Abstract
Mast cells are secretory cells that play an important role in host defense by discharging various intragranular contents, such as histamine and serotonin, upon stimulation of Fc receptors. The granules also contain spermine and spermidine, which can act as modulators of mast cell function, although the mechanism underlying vesicular storage remains unknown. Vesicular polyamine transporter (VPAT), the fourth member of the SLC18 transporter family, is an active transporter responsible for vesicular storage of spermine and spermidine in neurons. In the present study, we investigated whether VPAT functions in mast cells. RT-PCR and Western blotting indicated VPAT expression in murine bone marrow-derived mast cells (BMMCs). Immunohistochemical analysis indicated that VPAT is colocalized with VAMP3 but not with histamine, serotonin, cathepsin D, VAMP2, or VAMP7. Membrane vesicles from BMMCs accumulated spermidine upon the addition of ATP in a reserpine- and bafilomycin A1-sensitive manner. BMMCs secreted spermine and spermidine upon the addition of either antigen or A23187 in the presence of Ca2+, and the antigen-mediated release, which was shown to be temperature-dependent and sensitive to bafilomycin A1 and tetanus toxin, was significantly suppressed by VPAT gene RNA interference. Under these conditions, expression of vesicular monoamine transporter 2 was unaffected, but antigen-dependent histamine release was significantly suppressed, which was recovered by the addition of 1 mm spermine. These results strongly suggest that VPAT is expressed and is responsible for vesicular storage of spermine and spermidine in novel secretory granules that differ from histamine- and serotonin-containing granules and is involved in vesicular release of these polyamines from mast cells.
Keywords: histamine, mast cell, polyamine, transporter, vesicles, secretory granules, spermine, vesicular polyamine transporter
Introduction
Mast cells are hematopoietic cells that play important roles in the immune system. These cells exhibit unique morphological features, with electron-dense secretory granules containing large amounts of preformed and preactivated immunomodulatory compounds, such as histamine, serotonin, several mast cell-specific proteases (tryptases and chymases), tumor necrosis factor (TNF), etc. (1–3). When mast cells are activated in response to various external stimuli, such as cross-linking of IgE bound to the high affinity IgE receptor, the intragranular contents are discharged into the extracellular space through degranulation and trigger various immunoreactions involved in allergy, asthma, atherosclerosis, inflammatory arthritis, multiple sclerosis, and cancer (1, 3).
In addition to biogenic amines, such as histamine and serotonin, mast cells contain spermine and spermidine, polycations that are known to play essential roles in cell survival and proliferation in all organisms (4–6). These polyamines are stored in mast cell secretory granules, appear in the extracellular space through A23187-evoked Ca2+-dependent processes, and are thought to be involved in biogenesis and homeostasis of secretory granules (4). Furthermore, it has been reported that spermine and spermidine, when applied to mast cells extracellularly, facilitate synthesis and release of histamine from mast cells (7–11). Spermine and spermidine are also involved in storage and/or release of histamine and serotonin in the mast cell granules (4, 12). Although there are numerous studies on polyamine function in mast cells, it remains unclear how these polyamines are loaded into mast cell granules and discharged into the extracellular space upon stimulation.
Vesicular storage of neurotransmitters is a charged process of classical neurotransmitters in neuronal and neuroendocrine cells, which consists of active transport that is energetically coupled with a vacuolar proton pump. Vesicular neurotransmitter transporters specific to the transmitters are responsible for the storage of the respective neurotransmitters (13). Essentially all transporters for the classical neurotransmitters have been identified (i.e. vesicular monoamine transporters (SLC18A1 and -A2), vesicular acetylcholine transporter (SLC18A3), vesicular GABA transporter (SLC32A1), vesicular glutamate transporters (SLC17A6–A8), vesicular excitatory amino acid transporter (SLC17A5), and vesicular nucleotide transporter (SLC17A9), in order of identification) (14, 15).
Vesicular polyamine transporter (VPAT)3 encoded by the SLC18A4 (or SLC18B1) gene is the fourth member of the SLC18 family. VPAT is present in secretory vesicles and is responsible for vesicular storage of spermine and spermidine followed by vesicular release of these polyamines from astrocytes (16). In the present study, we investigated whether VPAT is expressed in mast cells and whether it contributes to the storage of polyamines in mast cell granules. We also examined whether VPAT-dependent polyamine release contributes to the release of other biogenic amine from mast cells. Here, we report that polyamine storage in mast cells represents typical chemical transmission mediated by VPAT.
Results
Expression of VPAT in Mast Cells
First, we investigated whether bone marrow-derived mast cells (BMMCs) express the VPAT gene. Maturation of BMMCs was confirmed by toluidine blue staining and flow cytometry according to the published procedures. More than 93% of cells in which the mast cell markers, c-Kit and FcϵRI, were co-expressed were judged to be mature BMMCs. As shown in Fig. 1a, a 179-kb VPAT transcript was detected in BMMCs, whereas no such transcript was detected in the −RT control. Furthermore, VMAT2 gene expression was also observed in BMMCs. Consistent with the results reported previously, marginal levels of VMAT1 expression were observed (17). Subsequently, we investigated whether VPAT protein is actually expressed in BMMCs. Immunoblotting with specific antibodies against mouse VPAT indicated that the BMMC-derived membrane fraction contained an immunoreactive polypeptide with an apparent molecular mass of 55.6 kDa, which disappeared with preabsorption of anti-VPAT antisera (Fig. 1b). The anti-VPAT antibody detected immunoreactive protein bands in the astrocyte membrane fraction, as reported previously (16) (Fig. 1b). Furthermore, immunohistochemical analysis with anti-VPAT antibody indicated that BMMCs were immunoreactive, and internal particle-like structures showed strong staining, whereas preabsorbed antibodies did not recognize these structures (Fig. 1c). In astrocytes, VPAT immunoreactivity was present in the cytoplasm, as reported previously (16) (Fig. 1c). Taken together, these results indicated that BMMCs express not only VMAT2 but also VPAT.
FIGURE 1.
