Abstract
Tandem mass spectrometry was applied to tissues from targeted mutant mouse models to explore the collagen substrate specificities of individual members of the prolyl 3-hydroxylase (P3H) gene family. Previous studies revealed that P3h1 preferentially 3-hydroxylates proline at a single site in collagen type I chains, whereas P3h2 is responsible for 3-hydroxylating multiple proline sites in collagen types I, II, IV, and V. In screening for collagen substrate sites for the remaining members of the vertebrate P3H family, P3h3 and Sc65 knock-out mice revealed a common lysine under-hydroxylation effect at helical domain cross-linking sites in skin, bone, tendon, aorta, and cornea. No effect on prolyl 3-hydroxylation was evident on screening the spectrum of known 3-hydroxyproline sites from all major tissue collagen types. However, collagen type I extracted from both Sc65−/− and P3h3−/− skin revealed the same abnormal chain pattern on SDS-PAGE with an overabundance of a γ112 cross-linked trimer. The latter proved to be from native molecules that had intramolecular aldol cross-links at each end. The lysine under-hydroxylation was shown to alter the divalent aldimine cross-link chemistry of mutant skin collagen. Furthermore, the ratio of mature HP/LP cross-links in bone of both P3h3−/− and Sc65−/− mice was reversed compared with wild type, consistent with the level of lysine under-hydroxylation seen in individual chains at cross-linking sites. The effect on cross-linking lysines was quantitatively very similar to that previously observed in EDS VIA human and Plod1−/− mouse tissues, suggesting that P3H3 and/or SC65 mutations may cause as yet undefined EDS variants.
Keywords: bone, collagen, endoplasmic reticulum (ER), mass spectrometry (MS), post-translational modification (PTM), skin, cross-links
Introduction
The collagen family of proteins has evolved into many different genes and gene translational products each with variably regulated post-translational modifications (1, 2). Even within a single genetic type of collagen, the post-translational quality of the protein is known to be regulated with a high degree of tissue specificity (3, 4). The functional significance of this post-translational variability is still not fully understood, although for fibril-forming collagens the number, placement, and chemistry of covalent intermolecular cross-links seem to be critically important regulators of tissue material properties and function (5–7).
In the last decade, new insights on the significance of a relatively rare collagen modification, prolyl 3-hydroxylation, came from the discovery that recessive forms of osteogenesis imperfecta (OI)2 are caused by biallelic mutations in prolyl 3-hydroxylase 1 (P3H1; Lepre1), CRTAP (Leprel3), or CypB (PPIB) (8). These proteins, which form a P3H1 enzyme complex, act on nascent collagen chains in the ER (9). Mutations in at least 6 further genes that encode either enzymes (e.g. PLOD2 encoding lysyl hydroxylase-2 (LH2) (10)) or chaperones (e.g. FKBP10 encoding FKBP65 (11, 12)), needed for collagen modification, folding, transport, and normal mineralization, have been shown to cause OI variants. We have gained insights on the disease-causing mechanisms by analyzing tissue collagens from OI patients and mouse models of OI. For example, P3H1, CRTAP, and PPIB mutations all cause telopeptide lysine over-hydroxylation (5), whereas PLOD2 or FKBP10 mutations cause telopeptide lysine under-hydroxylation (10–12). A common effect from all these mutations is an altered collagen cross-linking chemistry. These findings suggest an interplay in the ER between the prolyl 3-hydroxylation complex and the lysyl hydroxylation machinery.
Human mutations in P3H2 (Leprel1) have been shown to cause the eye disorder high myopia (13–15). Two different P3H2 knock-out mice have so far been generated by separate laboratories, the first was embryonic lethal (16), whereas the second had no obvious phenotype (17). Mass spectral analysis of multiple tissues from the latter P3H2 knock-out mouse revealed that collagen types I, II, V, and IV lack 3-hydroxyproline at all sites other than the few sites acted on by P3H1 (17). But so far, neither substrate specificity nor disease-associated mutations have been identified for P3H3. Recombinant P3H3 protein expressed in bacteria was reported to form insoluble aggregates with no detectable hydroxylating activity (18). The fifth remaining member of the gene family, synaptonemal complex 65 (Sc65), is a close homologue of Crtap with an apparent function in bone homeostasis (19), as well as a suspected role as an autoimmune target in membranous nephropathy (20). We recently reported suppressed collagen lysine hydroxylation in the Sc65-null mouse and evidence for complex formation between Sc65, P3H3, and potentially lysyl hydroxylase-1 (LH1) and CypB (21).
To understand more about the collagen substrate specificity of P3H3 and the function of Sc65, we screened tissues from Sc65- and P3h3-null mice using tandem mass spectrometry for collagen post-translational effects. The aim was to see if any known sites of 3-hydroxyproline in the various collagen types were under-hydroxylated and also if other post-translational modifications, in particular lysine modification and collagen cross-linking chemistry were affected. The results showed that both P3h3- and Sc65-null mice displayed a similar pattern of severe under-hydroxylation of lysines, particularly at cross-linking sites in type I collagen from bone, skin, dentin, cornea, and aorta. No loss of prolyl 3-hydroxylation was evident at any site accessible to tryptic peptide mass spectral screening of collagen types I, II, IV, and V from several tissue sources. The findings point to a complex evolution and interplay between the proteins forming the machinery of collagen prolyl 3-hydroxylation and lysyl hydroxylation in the ER.
Results
Generation of Sc65 and P3H3 Knock-out Mice
The murine Leprel4 (Sc65) gene was inactivated by homologous recombination in ES cells using two approaches. The first mouse model was established via gene trap insertional mutagenesis (19), whereas the second was generated by introducing flanking loxP sites in the Leprel4 gene (floxed) and then bred with a mouse ubiquitously expressing Cre-recombinase (21). Generation and initial characterization of each of these Sc65-null mouse models has been described (19, 21). The murine Leprel2 (P3h3) gene was inactivated by homologous recombination in ES cells using BAC technology (Fig. 1A). Cross-breeding of heterozygous mice (P3h3+/−) produced the null genotypes. Immunoblot analysis using a polyclonal P3H3 antibody against fractionated renal protein extracts showed absence of protein expression in the P3h3−/− mice (Fig. 1B). A 4 m guanidine HCl extract of fetal human cartilage was used as the positive control.
FIGURE 1.
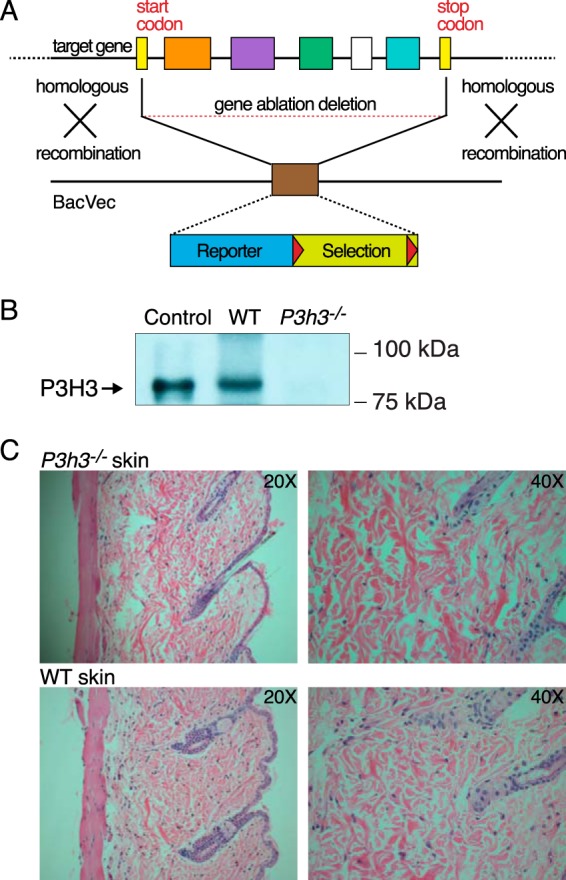
Generation of P3h3−/− mice. A, the Leprel2 gene was inactivated by homologous recombination in ES cells using BAC technology. Mice were maintained on a C57/BL6J background. B, immunoblot analysis using a polyclonal P3H3 antibody against total protein extracts from whole kidneys of WT and P3h3−/− mice supported the loss of protein in the knock-out mice. Human fetal cartilage extract was loaded as a positive control marker for P3H3. C, representative H&E images of skin sections from P3h3−/− and WT mice.
Both homozygous mouse strains, Sc65−/− and P3h3−/−, were viable and showed no skeletal growth defects grossly or histologically or any other abnormalities when observed in the cage environment. P3h3−/− skin sections on H&E staining (Fig. 1C) showed some evidence of collagen fabric fragility (Fig. 1C), similar to that observed in Sc65−/− skin sections (21). Furthermore, on dissection, the skin of both homozygous null strains appeared to lack the structural integrity of normal mouse skin. Skin from Sc65−/− mice also proved on mechanical testing to fail at lower loads (21). Unless otherwise stated, the WT mouse genotype used in this study was Sc65+/+. Tissue collagens from the heterozygote P3h3+/− mouse, which were determined to be indistinguishable from Sc65+/+ mouse (WT) by collagen SDS-PAGE and mass spectral analysis, were also used as a control in this study where indicated.
