Abstract
Caveolae are protein-dense plasma membrane domains structurally composed of caveolin-1 or -3 along with other proteins. Our previous studies have shown that caveolae enhance calcium signals generated through the Gαq/phospholipase Cβ signaling pathway and that subjecting cells to hypo-osmotic stress reverses this enhancement. In this study, we have used super-resolution fluorescence microscopy supplemented by fluorescence correlation studies to determine the structural factors that underlie this behavior. We find similar and significant population of Gαq and one of its receptors, bradykinin type 2 receptor (B2R), as well as a significant population of Gαi and its coupled β2-adrenergic receptor (βAR), are localized to caveola domains. Although mild osmotic stress deforms caveolae and alters interactions between the caveolae and these proteins, the general structure and the localization of caveola components remain largely unchanged. This deformation eliminates the ability of caveolae to stabilize calcium signals mediated through Gαq-B2R, but does not affect cAMP signals mediated through Gαi and βAR. Structurally, we find that mild osmotic stress corresponding roughly to a pressure of 3.82 newtons/m2 increases the domain diameter by ∼30% and increases the fluorescence intensity in the center of the domain mouth suggesting a flattening of the invagination. Approximate calculations show that caveolae in muscle tissue have the strength to handle the stress of muscle movement.
Keywords: caveolae, fluorescence, G protein, membrane structure, osmotic swelling
Introduction
Many cells undergo mechanical deformation in their normal function. The plasma membrane of several types of cells, and muscle cells in particular, contains protein-dense invaginations called caveolae. Caveolae are thought to confer mechanosensation and possibly play a role in endocytosis and cell signaling (1). Caveolae are structurally composed of ∼140 copies of either caveolin-1 or caveolin-3 (Cav1 or Cav3), which form a thimble-like structure in the inner leaflet of the plasma membrane promoting membrane curvature and leading to the invaginations seen by electron microscopy (2). Although many functions have been attributed to caveolae, recently Sinha et al. (3) revisited the idea that caveolae may provide mechanical strength to the host cell. They found that when cells were either mechanically stressed or when they reduced the osmotic strength by a factor of 10, caveola invaginations flatten and disassemble to increase the membrane area to accommodate the stress as indicated by electron microscopy and corroborating methods.
Our laboratory has shown that the presence of caveolae directly affects Ca2+ signals mediated through the Gαq/phospholipase Cβ (PLCβ)2 pathway (4, 5). Receptors that activate this pathway bind dopamine, acetylcholine, bradykinin, and angiotensin II as well as other hormones and neurotransmitters. Once activated by receptors, Gαq subunits stimulate PLCβ, which catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate leading to Ca2+ release from intracellular stores (6, 7). Based on previous work (8, 9), we found that Cav-1 or -3 specifically binds Gαq, and the affinity between the two proteins increases when Gαq is activated (4). The stabilization of activated Gαq by Cav1/3, coupled with release of Gβγ from caveola domains during the activation cycle, has the net effect of prolonging PLCβ activation and increasing the extent and duration of Ca2+ responses. The interaction between Cav1/3 not only localizes Gαq to caveola domains, but in turn it allows Gαq to scaffold its associated GPCRs (10) leading to a localized and potentially synergistic response.
Because caveolae may provide mechanical strength to cells, we determined whether their mechanical properties will alter their ability to affect Ca2+ signaling (11). These studies were carried out by subjecting cells to hypo-osmotic conditions by diluting the cell media with water. This treatment will cause the cells to exert an outward pressure (osmotic pressure) against the plasma membrane. Previous studies used very high osmotic stress (3). However, in the cell line used here (rat aortic smooth muscle cells), we find that this high stress causes a loss in eGFP-Cav1/mCherry-Cav1 FRET in accordance with caveola dissolution, but within minutes the cells begin to rupture (11). Therefore, in these studies we sought to understand changes in caveola structure under more physiological conditions. The studies reported here were carried out under conditions where the cell diameter is unchanged so that the actin cytoskeleton is largely unperturbed and the properties of the membrane should be unchanged as indicated by previous studies of brush-border membrane (12). We found that when rat aortic smooth muscle cells are subjected to relatively mild osmotic stress, Ca2+ signals mediated through Gαq/PLCβ are diminished suggesting a disruption of the interactions between Cav-1 and Gαq that stabilize the activated state. However, under these mild stress conditions, we could not detect dissociation of caveola domains (11). Although alterations in Gαq-Cav1 interactions were suggested, these changes were too minor to account for a loss in calcium signal. Thus, it is unclear how mild osmotic stress reduces Ca2+ signals without disassembling caveolae.
To understand how physiologically relevant stress conditions affect caveola structure and in turn Gαq/PLCβ-mediated calcium signals, we have used super-resolution fluorescence microscopy to visualize changes in caveola structure and in its interactions with associated proteins. Our studies support a model in which mild deformation of caveolae, such as those encountered during normal cell function, provides a mechanism for cells to control calcium signals and maintain membrane integrity. These structural measurements allow us to estimate the stability of caveolae and, in turn, the stability that caveola domains provide to cells.
Results
Visualization of Caveolae under Basal and Stress Conditions by Super-resolution Fluorescence Imaging
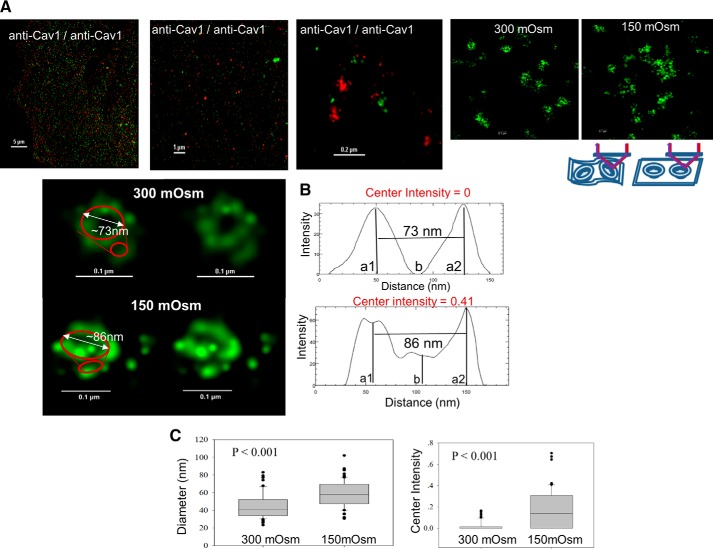
We carried out STORM measurements on fixed rat aortic smooth muscle cells that endogenously express Cav1, where Cav1 was visualized using Alexa488-tagged anti-Cav1. Here, cells were grown in glass bottom chambers that allow optical viewing, cross-linking the proteins by fixation, and permeabilizing the membranes to allow diffusion of the fluorescent-tagged antibodies into cell. In these studies, antibodies were directly labeled with the Alexa dyes at a labeling ratio of ∼4:1 probe/protein. Because the labeling is not on a specific site, there is an inherent uncertainty equal to the size of the antibody that is ∼10 nm. For hypo-osmotic conditions, cell media were diluted in half with water to lower the osmolarity from 300 to 150 mosm for 5 min prior to fixation. All images were taken in TIRF mode to view only the molecules on the surface. Typical images are shown in Fig. 1A, where the top panels show normal and super-resolution images of a single cell. Caveolae appear as varying clusters of intensity with sizes varying from 50 to 200 for single clusters, along with groups of multiple clusters. When the images are expanded, ∼74% (n = 65) display the “classic” caveola structure (13) as shown in Fig. 1B, where the surfaces appear as donut-like structures composed of a circular chain of bright spheres that are roughly 80 nm in diameter. Based on previous EM studies showing caveolae to be 50–100 nm in diameter, we interpret the typical structures shown in Fig. 1B to be a caveola. Typically, a dimmer arc of the same width but with a reduced intensity is seen ∼30 nm away the donut-like structures which, based on previous reports (2), are interpreted to be due to Cav1 molecules at the bottom of the invaginations (Fig. 1B). Other caveola domains (∼26%, n = 65) display a weak intensity in the center of the donuts. Although these are most likely natural variations of the domains, we note that they may also be due to variations in the incident light on the geometry of the structures especially if the membrane does not lie completely flat on the substrate (see schematic in the lower part of Fig. 1A). We note that previous EM studies support more uniform structures of the domains (13).
