Abstract
The c-MYC oncoprotein functions as a sequence-specific transcription factor. The ability of c-MYC to activate transcription relies in part on the recruitment of cofactor complexes containing the histone acetyltransferases mammalian GCN5 (mGCN5)/PCAF and TIP60. In addition to acetylating histones, these enzymes have been shown to acetylate other proteins involved in transcription, including sequence-specific transcription factors. This study was initiated in order to determine whether c-MYC is a direct substrate of mGCN5 and TIP60. We report here that mGCN5/PCAF and TIP60 acetylate c-MYC in vivo. By using nanoelectrospray tandem mass spectrometry to examine c-MYC purified from human cells, the major mGCN5-induced acetylation sites have been mapped. Acetylation of c-MYC by either mGCN5/PCAF or TIP60 results in a dramatic increase in protein stability. The data reported here suggest a conserved mechanism by which acetyltransferases regulate c-MYC function by altering its rate of degradation.
The c-myc oncogene is among the most frequently overexpressed genes in human cancer (35). Levels of c-myc are tightly regulated in cells, and even a twofold change can result in dramatic effects on cell cycle progression (32). c-myc encodes a sequence-specific transcription factor, c-MYC, which belongs to the basic, helix-loop-helix (HLH), leucine zipper (LZ) family (reviewed in reference 18). When dimerized with its obligate partner, MAX, through its HLH and LZ domains, c-MYC binds to CACGTG motifs in the genome to activate the transcription of adjacent genes (5, 6, 42, 43). Recent studies have linked the ability of c-MYC to activate transcription to its ability to recruit several cofactor complexes. These cofactors include histone acetyltransferases, ubiquitin ligases, and kinases (11, 17, 20, 27, 33, 34, 50). The modification of proteins at the promoters of target genes by these enzymes facilitates transcription through a variety of distinct but poorly defined mechanisms. c-MYC/MAX dimers are also capable of repressing transcription (51). In contrast to transcriptional activation, the biochemical mechanism of repression, the cofactors involved, and the DNA motifs through which it is mediated are not well understood.
The acetylation of nucleosomal histones has been known for several decades to be tightly correlated with transcriptional activation (38). However, it is only in recent years that the enzymes that catalyze this acetylation have been demonstrated to also modify nonhistone substrates (22, 45). The acetylation of nonhistone substrates regulates their function through a variety of means (28). For one of the most well-characterized nonhistone substrates, p53, acetylation has been variously reported to affect DNA binding, protein stability, and interaction with other proteins (4, 22, 29). Acetylation regulates these same functions in other transcription factors as well (24, 31, 39, 40). In addition, acetylation has been reported to regulate subcellular localization, protein-protein interactions, protein turnover rates, and other functions (3, 41). These pleiotropic effects have led to the suggestion that acetylation may be as broadly utilized a posttranslational modification as phosphorylation in the regulation of cellular physiology (28).
A number of studies have shown that one of the strategies used by c-MYC to regulate the transcription of target genes is the recruitment of multiprotein complexes containing either the mammalian GCN5 (mGCN5)/PCAF or TIP60 acetyltransferase (20, 21, 34). (mGCN5 and PCAF are paralogs which appear biochemically indistinguishable and exist in identical multiprotein complexes [36]). Existing experimental evidence suggests that recruitment of these enzymes to specific genes by c-MYC results in the acetylation of nucleosomal histones (7, 21). In most cases, histone acetylation is correlated with increased transcription of c-MYC targets. The present study was designed to examine whether one of these enzymes, mGCN5/PCAF or TIP60, directly acetylates the c-MYC protein itself. We report here the robust in vivo acetylation of c-MYC by both mGCN5/PCAF and TIP60. The sites of acetylation for mGCN5 have been mapped, with the major target residues residing within the nuclear localization signal (NLS) and the leucine zipper of c-MYC. Furthermore, c-MYC acetylation by either mGCN5/PCAF or TIP60 results in increased protein stability. These data help to define a novel pathway by which levels of the critical c-MYC oncoprotein are regulated.
MATERIALS AND METHODS
Cell culture, transfection, Western blotting, and acetylation reactions.
The human lung cancer cell line H1299 (American Type Culture Collection) (10) was maintained in Dulbecco's minimal essential medium (GIBCO) with 10% fetal calf serum (HyClone). Cells were transiently transfected using Lipofectamine-2000 (LTI). Cells were harvested for analysis 16 to 24 h posttransfection using an NP-40-based lysis buffer. Alternatively, where indicated, cells were lysed in radioimmunoprecipitation assay buffer containing sodium dodecyl sulfate (SDS). The cytomegalovirus-driven expression vectors for wild-type, FLAG-tagged c-MYC (33), mGCN5 (53), CBP (24), and TIP60 (14) have been described previously. Mutations in the acetylation sites of c-MYC were introduced using the QuikChange site-directed mutagenesis kit (Stratagene) and confirmed by DNA sequencing. Antibodies to FLAG (Sigma), mGCN5, and MAX (Santa Cruz) were obtained from commercial sources. Immunoprecipitations, nuclear and cytoplasmic fractionation, and Western blotting conditions have been described previously (1, 16, 34). In vitro acetylation reactions including recombinant c-MYC and the acetyltransferase domain of PCAF were performed as described previously (24). Luciferase reporter assays were performed as per the manufacturer's recommendation (Promega). Histone acetyltransferase assays were performed using purified core histones (Sigma) as substrates, as described previously (34).
Identification of c-MYC acetylation sites.
Acetylated c-MYC protein was purified from H1299 cells and digested with trypsin, and peptide sequence analysis was performed by microcapillary reverse-phase high-performance liquid chromatography nanoelectrospray tandem mass spectrometry (μLC-MS/MS) on a Finnigan LCQ DECA XP+ quadrupole ion trap mass spectrometer (Thermo Electron). The ion trap repetitively surveyed the range m/z 395 to 1,600, acquiring data-dependent MS/MS spectra for peptide sequence information on the four most abundant ions in each survey scan. MS/MS spectra were acquired with a relative collision energy of 30%, 2.5-Da isolation width, and recurring ions dynamically excluded. Preliminary sequencing of peptides was facilitated by database correlation with the algorithm SEQUEST (19). The discovery of peptides carrying acetylation and subsequent manual de novo interpretation of their MS/MS spectra were facilitated with the in-house programs MUQUEST and FUZZYIONS (12), respectively.
c-MYC half-life determination.
