukura Y., Ozaki H., Nishimura K., and Shindo K. (2013) Identification and quantification of the oxidation products derived from α-acids and β-acids during storage of hops (Humulus lupulus L.). J. Agric. Food Chem. 61, 3121–3130 [DOI] [PubMed] [Google Scholar]
- 39. Ano Y., Ozawa M., Kutsukake T., Sugiyama S., Uchida K., Yoshida A., and Nakayama H. (2015) Preventive effects of a fermented dairy product against Alzheimer's disease and identification of a novel oleamide with enhanced microglial phagocytosis and anti-inflammatory activity. PLoS One 10, e0118512. [DOI] [PMC free article] [PubMed] [Google Scholar]