FIGURE 1.
Structure of caveolae under basal and hypo-osmotic stress conditions. A, first three panels are images of A10 cells stained with green and red anti-Cav1 antibody used for at different optical resolutions. These types of images were used as positive controls for the colocalization studies described in the text. The two panels on the right show typical STORM images of caveolae at 300 and 150 mosm conditions. Just below these panels is a schematic showing how small microfolds or puckering of the plasma membrane (left) may alter the image as compared with caveolae on membranes that lie flat on the substrate (right) where the TIRF excitation and emission are depicted by purple and red arrows. B, left, enlarged image of a representative caveola imaged by STORM with and without a putative outline drawn in red. Right, corresponding intensity distributions along the cross-section profiles (see “Experimental Procedures”) through the center of the caveola where the Center Intensity is located (i.e. the ratio of center region intensity to the ring region as defined as b/((a1 + a2)/2) (bottom). C, compiled results of diameters (left) and the Center Intensities (right) of six cells from two to three independent experiments at 300 and 150 mosm, where 10–12 caveola domains were measured to give a total of 63–65 measurements.
Caveolae were shown to dissolve at a 10-fold reduction in osmolarity (3), but we find that subjecting A10 cells to this low osmolarity results in loss of eGFP-Cav1/mCherry-Cav1 FRET immediately preceding cell rupture. Therefore, we focused on milder and more physiological pressures where no changes in cell diameter or morphology were detected. When cells are subjected to mild osmotic stress, the population of the structures shifts from mostly clusters of intensities with spherical structures to less structured clusters of bright spots (Fig. 1A). We estimated the change in the integrity of the domains by fitting the cross-section of the domain profiles to two Gaussian curves (one for each side of the mouth of the domain that are 180° apart) (Fig. 1B), and we calculated the diameter of the domains from the distance between the centers of the two curves. Under osmotic stress, a significant amount of intensity is seen in the center of the domain mouths as compared with the normal osmolarity, and the distance between the centers of the two curves increases ∼33% as assessed by analysis of 63–65 caveolae as shown in Fig. 1C. The appearance of intensity in the center of the images suggests a significant flattening of the domains, which is consist with previous EM studies (3).
Organization of a B2R, Gαq, and Gαi in Caveolae
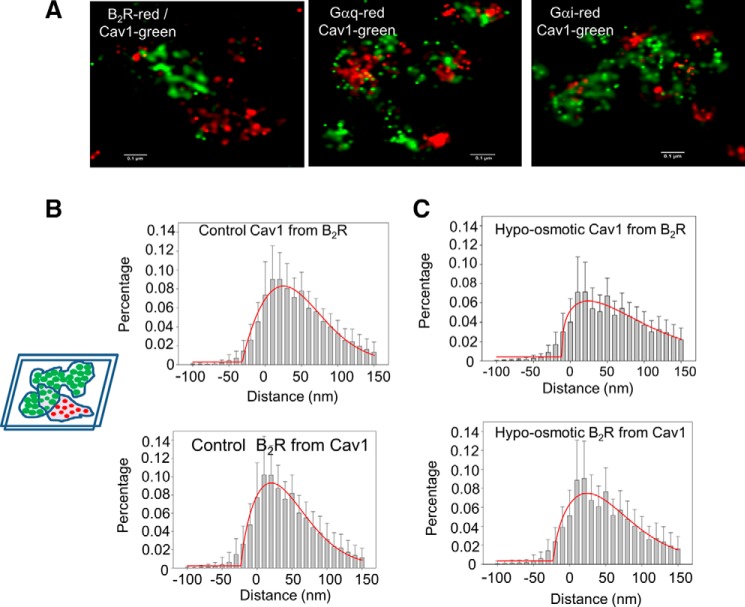
We imaged other proteins thought to be localized in caveolae by STORM (Fig. 2A). Initial studies were done for the Gαq-coupled receptor B2R, which mediates inflammation (14). B2R has been found to localize in caveola domains by biochemical studies as well as FRET studies in living cells (10, 15, 16). Cav1 was imaged using anti-Cav1 directly labeled with Alexa488, and B2R was imaged using anti-B2R labeled with Alexa647. In normal fluorescence imaging, colocalization is assessed by the appearance of two fluorophores with different colors in a given pixel. However, super-resolution imaging will be sensitive to the difference in length between the green and red emitted light (519 and 665 nm, respectively), and colocalization cannot be assessed by overlap between red and green pixels. To determine changes in the proximity of Cav1 and other proteins, we used an analysis developed by Soeller and co-workers (17, 18), which assesses the distance between the green and red pixels. In this analysis, masks are drawn separately around the green and red pixels, and the distance between the masks are calculated and plotted in histogram form as depicted in the simplified schematic in Fig. 2B. Some of the values are negative reflecting complete overlap between the green and red pixels, whereas positive values reflect the separation between the green and red pixels (Fig. 2B). From this analysis, we find that the majority of the populations of the two proteins are within the emission wavelength difference of the green and red fluorophores, suggesting a high degree of colocalization. Comparing the distance distributions of Cav1 from B2R (Fig. 2B, top) to B2R from Cav1 (Fig. 2B, bottom), we find a similar but slightly broader distribution. We believe this difference reflects non-uniformity in the number of Cav1 molecules surrounding B2R versus the number of B2R molecules surrounding Cav1 as depicted in the schematic insets in Fig. 2B. We note that the similar midpoints for the distances from Cav1 to B2R and from B2R to Cav1 reflect similar organization of the receptor in caveola domains.
FIGURE 2.