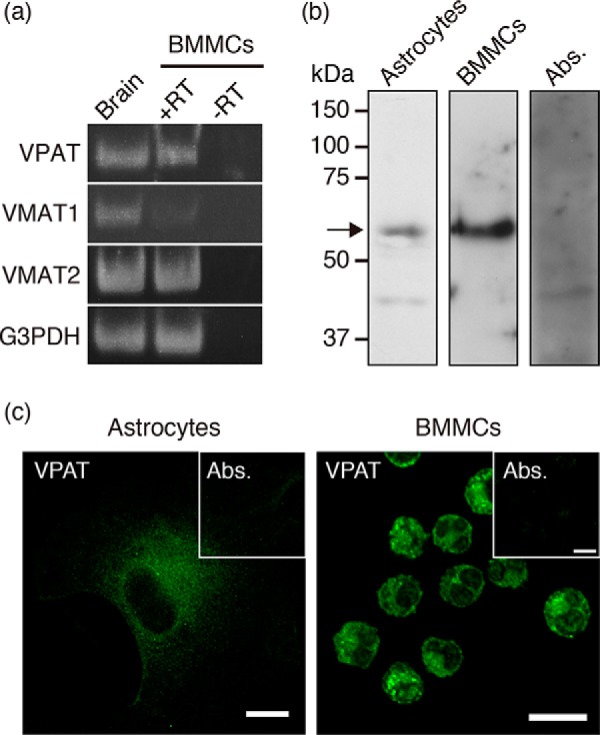
VPAT gene and protein expression in BMMCs. a, RT-PCR analysis was performed to examine the expression of VPAT (179 bp), VMAT1 (123 bp), and VMAT2 (141 bp) mRNAs in BMMCs. The PCR product from brain mRNA is shown as a positive control. Expression of the G3PDH gene is also shown as an RNA quality control (150 bp). b, Western blotting with anti-VPAT antibodies indicated the presence of VPAT protein in the BMMC granule fraction (4 μg). The astrocyte membrane fraction (30 μg) is also shown as a positive control. Preabsorbed antibodies were used in the BMMC granule fraction as a control. The position of VPAT protein is indicated by an arrow. c, indirect immunofluorescence microscopy revealed that VPAT was expressed in BMMCs. A picture of astrocytes is also shown as a positive control. Inset, control staining with preabsorbed antibodies. Bars, 10 μm.
Localization of VPAT in BMMCs
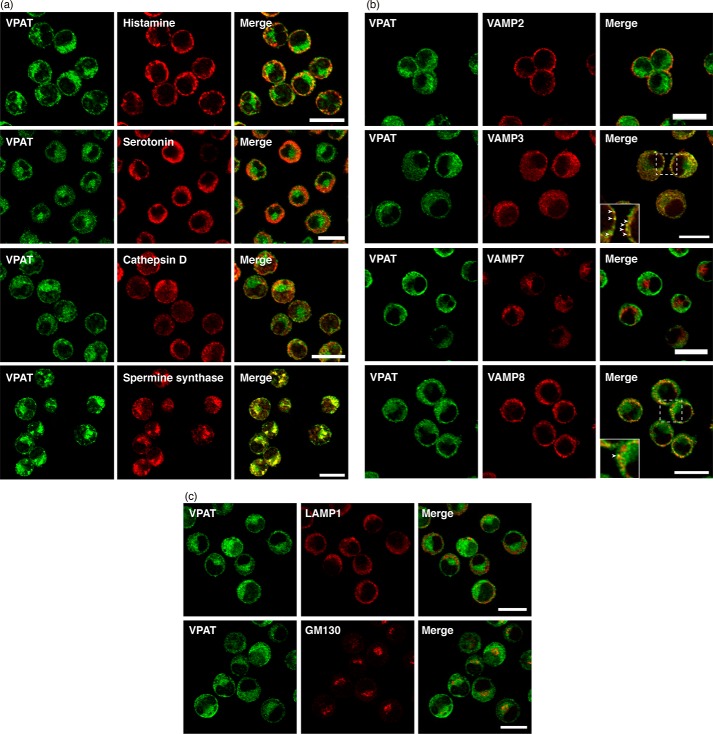
The observations that mast cells contain numerous secretory granules and BMMCs possess VPAT-immunoreactive particle-like structures suggest that VPAT is localized to secretory granules. Double immunofluorescence microscopy indicated that a major population of VPAT immunoreactivity was not co-localized with histamine, serotonin, and cathepsin D (Fig. 2a). VPAT was colocalized with spermine synthase, as confirmed by staining with specific antibodies against spermine synthase (Fig. 2a). Another series of double immunoreactivity experiments indicated that VPAT immunoreactivity is co-localized with VAMP3 but not with VAMP2 and VAMP7 (Fig. 2b). Partial co-localization was observed with VAMP8 (Fig. 2b). Furthermore, VPAT was not co-localized with LAMP1 or GM130, markers of the lysosome and Golgi apparatus, respectively (Fig. 2c). These results suggested that VPAT-containing organelles do not match histamine-containing secretory granules and serotonin-containing secretory granules.
FIGURE 2.
Localization of VPAT in BMMCs. a–c, BMMCs were fixed and subjected to double immunostaining with antibodies to VPAT (green, left), histamine, serotonin, cathepsin D, and spermine synthase (a); VAMP2, VAMP3, VAMP7, and VAMP8 (b); and LAMP1 and GM130 (c) (red, middle). Merged images (right) are also shown. Areas surrounded by dotted lines are enlarged in insets. Arrowheads, merged regions. Bars, 10 μm.
Vesicular Polyamine Storage
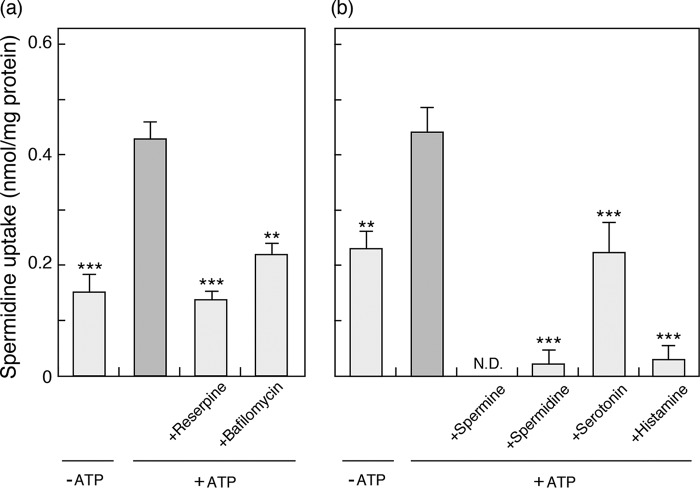
Like other classical neurotransmitter transporters, VPAT mediates active transport of spermine and spermidine at the expense of a proton motive force established by vacuolar proton ATPases (16). To investigate whether VPAT in BMMCs is functional, we prepared membrane fraction of postnuclear fraction of BMMCs and measured ATP-dependent spermidine uptake. As shown in Fig. 3, the addition of ATP facilitated spermidine uptake, which was inhibited by 1 μm reserpine, an inhibitor of VMATs and VPAT, and by 0.5 μm bafilomycin A1, a specific inhibitor of vacuolar proton ATPase (Fig. 3a). Furthermore, the ATP-dependent spermidine uptake was inhibited by an excess (5 mm) of spermine, spermidine, and histamine. Serotonin had a weak inhibitory effect compared with the other amines (Fig. 3b).
FIGURE 3.
ATP-dependent spermidine uptake by membrane fraction of BMMCs. a, the effects of 1 μm reserpine (an inhibitor of VMATs) and 0.5 μm bafilomycin A1 (an inhibitor of V-ATPase) on spermidine uptake by membrane fraction of BMMCs (25 μg/assay) after 10 min. b, cis-inhibition of spermidine uptake after 10 min. The additions were spermine, spermidine, serotonin, and histamine (all at 5 mm). Spermidine uptake in the absence of ATP is shown as a negative control. Data are means ± S.E. (error bars); n = 4–7 technical replicates. Essentially similar data were obtained from biological replicates (data not shown). **, p < 0.01; ***, p < 0.001; N.D., not detected.