Collagen Chain Profile on SDS-PAGE
Acid-extracted collagens from skin, bone, and tendon were resolved on SDS-PAGE to assess potential tissue-specific differences in the collagen cross-linking profiles. Reproducible and similar differences in collagen chain patterns were evident from skin of both Sc65−/− and P3h3−/− mice compared with their respective WT littermates (the latter patterns being indistinguishable). Significantly, a band we previously identified as the chain trimer, γ112 (12, 22), was reproducibly more prominent in the null mouse collagen extracts relative to the α and β bands (Fig. 2). The β12 component from both null strains was also noticeably depleted compared with WT in proportion with the increase in γ112. From normal skin and tendon, the β-dimers are known to be the result of intramolecular aldol cross-link formation between allysines in α1-α1 and α1-α2 N-telopeptides. The shift from β12 to γ112 observed with the null mice suggested an effect on intermolecular cross-linking. We reported a similar banding pattern from fibromodulin null mouse tendons and concluded the cause was α1-α1 C-telopeptide aldol formation due to enhanced lysyl oxidase oxidation of α1-C-telopeptide lysines (22). Further studies suggested that fibromodulin interacts with lysyl oxidase supporting this conclusion (23). Interestingly, the shift in band intensity in the current mice was most prominent in skin, less so in tendon, and absent in bone collagen extracts.
FIGURE 2.
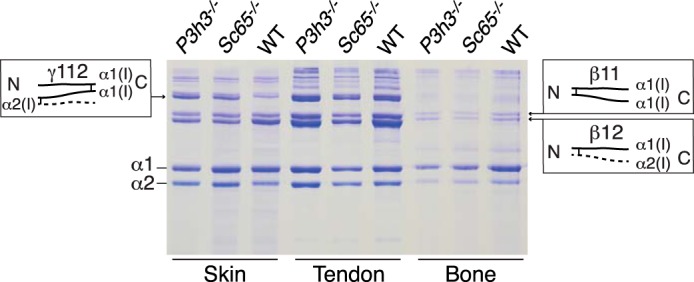
SDS-PAGE reveals altered cross-linking in skin type I collagen from P3h3−/− and Sc65−/− mice. As visualized by 6% SDS-PAGE, type I collagen from P3h3−/− and Sc65−/− mouse skin acid extracts reproducibly yielded more γ112 relative to WT. Accompanying this increased band intensity in knock-out mice skin is the decrease in β12 relative to WT mice. This shift in banding pattern is not observed in acid extracts of bone and tendon.
Differences in native collagen extractability in 3% acetic acid from lyophilized tissue samples supported an effect on stable intermolecular cross-linking in non-mineralized P3h3−/− tissue collagens. Densitometry of the combined stained α-, β-, and γ-chains resolved on SDS-PAGE revealed that P3h3−/− skin collagen (n = 4) was 41% more extractable than control skin collagen (n = 2). Similarly, P3h3−/− tendon collagen (n = 3) was 39% more extractable than control tendon collagen (n = 2).
Lack of Effect on Prolyl 3-Hydroxylation
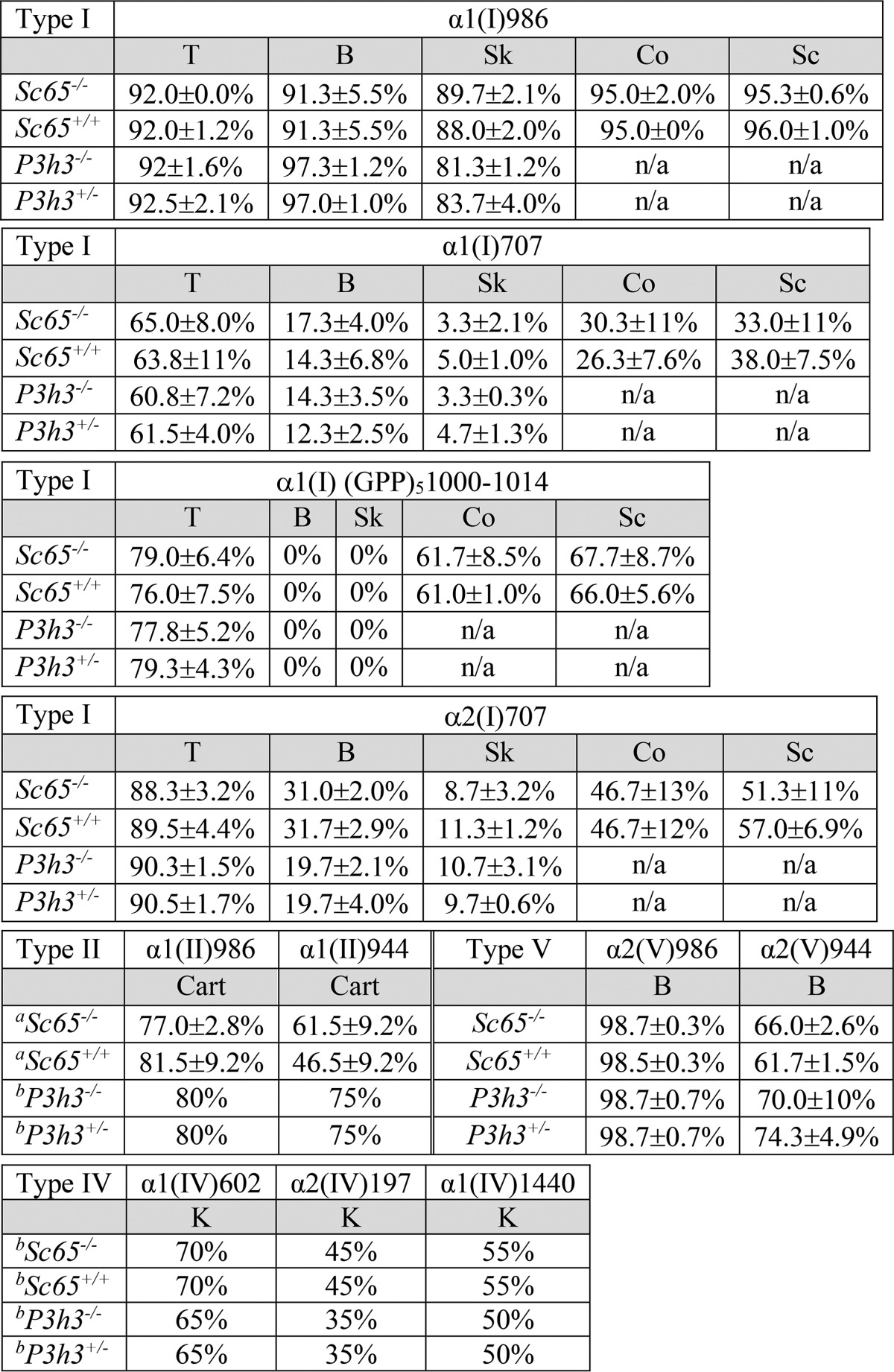
Tandem mass spectrometry was used as a screen to survey the degree of prolyl 3-hydroxylation at all known sites we could readily access as tryptic peptides from collagen types I, II, IV, and V. No significant evidence of suppression was observed at any site in Sc65−/− or P3h3−/− tissues (Table 1 details the findings). This was surprising, particularly for P3h3−/−, which is a close homolog of P3h1 and P3h2, including a potentially active catalytic domain. Both P3h1 and P3h2 have evolved preferred GPP substrate sequences in a range of collagen types (17, 24, 25).
TABLE 1.
Comparison of 3Hyp occupancy in collagens from Sc65-/-, P3h3-/-, and control mouse tissues
Percentage of 3Hyp at each major substrate site in type I collagen in tendon (T), bone (B), skin (Sk), kidney (K), cornea (Co), and sclera (Sc); type II collagen in cartilage (Cart), type IV collagen in kidney (K), and type V collagen in bone (B). Each percentage was determined based on the ratio of the m/z peaks of each post-translational variant from a minimum of three mice unless indicated. (atwo mice; bone mouse).
Identification of the Molecular Structure of the γ112 Trimer
The molecular nature of the γ112 trimer was investigated using in-gel trypsin mass spectrometry. Mass spectrometric analysis revealed a prominent dimeric α1(I) C-telopeptide with its cross-linking residue fitting exactly an α,β-unsaturated allysine aldol (Fig. 3A). As previously shown, trypsin does not cleave after lysines that have been converted to lysine aldehydes, resulting in 6-residue longer tryptic telopeptides cleaved after arginine (22). Notably, MS after in-gel trypsin digest of the γ112 trimer revealed no monomeric α1(I) C-telopeptide, only the cross-linked dimer. MS-MS confirmed the identity of the dimeric cross-linked tryptic peptide (Fig. 3B). The +16 Da mass ladder given by the parent ion is the result of variable 4-hydroxylation of the Yaa position Pro in the C-terminal GPP of the triple-helical domain of each α1-chain of the dimer.
FIGURE 3.