H1299 cells were seeded onto 100-mm-diameter dishes at 106 cells per plate and allowed to incubate at 37°C overnight. They were then transfected with 5 μg of FLAG-c-MYC and 10 μg of either pcDNA (two plates) or FLAG-GCN5 using Lipofectamine-2000 (Invitrogen). The transfection solution was kept on the cells for 4 h at 37°C, after which each plate was evenly split among the six wells of a six-well cluster plate and allowed to incubate at 37°C overnight. One of the FLAG-c-MYC/pcDNA sample groups was treated with 20 μM MG-132 (dissolved in dimethyl sulfoxide [Calbiochem]) for 1 h prior to cycloheximide treatment. Each well of cells from each group was treated with cycloheximide (100 μg/ml; Sigma) for the durations indicated in the text. Cells were harvested in 50 μl of NP-40-based lysis buffer containing protease inhibitors (34). Twenty-five micrograms of each lysate was run on 4 to 12% Tris-glycine SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gels (Invitrogen) and used to carry out Western blotting as described above. Autoradiography was performed, and the films were scanned and digitized. Individual c-MYC bands were quantified using Kodak 1D 3.5.3 imaging software. Individual bands were quantitated, and the mean intensity values were exported to Microsoft Excel. In addition to c-MYC, tubulin levels were also determined by Western blotting, and all c-MYC levels were normalized to tubulin. Normalized densitometric data for each time course was plotted, and curves were generated using linear regression. The point at which a given curve reached a c-MYC value equal to one-half of its starting value was determined and referred to as the half-life. Different exposures of films from different experimental groups were chosen for scanning based on similar intensity of c-MYC signals at the zero time point. This was necessary since coexpression of mGCN5 led to an overall increase in steady-state levels of c-MYC, as discussed in Results.
RESULTS
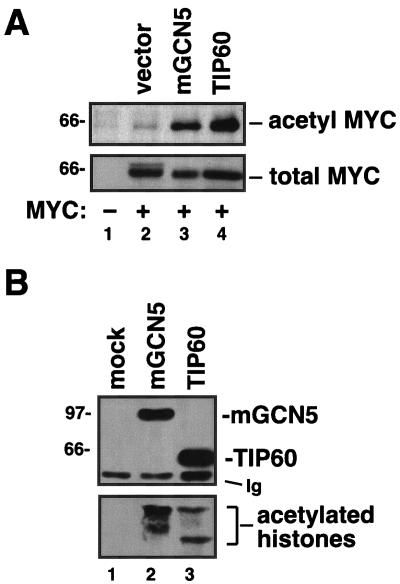
To test whether the c-MYC cofactors mGCN5/PCAF or TIP60 acetylate c-MYC itself, the proteins were coexpressed by transfection of the human lung carcinoma line H1299. After transfection, cells were lysed, and acetylated proteins were immunoprecipitated with antibodies that specifically recognize acetylated lysines. These precipitates were subjected to Western blotting for the c-MYC protein. Remarkably, this analysis revealed that both mGCN5/PCAF and TIP60 acetylate c-MYC in vivo (Fig. 1A, lanes 3 and 4). To confirm the enzymatic activity of the acetyltransferases used in this study, parallel experiments were carried out in which lysates from transfected H1299 cells were subjected to immunoprecipitation with anti-FLAG antibodies that recognize the epitope tag common to mGCN5/PCAF and TIP60. These precipitates were either tested for their ability to acetylate purified histones in vitro or subjected to Western blot analysis to document expression of the acetyltransferases (Fig. 1B). The proteins were expressed as predicted, and analysis of the enzymatic activity of the proteins revealed that they acetylate purified histones, as previously reported (9, 55).
FIG. 1.
The c-MYC oncoprotein is acetylated in vivo by its cofactors mGCN5 and TIP60. (A) The human lung cancer cell line H1299 was transfected with an expression vector for c-MYC (lanes 2 to 4). Mock transfected cells served as a negative control (lane 1). In addition, transfections included expression vectors for the acetyltransferases mGCN5 and TIP60, as indicated. Twenty-four hours posttransfection, cell lysates were produced, and acetylated proteins were precipitated with a universal antiacetyllysine antibody. Precipitates (top panel) and lysates (bottom panel) were resolved by SDS-PAGE and Western blotted for c-MYC. (B) H1299 cells were transfected with expression vectors for mGCN5 or TIP60, as indicated. Lysates were subjected to immunoprecipitation for the FLAG epitope present on the acetyltransferases. Precipitates were either resolved by SDS-PAGE and Western blotted for the FLAG epitope (top panel) or subjected to an in vitro acetyltransferase assay using purified histones as the substrate (bottom panel). The migration of molecular weight markers is indicated at the left. The migration of the immunoglobulin heavy chain from the immunoprecipitating antibody is indicated (Ig).
A number of well-characterized transcription factors are acetylated by mGCN5/PCAF, often with profound functional consequences (8, 13, 24, 26, 45, 47). We therefore focused the majority of our studies on mGCN5/PCAF-mediated acetylation of c-MYC. c-MYC is a member of a family of oncoproteins that also includes L-MYC and N-MYC in humans (reviewed in reference 35). Like c-MYC, L-MYC and N-MYC are overexpressed in cancer, although their tissue distribution is more restricted. Based on the high degree of structural and functional conservation among the MYC family members, we examined whether mGCN5/PCAF acetylates L-MYC and N-MYC as well as c-MYC. Coexpression of wild-type mGCN5/PCAF with either L-MYC or N-MYC resulted in significant acetylation (Fig. 2). As observed above, c-MYC was also robustly acetylated by mGCN5/PCAF. All MYC family proteins utilized in this analysis contained an N-terminal FLAG epitope tag. For N-MYC, basal levels of acetylation were higher than those observed for c-MYC or L-MYC, suggesting that N-MYC might be a preferred substrate for unknown endogenous acetyltransferases.