Effect of hypo-osmotic stress on Cav1 and B2R colocalization. A, STORM colocalization images of Cav1 and B2R, Gαq, and Gαi. B and C, distance of histograms showing the percent of Cav1 intensity from the edge of the nearest B2R intensity (upper panels) and the percent of B2R intensity from the edge of the nearest Cav1 intensity (lower panels) at 300 mosm (B) and at 150 mosm (C). The schematic illustrates the masks around the green (top) and red (bottom) pixels where the amount of labeling in one channel at a given distance from the edge of the other channel's mask was calculated to obtain the histograms in the figures. Negative values are obtained when the masks overlap. B and C, total of 126–145 images were measured from 10 to 40 images of six cells in two to three experimental groups with ∼20 to ∼30 caveolae in each image. The image size is ∼1.5 × 1.5 μm. Comparison of the histobars for 300 and 150 mosm at each distance differed with an error of p < 0.001 except for distance <0 where p = 0.227. The red lines correspond to the Gaussian fit of the data.
When we subjected the cells to osmotic stress, we found a significant shift of the histogram distribution to higher separation distances as seen by a more skewed Gaussian curve (Fig. 2C). These results support the idea that the flattening of caveolae due to osmotic stress increases the separation of Cav1 molecules at the mouth of the domain, and that a large population of B2R molecules remain associated with the domains.
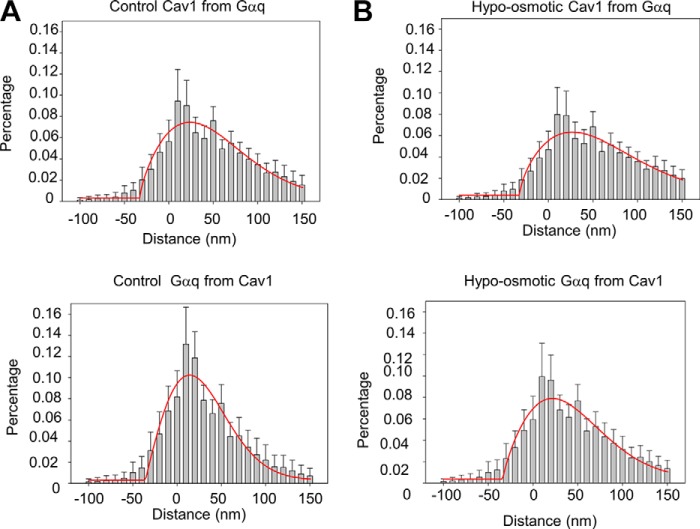
These studies were repeated using Gαq, whereas previous FRET studies indicated a high degree of caveola association (10). Super-resolution images (Fig. 3A), and analysis of their Gαq/Cav1 colocalization (Fig. 3B) support the idea that a portion of Gαq resides in caveola domains. Although not significant, it appears that the average distance between Gαq and Cav1 may be closer than B2R and Cav1, which is consistent with the idea that Gαq is helpful in scaffolding its associated receptors to caveola (10). Additionally, we note that the Gαq/Cav1 distance is smaller than the diameter of caveolae due to the contribution of Gαq from the sides and bottom of the domain invaginations. Interestingly, we find that the average distance from the G proteins to Cav1 molecules is longer than the distance from Cav1 to the G proteins. Although the most probable reason is experimental and due to differences in the handling of red versus green light, we cannot discount the idea that these differences lie in the non-uniformity in the number of Cav1 molecules surrounding G proteins versus the number of G proteins surrounding Cav1 as seen by the non-uniformity of the G proteins versus Cav1 in our images. By this reasoning, G proteins localized within or on the periphery of caveolae will have many more Cav1 surrounding them as compared with Cav1 and Gα subunits, which will skew the distances toward shorter values.
FIGURE 3.
Effect of hypo-osmotic stress on Cav1 and Gαq colocalization. A, distance histogram of the percentage of Cav1 intensity from the edge of the nearest Gαq intensity (upper panel), and the percentage of Gαq intensity from the edge of the nearest Cav1 intensity (lower panel) at 300 mosm; B, at 150 mosm. Data were collected from two to three sets of experiments with 9–38 images measured in each cell, and six to seven cells were measured in each group to give a total of 132–158 images. The image size is ∼1.5 × 1.5 μm. Comparison of the histobars for 300 and 150 mosm at each distance differed with an error of p < 0.001 except for Cav1 from Gαq distance <0 where p = 0.054. The red lines correspond to the Gaussian fit of the data.
Subjecting the cells to osmotic pressure did not significantly increase the distance from Gαq to Cav1 consistent with an increased diameter of the domains. When compared with our previous FRET data showing a small but significant reduction in Gαq-Cav1 FRET under hypo-osmotic conditions (11), these results suggest that that the interaction between Cav1 and Gαq may change under osmotic stress but that Gαq remains associated with caveolae.
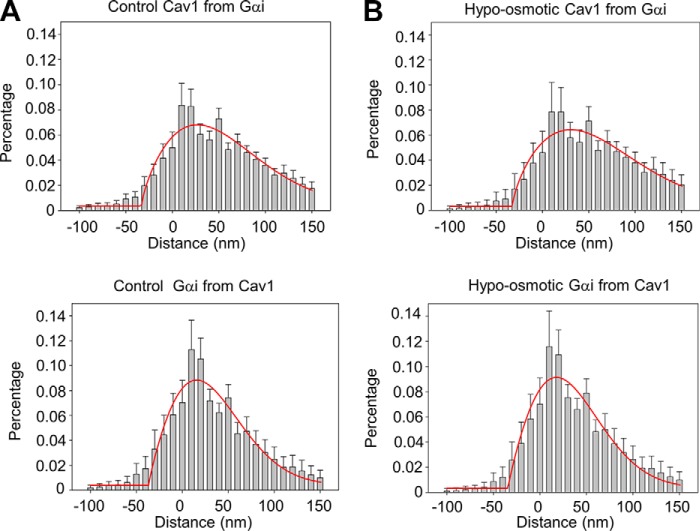
We repeated this study using Gαi, where previous FRET studies indicated a much smaller colocalization as compared with Gαq (10). However, STORM images suggest that the distances between Gαi-Cav1 and Cav1-Gαi are slightly longer but within error of those for Gαq and are shorter than B2R. These data suggest that a significant population of Gαi, like Gαq, localizes to caveola domains (Fig. 4, A and B). Under osmotic stress, the distance between Gαi and Cav1 changes, but not significantly (Fig. 4, A and B).
FIGURE 4.
Effect of hypo-osmotic stress on Cav1 and Gαi colocalization. A, distance histogram of the percentage of Cav1 from the edge of the nearest Gαi labeling (upper) and the percentage of Gαi from the edge of the nearest Cav1 labeling (lower) at 300 mosm. B, distance histogram of the percentage of Cav1 from the edge of the nearest Gαi labeling (upper) and the percentage of Gαi from the edge of the nearest Cav1 labeling (lower) at 150 mosm. Data were derived from two independent experiments in which 11–15 images were measured in each cell, and five cells were measured in each group to give a total of 56–66 images per group. The red lines correspond to the Gaussian fit of the data.