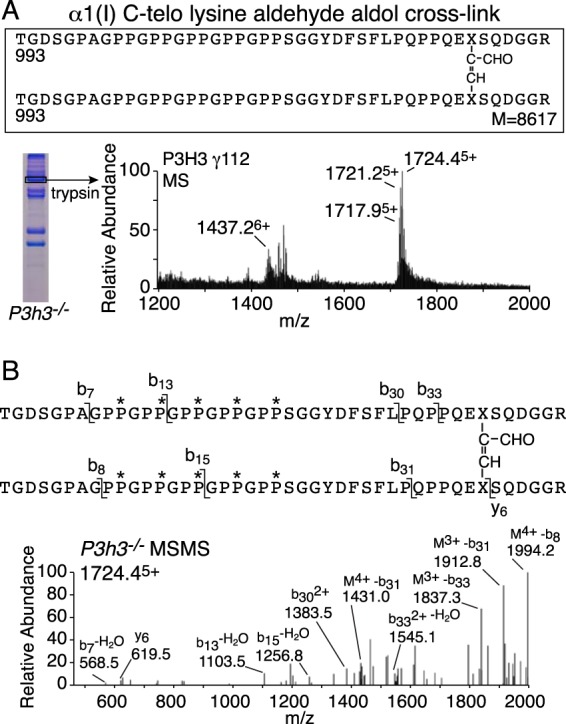
Increased intramolecular C-telopeptide aldol cross-links in knock-out skin type I collagen. The γ112 trimer from P3h3−/− mouse skin has a distinct tryptic peptide profile from that of the α1(I) monomer when analyzed using mass spectrometry. A, the LC-MS profile of in-gel trypsin digests of the γ112 band from P3h3−/− mouse skin confirmed the presence of the α1(I) C-telopeptide lysine aldehyde aldol dimer in the γ112 band. B, MS/MS fragmentation spectrum of the parent ion (1724.45+) from the P3h3−/− γ112 band. The trypsin-digested dimeric peptide is shown with P* indicating 4Hyp.
Tandem Mass Spectrometry Targeted at α1(I) and α2(I) Cross-linking Lysines Shows Under-hydroxylation
Lysine hydroxylation at the cross-linking site, residue Lys-87, was investigated using tandem mass spectrometry of type I collagen α1(I) chains extracted from bone, skin, tendon, aorta, and cornea. A similar pattern of reduced hydroxylation was observed from both Sc65−/− and P3h3−/− mouse tissues, but the effect was consistently greater with Sc65−/− mice. As previously reported for the Sc65−/− mouse, the post-translational shift was more pronounced in skin at helical domain cross-linking residue Lys-87 (Fig. 4) compared with bone. Type I collagen from all soft tissues (skin, tendon, and aorta) showed almost complete loss of post-translational modification of Lys-87 (Table 2). As in the Sc65−/− mouse, all helical cross-linking sites appear to be under-hydroxylated in the P3h3−/− mouse. Indeed, the α1(I) Lys-930 residue, which is completely hydroxylated in P3h3+/− skin, is unmodified in the P3h3−/− mouse skin (Fig. 5). A similar loss of hydroxylation was found at Lys-930 in skin, bone, and tendon from both P3h3−/− and Sc65−/− mice. WT tendon was unique among soft tissues with 100% hydroxylated and non-glycosylated Lys-87 on both α-chains of type I collagen. The effect on Lys-87 hydroxylation was greater in Sc65−/− tendon compared with P3h3−/− tendon. The data are compiled in Table 2.
FIGURE 4.
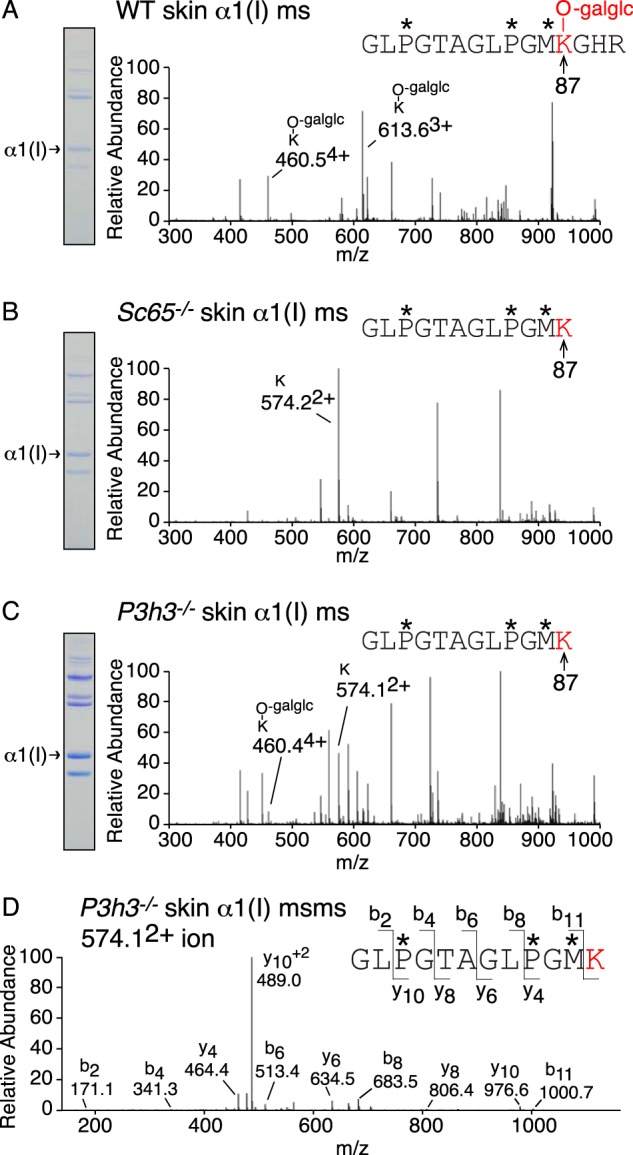
Under-hydroxylation at cross-linking Lys-87 in P3h3−/− and Sc65−/− skin collagen. LC-MS profiles of in-gel trypsin digests of the α1(I) collagen chains from WT, Sc65−/−, and P3h3−/− mouse skin. A, MS profile of α1(I) from WT mouse skin reveals 100% glucosyl-galactosyl-Hyl (460.54+ and 613.63+) at residue α1(I) Hyl87. B, from Sc65−/− mouse skin, Lys-87 is 100% unmodified. Trypsin cleaves after unmodified Lys-87 yielding a smaller tryptic peptide (574.22+). C, from P3h3−/− mouse skin, Lys-87 is predominantly unmodified (80% Lys (574.12+); 20% glucosyl-galactosyl-Hyl (460.44+)). D, MS-MS fragmentation spectrum of the parent ion (574.12+) from P3h3−/− skin α1(I). The b and y ion masses reveal no modifications on α1(I) Lys-87. In-gel trypsin digests typically undergo complete oxidation of methionine residues yielding methionine sulfoxide. The trypsin-generated peptide is shown with P* indicating 4Hyp, M* indicating methionine sulfoxide, and -galglc indicating glucosyl-galactosyl-.
TABLE 2.
Comparison of type I collagen lysine 87 post-translational modifications between tissues
Modifications on lysine 87 from both α-chains of type I collagen in WT, Sc65−/−, and P3h3−/− mice were measured using mass spectrometry in skin, bone, tendon, and aorta. Lysine modifications include unmodified Lys, hydroxylysine (Hyl), galactosyl-hydroxylysine (G-Hyl), and glucosylgalactosyl-hydroxylysine (GG-Hyl). Each percentage was determined based on the ratio of the m/z peaks of each post-translational variant from a minimum of three mice unless indicated.
| Mouse | Modification |
||||
|---|---|---|---|---|---|
| Lys | Hyl | G-Hyl | GG-Hyl | ||
| Skin | |||||
| α1(I) Lys-87 | WT | 100 ± 0.0% | |||
| Sc65−/− | 100 ± 0.0% | ||||
| P3h3−/− | 80.3 ± 3.0% | 20.1 ± 1.7% | |||
| α2(I) Lys-87 | WT | 35.7 ± 2.3% | 64.3 ± 2.3% | ||
| Sc65−/− | 93.7 ± 3.2% | 6.3 ± 3.2% | |||
| P3h3−/− | 90.0 ± 2.0% | 10.0 ± 2.0% | |||
| Bone | |||||
| α1(I) Lys-87 | WT | 21.7 ± 3.8% | 78.3 ± 3.8% | ||
| Sc65−/− | 58.7 ± 12% | 15.5 ± 5.5% | 25.3 ± 6.4% | ||
| P3h3−/− | 51.0 ± 5.3% | 14.7 ± 2.5% | 34.3 ± 3.5% | ||
| α2(I) Lys-87 | WT | 22.5 ± 4.8% | 77.5 ± 4.8% | ||
| Sc65−/− | 92.7 ± 2.9% | 7.3 ± 2.9% | |||
| P3h3−/− | 42.7 ± 3.8% | 57.3 ± 3.8% | |||
| Tendon | |||||
| α1(I) Lys-87 | WT | 100 ± 0.0% | |||
| Sc65−/− | 100 ± 0.0% | ||||
| P3h3−/− | 47.3 ± 0.3% | 52.7 ± 3.8% | |||
| α2(I) Lys-87 | WT | 100 ± 0.0% | |||
| Sc65−/− | 85.5 ± 8.1% | 14.5 ± 8.1% | |||
| P3h3−/− | 32.3 ± 2.6% | 67.8 ± 2.6% | |||
| Aorta | |||||
| α1(I) Lys-87 | WT | 100 ± 0.0% | |||
| Sc65−/− | 100 ± 0.0% | ||||
| aP3h3−/− | 100 ± 0.0% | ||||
| α2(I) Lys-87 | WT | 86.7 ± 4.3% | 13.3 ± 4.2% | ||
| Sc65−/− | 100 ± 0.0% | ||||
| P3h3−/−a | 66.0 ± 4.2% | 34.0 ± 4.2% | |||
| Cornea | |||||
| α1(I) Lys-87 | WT | 6.7 ± 11% | 93.3 ± 11% | ||
| Sc65−/− | 51.7 ± 10% | 14.0 ± 4.6% | 35.0 ± 10% | ||
| α2(I) Lys-87 | WT | 3.7 ± 6.4% | 93.0 ± 6.1% | 3.3 ± 5.8% | |
| Sc65−/− | 57.7 ± 17% | 42.3 ± 17% | |||
a Two mice.