BMMCs Release Polyamines
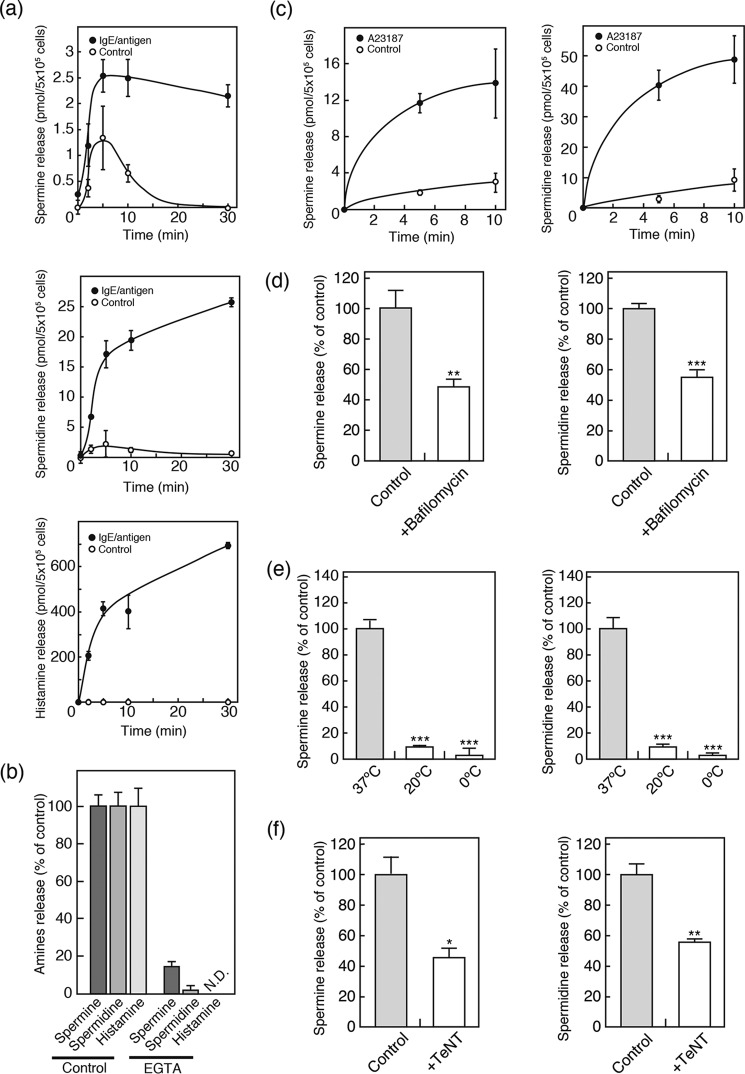
It is expected that vesicular polyamines are released from BMMCs upon stimulation. As shown in Fig. 4, IgE-mediated antigen stimulation facilitated release of spermine and spermidine in a time-dependent manner (Fig. 4a, closed circles). In the absence of stimulation, essentially no or low levels of spermine and spermidine release were observed (Fig. 4a, open circles). Time-dependent and IgE/antigen-dependent release of histamine was also observed (Fig. 4a). The pattern of spermidine release was similar to that of histamine but slightly different from that of spermine. In the presence of extracellular EGTA, both IgE/antigen-evoked spermine and spermidine release were abolished (Fig. 4b). These observations indicated that IgE/antigen-evoked spermine and spermidine release are Ca2+-dependent, as in the case of histamine release (Fig. 4b). Ca2+-dependent spermine and spermidine release were also observed with the addition of the Ca2+ ionophore, A23187 (Fig. 4c). Bafilomycin A1, a inhibitor of vacuolar proton ATPase, inhibited both IgE/antigen-evoked spermine and spermidine (51.5 and 45.2% inhibition, respectively) release compared with the control level (Fig. 4d). The polyamine release from BMMCs exhibited temperature dependence. Both IgE/antigen-evoked spermine and spermidine release were also significantly reduced when BMMCs were incubated at 20 or 4 °C (Fig. 4e). Furthermore, TeNT, a neurotoxin that degrades VAMP2 and VAMP3, inhibited both IgE/antigen-evoked spermine and spermidine release (53.7 and 43.5% inhibition, respectively) (Fig. 4f). These results are consistent with the characteristics of transmitter secretion through exocytosis and indicated the involvement of exocytotic mechanisms in polyamine secretion from BMMCs.
FIGURE 4.
Polyamines were released through exocytosis from BMMCs. a, time courses of spermine (top), spermidine (middle), and histamine (bottom) release from BMMCs in the presence (closed circles) or absence (open circles) of antigen. Data are means ± S.E. (error bars); n = 3–4 technical replicates. Essentially similar data were obtained from biological replicates (data not shown). b, calcium dependence of polyamines and histamine release. BMMCs were incubated in the presence or absence of 1 mm EGTA. c, time courses of spermine (left) and spermidine (right) release from BMMCs in the presence (closed circles) or absence (open circles) of 2 μm calcium ionophore (A23187). d, effects of 1 μm bafilomycin A1 on polyamine release from BMMCs. BMMCs were pretreated with inhibitors for 2 h, followed by assay of IgE/antigen-evoked spermine (left) and spermidine (right) secretion. e, temperature dependence of polyamine release. BMMCs were incubated and assayed for IgE/antigen-evoked spermine (left) and spermidine (right) secretion at the indicated temperature. f, effect of tetanus toxin (TeNT) on polyamine release from BMMCs. BMMCs were treated with 10 μg/ml TeNT for 36 h and assayed for IgE/antigen-evoked spermine (left) and spermidine (right) secretion. Data are means ± S.E.; n = 3–4 technical replicates. Essentially similar data were obtained from biological replicates (data not shown). *, p < 0.05; **, p < 0.01; ***, p < 0.001; N.D., not detected.