FIG. 2.
mGCN5 acetylates the three mammalian MYC proteins, c-MYC, L-MYC, and N-MYC. Expression vectors for FLAG epitope-tagged versions of the mammalian MYC family members c-MYC (lanes 3 and 4), L-MYC (lanes 5 and 6), and N-MYC (lanes 7 and 8) were transfected into H1299 cells. In addition, transfections included an expression vector for mGCN5 (lanes 2, 4, 6, and 8), as indicated. As a negative control, cotransfections including the empty expression vector were also performed (lanes 1, 3, 5, and 7). Lysates were resolved, blotted, and probed for the FLAG epitope common to c-MYC, L-MYC, and N-MYC (middle panel) or for mGCN5 (top panel). Antiacetyllysine immunoprecipitates (i.p.) were also resolved and blotted for FLAG to detect acetylated forms of the MYC family proteins (bottom panel).
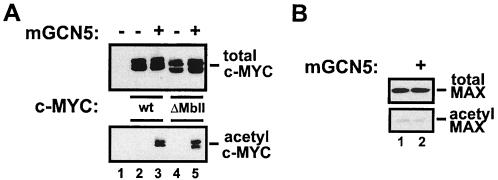
A highly conserved domain within the amino terminus of c-MYC termed MbII is critical for recruitment of the mGCN5/PCAF cofactor complexes (33, 34). In order to assess whether recruitment through this domain is essential for c-MYC to serve as a substrate for mGCN5/PCAF in vivo, a c-MYC mutant lacking MbII (amino acids 129 to 145) was utilized. As shown in Fig. 3, H1299 cells were transfected with an expression vector for mGCN5/PCAF and FLAG epitope-tagged versions of either wild-type c-MYC or the MbII domain mutant. In the presence of mGCN5/PCAF, the wild type and the MbII deletion mutant of c-MYC were acetylated to comparable levels. This suggests that stable recruitment of mGCN5/PCAF through the MbII domain of c-MYC is not required for acetylation under the conditions assayed here. It remains possible that at endogenous levels of mGCN5/PCAF and c-MYC, the physical interaction mediated by MbII contributes to increased local concentrations of the two proteins, leading to more efficient acetylation. In vivo, c-MYC exists primarily in a dimeric complex with MAX (6, 42). We therefore examined whether MAX was a substrate for mGCN5/PCAF as well. Coexpression of MAX with mGCN5/PCAF failed to result in MAX acetylation (Fig. 3B). Thus, MAX does not appear to be a substrate for mGCN5/PCAF despite its association with and structural similarity to another substrate, c-MYC. In addition, this result suggests that under the conditions used in these studies, mGCN5/PCAF retains its substrate specificity.
FIG. 3.
mGCN5-mediated acetylation of c-MYC does not require the MbII domain (A) H1299 cells were transfected with expression vectors encoding either wild-type c-MYC (wt, lanes 2 and 3) or a mutant lacking amino acids 129 to 145 (ΔMbII, lanes 4 and 5). Transfections also included the expression vector for mGCN5 (lanes 3 and 5), as indicated. After blotting, both lysates (top panel) and antiacetyl- lysine precipitates (bottom panel) were probed for the FLAG epitope present on c-MYC. (B) In parallel with the transfections shown in panel A, H1299 cells were transfected with an expression vector for the c-MYC partner MAX in the presence (lane 2) or absence (lane 1) of the mGCN5 expression vector. Lysates (top panel) and antiacetyl- lysine precipitates (bottom panel) were resolved and blotted for MAX.
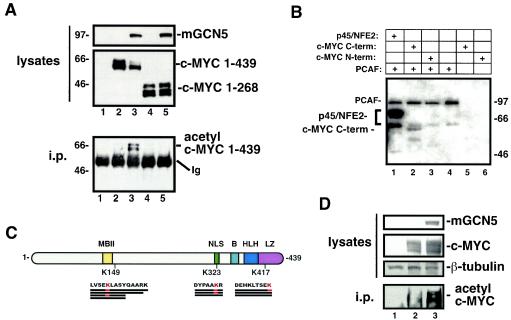
The c-MYC protein can be roughly divided into an N-terminal transactivation domain and a C-terminal DNA binding and dimerization domain (25). A truncation mutant of c-MYC lacking the C terminus was used to determine which domain of the protein is acetylated by mGCN5/PCAF. In this mutant a premature translational stop codon was incorporated after codon 268. The wild-type and mutant c-MYC proteins utilized in these studies contained an N-terminal FLAG tag. As seen in Fig. 4A, coexpression of wild-type c-MYC with mGCN5/PCAF in H1299 cells resulted in acetylation, while the truncated c-MYC protein was not acetylated. This suggested that the major site of mGCN5/PCAF-inducible acetylation is located between amino acids 269 and 439.
FIG. 4.
Mapping of c-MYC acetylation sites in vitro and in vivo. (A) H1299 cells were transfected with expression vectors for FLAG epitope-tagged versions of either wild-type c-MYC (amino acids 1 to 439, lanes 2 and 3) or a truncation mutant encoding only amino acids 1 to 268 (lanes 4 and 5), as indicated. Mock-transfected cells served as a control (lane 1). Lysates and antiacetyllysine immunoprecipitates were resolved and probed for the FLAG epitope common to the wild-type and mutant c-MYC proteins or mGCN5, as indicated. The migration of molecular weight markers is indicated at the left. Ig, immunoglobulin. (B) An in vitro acetylation assay was performed on glutathione S-transferase fusion proteins containing either the amino (amino acids 1 to 204) or carboxy (amino acids 200 to 439) terminal (term) region of c-MYC produced and purified from E. coli. The catalytic domain of the mGCN5 paralog PCAF was also produced and purified from E. coli. Purified proteins were assayed in the combinations indicated in an in vitro acetylation assay. The p45 subunit of NFE2, a known substrate for mGCN5/PCAF, was included as a positive control. The migration of molecular weight markers is indicated at the right. (C) FLAG epitope-tagged c-MYC expressed in either the presence or the absence of mGCN5 was purified from transfected H1299 cells. These proteins were subjected to μLC-MS/MS analysis to identify in vivo sites of mGCN5-mediated acetylation. This analysis revealed acetylation at lysine 149 of c-MYC in both the presence and the absence of mGCN5 overexpression. Two additional sites of acetylation were observed in the presence of mGCN5. The first of these lies within the c-MYC NLS at amino acid 323, and the second lies within the LZ domain at amino acid 417, as indicated. The individual peptides recovered for each region are indicated by thick black lines beneath the amino acid sequence of the region. Acetylated lysines within these peptides are indicated in red. Other functional domains of the c-MYC protein are indicated as follows: MbII, the highly conserved MYC homology box II region; B, the basic DNA binding motif; HLH, the helix-loop-helix domain essential for dimerization with MAX. (D) H1299 cells were transfected with the c-MYC expression vector in the presence or absence of mGCN5 expression, as indicated. Cell lysates were generated under denaturing conditions. These lysates and antiacetyllysine immunoprecipitates (i.p.) were blotted and probed for c-MYC. Lysates were also probed for mGCN5 and for β-tubulin.