FIGURE 5.
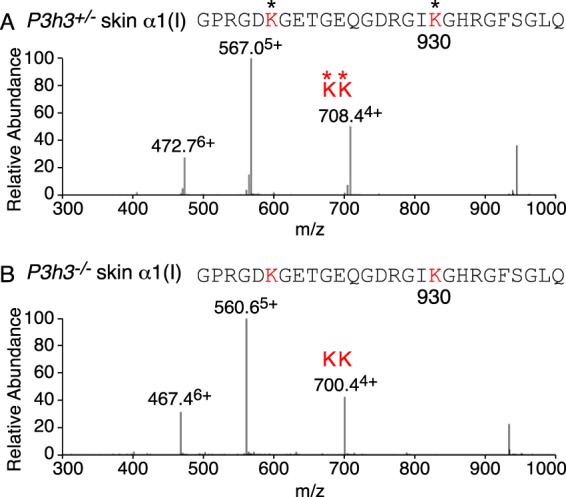
Complete loss of hydroxylation at cross-linking Lys-930 in P3h3−/− skin collagen. LC-MS profiles of peptides containing the helical domain Lys-930 cross-linking site from collagen α1(I) prepared by bacterial collagenase digestion of total dermal collagen. A, MS profile from P3h3+/− mouse skin reveals 100% lysyl hydroxylation at residue 930 (472.76+, 567.05+, and 708.44+). B, in P3h3−/− mouse skin, Lys-930 is 100% unmodified (467.46+, 560.65+, and 700.44+). Identical profiles were found at this site in skin, bone, and tendon from both P3h3−/− and Sc65−/− mice.
Type V Collagen Under-hydroxylation at Cross-linking Sites Is Restricted to Soft Tissue
The skin dominated reduction in hydroxylation and glycosylation of the helical domain cross-linking lysine 87 was also evident in type V collagen but to a lesser extent than in type I (Table 3). Lys-87 of α1(V) and α2(V) from WT skin was fully hydroxylated and glycosylated as glcgal-Hyl. A mild decrease in these modifications is seen from skin of Sc65−/− and P3h3−/− mice. As with type I collagen, the effect appeared to be more pronounced in the Sc65−/− mouse. In contrast, no post-translational variances were observed in the type V collagen α-chains from bone between WT and Sc65−/− or P3h3−/− (Table 3).
TABLE 3.
Summary of posttranslational variances in linear cross-linking lysine 87 from type V collagens
Modifications on lysine 87 from both α-chains of type V collagen in WT, Sc65−/−, and P3h3−/− mice were measured using mass spectrometry in skin and bone. Lysine modifications include unmodified Lys, hydroxylysine (Hyl), galactosyl-hydroxylysine (G-Hyl), and glucosylgalactosyl-hydroxylysine (GG-Hyl). Each percentage was determined based on the ratio of the m/z peaks of each post-translational variant from a minimum of three mice unless indicated.
| Mouse | Modification |
||||
|---|---|---|---|---|---|
| Lys | Hyl | G-Hyl | GG-Hyl | ||
| Skin | |||||
| α1(V) Lys-87 | WT | 100 ± 0.0% | |||
| Sc65−/− | 33.7 ± 14% | 66.3 ± 14% | |||
| P3h3−/−a | 23.0 ± 4.2% | 77.0 ± 4.2% | |||
| α2(V) Lys-87 | WT | 25.3 ± 4.9% | 74.7 ± 4.9% | ||
| Sc65−/− | 19.3 ± 10% | 26.0 ± 5.2% | 54.7 ± 5.7% | ||
| P3h3−/−a | 17.5 ± 0.7% | 24.0 ± 7.1% | 59.0 ± 8.5% | ||
| Bone | |||||
| α1(V) Lys-87 | WT | 100 ± 0.0% | |||
| Sc65−/− | 100 ± 0.0% | ||||
| P3h3−/−a | 100 ± 0.0% | ||||
| α2(V) Lys-87 | WT | 15 ± 2.6% | 85.0 ± 2.6% | ||
| Sc65−/− | 17.7 ± 10% | 82.3 ± 10% | |||
| P3h3−/−a | 18.5 ± 4.9% | 81.5 ± 4.9% | |||
a Two mice.
Altered Aldimine Divalent Cross-linking in Mutant Skin
Bacterial collagenase digests of borohydride-reduced skin were analyzed by mass spectrometry to target the pool of aldimine divalent cross-links in the whole tissue collagen. This approach is useful for tracking structural intermediates in the lysine aldehyde cross-linking pathway. The LC-MS profile of the C-telopeptide to Lys-87 cross-linked peptide from collagenase-digested WT skin shows a fully glycosylated reduced aldimine cross-linked structure between α1(I) Lys-87 and an α1(I) C-telopeptide lysine (Fig. 6A). This is consistent with the predominance of glcgal-Hyl at Lys-87 in the linear tryptic peptide from the α1(I) chain extracted from WT skin. From P3h3−/− mouse skin, about equal amounts of the fully glycosylated and a non-glycosylated reduced aldimine cross-link structure were recovered (Fig. 6, B and C). This finding suggests that glcgal-Hyl at Lys-87 is favored chemically and sterically over Lys and non-glycosylated Hyl as an aldimine end product on the lysine aldehyde cross-linking pathway, as only ∼20% of the α1(I) chains from P3h3−/− skin were glycosylated at Lys-87 (Fig. 4; Table 2). The total ion current, an index of the amount of the cross-linked peptide in the MS scan, was ∼10 times higher for the WT aldimine product compared with the equivalent P3h3−/− aldimine products. Although this should be considered only semiquantitative, it suggests that the mutant skin has significantly fewer aldimine cross-links at this site than WT skin. The cross-link between α1(I) Lys-930 and the α1(I)N-telopeptide was fully hydroxylated in WT skin (hydroxylysino-norleucine on reduction) (Fig. 7A); however, in P3h3−/− mouse skin only the non-hydroxylated aldimine cross-link was identified (lysino-norleucine on reduction) (Fig. 7B).
FIGURE 6.
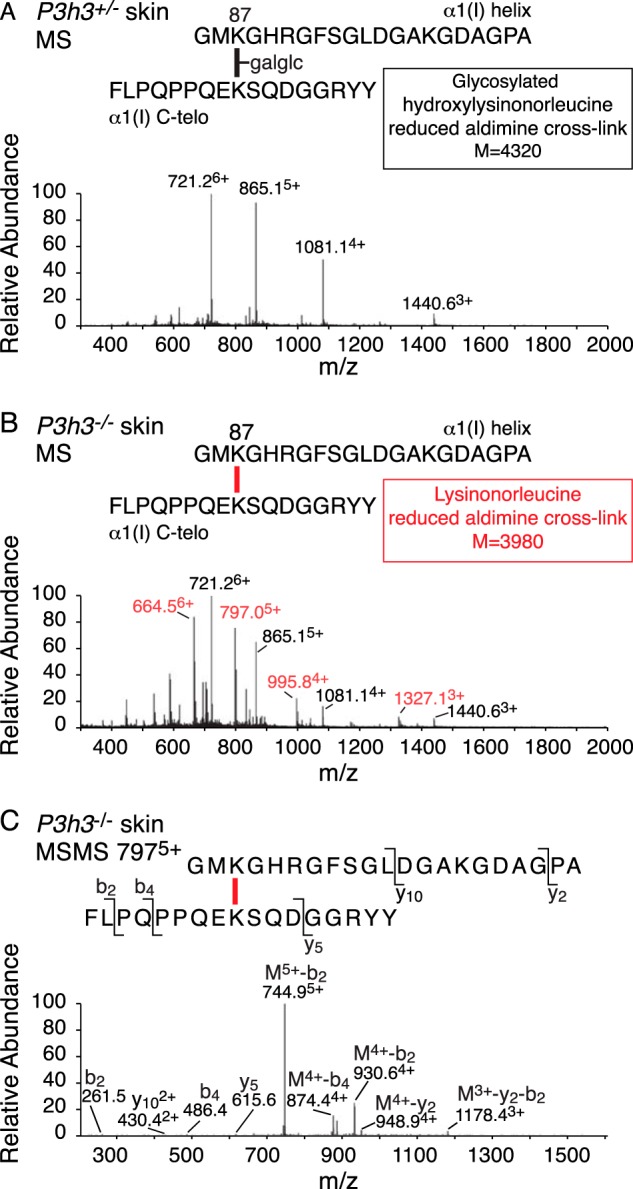
Altered divalent cross-link structure (Lys-87 to C-telopeptide) from P3h3−/− mouse skin. LC-MS profiles of type I collagen divalent cross-linking structures prepared by bacterial collagenase digestion of sodium borohydride-treated skin. A, MS profile of a fully glycosylated (glucosyl-galactosyl-) reduced aldimine structure from P3h3+/− mouse skin (721.26+, 865.15+, 1081.14+, and 1440.63+). B, from P3h3−/− mouse skin, two structures were identified in equal amounts. The same fully glycosylated reduced aldimine found in P3h3+/− (721.26+, 865.15+, 1081.14+, and 1440.63+) plus a non-glycosylated reduced aldimine (664.56+, 797.05+, 995.84+, and 1327.13+). C, MS/MS fragmentation spectrum of the parent ion (7975+) from P3h3−/− confirm the mass of the lysine variant from mutant skin. Molecular ions from the lysine and glycosylated hydroxylysine variants are distinguished in red and black, respectively.