Involvement of VPAT in Vesicular Polyamine Storage and Release
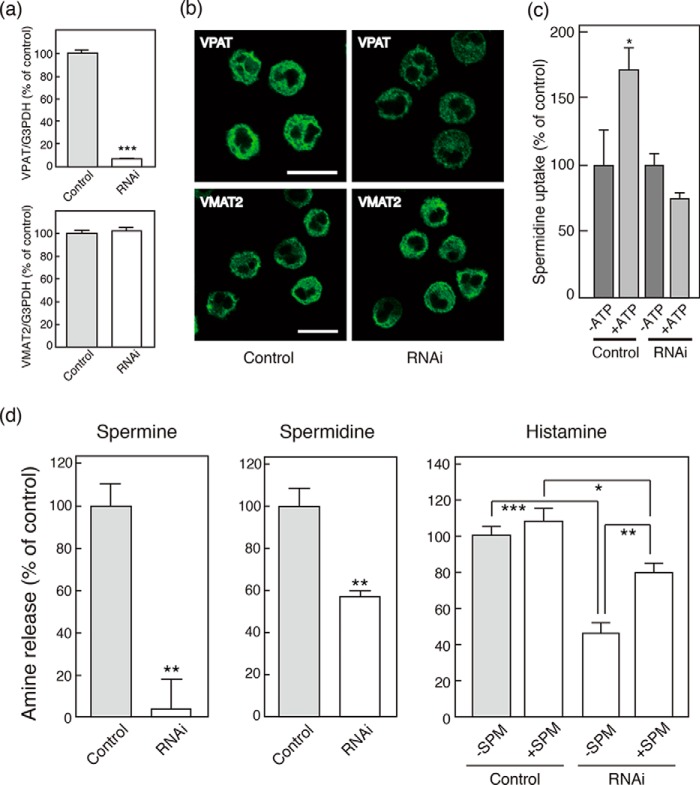
Next, we investigated whether VPAT is involved in vesicular polyamine release using gene knockdown techniques. As shown in Fig. 5a, gene knockdown specific for VPAT suppressed its gene expression by 95% without affecting VMAT2 gene expression in BMMCs (Fig. 5a). Consistent with the suppression of gene expression, VPAT immunoreactivity in BMMCs was decreased, whereas immunoreactivity for VMAT2 showed no changes (Fig. 5b). Under these conditions, we prepared the membrane fraction from siRNA-treated BMMCs and assayed spermidine uptake. As shown in Fig. 5c, ATP-dependent spermidine uptake by the siRNA-treated membrane fraction was abolished, suggesting that the VPAT expressed in BMMCs is functional. We also found that >95% of IgE/antigen-evoked release of spermine was inhibited in siRNA-treated BMMCs (Fig. 5d, left). IgE/antigen-evoked release of spermidine was also inhibited by gene knockdown, although the degree of inhibition was not as strong as that of spermine release (Fig. 5d). Unexpectedly, however, IgE/antigen-evoked release of histamine was also inhibited by 50% upon suppression of VPAT gene expression (Fig. 5d, right). This inhibition was partially blocked when exogenous spermine was included in the culture medium at a concentration of 1 mm (Fig. 5d, right). These results clearly indicated that VPAT gene expression is positively related to vesicular spermine and spermidine storage and release.
FIGURE 5.
Effects of VPAT gene knockdown on endogenous expression of VPAT, polyamine storage, and release from BMMCs. For the VPAT gene knockdown experiment, BMMCs were treated with VPAT-specific siRNA (RNAi) or AllStars negative control siRNA (control) as described under “Experimental Procedures.” a, quantitative PCR analysis of VPAT (top) and VMAT2 (bottom) mRNA expression. Values are shown relative to G3PDH. Data are means ± S.E. (error bars); n = 3 technical replicates. Essentially similar data were obtained from biological replicates (data not shown). ***, p < 0.001. b, immunohistochemical detection of VPAT and VMAT2 in control or RNAi-treated BMMCs. Bars, 10 μm. c, ATP-dependent spermidine uptake by membrane fraction derived from RNAi-treated BMMCs. Spermidine uptake is shown relative to that in the absence of ATP (0.20 nmol/mg protein) as 100%. d, polyamine release from BMMCs stimulated with IgE/antigen (left and middle). Histamine release from BMMCs stimulated with IgE/antigen in the presence or absence of 1 mm spermine (right). Data are means S.E.; n = 3–4 technical replicates. Essentially similar data were obtained from biological replicates (data not shown). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
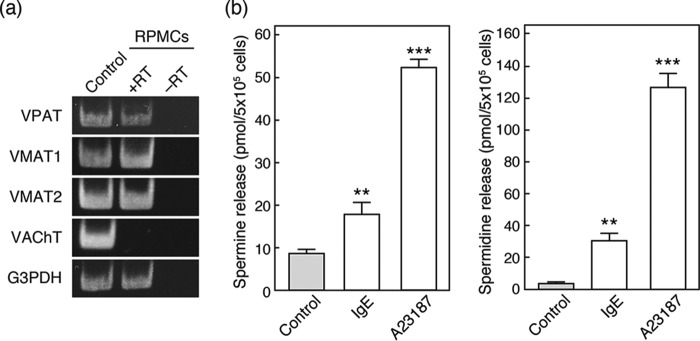
Expression of VPAT and Vesicular Polyamine Release from Rat Peritoneal Mast Cells (RPMCs)
Finally, we assessed the expression of VPAT in RPMCs to determine whether its expression is specific to BMMCs. As shown in Fig. 6, RT-PCR analysis indicated that RPMCs also express VPAT as well as VMAT2. RPMCs also express VMAT1. Furthermore, both IgE/antigen treatment and the addition of A23187 triggered RPMCs to release spermine and spermidine. Thus, it is reasonable to suggest that RPMCs are equipped with VPAT-mediated machinery for storage and release of polyamines, similar to BMMCs.
FIGURE 6.
VPAT gene expression and polyamine release from RPMCs. a, RT-PCR analysis of SLC18 family and G3PDH mRNA expression in RPMCs. The PCR product from brain mRNA is shown as a positive control. b, spermine (left) and spermidine (right) release from RPMCs stimulated with IgE/antigen or 2 μm calcium ionophore (A23187). Data are means S.E. (error bars); n = 3–6 technical replicates. Essentially similar data were obtained from biological replicates (data not shown). **, p < 0.01; ***, p < 0.001.
Discussion
In chemical transmission, classical neurotransmitters, such as serotonin and glutamate, are first stored in the secretory vesicles via vesicular neurotransmitter transporters and then discharged through exocytosis upon stimulation. In addition to secretion of various cytokines and proteases, mast cells are known to be histaminergic and serotonergic in nature, store these monoamines in secretory granules, and release them upon stimulation (18). The secretion of biogenic amines is involved in bronchoconstriction, vasodilation, and vascular permeability, which are the chief physiological and pathological roles of mast cells (1). In the present study, we added a novel feature of mast cells (i.e. mast cells accumulate polyamines in the subpopulation of secretory granules through VPAT-mediated active transport and subsequently secrete them upon stimulation).
This conclusion was based on the following observations. First, VPAT is expressed and associated with secretory granules in mast cells. Second, the membrane vesicles prepared from BMMCs can take up spermidine in an ATP-dependent and reserpine-sensitive manner, a known property of VPAT. Third, inhibition of VPAT expression decreases the storage and release of polyamines. Thus, VPAT is responsible for vesicular storage of spermine and spermidine, followed by vesicular release of these polyamines. Both spermine and spermidine are synthesized from putrescine by spermidine synthase and spermine synthase in a successive cascade. Because spermine synthase and VPAT are co-localized in mast cells, these polyamines formed in close proximity to VPAT-containing secretory granules may be taken up effectively by VPAT and accumulate inside granules.