The evidence for c-MYC acetylation by mGCN5/PCAF presented thus far has relied on in vivo coexpression of the two proteins. This strategy leaves open the possibility that mGCN5/PCAF-induced acetylation of c-MYC is not a direct effect but instead is catalyzed by an mGCN5/PCAF-responsive intermediate. In order to determine whether c-MYC is a direct substrate of mGCN5/PCAF, an in vitro acetylation assay was established. For this purpose, the N terminus (amino acids 1 to 204) and C terminus (amino acids 200 to 439) of c-MYC were expressed separately in Escherichia coli as glutathione S-transferase fusions (23). The catalytic domain of PCAF was also expressed and purified from bacteria. Purified proteins were incubated in the presence of radiolabeled acetyl coenzyme A and then resolved by SDS-PAGE. Autoradiography revealed that the C-terminal portion of c-MYC was specifically acetylated by purified PCAF, while no acetylation of the N terminus was observed (Fig. 4B). This suggests that c-MYC is a direct substrate of PCAF. In addition, reactions lacking PCAF displayed no acetylation of either c-MYC protein, eliminating the possibility that c-MYC can be autoacetylated, as has been shown for other transcription factors. As a positive control, the p45 subunit of the NFE2 transcription factor was included in a parallel reaction. Our previous studies have shown that p45 is a substrate of PCAF (G. A. Blobel et al., unpublished observations). As expected, PCAF also exhibited autoacetylation (30).
To define the site of acetylation, direct peptide sequence analysis was performed on c-MYC protein purified from H1299 cells. For this analysis, c-MYC was expressed alone or in combination with mGCN5, so that inducible sites of acetylation could be distinguished from those sites constitutively acetylated by endogenous enzymes. The FLAG epitope tag on c-MYC allowed the isolation of sufficient quantities of mGCN5-acetylated c-MYC from human cells for this analysis. This material was subjected to sequence analysis by microcapillary reverse-phase high-performance liquid chromatography and μLC-MS/MS. The c-MYC peptides that were captured and analyzed by this method cover approximately 65% of the protein and included 17 of 27 lysines. This analysis revealed constitutive acetylation on lysine 149, even in the absence of mGCN5 coexpression. Based on the in vitro assay illustrated in Fig. 4B, acetylation of lysine 149 is not likely to be targeted by mGCN5/PCAF. mGCN5-induced acetylation of c-MYC was observed at lysines 323 and 417 (Fig. 4C). As predicted by the in vivo and in vitro mapping studies described above, both of these sites reside within the C-terminal half of c-MYC. More specifically, lysine 323 resides within the major nuclear localization sequence of c-MYC (15), while lysine 417 is located within the leucine zipper motif (6). Of the three peptides spanning lysine 323 that were recovered and sequenced by μLC-MS/MS, two were acetylated. While this does not provide quantitative information about the level of c-MYC acetylation achieved in these studies, it does suggest that the acetylated form of c-MYC represents a significant fraction of the total c-MYC pool. In addition, a comparison of immunoprecipitates performed using antiacetyllysine or anti-c-MYC demonstrated that 40 to 70% of the total c-MYC pool is acetylated under the conditions used here (data not shown).
To confirm that the acetylation observed in vivo was occurring on c-MYC rather than on a c-MYC-associated protein, antiacetyllysine immunoprecipitates were generated under denaturing conditions. Probing of these precipitates for c-MYC revealed that c-MYC was directly recognized by the antiacetyllysine antisera (Fig. 4D).
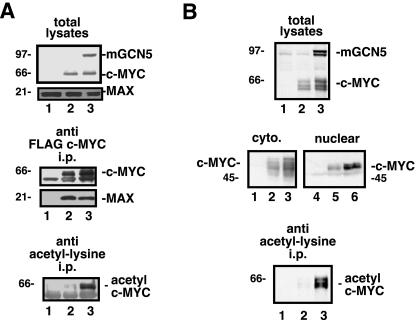
As the mGCN5 acetylation sites mapped to regions of c-MYC with well-defined function, studies were conducted to assess whether acetylation affects these functions. The leucine zipper of c-MYC cooperates with the adjacent helix-loop-helix motif to mediate dimerization with MAX (6). H1299 cells were transfected with the c-MYC expression vector in either the presence or the absence of the mGCN5 expression vector. Efficient mGCN5-induced acetylation of c-MYC was confirmed by performing an antiacetyllysine immunoprecipitation on lysates from a portion of these cells (Fig. 5A, bottom panel). To test for dimerization with MAX, lysates from the same cell pools were precipitated for the FLAG epitope on c-MYC. Precipitates and lysates were then probed for FLAG to detect c-MYC and mGCN5 (Fig. 5A, top and middle panels). Precipitates were also probed for MAX to assess the level of endogenous MAX associated with the acetylated and nonacetylated forms of c-MYC. The level of MAX binding was not significantly different between the two forms of c-MYC, suggesting that acetylation within the leucine zipper neither enhanced nor inhibited the dimerization of c-MYC and MAX. The lack of a significant effect on this critical c-MYC function was partially predicted by the observation that lysine 417 is not entirely conserved among c-MYC proteins of different species. Since lysine 323 lies within the main nuclear localization signal in c-MYC (15), the affect of acetylation on localization was assessed. Differential cytoplasmic and nuclear extracts were generated from cells transfected with the c-MYC expression vector in the presence or the absence of mGCN5. Quantitation of the ratio of cytoplasmic to nuclear c-MYC revealed no change in the localization of c-MYC in response to acetylation by mGCN5 (Fig. 5B). Direct examination of the acetylated pool of c-MYC showed a subcellular localization pattern indistinguishable from that reported above for total c-MYC (data not shown).
FIG. 5.