FIGURE 7.
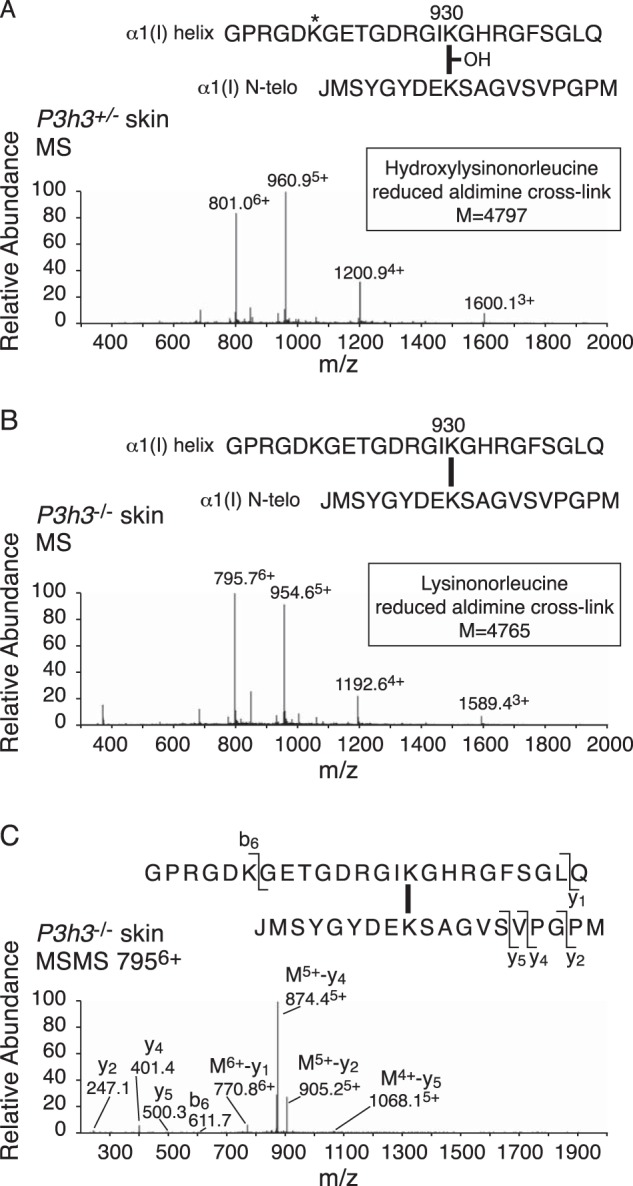
Altered divalent cross-link structure (Lys-930 to N-telopeptide) from P3h3−/− mouse skin. LC-MS profiles of type I collagen divalent cross-linking structures prepared by bacterial collagenase digestion of sodium borohydride-treated skin. A, MS profile of a hydroxylated reduced aldimine structure (hydroxylysinonorleucine) from P3h3+/− mouse skin (801.06+, 960.95+, 1200.94+, and 1600.13+). B, from P3h3−/− mouse skin, only the non-hydroxylated lysine variant (lysinonorleucine) was recovered (795.76+, 954.65+, 1192.64+, and 1589.43+). C, MS/MS fragmentation spectrum of the parent ion (7955+) from P3h3−/− mouse skin.
Pyridinoline (HP and LP) Cross-link Analysis
As an independent downstream measure of the effect of the loss of Sc65 or P3H3 on lysine cross-linking, pyridinoline cross-links were quantified after acid hydrolysis of samples of bone, aorta, lung, cartilage, and dentin collagen from each genotype (Table 4). The results showed a significantly lower ratio of HP/LP in bone, aorta, and lung in both knock-out mouse strains. A similar reversal in the HP/LP ratio of the cross-linking pyridinolines of bone and other tissues is seen in patients with Ehlers-Danlos syndrome (EDS) VI (26). A comparable but less marked shift in the HP/LP ratio was found in dentin and cartilage samples from the Sc65−/− mice. No genotypic significance can be attached to variations in total content (HP + LP mol/mol of collagen) between samples as shown in Table 4, which may be due to sampling site variability.
TABLE 4.
Altered mature cross-linking in P3h3−/− and Sc65−/− mouse tissues
Concentration of pyridinoline cross-linking residues in mouse bone, aorta, lung, dentin, and cartilage expressed as moles/mole of collagen (HP, hydroxylysylpyridinoline; LP, lysylpyridinoline). Values were calculated from a minimum of three mice unless indicated.
| HP + LP | HP/LP molar ratio | |
|---|---|---|
| Bone | ||
| WTa | 0.29 ± 0.19 | 7.75 ± 3.46 |
| Sc65−/− | 0.34 ± 0.02 | 0.27 ± 0.06 |
| P3h3−/−a | 0.57 ± 0.03 | 0.40 ± 0.00 |
| Aorta | ||
| WTa | 0.59 ± 0.01 | 12.5 ± 1.41 |
| Sc65−/−b | 0.78 | 0.40 |
| P3h3−/−a | 0.79 ± 0.19 | 0.60 ± 0.00 |
| Lung | ||
| WTb | 0.86 | 14.5 |
| Sc65−/−a | 0.97 ± 0.40 | 1.55 ± 1.06 |
| P3h3−/−a | 1.92 ± 0.86 | 4.10 ± 2.97 |
| Dentin | ||
| WTb | 0.85 | 2.6 |
| Sc65−/−b | 0.94 | 1.4 |
| Cartilage | ||
| WTb | 2.0 | 4.5 |
| Sc65−/−b | 2.1 | 2.8 |
a Two mice.
b One mouse.
Discussion
Although both construct strains of Sc65−/− (gene trap and floxed) and P3h3−/− mice were indistinguishable by gross physical appearance from their WT littermates, the mutant skins lacked the structural integrity of normal skin on dissection. It is notable that increased skin fragility and low bone mass were quantified in the Sc65−/− mouse strain compared with WT littermates (21). On mass spectrometric analysis, type I collagen from both Sc65−/− and P3h3−/− tissues revealed severe under-hydroxylation of lysine residues at triple-helical domain cross-linking sites for skin, tendon, cornea, and aorta. In addition to under-hydroxylation, the residual Hyl87 was under-glycosylated compared with WT for skin, aorta, and cornea. Type V collagen from null mouse skin and aorta exhibited a decrease in hydroxylation/glycosylation at Lys-87 compared with WT; however, no change was detected in type V collagen from null-bone versus WT bone. As collagen type V acts as a quantitatively minor but essential template for type I collagen heterofibril polymerization, it is possible that even a minor disturbance in collagen V cross-linking could be amplified in the mature fibril.
The present phenocopied effects on collagen lysine hydroxylation can best be explained if Sc65 and P3H3 form a complex in the ER that is required to facilitate access of LH1 to substrate lysine residues on newly made collagen chains. We proposed an interaction complex that at least transiently involves LH1, CypB, SC65, and P3H3 (21). Similar to the P3H1/CRTAP/CypB enzyme complex, which is required to 3-hydroxylate Pro-986, the primary site of 3Hyp in type I collagen, all four ER components LH1, CypB, SC65, and P3H3 are essential for normal triple-helix lysine hydroxylation. The requirement of CypB for collagen post-translational modification became clear from the effects of PPIB mutations that cause severe osteogenesis imperfecta. They result in loss of prolyl-hydroxylation at Pro-986 (27) and, in CypB knock-out mice, defective lysine hydroxylation in bone (28) and tendon (29) collagens. Furthermore, we know that FKBP65, another peptidyl prolyl isomerase and ER chaperone, can regulate the activity of LH2 the predicted telopeptide lysine hydroxylase (12, 30, 31).