Recently, it was reported that mast cells possess distinct secretory granule subsets (2, 19, 20). Biochemical and histochemical analyses clarified the presence of histamine-containing secretory granules and cathepsin D- and serotonin-containing secretory granules (20). Because VPAT can be regarded as a molecular marker of polyamine-containing secretory vesicles, identification of VPAT in mast cells would be very helpful to characterize secretory granule subsets. In fact, the localization of VPAT is different from those of histamine and serotonin, suggesting that VPAT-containing secretory granules (i.e. polyamine-containing secretory granules) is distinct from histamine and serotonin granules. Recent studies indicated that degranulation of secretory granule subsets is regulated by different SNARE isoforms (2, 20–23). In VAMP8 knock-out mice, serotonin and cathepsin D release from BMMCs are significantly reduced, whereas vesicular release of histamine is rather stimulated (20). Pairs of cathepsin D and serotonin and serotonin and VAMP 8 showed co-localization, whereas histamine and serotonin did not (20). Another study demonstrated reduced histamine release from BMMCs derived from VAMP8 knock-out mice (21). In addition to VAMP8, VAMP7 is also involved in histamine release, whereas VAMP3 is not required for histamine release from human mast cells (22). We found that both IgE/antigen-evoked spermine and spermidine release were sensitive to TeNT, a neurotoxin that degrades VAMP2 and VAMP3 but does not degrade VAMP7 or VAMP8, suggesting the involvement of VAMP2 or VAMP3 in exocytotic polyamine release from BMMCs (24–27). Our immunohistochemical analyses indicated that VPAT is co-localized with VAMP3 and partially co-localized with VAMP8, but not with VAMP2 or VAMP7, further supporting the suggestion that VPAT-containing granules (polyamine-containing granules) are distinct from histamine granules and serotonin granules. Further characterization of the VPAT-containing granules will be helpful to understand the mechanism for regulating biogenic amines release from mast cells.
It is well established that VMAT2 is specifically expressed in mast cells and is responsible for vesicular storage and release of histamine (17, 23). Furthermore, it is noteworthy that VMAT1 was also expressed in BMMCs and RPMCs (Figs. 1a and 6a). Taken together with the association of SNARE complex outlined above, these results suggest that mast cell secretory granules may be much more heterogeneous than previously anticipated. The secretory granules of mast cells should be classified from the viewpoint of the presence of VMAT1, VMAT2, and VPAT. Such studies are currently under way in our laboratory.
It should be stressed that although VPAT gene knockdown did not affect expression of VMAT2, it significantly suppressed antigen-evoked histamine release (Fig. 5c). Furthermore, the addition of spermine partially recovered the vesicular release of histamine (Fig. 5c). These results explain the mode of action of polyamines as autocrine or paracrine modulators; once released, spermine and spermidine may act as intercellular messengers or chemical transmitters and facilitate the synthesis and secretion of histamine. The action of polyamines implies the presence of polyamine receptors in mast cells. It is well known in other cell types, such as neuronal cells, that polyamines affect NMDA glutamate receptor activity (29, 30). In mast cells, the NMDA receptor should be a candidate polyamine receptor, but the presence of NMDA receptors in these cells is currently controversial (7, 9, 10). Another possibility is the presence of an as yet unidentified polyamine-specific receptor in mast cells. Compound 48/80, a polycationic histamine releaser, evokes histamine release through Mrgprb2 (human ortholog, MRGPRX2), which is inhibited by polyamines (31–34). Thus, it has been reported that polyamine acts as an autocrine or paracrine transmitter through some type of membrane protein and is associated with histamine release. Although the hypothetical polyamine receptor has yet to be identified, characterization of polyamine receptor may help to elucidate the molecular mechanism of action of polyamine in mast cells.
In conclusion, we have demonstrated that VPAT is responsible for vesicular storage and release of polyamines from mast cells and that polyamine-containing secretory granules are distinct from histamine- and serotonin-containing secretory granules. Thus, our findings strongly suggest that secretory granules are more heterogeneous in mast cells, and further functional characterization of SLC18 family members will elucidate more refined features of secretory granules in mast cells.
Experimental Procedures
Preparation of BMMCs and RPMCs
Male 8-week-old BALB/c mice and male 7-week-old Wistar/ST rats were obtained from Japan SLC (Shizuoka, Japan). All animal procedures and care were approved by the institutional animal care and use committee and were carried out according to the guidelines of Okayama University. BMMCs were prepared as described previously (35). Briefly, bone marrow cells were isolated from BALB/c mice. These cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, 100 units/ml streptomycin, 50 μm 2-mercaptoethanol, 0.1 mm non-essential amino acid solution, and 10 ng/ml mouse IL-3. Medium was changed every 4–5 days, and cells were used for experiments after 4–5 weeks of culture.
RPMCs were obtained from Wistar/ST rats as described previously (36). Briefly, rat peritoneal cells were recovered with Tyrode-HEPES buffer (137 mm NaCl, 2.7 mm KCl, 1.6 mm CaCl2, 1 mm MgCl2, 5.6 mm glucose, 10 mm HEPES-NaOH, pH 7.4) containing 0.41 mm NaH2PO4 and 0.1% gelatin (THG buffer) and centrifuged at 800 × g for 3 min at 4 °C. The peritoneal cells were resuspended in 3 ml of THG buffer, layered onto 1.5 ml of Tyrode-HEPES buffer containing 0.05% gelatin and 22.5% (w/w) Histodenz (Sigma-Aldrich), and centrifuged at 45 × g for 15 min at 4 °C. The cell pellet was washed twice with PBS. Over 96% of the cells were confirmed to be RPMCs by toluidine blue staining.
Flow Cytometry and Toluidine Blue Staining
Maturation of BMMCs was assessed by analyzing the expression of mast cell markers, FcϵRI and c-Kit, using flow cytometry and by toluidine blue staining. For flow cytometric analysis, the cells were washed and resuspended in staining medium (PBS supplemented with 7.7 mm sodium azide and 2% FBS) and blocked with 16.7 μg/ml rat anti-mouse FcγRIII/FcγRII Ab (clone 2.4G2; BD Biosciences) for 10 min on ice. After incubation with 12.5 μg/ml mouse anti-dinitrophenyl mAb (clone SPE-7; Sigma-Aldrich) for 50 min on ice, cells were washed and incubated with 2.5 μg/ml rat anti-mouse IgE Ab conjugated with FITC and 1 μg/ml rat anti-mouse c-Kit Ab conjugated with PE or isotype control Abs (all from BD Biosciences) for 25 min on ice. Cells were washed and resuspended in staining medium containing 50 μg/ml propidium iodide for analysis with a FACSCalibur flow cytometer using CellQuest software (BD Biosciences). Over 93% of BMMCs showed coexpression of both mast cell markers. For toluidine blue staining, cells were stained with 2.5% toluidine blue solution (pH 3.3) for 15 min at room temperature.