Caveola Domain Remains Intact under Osmotic Stress
Previous EM studies of caveolae under acute osmotic stress (i.e. 300 to 30 mosm) showed dissolution of the domains as seen by the appearance of free Cav1 molecules (3). To determine whether this is the case under milder stress conditions, we calculated the distances between identical Cav1, B2R, Gαq, and Gαi molecules at 300 and 150 mosm. Under normal osmotic conditions, we find that the distributions of all proteins consisted of a single population (Fig. 5). As expected, Cav1's nearest neighbors occurred at much shorter distances than the other proteins because the domains consist of multiple copies of the molecule. Additionally, the nearest neighbor distances of B2R, Gαq, and Gαi were almost identical indicating that these proteins organize in caveolae in a similar way. In the case of Cav1, we find that at 150 mosm, the curve of the distance between the molecules broadened in accordance with increased caveola diameter. However, osmotic stress does not change the distances between the other caveola components.
FIGURE 5.
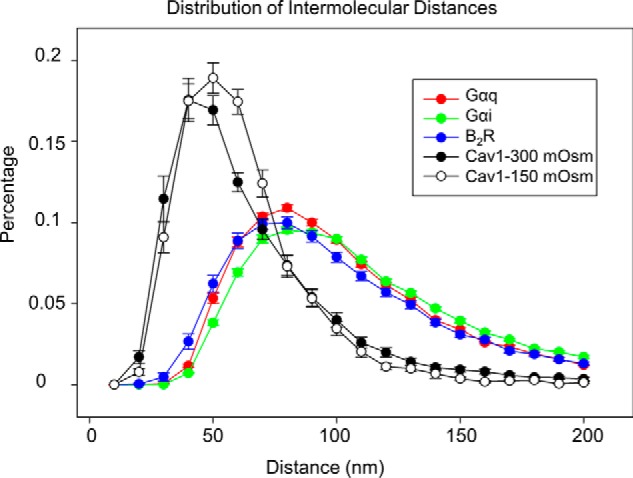
Changes in the nearest neighbor distances between identical molecules under osmotic stress. Distances between identical Cav1 B2R, Gαq, and Gαi molecules under basal and stress conditions were calculated using ImageJ NND plug-in. Data were derived from two to three independent experiments in which 6–18 images were measured in each cell and five to seven cells were measured in each group, to give a total of 53–74 images per group.
Amounts of Gαi and Gαq Associated with Caveola Domains Differ
We quantified the percent of B2R, Gαq, and Gαi that localize to caveola domains. This was accomplished by determining the overlap in the green and red channels of the images described in Figs. 2–4 and dividing by the total amount of red area using ImageJ. In this analysis, higher values will indicate a larger percentage of protein localized in caveolae. Our data show that a much higher amount of Gαi intensity is associated with caveola domains as compared with Gαq and B2R (Fig. 6).
FIGURE 6.
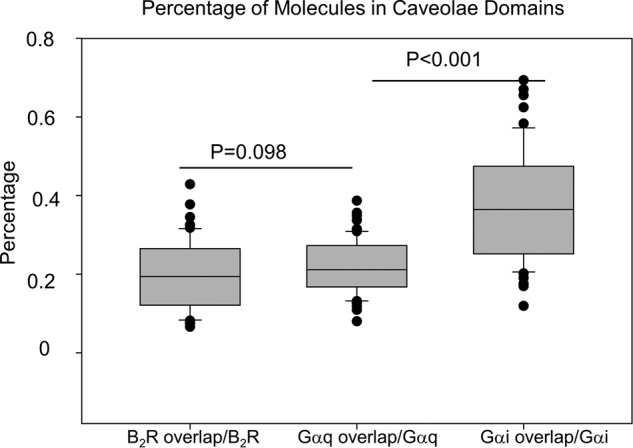
Overlap between Cav1 and B2R, Gαq, or Gαi intensities suggests that minor populations are confined in the domains. Percentage of B2R, Gαq, or Gαi area in caveola domains is indicated by the overlap between the intensity areas of these molecules with Cav1 versus their total area. Data were derived from two to three independent experiments in which 7–15 images were measured in each cell, and five to seven cells were measured in each group, to give a total of 52–61 images per group.
Caveolae Do Not Regulate Gαi/β2AR Signals
Our previous work showed that caveolae stabilize Ca2+ signals generated from Gαq activated by B2R or acetylcholine but had no effect on morphine signals mediated through Gαi (10). These latter studies were done by comparing cAMP signals in Fischer rat thyroid cells that lacked Cav1 versus a stable Cav1-transfected line (19). It is possible that the caveolae formed in this cell line lack factors that allow for Gαi interactions and that natively expressed caveolae may affect cAMP in a manner similar to Ca2+ signal through stabilization of the activated state of Gαi.
We tested this idea using a fluorescent cAMP sensor (ICUE3) that monitors the amount of cAMP by eCFP/eYFP FRET (20). We stimulated a GPCR that activates this pathway, β2AR, and monitored the decrease in cAMP levels by the decrease in CFP/YFP ratio of cells at 300 and 150 mosm (Fig. 7). These studies clearly show that osmotic stress does not impact cAMP signals through the β2AR.
FIGURE 7.
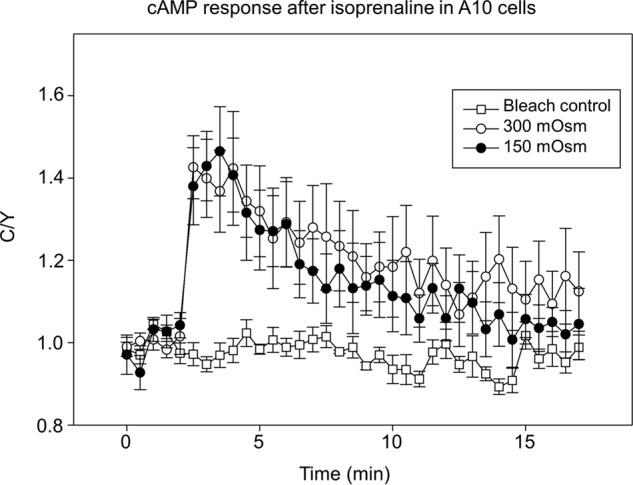
Hypo-osmotic stress does not affect intracellular cAMP response after βAR stimulation. Normalized emission intensity ratio (eCFP/FRET) changes of the cAMP sensor ICUE3 was measured in A10 cells treated with 5 μm isoprenaline under 300 and 150 mosm conditions. Bleach control cells were transfected with ICUE3 but not treated with isoprenaline. Six to seven cells were measured in each group.