Tissues from both P3h2−/− and Crtap−/− mice showed no effect on Lys-87 hydroxylation/glycosylation, ruling out any potential redundancy or compensatory function between P3H3, P3H2, and P3H1 subunits in the ER.3 In contrast to the functionally interdependent complex P3H1/CRTAP required for Pro-986 3-hydroxylation, P3H3/Sc65 appears to be redundant as a prolyl 3-hydroxylase. We had anticipated that P3H3 would act as a prolyl 3-hydroxylase somewhere on collagen, but despite a broad screen of candidate sites in the common collagens no evidence of an affected site was found (Table 1). Given the recent identification of many low occupancy 3-hydroxyproline sites in type IV (32) and type V (33) collagens, and a long list of genetic types of collagen with GPP triplets, it is possible that a proline substrate for P3H3 remains to be identified. Equally possible is lost enzyme activity over the course of evolution but a retained collagen chaperone function in the ER that optimizes LH1-mediated lysine hydroxylation particularly at helical domain cross-linking sites.
The pathogenic significance of the loss of Pro-986 3-hydroxylation in recessive cases of osteogenesis imperfecta caused by P3H1, CRTAP, or CypB mutations is still not clear, although absence of the complex as a procollagen chaperone rather than absence of 3Hyp is indicated (34, 35). In this context, the over-hydroxylation of telopeptide lysines seen in the bone collagen of OI patients and mouse models caused by mutations in P3H1, CRTAP, and CypB should be noted.
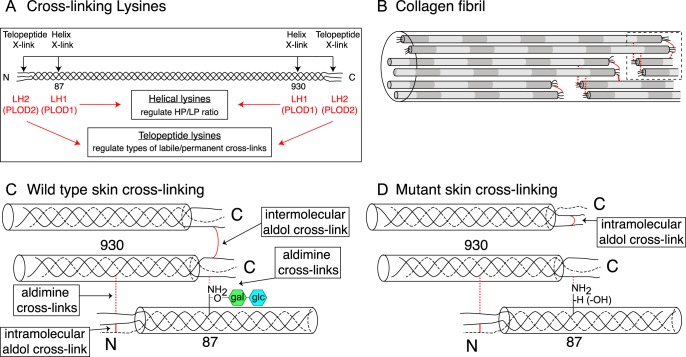
Hydroxylysines are functionally important in collagen for covalent cross-linking. In type I collagen four triple-helical domain lysine sites (α1(I) Lys-87 and Lys-930; α2(I) Lys-87 and Lys-933) can interact with C- and N-telopeptide lysines after the latter have been converted to aldehyde residues by lysyl oxidase. Tissue-specific differences in collagen cross-linking chemistry and maturation are largely determined in the ER by the lysine hydroxylation state of these specific lysines (Fig. 8A).
FIGURE 8.
Model speculating how collagen cross-link formation is affected in P3h3−/− and Sc65−/− mouse skin. Under normal conditions, LH1 catalyzes the hydroxylation of helical lysines 87 and 930; and LH2 catalyzes the hydroxylation of the N- and C-telopeptide lysines (A). In the fibril, collagen molecules are spatially arranged such that intermolecular cross-link placement is optimal (B). In WT skin, fully glycosylated Hyl87 preferentially forms an intermolecular aldimine cross-link with a C-telopeptide lysine aldehyde (C). In the P3h3−/− and Sc65−/− mouse tissues the LH1 substrates are under-hydroxylated and subsequently under-glycosylated, which alters collagen cross-linking chemistry (D). From mutant skin the results are consistent with the C-telopeptide lysine aldehydes preferentially forming intramolecular aldol cross-links (as opposed to intermolecular aldols). We predict that under normal conditions the presence of the disaccharide on Hyl87 favors aldimine formation with a single C-telopeptide aldehyde and hinders intramolecular aldol formation with a second α1(I) C-telopeptide from the same molecule as the first one, so favoring intermolecular interactions. The net effect of under-hydroxylated Lys-87 then would be fewer stable aldol intermolecular cross-links within and between fibrils.
Based on the present findings, what mechanism can explain the altered pattern (Fig. 2) of cross-linked β- and γ-chains seen on SDS-PAGE of skin collagen from Sc65−/− and P3h3−/− mice? We believe the latter effect is caused by the lack of glcgal-Hyl at Lys-87 in both α-chains of skin type I collagen. In WT skin, aorta and cornea (>90%), type I collagen α1(I) chains all have fully glycosylated glcgal-Hyl at Lys-87 to which α1(I) C-telopeptide allysines in neighboring four-dimensional staggered molecules normally interact to form aldimine cross-links. When α1(I) and α2(I) Lys-87 are not hydroxylated and so cannot be glycosylated the interaction chemistry changes. If in the same molecule both α1-chain C-telopeptide lysines are converted to aldehydes, then they interact to form an intramolecular aldol cross-link in lieu of the typical intermolecular glycosylated aldimine cross-link present in WT tissue (Fig. 8). Such an abnormal cross-link arrangement for skin can explain the marked increase in γ112 trimer and decrease in β12 dimer seen on SDS-PAGE (Figs. 2 and 3). It can also explain the increased extractability of type I collagen in non-denaturing 3% acetic acid at 4 °C (∼40% more extractable in P3h3−/− skin and tendon), if in essence intermolecular C-telopeptide aldols are replaced by intramolecular aldols. Although beyond the scope of the present study it should be noted that major complex cross-links on the allysine aldehyde pathway were reported to be the product of an allysine aldol linked to a histidine by Michael addition and a hydroxylysine by aldimine addition (dehydrohistidinohydroxymerodesmosine (HHMD) on reduction) (36). The structure was based on analyses of acid-hydrolyzed collagen after sodium borohydride reduction, so the native state of the mature aldol cross-linking structures in tissue remains uncertain (37).
The reason why skin appears to be more affected than tendon collagen is probably related to the lack of glcgal-Hyl at Lys-87 in normal mature tendon type I collagen. To speculate, the combined loss of hydroxylation and glycosylation at Lys-87 of skin, aorta, and cornea may have the above summarized effect on C-telopeptide aldol placement but not in tendon, which normally has Hyl alone at Lys-87 with no attached sugars. It is notable that the band pattern of WT tendon collagen on SDS-PAGE (Fig. 2) resembles that of Sc65−/− and P3h3−/− skin with a prominent γ112 trimer and with no obvious difference from Sc65−/− and P3h3−/− tendons. In a sense, therefore, the effect in null mice on skin collagen is to make it resemble normal tendon collagen at this cross-link site. However, with that in mind, normal tendon collagen in a study of fibromodulin-null mice was shown to have incomplete C-telopeptide lysine conversion to aldehydes, a restriction that was lifted in the fibromodulin-null mice (22). It was speculated that in fibromodulin-null mice an increase in collagen molecules with two C-telopeptide allysines produced an observed increase in C-telopeptide aldols and γ112 trimers (22).
The observed effects on collagen lysine hydroxylation and cross-linking closely resemble those observed in tissues of EDS VIA patients. Of the many defined clinical subtypes of EDS, most are characterized by skin hyper-extensibility, joint hypermobility, and connective tissue fragility. The kyphoscoliotic type, EDS VIA, is caused by homozygous or compound heterozygous mutations in the PLOD1 gene (encodes LH1). Clinical features of patients with this EDS subtype include progressive kyphoscoliosis, soft velvety skin, muscle hypotonia, arterial ruptures, and fragility of ocular tissues often leading to rupture of the globe and retinal detachment (38, 39). Similar to Sc65−/− and P3h3−/− mice, the LH1 knock-out (Plod1−/−) mouse seemed to have milder consequences than Plod1 human cases. For example, muscle hypotonia and aortic ruptures were reported in the Plod1−/− mouse but not kyphoscoliosis or skin laxity (40). Despite Sc65−/− and P3h3−/− mice having at this stage primarily a biochemical phenotype, the potential is clear for suppressed lysine hydroxylation to manifest as an EDS variant in humans.
Experimental Procedures
Production of Knock-out Mice
The P3h3 knock-out (P3h3−/−) mouse was generated using embryonic stem cells obtained from the Knock-out Mouse Project (KOMP) repository. Embryonic stem cells were injected in-house to generate the heterozygous knock-out mice (P3h3+/−). Mice were maintained on a C57/BL6J background and were housed in the Baylor College of Medicine vivarium. Genotyping was performed using primers for the WT allele, 5′-TGGAGAACGTGTCAAATTGGAATG-3′ and 5′-TCCATCTGCTCTTCGTATCTGAAAG-3′. The mutant allele was identified by primers, 5′-CGTGGGTAGATGAACGTGTG-3′ and 5′-CTGTCCTAGCTTCCTCACTG-3′.