Dimerization with MAX and nuclear localization are not affected by c-MYC acetylation. (A) To assess whether acetylation of c-MYC altered its potential for dimerization with MAX, H1299 cells were transfected with the FLAG c-MYC expression vector (lanes 2 and 3), in the presence (lane 3) or absence (lane 2) of mGCN5, as indicated. Lysates and anti-FLAG immunoprecipitates (i.p.) were resolved by SDS-PAGE and blotted for the FLAG epitope to detect the tagged c-MYC and mGCN5, as indicated. In addition, the blots were probed for endogenous MAX to determine the amount dimerized with c-MYC. To confirm that c-MYC was efficiently acetylated by mGCN5 in this experiment, a portion of the lysates were subjected to immunoprecipitation with antiacetyllysine antisera. After blotting, these precipitates were probed for the FLAG tag to visualize acetylated c-MYC (bottom panel). (B) The effect of acetylation on c-MYC's subcellular localization was assessed in H1299 cells transfected with the FLAG c-MYC expression vector (lanes 2 and 3), in the presence (lane 3) or absence (lane 2) of mGCN5. Whole-cell lysates (top panel) were produced from a portion of the transfected cells, while the remaining cells were subjected to differential extraction of the cytoplasmic (cyto.; left middle panel) and nuclear fractions (right middle panel), as indicated. The presence of c-MYC in the various fractions was detected by probing for the FLAG epitope. To confirm that c-MYC was efficiently acetylated by mGCN5 in this experiment, a portion of the lysates were subjected to immunoprecipitation with antiacetyllysine antisera. These precipitates were blotted and probed for the FLAG tag to visualize acetylated c-MYC (bottom panel). The migration of molecular weight markers is indicated at the left.
In several experiments, including those shown in Fig. 5, coexpression of mGCN5 resulted in higher steady-state levels of c-MYC. We therefore tested whether acetylation by mGCN5 might increase the half-life of c-MYC. For this purpose, H1299 cells expressing c-MYC, in the presence or absence of mGCN5, were treated with cycloheximide 24 h after transfection to block further protein translation. The decay rate of the existing pool of c-MYC protein was then determined by quantitation of Western blots of lysates from cells harvested at various time points after cycloheximide treatment. CBP served as a control in these studies since its expression was recently reported to increase c-MYC stability (49). In the absence of mGCN5, c-MYC had a half-life of 41 min (Fig. 6), consistent with previous reports (44). In the presence of mGCN5, the half-life of c-MYC increased to 132 min. As expected, expression of CBP increased c-MYC's half-life to 153 min. As mGCN5 (and CBP) dramatically increased the steady-state level of c-MYC, identical exposures of Western blots in the presence and absence of acetyltransferase were not directly compared. Instead, lighter exposures of the mGCN5- and CBP-expressing lysates were quantitated and compared to the longer exposures of c-MYC alone. This ensured that differences in film linearity were not contributing to observed differences in c-MYC half-life. Similar effects on c-MYC half-life produced by mGCN5 were obtained in several independent experiments. To ensure that cycloheximide treatment was not affecting the results, we repeated the studies illustrated in Fig. 6 using the pulse-chase method to determine c-MYC stability. The results of this analysis paralleled those obtained using cycloheximide, with mGCN5-induced acetylation of c-MYC resulting in a substantial increase in both the steady-state levels and the half-life of c-MYC (data not shown).
FIG. 6.
Acetylation by mGCN5 stabilizes c-MYC. In order to determine the effect of acetylation on the stability of the c-MYC protein, half-life studies were conducted with H1299 cells transfected with the FLAG epitope-tagged c-MYC expression vector. In addition, transfections included vectors encoding either mGCN5 or CBP, as indicated. After transfection, cells were treated with cycloheximide to block further protein synthesis. Cells were harvested at the times indicated, and c-MYC levels were determined by Western blotting. Half-life values were determined by densitometric quantitation of Western blots. Signals for c-MYC levels at each time point were plotted using a logarithmic curve-fit algorithm, and the time point at which c-MYC levels decreased to 50% of their original value was determined and reported as the half-life. Because acetylation dramatically increased the overall steady-state levels of c-MYC, shorter exposures of c-MYC blots from the cells coexpressing mGCN5 or CBP were used for densitometry. Cells transfected with c-MYC in the absence of either acetyltransferase served as the source for determining the c-MYC basal half-life.
As shown in Fig. 1, the c-MYC cofactor TIP60 also acetylates c-MYC. To determine whether TIP60-mediated acetylation, like that of the mGCN5 cofactor, increased c-MYC stability, half-life studies similar to those shown in Fig. 6 were performed. In this experiment, the observed half-life of c-MYC was approximately 66 min. Inhibiting proteasome function with MG132 (2) extended the half-life of c-MYC to more than 360 min (Fig. 7). Remarkably, acetylation of c-MYC by TIP60 increased its half-life from 66 to 201 min, demonstrating that acetylation of c-MYC by multiple acetyltransferases has a similar functional outcome.
FIG. 7.
TIP60 increases c-MYC half-life. To determine the effect of acetylation by TIP60 on the stability of the c-MYC protein, half-life studies were conducted with H1299 cells as described for Fig. 6. Briefly, cells were transfected with the FLAG epitope-tagged c-MYC expression vector, in the presence or absence of TIP60, as indicated. Cell lysates were examined for c-MYC levels, and half-life values were determined as described in the legend to Fig. 6. As a control, one group of cells was treated with the proteasome inhibitor MG-132 to artificially stabilize c-MYC.
The data reported here suggest a model in which acetylation of c-MYC results in its stabilization. This model predicts that mutation of the acetylation sites should result in a c-MYC protein that is not stabilized by mGCN5. In order to test this prediction, mutations in c-MYC were introduced at the sites of mGCN5-induced acetylation mapped in our μLC-MS/MS analysis. Lysines 323 and 417 were converted to arginine in order to preserve charge but eliminate acetylation potential. As shown in Fig. 8, mGCN5 expression induced a 2.4- to 3.2-fold increase in the stability of wild-type c-MYC, consistent with results from previous experiments. Elimination of the two acetylation sites (K323 and K417) resulted in a decrease in mGCN5-induced stability to only 1.6-fold. These data provide support for the model in which acetylation by mGCN5 at lysines 323 and 417 dramatically stabilizes the c-MYC oncoprotein.
FIG. 8.