RT-PCR Analysis
Mouse and rat brain total RNAs were purchased from Unitech (Chiba, Japan). Total RNAs were isolated from BMMCs and RPMCs using an RNeasy minikit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. cDNA was generated from 1 μg of total RNA using a transcriptase kit (Toyobo, Osaka, Japan). Real-time PCR was carried out with 400 nmol/liter specific forward and reverse primers and 5 units/ml SYBR Premix ExTaq (Takara Bio, Otsu, Japan). Thirty-five cycles of amplification were performed with denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 30 s. The primer sets were as follows: mVPAT, 5′-TCGAGTGGGCAGCAGCTATG-3′ and 5′-CCAGAGCTCTCAAGGCTAGGTGTC-3′ (product size 179 bp); mVMAT1, 5′-GGACAATATGCTGCTCACTGTGG-3′ and 5′-GTGAGAGCTTGCTGGGAGCTTAC-3′ (product size 123 bp); mVMAT2, 5′-TACGGACTCATCGCTCCCAAC-3′ and 5′-GGCTACATCTGCAATGGCATACAC-3′ (product size 141 bp); rVPAT, 5′-ACGGAATAAGTACGCTGGGACTTG-3′ and 5′-TAGCTGCTGCCCACTCGAAAC-3′ (product size 126 bp); rVMAT1, 5′-TTGGCTCATGGTCATCATTGG-3′ and 5′-CTGTGTACATCTGGGTCTCTGTGG-3′ (product size 137 bp); rVMAT2, 5′-CCTTCGAAGTCCACCTGCTAA-3′ and 5′-CATCACCGATGGGATATGACTG-3′ (product size 116 bp); rVAChT, 5′-CGTGGATGAAGCACACAATGG-3′ and 5′-CCTAACACGTGTGGCACGAAAG-3′ (product size 82 bp). Mouse VPAT and VMAT2 expression were evaluated relative to that of the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (G3PDH).
Antibodies
The immunological specificities of rabbit polyclonal antibodies against Glu429–Thr457 of mouse VPAT polypeptide prepared in house were determined previously (16). Rat polyclonal antibodies against mouse spermine synthase were prepared by repeatedly injecting GST fusion polypeptides encoding Ala178–Pro366 of mouse spermine synthase polypeptides into rats. The following antibodies were obtained commercially: anti-histamine mouse monoclonal (Abcam, Cambridge, UK), anti-serotonin mouse monoclonal (Dako, Glostrup, Denmark), anti-cathepsin D goat polyclonal (Santa Cruz Biotechnology, Inc., Dallas, TX), anti-VAMP2 mouse monoclonal (Synaptic Systems, Göttingen, Germany), anti-VAMP3 sheep polyclonal (Abcam), anti-VAMP7 mouse monoclonal (Abcam), anti-VAMP8 mouse monoclonal (Santa Cruz Biotechnology), anti-LAMP1 mouse monoclonal (StressMarq Biosciences, Victoria, Canada), anti-GM130 mouse monoclonal (BD Biosciences), anti-spermine rabbit polyclonal (Novus Biologicals, Littleton, CO), and anti-spermidine rabbit polyclonal (Novus Biologicals) antibodies.
Immunofluorescence Microscopy
Indirect immunofluorescence microscopy was performed as described previously (37). Briefly, BMMCs on poly-l-lysine-coated coverslips were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature. The cells were washed with PBS and incubated with the same buffer containing 0.02% saponin, 1% BSA, and 2% goat serum or 0.02% saponin and 1% BSA for 15 min at room temperature. The first antibody treatment was performed with anti-mouse VPAT antibodies diluted 1:200 in PBS containing 0.5% BSA for 1 h at room temperature. Washing and secondary antibody treatment were performed according to the published procedures (37). The specimens were observed using an Olympus FV300 confocal laser microscope.
Preparation of Granule Fraction and Membrane Fraction from BMMCs
BMMCs were suspended in 20 ml of 10 mm MOPS-Tris (pH 7.0) containing 0.3 m sucrose, 5 mm EDTA, 10 μg/ml pepstatin A, and 10 μg/ml leupeptin (SME buffer) and then homogenized with a Dounce homogenizer. The homogenate was disrupted by nitrogen cavitation after equilibration at 1000 p.s.i. for 10 min and centrifuged at 900 × g for 8 min to remove cell debris. To obtain the granule fraction, the resulting supernatant was centrifuged at 10,000 × g for 15 min. The pellet was suspended in 1 ml of SME buffer and diluted with 19 ml of 8 mm MOPS/Tris (pH 7.0) buffer. After incubation on ice for 30 min, the suspension was centrifuged at 10,000 × g for 15 min. The resulting supernatant was centrifuged at 150,000 × g for 1 h. The pellet (granule fraction) was suspended in SME. To obtain the membrane fraction, the supernatant obtained after centrifugation at 900 × g for 8 min was centrifuged at 100,000 × g for 1 h. The pellet (membrane fraction) was suspended in SME.
Spermidine Uptake
The membrane fraction (25 μg of protein/assay) was suspended in 120 μl of 20 mm MOPS-Tris (pH 7.0), 0.1 m KCl, 0.2 m sucrose, and 5 mm magnesium acetate and incubated for 1 min at 30 °C in the absence or presence of inhibitors. After incubation with 5 mm ATP for 1 min at 30 °C, the assay was initiated by the addition of 100 μm [3H]spermidine (0.5 MBq/μmol); aliquots of 100 μl were taken at the indicated times and filtered through 0.45-μm nitrocellulose filters (Millipore, Billerica, MA). After washing with 6 ml of cold buffer containing 20 mm MOPS-Tris (pH 7.0), 0.1 m KCl, 0.2 m sucrose, and 5 mm magnesium acetate, the radioactivity remaining on the filters was counted in a liquid scintillation counter (PerkinElmer Life Sciences).
RNAi
AllStars negative control siRNA (Qiagen) or mVPAT siRNA (SI01708707; Qiagen) at a concentration of 1 μm was transfected using a Nucleofector I device (Lonza, Basel, Switzerland) set at program Y-01 with an Amaxa mouse macrophage nucleofector kit (Lonza) according to the manufacturer's instructions. mVPAT gene expression was assayed after 72 h.
Degranulation Assay
The cells were stimulated with antigen or the calcium ionophore A23187. For antigen stimulation, 1 × 106 cells/ml of BMMCs were resuspended in RPMI medium containing 1 μg/ml mouse anti-dinitrophenyl mAb (SPE-7) and incubated at 37 °C, 5% CO2 for 16 h. After washing with Krebs-Ringer solution (128 mm NaCl, 26 mm NaHCO3, 10 mm HEPES/Tris, pH 7.4, 2.4 mm CaCl2, 1.9 mm KCl, 1.3 mm MgSO4, 1.2 mm KH2PO4) containing 0.2% BSA. The cells were stimulated with 100 ng/ml DNP-HSA for 10 min at 37 °C, 5% CO2. Supernatants were collected and stored at −80 °C until use.