Tissues from two strains of Sc65−/− mice (19, 21) were analyzed in comparison to P3h3−/− and respective WT tissues. Briefly, for the initial strain the embryonic stem cell clone (129X1/SvJ × 129S1) with a proviral insertion (gene-trap) in the Sc65 gene was purchased from the Gene Trap Resource of the Phenogenomics Toronto Center (Toronto, Canada). C57/BL6J blastocysts were injected with ES cells and chimeric mice were generated (19). The second Sc65−/− strain employed a conditional targeting construct (using the vector p-flrt-neo-2XloxP) that was designed to introduce loxP sites flanking the last 2 exons (7 and 8) of Sc65 (floxed mouse). Chimeric mice were generated by electroporating the construct into mouse ES cells derived from F1(C57B6/jx129sv) embryos. Global Sc65 excision was achieved by mating male Sc65 mice carrying a floxed allele with Hprt-Cre females (21). The use of the latter laboratory mice was approved by the University of Arkansas for Medical Sciences IACUC committee. All mice used in this study had the same genetic background. The reported post-translational variances did not appear to be related to the age or sex of the animals tested.
Collagen Extraction
Type I collagen was solubilized from bone, skin, tendon, aorta, sclera, and cornea by heat denaturation for 5 min at 100 °C in Laemmli buffer (SDS extraction), 3% acetic acid at 4 °C for 24 h, or cyanogen bromide digestion in 70% formic acid at room temperature for 24 h. Type II collagen (rib cage cartilage), type IV collagen (kidney cortex), and type V collagen (bone and skin) α-chains were solubilized by pepsin digestion or cyanogen bromide digestion as previously described (17, 41). Collagen α-chains were resolved by SDS-PAGE and stained with Coomassie Blue R-250. Collagen extractability was determined using NIH ImageJ software as previously described (22).
P3h3 Immunoblot
Human fetal control cartilage tissue was obtained from a National Institutes of Health-sponsored institutional resource (Birth Defects Research Laboratory, University of Washington) (42). P3h3−/− and control whole mouse kidneys were homogenized in saline with protease inhibitors (50 mm Tris-HCl, pH 7.5, 0.15 m NaCl, 2 mm PMSF, 10 mm phenanthroline). An intracellular fraction was solubilized from this tissue extract in SDS sample buffer at 95 °C. Human fetal control cartilage was 4 m guanidine-HCl extracted as previously described (42). Equal loads of total protein were resolved on a 7.5% SDS-PAGE and transblotted to PVDF membrane. Membranes were blocked in 5% milk, probed with P3h3 polyclonal antibody (1:500 dilution, ProteinTech, catalog number 16023-1-AP), and detected with an HRP-conjugated secondary antibody (1:5000, anti-rabbit). Blots were developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) and exposed on autoradiography film.
H&E Staining
Skins and femurs were harvested from a 2-month-old mouse and fixed in 10% buffered formalin. Femurs were decalcified with 14% EDTA for 2 weeks before paraffin embedding processing. Paraffin-embedded skin and femur were sectioned (6 micrometer) and the sections were used for H&E staining.
Mass Spectral Analysis of Site-specific Post-translational Modifications
The post-translational modifications 3-hydroxyproline, 5-hydroxylysine (Hyl), glcgal-Hyl, and gal-Hyl glycosides were quantified at specific sites in collagen α-chains as previously described (41). Collagen α-chains were cut from SDS-PAGE gels and subjected to in-gel trypsin digestion. Skin was also digested with bacterial collagenase with and without borohydride reduction as previously described (21) and total collagenase digests were resolved into peptide fractions by C8 reverse-phase HPLC and individual fractions were analyzed by mass spectrometry (43). Electrospray mass spectrometry was carried out on the trypsin- and collagenase-digested peptides using an LTQ XL linear quadrapole ion-trap mass spectrometer equipped with in-line Accela 1250 liquid chromatography and automated sample injection (ThermoFisher Scientific). Proteome Discoverer software (ThermoFisher Scientific) was used for peptide identification. Tryptic peptides were also identified manually by calculating the possible MS-MS ions and matching these to the actual MS-MS spectrum using Thermo Xcalibur software. Hydroxyl differences were determined manually by averaging the full scan MS over several LC-MS minutes to include all the post-translational variations of a given peptide. Protein sequences used for MS analysis were obtained from the Ensembl genome database.
Collagen Cross-linking Analysis
The pyridinoline cross-link content of collagen in bone, dentin, cartilage, and aorta samples was determined by HPLC after acid hydrolysis in 6 m HCl for 24 h at 108 °C. Dried samples were dissolved in 1% (v/v) n-heptafluorobutyric acid for quantitation of lysyl pyridinoline (LP) and hydroxylysyl pyridinoline (HP) by reverse-phase HPLC and fluorescence monitoring as previously described (7).
Author Contributions
D. R. E., B. L., and R. M. conceived the idea for the project. J. R., D. M. H., and M. A. W. conducted most of the experiments. K. S. J. generated the P3h3−/− mouse. R. M. and M. D. generated the Sc65−/− mouse. D. M. H., M. A. W., and D. R. E. analyzed the results. D. M. H. and D. R. E. wrote the initial draft of the paper and all authors contributed to the revisions and reviewed the final submitted version.
This work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and Eunice Kennedy Shriver NICHD, National Institutes of Health NIAMS Grants AR037318 (to D. E.), AR036794 (to D. E.), AR060823 (to R. M.), and NICHD Grant HD070394 (to B. L. and D. E.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
D. M. Hudson and D. R. Eyre, unpublished data.
- OI
- osteogenesis imperfecta
- ER
- endoplasmic reticulum
- Sc65
- synaptonemal complex 65
- P3H1
- P3H2, P3H3, prolyl 3-hydroxylases
- LH1
- LH2, lysyl hydroxylases
- EDS
- Ehlers-Danlos syndrome
- 3Hyp
- 3-hydroxyproline
- Hyl
- hydroxylysine
- LP
- lysyl pyridinoline
- HP
- hydroxylysyl pyridinoline.
References
- 1. Myllyharju J., and Kivirikko K. I. (2004) Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 20, 33–43 [DOI] [PubMed] [Google Scholar]
- 2. Bella J. (2016) Collagen structure: new tricks from a very old dog. Biochem. J. 473, 1001–1025 [DOI] [PubMed] [Google Scholar]
- 3. Hudson D. M., and Eyre D. R. (2013) Collagen prolyl 3-hydroxylation: a major role for a minor post-translational modification? Connect. Tissue Res. 54, 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamauchi M., and Sricholpech M. (2012) Lysine post-translational modifications of collagen. Essays Biochem. 52, 113–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eyre D. R., and Weis M. A. (2013) Bone collagen: new clues to its mineralization mechanism from recessive osteogenesis imperfecta. Calcif. Tissue Int. 93, 338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eyre D. R., Paz M. A., and Gallop P. M. (1984) Cross-linking in collagen and elastin. Annu. Rev. Biochem. 53, 717–748 [DOI] [PubMed] [Google Scholar]
- 7. Eyre D. (1987) Collagen cross-linking amino acids. Methods Enzymol. 144, 115–139 [DOI] [PubMed] [Google Scholar]
- 8. Marini J. C., Reich A., and Smith S. M. (2014) Osteogenesis imperfecta due to mutations in non-collagenous genes: lessons in the biology of bone formation. Curr. Opin. Pediatr. 26, 500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishikawa Y., Wirz J., Vranka J. A., Nagata K., and Bächinger H. P. (2009) Biochemical characterization of the prolyl 3-hydroxylase 1·cartilage-associated protein·cyclophilin B complex. J. Biol. Chem. 284, 17641–17647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ha-Vinh R., Alanay Y., Bank R. A., Campos-Xavier A. B., Zankl A., Superti-Furga A., and Bonafé L. (2004) Phenotypic and molecular characterization of Bruck syndrome (osteogenesis imperfecta with contractures of the large joints) caused by a recessive mutation in PLOD2. Am. J. Med. Genet. Part A. 131, 115–120 [DOI] [PubMed] [Google Scholar]
- 11. Barnes A. M., Cabral W. A., Weis M., Makareeva E., Mertz E. L., Leikin S., Eyre D., Trujillo C., and Marini J. C. (2012) Absence of FKBP10 in recessive type XI osteogenesis imperfecta leads to diminished collagen cross-linking and reduced collagen deposition in extracellular matrix. Hum. Mutat. 33, 1589–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwarze U., Cundy T., Pyott S. M., Christiansen H. E., Hegde M. R., Bank R. A., Pals G., Ankala A., Conneely K., Seaver L., Yandow S. M., Raney E., Babovic-Vuksanovic D., Stoler J., Ben-Neriah Z., et al. (2013) Mutations in FKBP10, which result in Bruck syndrome and recessive forms of osteogenesis imperfecta, inhibit the hydroxylation of telopeptide lysines in bone collagen. Hum. Mol. Genet. 22, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mordechai S., Gradstein L., Pasanen A., Ofir R., El Amour K., Levy J., Belfair N., Lifshitz T., Joshua S., Narkis G., Elbedour K., Myllyharju J., and Birk O. S. (2011) High myopia caused by a mutation in LEPREL1, encoding prolyl 3-hydroxylase 2. Am. J. Hum. Genet. 89, 438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo H., Tong P., Peng Y., Wang T., Liu Y., Chen J., Li Y., Tian Q., Hu Y., Zheng Y., Xiao L., Xiong W., Pan Q., Hu Z., and Xia K. (2014) Homozygous loss-of-function mutation of the LEPREL1 gene causes severe non-syndromic high myopia with early-onset cataract. Clin. Genet. 86, 575–579 [DOI] [PubMed] [Google Scholar]