Live Cell Studies Support the Stability of Caveola Domains
We complemented the structural studies of caveolae with live cell fluorescence imaging of the receptors by following the diffusion of the two proteins by fluorescence correlation spectroscopy (FCS). Previous studies using Fischer rat thyroid cells showed that the presence of caveolae does not greatly affect the mobility of Gαq, μOR, or B2R but did affect the amount of μOR and B2R in the mobile fraction (21). Based on these studies, we focused on the diffusion of the two GPCRs, i.e. B2R and β2AR. FCS data for B2R and β2AR fit best to a model where two populations diffused on a two-dimensional surface (see under “Experimental Procedures”). Unlike super-resolution imaging where we viewed the cells in TIRF mode, here we measured FCS on the apical (top) side of the cell membrane that contains less caveolae, and we compared these to the basal (bottom) of the cell membrane, which contains more caveolae (10). This difference in the amount of caveolae in the two membranes is reflected in the higher mobility of the receptors diffusing in the apical as opposed to the basal membrane under normal osmotic conditions (Table 1). Under hypo-osmotic conditions, the diffusion of receptors on the more caveola-rich basal membranes suggests a reduction in diffusion, although we could only detect significant differences in diffusion between the 300 and 150 mosm samples for B2R. The lack of significant effect of osmotic stress on the slow diffusion of the receptors is consistent with a minor population of the receptors being localized in the periphery but not inside caveola domains. Additionally, these results support the idea that the receptors remain associated with caveolae during mild osmotic stress.
TABLE 1.
Comparison of FCS diffusion coefficients for two GPCRs
5–11 cells were measured in each group; 1–5 positions were measured in each cell, and 13–30 positions were measured in each group. Media (Q) were used to show the diffusion coefficients in each group. The statistical differences of B2R in the apical and basal membranes at 300 mosm is p = 0.16 and for β2AR is 0.07.
| Protein | Position | 300 mosm | 150 mosm | p value |
|---|---|---|---|---|
| μm2/s | μm2/s | |||
| B2R | Apical | 0.323(0.308) | 0.224(0.163) | 0.09 |
| Basal | 0.256(0.264) | 0.292(0.253) | 0.59 | |
| β2AR | Apical | 0.346(0.794) | 0.296(0.231) | 0.41 |
| Basal | 0.238(0.123) | 0.301(0.669) | 0.35 |
Discussion
Caveolae have the potential to undergo regular deformation during normal biological function, and this deformation directly affects Gαq-mediated Ca2+ signals (4, 11). In this study, we have used super-resolution fluorescence imaging to view caveola deformation and to determine the underlying cause of the changes in calcium signals.
The structure of caveolae has been characterized by EM (2), and the dimensions of the domains measured here by super-resolution match these previous studies as well as those measured by near field imaging (22). To date, super-resolution fluorescence studies of caveolae have been used to measure the localization of ryanodine receptors (18) and the promotion of antigen aggregation by caveolae (23). A clear advantage of super-resolution studies is that the method can be used to visualize components in intact cells without the need for cell disruption or membrane separation. It is important to note that fluorescence intensities of these microscopy measurements may vary with the incidence angle of the exciting laser light with respect to the position of the fluorophore in the caveola invagination. The specific geometric orientation of the fluorophore may explain why, for caveolae whose invaginations are perpendicular to the surface, only the membrane surface and back regions of the domains appear to be more readily seen, whereas the side regions are not as visible. Nevertheless, our estimation of the domain depth (∼30 nm) is similar to those suggested by EM.
Previous studies found that caveolae will flatten and dissolve when subjected to a 10-fold reduction in osmolarity (3). However, in our studies, subjecting A10 cells to this low osmolarity results in loss of eGFP-Cav1/mCherry-Cav1 FRET, which in turn results in cell rupture, suggesting that when the domains dissociate the cells are no longer stable. Here, we used a 2-fold reduction in osmolarity in which the diameter of the cell remains constant to avoid disruption of the actin cytoskeleton. This low change in osmolarity better mimics physiological conditions. As noted, this level of stress is sufficient to completely reverse the increase in Ca2+ response due to stabilization of the activated state of Gαq by Cav1 (11). We find that rather than completely dissolving as seen at 30 mosm, the caveolae deform to a somewhat flatter configuration at 150 mosm to provide more surface area and to accommodate the increased cell volume. This flattening is seen by the ∼30% increase in the diameter of the domain mouth and an increase in intensity in the center region of the domain surface. We could not detect large scale disruption or dissolution of the domains under these conditions. These changes support our previous studies in A10 cells showing a small but reversible loss in GFPCav1/mCherryCav1 FRET with osmotic stress (11).
We can use the van't Hoff equation relating to osmotic pressure to estimate the pressure exerted on the cell membrane by the change in osmolarity without assuming the molecular nature of the membrane itself: π = MRT, where π is the osmotic pressure; M is the molar concentration of solutes in moles/liter; R is the gas constant, and T is the absolute temperature. Lowering the osmolarity from 300 to 150 mosm results in a pressure change of Δπ = 150 mol/liter·0.0831 liters·atm/mol/K·298 K, or 3710 atms, or the pressure exerted on the membrane by the osmotic stress. Although this pressure is directional, there is a wealth of previous studies on the behavior of lipid and pressure with hydrostatic pressure, where the force on the samples is uniform. The application of a hydrostatic pressure of ∼3710 atms will promote the dissociation of protein oligomers in solution and increase the packing of lipid molecules in membrane bilayers (24). Based on previous hydrostatic pressure studies, and osmotic pressure studies on brush-border membranes (12), we can offer a molecular interpretation of our results. Our images do not indicate large scale caveola dissociation suggesting a fairly incompressible structure, and the analysis described below suggests that these domains are very strong. Because lipid chains are much more compressible than proteins, we hypothesize that increased osmotic pressure allows for better packing of chains around the Cav1 as the caveolae deform due to stress. High osmotic stress further forces lipids or small molecules, such as cholesterol, into regions between Cav1 molecules better promoting deformation until eventually dissociating the domains. The binding of the peripherally bound Gαq, which is anchored to membranes by two palmitoyl chains, is not affected by mild osmotic stress. We believe that a similar mechanism occurs with mechanical deformation in which of lipids and small membrane molecules act as a solvent to fill in the voids between Cav1 molecules that accompany deformation.
The amount of deformation of a caveola at the osmotic pressure used here allows us to roughly estimate the stability to cells provided by these domains. The osmotic pressure change of 3710 atms in going from 300 to 150 mosm (see above) corresponds to a force/area of 3.82 × 108 N/m2. Our results show an ∼30% increase in caveola diameter, which will correspond to an increased area of ∼478 nm2 for a caveola that is 100 nm in length. A simplified analysis suggests that a caveola has a Young's modulus, which relates to the stiffness of a material, of ∼1.2 GPa, putting it in range of stiffer rubber materials and bacteriophage capsids. Because pressure is force per unit area, we can divide the osmotic pressure by the change in area (3.82 × 108 N/m2/478 × 10−18 m2) to obtain the force used to deform caveola of 182 nN. Considering that normal muscle movement is 0.1–100 N, and for a 50 × 50 × 50-μm cell in which caveolae take up 5% of the surface area to give ∼300,000 copies of the domain, then each cell can handle ∼0.05 N. Thus, the caveolae in large muscle can easily handle this force and augment the stability of muscle fibers.