Mutation of c-MYC acetylation sites inhibits mGCN5-induced stabilization. In experiments (EXPT) identical to those presented in Fig. 6 and 7, wild-type (wt) and acetylation site mutant (K323/417R) versions of c-MYC were compared for their stabilization in response to mGCN5. (A) In two independent experiments, the loss of acetylation inhibited the ability of mGCN5 to stabilize c-MYC from 2.4- to 3.2-fold down to 1.6-fold (data are displayed as half-lives in minutes). (B) Western blots from experiment #2 are shown.
DISCUSSION
The c-MYC oncoprotein functions by regulating the transcription of downstream target genes (37). The recruitment of acetyltransferase complexes containing the enzymes mGCN5/PCAF and TIP60 is important for c-MYC's ability to regulate transcription (20, 21, 34). While much of the function ascribed to these enzymes comes from their ability to acetylate nucleosomal histones associated with target genes, it is now clear that the acetylation of nonhistone substrates is also critical for accurate transcriptional regulation. This study was initiated to determine whether the cofactors recruited by c-MYC, mGCN5/PCAF and TIP60, directly acetylate c-MYC. Our data indicate that both enzymes indeed acetylate c-MYC in vivo and that this acetylation has profound functional consequences. For mGCN5, two of the major acetylation sites have been mapped to lysine residues within the NLS and LZ motifs. The major effect of c-MYC acetylation identified in this study is increased protein stability.
To date, the mGCN5 sites reported here are the only sites of acetylation on c-MYC. While these sites lie within the NLS and leucine zipper, acetylation does not appear to play a major role in regulating the function of either of these domains. Instead, acetylation by mGCN5 (and TIP60) results in increased stability of the c-MYC protein. This is similar to the effect of c-MYC acetylation by CBP shown here and recently reported by others (49). A major unresolved issue, therefore, is whether all three families of enzymes increase protein stability by targeting the same residues on c-MYC. It should also be pointed out that our μLC-MS/MS data only allowed us to examine ∼65% of the c-MYC polypeptide, leaving open the possibility that residues in addition to lysines 323 and 417 are acetylated. Lysine 323 is highly conserved among c-MYC proteins from different species, and lysines in a similar position are also present in N-MYC and L-MYC proteins. Lysine 417 is also highly conserved among c-MYC, N-MYC, and L-MYC proteins, with the notable exceptions of human and Xenopus laevis c-MYC, where lysine 417 is not conserved. (Our data were obtained by expressing mouse c-MYC in human cells and therefore included lysine 417.) What is clear from this study and the previous study with CBP (49) is that the posttranslational modification of c-MYC by acetylation is a conserved mechanism by which levels of c-MYC may be regulated in vivo.
The mechanism by which acetylation increases c-MYC stability remains unclear. c-MYC has recently been reported to exist within two distinct cellular pools (48). The first of these is a detergent-soluble pool termed S1, containing 90 to 95% of cellular c-MYC and displaying a relatively short half-life. The remaining 5 to 10% of c-MYC resides in a detergent-insoluble pool termed S2, where it displays a much longer half-life. One potential mechanism by which c-MYC acetylation might increase its half-life is the relocalization of c-MYC from the unstable S1 pool to the more stable S2 pool. This possibility seemed particularly plausible given that one of the major acetylation sites identified here resides within the nuclear localization signal. However, this mechanism does not appear to be responsible for the acetylation-induced increase in c-MYC stability observed here, since acetylation did not result in a significant increase in the percentage of c-MYC in the stable S2 pool (data not shown).
Recently, c-MYC has been shown to be a substrate for two ubiquitin ligases, termed SKP2 and Fbw7 (27, 50, 52, 54). Ubiquitylation of c-MYC by these enzymes targets it for proteasome-mediated degradation. In an attractive model for the mechanism by which acetylation of c-MYC results in increased protein stability, acetylation of lysine 323 and/or 417 blocks these residues from becoming ubiquitylated. Thus, acetylated c-MYC would not be targeted for degradation. Ubiquitylation of c-MYC is also required for its ability to transactivate target genes (46). These data establish a seemingly paradoxical scenario in which the modification that marks the c-MYC protein for destruction is also required for its activity. If acetylation regulates c-MYC ubiquitylation, then it would also be predicted to have an impact on transcriptional activity. Our data show that mutation of the acetylation sites results in decreased transactivation potential (data not shown). This effect may result from the fact that mutant c-MYC cannot be acetylated and therefore can not be stabilized. Alternatively, if the acetylated lysines are also the sites of ubiquitylation, then their mutation would result in an inactive protein.
It is tempting to speculate that the fraction of c-MYC associated with its cofactors mGCN5/PCAF and TIP60 is preferentially stabilized as a result of direct acetylation by these same cofactors. By virtue of its increased stability, the transcriptionally active, cofactor-bound pool of c-MYC could thereby gain a functional advantage over other pools of c-MYC in the cell. However, at the levels of ectopic expression examined here, the stable recruitment of mGCN5/PCAF and TIP60 through the MbII domain of c-MYC is not required for its acetylation. This suggests a model in which the stable recruitment of these enzymes as cofactors for c-MYC is separable from their ability to recognize c-MYC as a substrate. Other issues that remain to be resolved include defining whether c-MYC is preferentially acetylated during specific cellular responses or stages of the cell cycle.
Levels of the c-MYC oncoprotein are tightly regulated in cells. A number of studies have demonstrated that cell cycle progression rates are tightly coupled to c-MYC levels, with even twofold changes in c-MYC having profound effects on doubling times (32). In fact, a modest increase in the levels of c-MYC may be an initiating event in the various forms of human cancer where the c-myc oncogene is amplified (35). The results described here identify a novel mechanism by which the acetyltransferases mGCN5/PCAF and TIP60 increase c-MYC levels in cells.
Acknowledgments
We thank Michael Cole for generously providing plasmids used in this study. In addition, we thank Renee A. Robinson and John M. Neveu for expert LC-MS/MS.
This work was supported by the following grants from the NIH:CA090465 and CA098172 (to S.B.M.), DK58044 (to G.A.B.), and CA085678 (to P.M.L.). In addition, this work was partially supported by funds from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health.