RPMCs were resuspended in Ringer's solution containing 1 μg/ml mouse anti-dinitrophenyl mAb (SPE-7) and incubated for 1 h at 37 °C, 5% CO2. Then 2 × 105 cells were stimulated with 100 ng/ml DNP-HSA and 2 μm 1-oleoyl-2-hydroxy-sn-glycero-3-(phospho-l-serine) (lysophosphatidylserine; Avanti Polar Lipids, Alabaster, AL) in Krebs-Ringer solution for 10 min at 37 °C, 5% CO2. For A23187 stimulation, 5 × 105 cells were stimulated with Krebs-Ringer solution containing 2 μm A23187 for 10 min at 37 °C, 5% CO2.
Quantification of Polyamine and Histamine Release
Polyamines were quantified by dansylation and HPLC as reported previously with some modifications (4, 38, 39). Briefly, 400 μl of supernatant was incubated with 150 nm 1,8-diaminooctane as an internal standard and 4.3% perchloric acid for 30 min on ice. After centrifugation at 120,000 × g for 15 min at 4 °C, the resulting supernatant was incubated with 600 μl of 10 mg/ml dansyl chloride and saturated Na2CO3 for 30 min at 60 °C and then incubated with 10 μl of 100 mg/ml l-proline for 30 min at 60 °C. Dansyl derivatives were extracted with 1.5 ml of toluene and evaporated in a centrifugal vacuum concentrator. The resulting pellet was resuspended with 100 μl of solvent (acetonitrile/water = 7:3) and injected into the CAPCELL PAK 4.6 × 250-mm column (5 μm, SHISEIDO, Tokyo, Japan).
The amount of histamine was determined as described previously (40). Briefly, cells were homogenized in PBS containing 2 m NaCl and 0.5% Triton X-100, and the soluble fraction was obtained by centrifugation at 1000 × g for 15 min at 4 °C. Perchloric acid (final concentration 3%) was added to the resulting supernatant for deproteinization. The sample was subjected to HPLC with a cation exchange column (WCX-1; Shimadzu, Kyoto, Japan), and histamine was detected fluorometrically through post-column derivatization with o-phthalaldehyde.
Miscellaneous Methods
SDS-PAGE and Western blotting were performed as described (41). Protein concentrations were determined using BSA as a standard (28). Hippocampal astrocyte cultures were obtained from embryonic Wistar rats (embryonic day 17) as described (16).
Data Analysis
All numerical values were shown as the means ± S.E. unless otherwise specified. Statistical significance was determined by two-tailed paired Student's t test, Dunnett's test for multiple comparisons after analysis of variance, or one-way repeated-measures analysis of variance followed by Dunnett's test were performed using GraphPad Prism version 6 software (GraphPad Software, La Jolla, CA). Differences were considered significant at p < 0.05.
Author Contributions
K. F., S. T., T. M., H. O., Y. M., and M. H. designed the experiments, analyzed data, and wrote the paper. T. T., Y. H., S. M., and M. H. performed the experiments.
This work was supported in part by Grants-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology of Japan 22790067 (to T. M.), 25253008 (to H. O. and Y. M.), and 16K08230 (to M. H.); by the Uehara Memorial Foundation and the Smoking Research Foundation (to T. M.); and by the Takeda Science Foundation and the Astellas Foundation for Research on Metabolic Disorders (to T. M. and M. H.). The authors declare that they have no conflicts of interest with the contents of this article.
- VPAT
- vesicular polyamine transporter
- BMMC
- bone marrow-derived mast cell
- RPMC
- rat peritoneal mast cell
- VAMP
- vesicle-associated membrane protein
- VMAT
- vesicular monoamine transporter
- mVPAT
- mouse VPAT
- Ab
- antibody
- G3PDH
- glyceraldehyde 3-phosphate dehydrogenase.
References
- 1. Metcalfe D. D., Baram D., and Mekori Y. A. (1997) Mast cells. Physiol. Rev. 77, 1033–1079 [DOI] [PubMed] [Google Scholar]
- 2. Wernersson S., and Pejler G. (2014) Mast cell secretory granules: armed for battle. Nat. Rev. Immunol. 14, 478–494 [DOI] [PubMed] [Google Scholar]
- 3. Kalesnikoff J., and Galli S. J. (2008) New developments in mast cell biology. Nat. Immunol. 9, 1215–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. García-Faroldi G., Rodríguez C. E., Urdiales J. L., Pérez-Pomares J. M., Dávila J. C., Pejler G., Sánchez-Jiménez F., and Fajardo I. (2010) Polyamines are present in mast cell secretory granules and are important for granule homeostasis. PLoS One 5, e15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medina M. A., Urdiales J. L., Rodríguez-Caso C., Ramírez F. J., and Sánchez-Jiménez F. (2003) Biogenic amines and polyamines: similar biochemistry for different physiological missions and biomedical applications. Crit. Rev. Biochem. Mol. Biol. 38, 23–59 [DOI] [PubMed] [Google Scholar]
- 6. Gerner E. W., and Meyskens F. L. Jr. (2004) Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer 4, 781–792 [DOI] [PubMed] [Google Scholar]
- 7. Bueb J. L., Mousli M., and Landry Y. (1991) Molecular basis for cellular effects of naturally occurring polyamines. Agents Actions 33, 84–87 [DOI] [PubMed] [Google Scholar]
- 8. Kurosawa M., Uno D., and Kobayashi S. (1991) Naturally occurring aliphatic polyamines-induced histamine release from rat peritoneal mast cells. Allergy 46, 349–354 [DOI] [PubMed] [Google Scholar]
- 9. Purcell W. M., Doyle K. M., Bagga L., and Derks M. (1994) Histamine release from mast cells by polyamines: an NMDA receptor-mediated event? Biochem. Soc. Trans. 22, 398S. [DOI] [PubMed] [Google Scholar]
- 10. Purcell W. M., Doyle K. M., Westgate C., and Atterwill C. K. (1996) Characterisation of a functional polyamine site on rat mast cells: association with a NMDA receptor macrocomplex. J. Neuroimmunol. 65, 49–53 [DOI] [PubMed] [Google Scholar]
- 11. Vliagoftis H., Mak L., Boucher W., and Theoharides T. C. (1999) Dual effect of spermine on mast cell secretion exhibits different calcium and temperature requirements. Int. J. Immunopharmacol. 21, 547–559 [DOI] [PubMed] [Google Scholar]
- 12. Kanerva K., Lappalainen J., Mäkitie L. T., Virolainen S., Kovanen P. T., and Andersson L. C. (2009) Expression of antizyme inhibitor 2 in mast cells and role of polyamines as selective regulators of serotonin secretion. PLoS One 4, e6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Omote H., Miyaji T., Hiasa M., Juge N., and Moriyama Y. (2016) Structure, function, and drug interactions of neurotransmitter transporters in the postgenomic era. Annu. Rev. Pharmacol. Toxicol. 56, 385–402 [DOI] [PubMed] [Google Scholar]