Many types of GPCRs have been associated with caveolae (25). Our previous work suggested that Gαq scaffolds its associated receptors to caveolae but that, in general, GPCRs do not have intrinsic affinity for caveolae (4, 10). Thus, it is not surprising that the distances between Cav1 and Gαq are within the error of the distances between Cav-1 and B2R, and the amount of Gαq and B2R that we estimate to be localized in caveolae are in accord with FRET studies (10). However, these previous FRET studies along with previous studies by coimmunofluorescence (8) and pulldown studies (9) suggested that Gαi does not localize in caveolae. Here, we find that a significant population resides in or close to caveola domains. The basis for caveolae localization of Gαi is unclear because Gαi, unlike Gαq, does not have specific affinity for caveolin molecules (10). It is notable that there are many studies showing that a significant amount of the Gαi-coupled receptor of β2AR is localized to caveola domains (26, 27). The factors that are responsible for this localization are unclear and may be primarily due to its association with β2AR along with ion channels or other proteins. Unlike Gαq-mediated signals, we find that changes in cAMP levels are not sensitive to caveolae and so the functional basis for the localization of β2AR/Gαi is unclear. This result argues against the idea that aggregation of receptors in caveola domains enhances signaling.
As mentioned, when A10 cells are subjected to osmotic pressure, the enhancement of Ca2+ signals due to Gαq-Cav1 interactions is ablated (10). Our data suggest that only 20% of Gαq is localized to caveola domains and thus stabilized by caveolae. Thus, only this population is affected by osmotic stress. Additionally, our results suggest that osmotic stress does not release Gαq from caveolae but instead alters their interaction most likely due to caveola deformation. This estimate is consistent with the minor change in FRET between Cav1 and Gαq seen under hypo-osmotic conditions in earlier studies (5). This population is consistent with the population displaying enhanced Ca2+ signals. We note also that our studies indicate that the subpopulations of Gαq, B2R, or Gαi localized to caveolae are not kinetically trapped but can undergo exchange with diffusing populations. These observations suggest that cells have varying caveolae that may be involved in different functions. This idea is currently being tested.
Experimental Procedures
Materials
Rat aortic smooth muscle (A10) cells were purchased from American Tissue Culture Collection. B2R-GFP DNA was a gift from Dr. Fredrik Leeb-Lundberg (Lund University) and constructed into B2R-eYFP as described (28). β2AR-YFP DNA was a gift from Dr. Catherine Berlot (Geisinger Clinic) (29), and the DNA for the fluorescent cAMP sensor, ICUE3, DNA was a gift from Dr. Jin Zhang (The Johns Hopkins University) (30). Primary antibodies to Cav1 (mouse or rabbit), B2R (goat), Gαq (goat), and Gαi (rabbit) were from Santa Cruz Biotechnology (Santa Cruz, CA). Alexa Fluor 488 5-tetrafluorophenol ester and Alexa Fluor 647 succinimidyl ester were from Thermo Fisher Scientific.
Cell Culture and Transfection
A10 cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Gibco 11965, 320–355 mosm) supplemented with 1 mm sodium pyruvate, 10% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin sulfate as described previously (11). All cells were maintained at 37 °C in a 5% CO2 incubator. Cells were transfected with ICUE3 DNA (1 μg/0.5 × 106 cells in a 35-mm dish) or other fluorescent-tagged proteins as indicated using LipofectamineTM 3000 transfection reagent (Invitrogen) according to the manufacturer's protocol. For measurements, cells were transferred to Hanks' balanced salt solution (HBSS, Gibco 14025, 300 mosm) or treated with 150 mosm (1:1 dilution) HBSS for 5 min at room temperature for hypo-osmotic shock.
Immunostaining for Super-resolution Studies
The procedure for immunostaining was taken directly from the N-STORM sample protocol procedure from the super-resolution microscope supplier (Nikon). Briefly, A10 cells were seeded onto 35-mm glass bottom dishes (MatTek) 1 day before immunostaining. The cells were fixed in 4% paraformaldehyde for 20 min at room temperature and reduced with 0.1% sodium borohydride (Sigma) for 7 min. The fixed cells were washed three times with PBS and incubated with 0.2% Triton X-100 in PBS for 15 min and then blocked in PBS containing 10% horse serum and 0.05% Triton X-100 for at least 3 h. Then, the cells were incubated with primary antibody directly labeled with fluorophore. Anti-Cav1, Gαq, B2R, or Gαi were diluted 1:100 in PBS containing 5% horse serum and 0.05% Triton X-100 for 1 h. The normal resolution of confocal Cav-1-immunostained images in A10 using this procedure appeared similar to previous images (10). The antibodies were labeled with Alexa Fluor 488 or Alexa Fluor 647 as in a previous study (32). The labeling stoichiometry was ∼4 fluorophores per antibody. Cav1 antibodies were labeled directly with Alexa Fluor 488. B2R, Gαq, and Gαi antibodies were labeled with Alexa Fluor 647. Cells were then washed five times for 15 min with PBS, followed by post-fixation with 4% paraformaldehyde and incubation at room temperature for 10 min. The cells were then washed three times with PBS and transferred into an imaging buffer containing 10% (w/v) glucose, 100 mm cysteamine, 0.5 mg/ml glucose oxidase, and 40 μg/ml catalase before measurement.
STORM Imaging
STORM imaging was performed on a Nikon Eclipse Ti-E motorized inverted microscope equipped with a CFI apo TIRF ×100 oil objective (1.49 NA) located at Stony Brook University. The setup was controlled by Nikon NIS-Element AR software (version 4.30.02) with N-STORM module. Samples were imaged near the cell surface and excited by an Agilent MLC monolithic laser combiner system (405/488/561/647 nm, Agilent Technology, MLC 400B). The 488- and 647-nm laser lines were used for excitation of Alexa Fluor 488- and Alexa Fluor 647-labeled structures. Data were recorded simultaneously from both 488- and 647-nm channels via Andor iXon Ultra DU-897 EMCCD camera (Andor Technology). A series of ∼50,000 raw frames were acquired at ∼40 ms per frame at a pixel size of 2–3 or 2–4 pixels/nm. The TIRF angle was adjusted to minimize out-of-focus background, and the focus was kept stable during acquisition using Nikon's perfect focus system. The images were reconstructed using NIS-A N-STORM analysis software and exported at 2–3 nm/pixel for the following analysis. Full width at half-maximum of each Gaussian function was used to calculate the imaging resolution. Note that because this method involves collecting a large number (50,000) images where only a few fluorophores are detected, sporadic points do not contribute.