REFERENCES
- 1.Ard, P. G., C. Chatterjee, S. Kunjibettu, L. R. Adside, L. E. Gralinski, and S. B. McMahon. 2002. Transcriptional regulation of the mdm2 oncogene by p53 requires TRRAP acetyltransferase complexes. Mol. Cell. Biol. 22:5650-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee, D., and A. Liefshitz. 2001. Potential of the proteasomal inhibitor MG-132 as an anticancer agent, alone and in combination. Anticancer Res. 21:3941-3947. [PubMed] [Google Scholar]
- 3.Bannister, A. J., E. A. Miska, D. Gorlich, and T. Kouzarides. 2000. Acetylation of importin-alpha nuclear import factors by CBP/p300. Curr. Biol. 10:467-470. [DOI] [PubMed] [Google Scholar]
- 4.Barlev, N. A., L. Liu, N. H. Chehab, K. Mansfield, K. G. Harris, T. D. Halazonetis, and S. L. Berger. 2001. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell 8:1243-1254. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell, T. K., L. Kretzner, E. M. Blackwood, R. N. Eisenman, and H. Weintraub. 1990. Sequence-specific DNA binding by the c-Myc protein. Science 250:1149-1151. [DOI] [PubMed] [Google Scholar]
- 6.Blackwood, E. M., and R. N. Eisenman. 1991. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science 251:1211-1217. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard, C., O. Dittrich, A. Kiermaier, K. Dohmann, A. Menkel, M. Eilers, and B. Luscher. 2001. Regulation of cyclin D2 gene expression by the Myc/Max/Mad network: Myc-dependent TRRAP recruitment and histone acetylation at the cyclin D2 promoter. Genes Dev. 15:2042-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyes, J., P. Byfield, Y. Nakatani, and V. Ogryzko. 1998. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature 396:594-598. [DOI] [PubMed] [Google Scholar]
- 9.Brownell, J. E., J. Zhou, T. Ranalli, R. Kobayashi, D. G. Edmondson, S. Y. Roth, and C. D. Allis. 1996. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84:843-851. [DOI] [PubMed] [Google Scholar]
- 10.Chen, J. Y., W. D. Funk, W. E. Wright, J. W. Shay, and J. D. Minna. 1993. Heterogeneity of transcriptional activity of mutant p53 proteins and p53 DNA target sequences. Oncogene 8:2159-2166. [PubMed] [Google Scholar]
- 11.Cheng, S. W., K. P. Davies, E. Yung, R. J. Beltran, J. Yu, and G. V. Kalpana. 1999. c-MYC interacts with INI1/hSNF5 and requires the SWI/SNF complex for transactivation function. Nat. Genet. 22:102-105. [DOI] [PubMed] [Google Scholar]
- 12.Chittum, H. S., W. S. Lane, B. A. Carlson, P. P. Roller, F. D. Lung, B. J. Lee, and D. L. Hatfield. 1998. Rabbit beta-globin is extended beyond its UGA stop codon by multiple suppressions and translational reading gaps. Biochemistry 37:10866-10870. [DOI] [PubMed] [Google Scholar]
- 13.Col, E., C. Caron, D. Seigneurin-Berny, J. Gracia, A. Favier, and S. Khochbin. 2001. The histone acetyltransferase, hGCN5, interacts with and acetylates the HIV transactivator, Tat. J. Biol. Chem. 30:30. [DOI] [PubMed] [Google Scholar]
- 14.Creaven, M., F. Hans, V. Mutskov, E. Col, C. Caron, S. Dimitrov, and S. Khochbin. 1999. Control of the histone-acetyltransferase activity of Tip60 by the HIV-1 transactivator protein, Tat. Biochemistry 38:8826-8830. [DOI] [PubMed] [Google Scholar]
- 15.Dang, C. V., and W. M. F. Lee. 1988. Identification of the human c-myc protein nuclear translocation signal. Mol. Cell. Biol. 8:4048-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eberhardy, S. R., and P. J. Farnham. 2002. Myc recruits P-TEFb to mediate the final step in the transcriptional activation of the cad promoter. J. Biol. Chem. 277:40156-40162. [DOI] [PubMed] [Google Scholar]
- 18.Eisenman, R. N. 2001. Deconstructing myc. Genes Dev. 15:2023-2030. [DOI] [PubMed] [Google Scholar]
- 19.Eng, J., A. McCormick, and J. I. Yates. 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5:976-989. [DOI] [PubMed] [Google Scholar]
- 20.Frank, S. R., T. Parisi, S. Taubert, P. Fernandez, M. Fuchs, H. M. Chan, D. M. Livingston, and B. Amati. 2003. MYC recruits the TIP60 histone acetyltransferase complex to chromatin. EMBO Rep. 4:575-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank, S. R., M. Schroeder, P. Fernandez, S. Taubert, and B. Amati. 2001. Binding of c-Myc to chromatin mediates mitogen-induced acetylation of histone H4 and gene activation. Genes Dev. 15:2069-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu, W., and R. G. Roeder. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90:595-606. [DOI] [PubMed] [Google Scholar]
- 23.Hateboer, G., H. T. M. Timmers, A. K. Rustgi, M. Billaud, L. J. van'T Veer, and R. Bernards. 1993. TATA-binding protein and the retinoblastoma gene product bind to overlapping epitopes on c-Myc and adenovirus E1A protein. Proc. Natl. Acad. Sci. USA 90:8489-8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung, H. L., J. Lau, A. Y. Kim, M. J. Weiss, and G. A. Blobel. 1999. CREB-binding protein acetylates hematopoietic transcription factor GATA-1 at functionally important sites. Mol. Cell. Biol. 19:3496-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato, G. J., J. Barrett, M. Villa-Garcia, and C. V. Dang. 1990. An amino-terminal c-Myc domain required for neoplastic transformation activates transcription. Mol. Cell. Biol. 10:5914-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiernan, R. E., C. Vanhulle, L. Schiltz, E. Adam, H. Xiao, F. Maudoux, C. Calomme, A. Burny, Y. Nakatani, K. T. Jeang, M. Benkirane, and C. Van Lint. 1999. HIV-1 tat transcriptional activity is regulated by acetylation. EMBO J. 18:6106-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, S. Y., A. Herbst, K. A. Tworkowski, S. E. Salghetti, and W. P. Tansey. 2003. Skp2 regulates Myc protein stability and activity. Mol. Cell 11:1177-1188. [DOI] [PubMed] [Google Scholar]