- 14. Omote H., Miyaji T., Juge N., and Moriyama Y. (2011) Vesicular neurotransmitter transporter: bioenergetics and regulation of glutamate transport. Biochemistry 50, 5558–5565 [DOI] [PubMed] [Google Scholar]
- 15. Omote H., and Moriyama Y. (2013) Vesicular neurotransmitter transporters: an approach for studying transporters with purified proteins. Physiology 28, 39–50 [DOI] [PubMed] [Google Scholar]
- 16. Hiasa M., Miyaji T., Haruna Y., Takeuchi T., Harada Y., Moriyama S., Yamamoto A., Omote H., and Moriyama Y. (2014) Identification of a mammalian vesicular polyamine transporter. Sci. Rep. 4, 6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anlauf M., Schafer M. K., Depboylu C., Hartschuh W., Eiden L. E., Kloppel G., and Weihe E. (2004) The vesicular monoamine transporter 2 (VMAT2) is expressed by normal and tumor cutaneous mast cells and Langerhans cells of the skin but is absent from Langerhans cell histiocytosis. J. Histochem. Cytochem. 52, 779–788 [DOI] [PubMed] [Google Scholar]
- 18. Sjoerdsma A., Waalkes T. P., and Weissbach H. (1957) Serotonin and histamine in mast cells. Science 125, 1202–1203 [DOI] [PubMed] [Google Scholar]
- 19. Theoharides T. C., Bondy P. K., Tsakalos N. D., and Askenase P. W. (1982) Differential release of serotonin and histamine from mast cells. Nature 297, 229–231 [DOI] [PubMed] [Google Scholar]
- 20. Puri N., and Roche P. A. (2008) Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc. Natl. Acad. Sci. U.S.A. 105, 2580–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tiwari N., Wang C. C., Brochetta C., Ke G., Vita F., Qi Z., Rivera J., Soranzo M. R., Zabucchi G., Hong W., and Blank U. (2008) VAMP-8 segregates mast cell-preformed mediator exocytosis from cytokine trafficking pathways. Blood 111, 3665–3674 [DOI] [PubMed] [Google Scholar]
- 22. Sander L. E., Frank S. P., Bolat S., Blank U., Galli T., Bigalke H., Bischoff S. C., and Lorentz A. (2008) Vesicle associated membrane protein (VAMP)-7 and VAMP-8, but not VAMP-2 or VAMP-3, are required for activation-induced degranulation of mature human mast cells. Eur. J. Immunol. 38, 855–863 [DOI] [PubMed] [Google Scholar]
- 23. Lorentz A., Baumann A., Vitte J., and Blank U. (2012) The SNARE machinery in mast cell secretion. Front. Immunol. 3, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niemann H., Blasi J., and Jahn R. (1994) Clostridial neurotoxins: new tools for dissecting exocytosis. Trends Cell Biol. 4, 179–185 [DOI] [PubMed] [Google Scholar]
- 25. McMahon H. T., Ushkaryov Y. A., Edelmann L., Link E., Binz T., Niemann H., Jahn R., and Südhof T. C. (1993) Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature 364, 346–349 [DOI] [PubMed] [Google Scholar]
- 26. Galli T., Zahraoui A., Vaidyanathan V. V., Raposo G., Tian J. M., Karin M., Niemann H., and Louvard D. (1998) A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol. Biol. Cell 9, 1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paumet F., Le Mao J., Martin S., Galli T., David B., Blank U., and Roa M. (2000) Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment. J. Immunol. 164, 5850–5857 [DOI] [PubMed] [Google Scholar]
- 28. Schaffner W., and Weissmann C. (1973) A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal. Biochem. 56, 502–514 [DOI] [PubMed] [Google Scholar]
- 29. Williams K. (1997) Modulation and block of ion channels: a new biology of polyamines. Cell. Signal. 9, 1–13 [DOI] [PubMed] [Google Scholar]
- 30. Ogden K. K., and Traynelis S. F. (2011) New advances in NMDA receptor pharmacology. Trends Pharmacol. Sci. 32, 726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Read G. W., Hong S. M., and Kiefer E. F. (1982) Competitive inhibition of 48/80-induced histamine release by benzalkonium chloride and its analogs and the polyamine receptor in mast cells. J. Pharmacol. Exp. Ther. 222, 652–657 [PubMed] [Google Scholar]
- 32. Lagunoff D., Martin T. W., and Read G. (1983) Agents that release histamine from mast cells. Annu. Rev. Pharmacol. Toxicol. 23, 331–351 [DOI] [PubMed] [Google Scholar]
- 33. Tatemoto K., Nozaki Y., Tsuda R., Konno S., Tomura K., Furuno M., Ogasawara H., Edamura K., Takagi H., Iwamura H., Noguchi M., and Naito T. (2006) Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 349, 1322–1328 [DOI] [PubMed] [Google Scholar]
- 34. McNeil B. D., Pundir P., Meeker S., Han L., Undem B. J., Kulka M., and Dong X. (2015) Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rottem M., Goff J. P., Albert J. P., and Metcalfe D. D. (1993) The effects of stem cell factor on the ultrastructure of Fc epsilon RI+ cells developing in IL-3-dependent murine bone marrow-derived cell cultures. J. Immunol. 151, 4950–4963 [PubMed] [Google Scholar]
- 36. Inagaki N., Kawasaki H., Ueno M., Nagai H., and Koda A. (1994) Potentiation of antigen-induced histamine release from rat peritoneal mast cells through a direct interaction between mast cells and non-mast cells. Life Sci. 54, 1403–1409 [DOI] [PubMed] [Google Scholar]
- 37. Hayashi M., Yamada H., Uehara S., Morimoto R., Muroyama A., Yatsushiro S., Takeda J., Yamamoto A., and Moriyama Y. (2003) Secretory granule-mediated co-secretion of l-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J. Biol. Chem. 278, 1966–1974 [DOI] [PubMed] [Google Scholar]
- 38. García-Faroldi G., Correa-Fiz F., Abrighach H., Berdasco M., Fraga M. F., Esteller M., Urdiales J. L., Sánchez-Jiménez F., and Fajardo I. (2009) Polyamines affect histamine synthesis during early stages of IL-3-induced bone marrow cell differentiation. J. Cell Biochem. 108, 261–271 [DOI] [PubMed] [Google Scholar]
- 39. Smith M. A., and Davies P. J. (1985) Separation and quantitation of polyamines in plant tissue by high performance liquid chromatography of their dansyl derivatives. Plant Physiol. 78, 89–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamatodani A., Fukuda H., Wada H., Iwaeda T., and Watanabe T. (1985) High-performance liquid chromatographic determination of plasma and brain histamine without previous purification of biological samples: cation-exchange chromatography coupled with post-column derivatization fluorometry. J. Chromatogr. 344, 115–123 [DOI] [PubMed] [Google Scholar]
- 41. Moriyama Y., and Yamamoto A. (1995) Vesicular l-glutamate transporter in microvesicles from bovine pineal glands: driving force, mechanism of chloride anion activation, and substrate specificity. J. Biol. Chem. 270, 22314–22320 [DOI] [PubMed] [Google Scholar]