Super-resolution Image Analysis
The diameters of caveolae were measured using ImageJ by constructing circles through the middle of the intensities of the mouth of the domains and measuring the diameters using the distance between the center of the intensities at the ends of the circle. To assess flattening of the caveolae, we used a cross-section profiling approach in which we fit the intensities of the two outer ends of the mouths of the caveolae to two Gaussian distributions. For well formed caveola invaginations, this treatment resulted in two Gaussian curves whose midpoints are separated by a distance equal to the diameter of the caveolae with no visible intensity between the curves, as seen in Fig. 1B. As described under the “Results,” caveola deformation causes intensity to appear in the center of the domain mouth distorting structure and making fitting of the intensity distributions on the ends of the diameters to Gaussian curves difficult. Therefore, if it was not possible to fit the outer-most intensities to a Gaussian curve, we simply approximated the midpoint of the intensity to allow the distance between these midpoints to be calculated as exemplified in Fig. 1B.
Colocalization analysis was performed using a python-written Anaconda software (windows 64 bit version of python 2.7). As described in Soeller et al. (33), images were thresholded using the isodata algorithm to obtain binary masks for the red- and green-labeled structures (17). After a distance transform-based analysis was performed, the amount of labeling in one channel at a given distance from the edge of the other channel's mask was calculated. By calculating the amount of labeling at a given distance from the edge of the masks, using the convention that distances within the cluster mask were negative and distances outside the mask were positive, the fraction of total labeling as a function of distance was determined. Thus, negative distances reflect complete overlap between the red and green channels, whereas positive distances refer to the distances between the pixels outside of the overlap region. The fractions were summarized in distance histograms. The resulting distance histograms were normalized. Then the distance histogram was fitted with a weibull function to determine the centroid of distance distribution.
The distribution of Cav1, Gαq, B2R, and Gαi protein on the cell membrane was analyzed using Nearest Neighbor Distance (NND) plugin for ImageJ. The image was set to scale, thresholded, and then the x and y coordinates of each particle in the image was analyzed. The NND plugin was then used to get the distance to the nearest neighbor particle for each particle, and the data were summarized in a distance histogram.
Overlap of Cav1 and caveola components were analyzed by ImageJ as well. Areas of green and red in the image were analyzed, respectively, and then the area of composite image with two colors was analyzed. The overlap area was defined as the green area plus the red area minus the composite area, and then the overlap area was divided by the total amount of green area or red area, respectively.
ICUE3 Imaging
ICUE3 experiments were performed on a Zeiss LSM 510 Meta instrument (Jena, Germany) equipped with a ×40 NA 1.2 C-Apochromat water immersion objective. eCFP and YFP were excited by 458- and 514-nm argon ion laser lines, respectively, and 475–525- and 560–615-nm bandpass filters were used to collect emission images, respectively. FRET images were obtained by exciting samples using a 458-nm laser line and collecting images through 560–615-nm bandpass filter. Cells were transferred into HBSS buffer 48 h after transfection and treated with 5 μm isoprenaline (Sigma). Images were taken every 30 s with an exposure time of 200 ms for ∼17.5 min. Fluorescent images were analyzed by ImageJ, and the intensity ratios of eCFP to FRET images were calculated at different time points and normalized by dividing all ratios by the intensity ratio before stimulation. The binding of cAMP to ICUE3 led to increases in the intensity ratio of eCFP to FRET images. Intracellular cAMP concentration changes were measured under normal or hypo-osmotic conditions. Bleach control samples were measured identically but not treated by isoprenaline.
FCS
FCS measurements were performed on the dual-channel confocal fluorescence correlation spectrometer (Alba version 5, ISS Inc.) equipped with avalanche photodiodes and a Nikon Eclipse Ti-U inverted microscope. A ×60 Plan Apo (1.2 NA, water immersion) objective and a mode-locked two-photon titanium-sapphire laser (Tsunami; Spectra-Physics) was used in this study. The waist (ω0) of the excitation beam was calibrated every day before experiments by measuring the diffusion of 10 nm Alexa Fluor 488 in water with a diffusion coefficient of 435 μm2/s (31). The typical ω0 values were 0.43–0.45 μm. Cells expressing low amounts of B2R-eYFP and β2AR-YFP proteins were selected for viewing. The samples were excited at 930 nm, and emission spectra were collected through a 547/27 bandpass filter. The data were acquired in the time mode for 200 s, and the sampling frequency was 10 kHz. Measurements that showed abrupt and significant changes in the count rate were neglected to avoid artifacts due to bleaching and/or cell movement. The data were stored and processed by Vista software (ISS Inc.). The autocorrelation functions were analyzed using a two-dimensional, two-component diffusion model provided by ISS software where fitting Model 1 is described.
All measurements were performed at room temperature. The diffusions of B2R-eYFP and β2AR-YFP proteins on both basal and apical membranes were measured under normal or hypo-osmotic conditions.
Author Contributions
L. Y. was responsible for preparing the samples and collecting and analyzing the data. S. S. was responsible for conceiving and planning the experiments, helping with the data analysis, and writing the manuscript.
Acknowledgments
We thank Dr. Christian Soeller for advice on handling the super-resolution data, Dr. Yuanjian Guo for help with some of the experimental methods, and Dr. Urszula Golebiewska for reading the manuscript. The purchase of the Nikon SIM (or STORM) was supported, in part, by National Institutes of Health Grant 1S10OD016405-01.
This work was supported by National Institutes of Health Grant GM116187. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- PLCβ
- phospholipase Cβ
- B2R
- bradykinin type 2 receptor
- βAR
- β2-adrenergic receptor
- GPCR
- G protein-coupled receptor
- eGFP
- enhanced GFP
- eCFP
- enhanced cyan fluorescent protein
- eYFP
- enhanced YFP
- FCS
- fluorescence correlation spectroscopy
- N
- newton
- STORM
- stochastic optical reconstruction microscopy
- NND
- Nearest Neighbor Distance
- HBSS
- Hanks' balanced salt solution.