- 28.Kouzarides, T. 2000. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 19:1176-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, M., J. Luo, C. L. Brooks, and W. Gu. 2002. Acetylation of p53 inhibits its ubiquitination by Mdm2. J. Biol. Chem. 277:50607-50611. [DOI] [PubMed] [Google Scholar]
- 30.Liu, Y., A. L. Colosimo, X. J. Yang, and D. Liao. 2000. Adenovirus E1B 55-kilodalton oncoprotein inhibits p53 acetylation by PCAF. Mol. Cell. Biol. 20:5540-5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Balbas, M. A., U. M. Bauer, S. J. Nielsen, A. Brehm, and T. Kouzarides. 2000. Regulation of E2F1 activity by acetylation. EMBO J. 19:662-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mateyak, M. K., A. J. Obaya, S. Adachi, and J. M. Sedivy. 1997. Phenotypes of c-Myc-deficient rat fibroblasts isolated by targeted homologous recombination. Cell Growth Differ. 8:1039-1048. [PubMed] [Google Scholar]
- 33.McMahon, S. B., H. A. VanBuskirk, K. A. Dugan, T. D. Copeland, and M. D. Cole. 1998. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell 94:363-374. [DOI] [PubMed] [Google Scholar]
- 34.McMahon, S. B., M. A. Wood, and M. D. Cole. 2000. The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc. Mol. Cell. Biol. 20:556-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nesbit, C. E., J. M. Tersak, and E. V. Prochownik. 1999. MYC oncogenes and human neoplastic disease. Oncogene 18:3004-3016. [DOI] [PubMed] [Google Scholar]
- 36.Ogryzko, V. V., T. Kotani, X. Zhang, R. L. Schiltz, T. Howard, J. Quin, and Y. Nakatani. 1998. Histone-like TAFs within the PCAF histone acetylase complex. Cell 94:35-44. [DOI] [PubMed] [Google Scholar]
- 37.Oster, S. K., C. S. Ho, E. L. Soucie, and L. Z. Penn. 2002. The myc oncogene: MarvelouslY Complex. Adv. Cancer Res. 84:81-154. [DOI] [PubMed] [Google Scholar]
- 38.Pogo, B. G., V. G. Allfrey, and A. E. Mirsky. 1966. RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc. Natl. Acad. Sci. USA 55:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Polesskaya, A., A. Duquet, I. Naguibneva, C. Weise, A. Vervisch, E. Bengal, F. Hucho, P. Robin, and A. Harel-Bellan. 2000. CREB-binding protein/p300 activates MyoD by acetylation. J. Biol. Chem. 275:34359-34364. [DOI] [PubMed] [Google Scholar]
- 40.Polesskaya, A., I. Naguibneva, A. Duquet, E. Bengal, P. Robin, and A. Harel-Bellan. 2001. Interaction between acetylated MyoD and the bromodomain of CBP and/or p300. Mol. Cell. Biol. 21:5312-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polevoda, B., and F. Sherman. 2002. The diversity of acetylated proteins. Genome Biol. 3:reviews0006.1-reviews0006.6. [DOI] [PMC free article] [PubMed]
- 42.Prendergast, G. C., D. Lawa, and E. B. Ziff. 1991. Association of Myn, the murine homolog of Max, with c-Myc stimulates methylation-sensitive DNA binding and Ras cotransformation. Cell 65:395-407. [DOI] [PubMed] [Google Scholar]
- 43.Prendergast, G. C., and E. B. Ziff. 1991. Methylation-sensitive sequence-specific DNA binding by the c-Myc basic region. Science 251:186-189. [DOI] [PubMed] [Google Scholar]
- 44.Ramsay, G., G. I. Evan, and J. M. Bishop. 1984. The protein encoded by the human proto-oncogene c-myc. Proc. Natl. Acad. Sci. USA 81:7742-7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakaguchi, K., J. E. Herrera, S. Saito, T. Miki, M. Bustin, A. Vassilev, C. W. Anderson, and E. Appella. 1998. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 12:2831-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salghetti, S. E., M. Muratani, H. Wijnen, B. Futcher, and W. P. Tansey. 2000. Functional overlap of sequences that activate transcription and signal ubiquitin-mediated proteolysis. Proc. Natl. Acad. Sci. USA 97:3118-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sartorelli, V., P. L. Puri, Y. Hamamori, V. Ogryzko, G. Chung, Y. Nakatani, J. Y. Wang, and L. Kedes. 1999. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol. Cell 4:725-734. [DOI] [PubMed] [Google Scholar]
- 48.Tworkowski, K. A., S. E. Salghetti, and W. P. Tansey. 2002. Stable and unstable pools of Myc protein exist in human cells. Oncogene 21:8515-8520. [DOI] [PubMed] [Google Scholar]
- 49.Vervoorts, J., J. M. Luscher-Firzlaff, S. Rottmann, R. Lilischkis, G. Walsemann, K. Dohmann, M. Austen, and B. Luscher. 2003. Stimulation of c-MYC transcriptional activity and acetylation by recruitment of the cofactor CBP. EMBO Rep. 4:484-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von der Lehr, N., S. Johansson, S. Wu, F. Bahram, A. Castell, C. Cetinkaya, P. Hydbring, I. Weidung, K. Nakayama, K. I. Nakayama, O. Soderberg, T. K. Kerppola, and L. G. Larsson. 2003. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol. Cell 11:1189-1200. [DOI] [PubMed] [Google Scholar]
- 51.Wanzel, M., S. Herold, and M. Eilers. 2003. Transcriptional repression by Myc. Trends Cell. Biol. 13:146-150. [DOI] [PubMed] [Google Scholar]
- 52.Welcker, M., A. Orian, J. Jin, J. A. Grim, J. W. Harper, R. N. Eisenman, and B. E. Clurman. 2004. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl. Acad. Sci. USA 101:9085-9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu, W., D. G. Edmondson, and S. Y. Roth. 1998. Mammalian GCN5 and P/CAF acetyltransferases have homologous amino-terminal domains important for recognition of nucleosomal substrates. Mol. Cell. Biol. 18:5659-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yada, M., S. Hatakeyama, T. Kamura, M. Nishiyama, R. Tsunematsu, H. Imaki, N. Ishida, F. Okumura, K. Nakayama, and K. I. Nakayama. 2004. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 23:2116-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto, T., and M. Horikoshi. 1997. Novel substrate specificity of the histone acetyltransferase activity of HIV-1-Tat interactive protein Tip60. J. Biol. Chem. 272:30595-30598. [DOI] [PubMed] [Google Scholar]