- 15. Khan A. O., Aldahmesh M. A., Alsharif H., and Alkuraya F. S. (2015) Recessive mutations in LEPREL1 underlie a recognizable lens subluxation phenotype. Ophthalmic Genet. 36, 58–63 [DOI] [PubMed] [Google Scholar]
- 16. Pokidysheva E., Boudko S., Vranka J., Zientek K., Maddox K., Moser M., Fässler R., Ware J., and Bächinger H. P. (2014) Biological role of prolyl 3-hydroxylation in type IV collagen. Proc. Natl. Acad. Sci. U.S.A. 111, 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hudson D. M., Joeng K. S., Werther R., Rajagopal A., Weis M., Lee B. H., and Eyre D. R. (2015) Post-translationally abnormal collagens of prolyl 3-hydroxylase-2 null mice offer a pathobiological mechanism for the high myopia linked to human LEPREL1 mutations. J. Biol. Chem. 290, 8613–8622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tiainen P., Pasanen A., Sormunen R., and Myllyharju J. (2008) Characterization of recombinant human prolyl 3-hydroxylase isoenzyme 2, an enzyme modifying the basement membrane collagen IV. J. Biol. Chem. 283, 19432–19439 [DOI] [PubMed] [Google Scholar]
- 19. Gruenwald K., Castagnola P., Besio R., Dimori M., Chen Y., Akel N. S., Swain F. L., Skinner R. A., Eyre D. R., Gaddy D., Suva L. J., and Morello R. (2014) Sc65 is a novel endoplasmic reticulum protein that regulates bone mass homeostasis. J. Bone Miner. Res. 29, 666–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cavazzini F., Magistroni R., Furci L., Lupo V., Ligabue G., Granito M., Leonelli M., Albertazzi A., and Cappelli G. (2012) Identification and characterization of a new autoimmune protein in membranous nephropathy by immunoscreening of a renal cDNA library. PloS One 7, e48845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heard M. E., Besio R., Weis M., Rai J., Hudson D. M., Dimori M., Zimmerman S. M., Kamykowski J. A., Hogue W. R., Swain F. L., Burdine M. S., Mackintosh S. G., Tackett A. J., Suva L. J., Eyre D. R., and Morello R. (2016) Sc65-null mice provide evidence for a novel endoplasmic reticulum complex regulating collagen lysyl hydroxylation. PLoS Genet. 12, e1006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalamajski S., Liu C., Tillgren V., Rubin K., Oldberg Å., Rai J., Weis M., and Eyre D. R. (2014) Increased C-telopeptide cross-linking of tendon type I collagen in fibromodulin-deficient mice. J. Biol. Chem. 289, 18873–18879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kalamajski S., Bihan D., Bonna A., Rubin K., and Farndale R. W. (2016) Fibromodulin interacts with collagen cross-linking sites and activates lysyl oxidase. J. Biol. Chem. 291, 7951–7960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hudson D. M., Werther R., Weis M., Wu J.-J., and Eyre D. R. (2014) Evolutionary origins of C-terminal (GPP)n 3-hydroxyproline formation in vertebrate tendon collagen. PLoS One 9, e93467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pokidysheva E., Zientek K. D., Ishikawa Y., Mizuno K., Vranka J. A., Montgomery N. T., Keene D. R., Kawaguchi T., Okuyama K., and Bächinger H. P. (2013) Posttranslational modifications in type I collagen from different tissues extracted from wild type and prolyl 3-hydroxylase 1 null mice. J. Biol. Chem. 288, 24742–24752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steinmann B., Eyre D. R., and Shao P. (1995) Urinary pyridinoline cross-links in Ehlers-Danlos syndrome type VI. Am. J. Hum. Genet. 57, 1505–1508 [PMC free article] [PubMed] [Google Scholar]
- 27. Barnes A. M., Carter E. M., Cabral W. A., Weis M., Chang W., Makareeva E., Leikin S., Rotimi C. N., Eyre D. R., Raggio C. L., and Marini J. C. (2010) Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. N. Engl. J. Med. 362, 521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cabral W. A., Perdivara I., Weis M., Terajima M., Blissett A. R., Chang W., Perosky J. E., Makareeva E. N., Mertz E. L., Leikin S., Tomer K. B., Kozloff K. M., Eyre D. R., Yamauchi M., and Marini J. C. (2014) Abnormal type I collagen post-translational modification and crosslinking in a cyclophilin B KO mouse model of recessive osteogenesis imperfecta. PLoS Genet. 10, e1004465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Terajima M., Taga Y., Chen Y., Cabral W. A., Hou-Fu G., Srisawasdi S., Nagasawa M., Sumida N., Hattori S., Kurie J. M., Marini J. C., and Yamauchi M. (2016) Cyclophilin-B modulates collagen cross-linking by differentially affecting lysine hydroxylation in the helical and telopeptidyl domains of tendon type I collagen. J. Biol. Chem. 291, 9501–9512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lietman C. D., Rajagopal A., Homan E. P., Munivez E., Jiang M.-M., Bertin T. K., Chen Y., Hicks J., Weis M., Eyre D., Lee B., and Krakow D. (2014) Connective tissue alterations in Fkbp10−/− mice. Hum. Mol. Genet. 23, 4822–4831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gjaltema R. A., van der Stoel M. M., Boersema M., and Bank R. A. (2016) Disentangling mechanisms involved in collagen pyridinoline cross-linking: the immunophilin FKBP65 is critical for dimerization of lysyl hydroxylase 2. Proc. Natl. Acad. Sci. U.S.A. 113, 7142–7147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Basak T., Vega-Montoto L., Zimmerman L. J., Tabb D. L., Hudson B. G., and Vanacore R. M. (2016) Comprehensive characterization of glycosylation and hydroxylation of basement membrane collagen IV by high-resolution mass spectrometry. J. Proteome Res. 15, 245–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang C., Park A. C., Davis N. A., Russell J. D., Kim B., Brand D. D., Lawrence M. J., Ge Y., Westphall M. S., Coon J. J., and Greenspan D. S. (2012) Comprehensive mass spectrometric mapping of the hydroxylated amino acid residues of the α1(V) collagen chain. J. Biol. Chem. 287, 40598–40610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hudson D. M., Kim L. S., Weis M., Cohn D. H., and Eyre D. R. (2012) Peptidyl 3-hydroxyproline binding properties of type I collagen suggest a function in fibril supramolecular assembly. Biochemistry 51, 2417–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Homan E. P., Lietman C., Grafe I., Lennington J., Morello R., Napierala D., Jiang M.-M., Munivez E. M., Dawson B., Bertin T. K., Chen Y., Lua R., Lichtarge O., Hicks J., Weis M. A., Eyre D., and Lee B. H. (2014) Differential effects of collagen prolyl 3-hydroxylation on skeletal tissues. PLoS Genet. 10, e1004121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tanzer M. L., Housley T., Berube L., Fairweather R., Franzblau C., and Gallop P. M. (1973) Structure of two histidine-containing crosslinks from collagen. J. Biol. Chem. 248, 393–402 [PubMed] [Google Scholar]
- 37. Robins S. P., and Bailey A. J. (1973) The chemistry of the collagen cross-links: the characterization of fraction C, a possible artifact produced during the reduction of collagen fibres with borohydride. Biochem. J. 135, 657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steinmann B., Royce P. M., and Superti-Furga A. (2002) The Ehlers-Danlos Syndrome. in Connective Tissue and Its Heritable Disorders, pp. 431–523, John Wiley & Sons, Inc., Hoboken, NJ [Google Scholar]
- 39. Steinmann B., Royce P. M., and Superti-Furga A. (2003) in Connective Tissue and Its Heritable Disorders (Royce P. M., and Steinman B., eds), pp. 431–523, John Wiley & Sons, Inc., New York [Google Scholar]
- 40. Takaluoma K., Hyry M., Lantto J., Sormunen R., Bank R. A., Kivirikko K. I., Myllyharju J., and Soininen R. (2007) Tissue-specific changes in the hydroxylysine content and cross-links of collagens and alterations in fibril morphology in lysyl hydroxylase 1 knock-out mice. J. Biol. Chem. 282, 6588–6596 [DOI] [PubMed] [Google Scholar]
- 41. Weis M. A., Hudson D. M., Kim L., Scott M., Wu J. J., and Eyre D. R. (2010) Location of 3-hydroxyproline residues in collagen types I, II, III, and V/XI implies a role in fibril supramolecular assembly. J. Biol. Chem. 285, 2580–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsui Y., Wu J.-J., Weis M. A., Pietka T., and Eyre D. R. (2003) Matrix deposition of tryptophan-containing allelic variants of type IX collagen in developing human cartilage. Matrix Biol. 22, 123–129 [DOI] [PubMed] [Google Scholar]
- 43. Hanson D. A., and Eyre D. R. (1996) Molecular site specificity of pyridinoline and pyrrole cross-links in type I collagen of human bone. J. Biol. Chem. 271, 26508–26516 [DOI] [PubMed] [Google Scholar]