References
- 1. Parton R. G., and Simons K. (2007) The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 8, 185–194 [DOI] [PubMed] [Google Scholar]
- 2. Stan R. V. (2005) Structure of caveolae. Biochim. Biophys. Acta 1746, 334–348 [DOI] [PubMed] [Google Scholar]
- 3. Sinha B., Köster D., Ruez R., Gonnord P., Bastiani M., Abankwa D., Stan R. V., Butler-Browne G., Vedie B., Johannes L., Morone N., Parton R. G., Raposo G., Sens P., Lamaze C., and Nassoy P. (2011) Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144, 402–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sengupta P., Philip F., and Scarlata S. (2008) Caveolin-1 alters Ca2+ signal duration through specific interaction with the Gαq family of G proteins. J. Cell Sci. 121, 1363–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo Y., Golebiewska U., and Scarlata S. (2011) Modulation of Ca2+ activity in cardiomyocytes through caveolae-Gαq Interactions. Biophys. J. 100, 1599–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rebecchi M. J., and Pentyala S. N. (2000) Structure, function and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 80, 1291–1335 [DOI] [PubMed] [Google Scholar]
- 7. Suh P. G., Park J. I., Manzoli L., Cocco L., Peak J. C., Katan M., Fukami K., Kataoka T., Yun S., and Ryu S. H. (2008) Multiple roles of phosphoinositide-specific phospholipase C isozymes. BMB Rep. 41, 415–434 [DOI] [PubMed] [Google Scholar]
- 8. Oh P., and Schnitzer J. E. (2001) Segregation of heterotrimeric G proteins in cell surface microdomains. Gq binds caveolin to concentrate in caveolae, whereas Gi and Gs target lipid rafts by default. Mol. Biol. Cell 12, 685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murthy K. S., and Makhlouf G. M. (2000) Heterologous desensitization mediated by G protein-specific binding to caveolin. J. Biol. Chem. 275, 30211–30219 [DOI] [PubMed] [Google Scholar]
- 10. Calizo R. C., and Scarlata S. (2012) A role for G-proteins in directing G-protein-coupled receptor–caveolae localization. Biochemistry 51, 9513–9523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo Y., Yang L., Haught K., and Scarlata S. (2015) Osmotic stress reduces Ca2+ signals through deformation of caveolae. J. Biol. Chem. 290, 16698–16707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohyashiki T., Taka M., and Mohri T. (1985) The effects of ionic strength on the protein conformation and the fluidity of porcine intestinal brush border membranes. Fluorometric studies using N-[7-dimethylamino-4-methylcoumarinyl]maleimide and pyrene. J. Biol. Chem. 260, 6857–6861 [PubMed] [Google Scholar]
- 13. Anderson R. G. (1998) The caveolae membrane system. Annu. Rev. Biochem. 67, 199–225 [DOI] [PubMed] [Google Scholar]
- 14. Golias Ch., Charalabopoulos A., Stagikas D., Charalabopoulos K., and Batistatou A. (2007) The kinin system–bradykinin: biological effects and clinical implications. Multiple role of the kinin system–bradykinin. Hippokratia 11, 124–128 [PMC free article] [PubMed] [Google Scholar]
- 15. Lamb M. E., De Weerd W. F., and Leeb-Lundberg L. M. (2001) Agonist-promoted trafficking of human bradykinin receptors: arrestin- and dynamin-independent sequestration of the B2 receptor and bradykinin in HEK293 cells. Biochem. J. 355, 741–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lamb M. E., Zhang C., Shea T., Kyle D. J., and Leeb-Lundberg L. M. (2002) Human B1 and B2 bradykinin receptors and their agonists target caveolae-related lipid rafts to different degrees in HEK293 cells. Biochemistry 41, 14340–14347 [DOI] [PubMed] [Google Scholar]
- 17. Jayasinghe I. D., Baddeley D., Kong C. H., Wehrens X. H., Cannell M. B., and Soeller C. (2012) Nanoscale organization of junctophilin-2 and ryanodine receptors within peripheral couplings of rat ventricular cardiomyocytes. Biophys. J. 102, L19–L21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong J., Baddeley D., Bushong E. A., Yu Z., Ellisman M. H., Hoshijima M., and Soeller C. (2013) Nanoscale distribution of ryanodine receptors and caveolin-3 in mouse ventricular myocytes: dilation of T-tubules near junctions. Biophys. J. 104, L22–L24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mora R., Bonilha V. L., Marmorstein A., Scherer P. E., Brown D., Lisanti M. P., and Rodriguez-Boulan E. (1999) Caveolin-2 localizes to the Golgi complex but redistributes to plasma membrane, caveolae, and rafts when co-expressed with caveolin-1. J. Biol. Chem. 274, 25708–25717 [DOI] [PubMed] [Google Scholar]
- 20. Sample V., DiPilato L. M., Yang J. H., Ni Q., Saucerman J. J., and Zhang J. (2012) Regulation of nuclear PKA revealed by spatiotemporal manipulation of cAMP. Nat. Chem. Biol. 8, 375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calizo R. C., and Scarlata S. (2013) Discrepancy between fluorescence correlation spectroscopy and fluorescence recovery after photobleaching diffusion measurements of G-protein-coupled receptors. Anal. Biochem. 440, 40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abulrob A., Lu Z., Brunette E., Pulla D., Stanimirovic D., and Johnston L. J. (2008) Near-field scanning optical microscopy detects nanoscale glycolipid domains in the plasma membrane. J. Microsc. 232, 225–234 [DOI] [PubMed] [Google Scholar]
- 23. Gabor K., Stevens C., Pietraszewski M., Gould T., Lam S. H., Gong Z., Hess S., and Kim C. (0000) Super resolution microscopy reveals that caveolin-1 is required for antiviral immune response. Biophys. J. 100, 21a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heremans K. (1982) High pressure effects upon proteins and other biomolecules. Annu. Rev. Biophys. Bioeng. 11, 1–21 [DOI] [PubMed] [Google Scholar]
- 25. Chini B., and Parenti M. (2004) G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J. Mol. Endocrinol. 32, 325–338 [DOI] [PubMed] [Google Scholar]
- 26. Rybin V. O., Xu X., Lisanti M. P., and Steinberg S. F. (2000) Differential targeting of β-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae: a mechanism to functionally regulate the cAMP signaling pathway. J. Biol. Chem. 275, 41447–41457 [DOI] [PubMed] [Google Scholar]
- 27. Steinberg S. F. (2004) β2-Adrenergic receptor signaling complexes in cardiomyocyte caveolae/lipid rafts. J. Mol. Cell. Cardiol. 37, 407–415 [DOI] [PubMed] [Google Scholar]
- 28. Philip F., Sengupta P., and Scarlata S. (2007) Signaling through a G protein-coupled receptor and its corresponding G protein follows a stoichiometrically limited model. J. Biol. Chem. 282, 19203–19216 [DOI] [PubMed] [Google Scholar]
- 29. Hynes T. R., Mervine S. M., Yost E. A., Sabo J. L., and Berlot C. H. (2004) Live cell imaging of Gs and the β2-adrenergic receptor demonstrates that both αs and β1γ7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the β2-adrenergic receptor. J. Biol. Chem. 279, 44101–44112 [DOI] [PubMed] [Google Scholar]
- 30. DiPilato L. M., and Zhang J. (2009) The role of membrane microdomains in shaping β2-adrenergic receptor-mediated cAMP dynamics. Mol. Biosyst. 5, 832–837 [DOI] [PubMed] [Google Scholar]
- 31. Petrásek Z., and Schwille P. (2008) Precise measurement of diffusion coefficients using scanning fluorescence correlation spectroscopy. Biophys. J. 94, 1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Philip F., and Scarlata S. (2006) Real-time measurements of protein affinities on membrane surfaces by fluorescence spectroscopy. Sci. STKE 2006, pl5. [DOI] [PubMed] [Google Scholar]
- 33. Wong J., Baddeley D., Bushong E. A., Yu Z., Ellisman M. H., Hoshijima M., and Soeller C. (2013) Nanoscale distribution of ryanodine receptors and caveolin-3 in mouse ventricular myocytes: dilation of t-tubules near junctions. Biophys. J. 104, L22–L24 [DOI] [PMC free article] [PubMed] [Google Scholar]