Summary
Background
Allergic rhinitis is a disease with a high global disease burden, but risk factors that contribute to this condition are not well understood.
Objective
To assess the prevalence and risk factors of allergic rhinitis in two Peruvian populations with disparate degrees of urbanization.
Methods
We conducted a population-based, cross-sectional study on 1441 children aged 13–15 years at enrollment (mean age 14.9 years, 51% boys) to investigate the prevalence of allergic disease. We used a standardized, Spanish validated questionnaire to determine the prevalence of allergic rhinitis and asked about sociodemographics and family history of allergies. Children also underwent spirometry, exhaled nitric oxide, allergy skin testing to 10 common household allergens and provided a blood sample for measurement of 25OH vitamin D and total serum IgE.
Results
Overall prevalence of allergic rhinitis was 18% (95% CI 16% to 20%). When stratified by site, the prevalence of allergic rhinitis was 23% Lima vs. 13% in Tumbes (P < 0.001); however, this difference was no longer significant after controlling for subject- specific factors (P = 0.95). There was a strong association with other allergic diseases: 53% of children with asthma had allergic rhinitis vs. 15% in those without asthma (P < 0.001) and 42% of children with eczema vs. 17% of those without eczema (P < 0.001). Important risk factors for allergic rhinitis were parental rhinitis (adjusted OR = 3.0, 95% CI 1.9–4.7 for 1 parent and adjusted OR = 4.4, 95% CI 1.5–13.7 for 2 parents); allergic sensitization to common household aeroallergens (1.6, 1.1–2.3); being overweight (1.5, 1.0–2.3); exhaled nitric oxide ≥20 ppb (1.9, 1.3–2.7); and total serum IgE ≥ 95th percentile (2.4, 1.2–4.8). Population attributable risk of important factors for allergic rhinitis were 25% for high exhaled nitric oxide, 22% for allergic sensitization to common household aeroallergens, 22% for paternal rhinitis, 10% for being overweight and 7% for an elevated total serum IgE.
Conclusion and Clinical Relevance
Allergic rhinitis was prevalent in both settings, and important risk factors include elevated exhaled nitric oxide, allergic sensitization to common household aeroallergens, parental rhinitis, being overweight and high total serum IgE. When considering subject-specific factors, the difference in prevalence between the urban and rural settings became non-important.
Keywords: allergic rhinitis, atopy, asthma, epidemiology
Introduction
Allergic rhinitis is characterized by inflammation of the nasal mucosa, resulting in symptoms of pruritus, rhinorrhea, sneezing and congestion. It affects nearly 400 million people worldwide [1, 2] and has deleterious effects on the performance of daily activities, quality of sleep, work and school performance and psychosocial well-being [3, 4]. Allergic rhinitis frequently coexists with other allergic diseases such as asthma and atopic dermatitis [5, 6]. Children with allergic rhinitis are more likely to have asthma and other respiratory symptoms and higher rates of conjunctivitis [6, 7]. The ISAAC study found that the prevalence of allergic rhinitis in children was 39% in some countries; however, there is great variation around the world [8]. Unlike asthma, the prevalence of allergic rhinitis does not follow the traditional distribution where high-income countries see a greater prevalence, as low- to middle-income countries are also significantly affected [8]. However, the reason for this finding is not well understood.
Well-recognized risk factors for allergic rhinitis include having atopy, asthma, eczema and other allergic diseases [9–12]. Parental history of allergic disease is also a well-documented risk factor. The risk of allergic rhinitis increases in children of parents with allergic rhinitis, asthma, hay fever and pollen allergies [13–15]. A recent study conducted in Sweden demonstrated that children born to parents with allergic disease had increased odds of developing allergic rhinitis anywhere from 1.8- to 8.8-fold, the highest odds belonging to children born to parents with both hay fever and pollen allergy [14]. Another recent study evaluating the prevalence of allergic rhinitis in Turkish adolescents found that children with a family history of atopic disease had a 7-fold increase in the odds of developing allergic rhinitis [16].
Factors that may contribute to disease risk but are not well understood include vitamin D, obesity, exposure to cigarette smoke, increased total serum IgE, increased blood eosinophils and other environmental exposures common in urban settings [7, 11, 17]. While vitamin D has a modulatory role in both innate and adaptive immune systems [18], current evidence of an association with allergic diseases remains conflicting [19, 20]. Comprehensive, population-based studies on allergic rhinitis are needed to better understand the relative importance of these multiple risk factors. We sought to assess the prevalence of allergic rhinitis and identify risk factors in two resource-limited settings in Peru with disparate degrees of urbanization.
Methods
Study setting
The Peru Urban to Rural Asthma (PURA) study was a population-based, cross-sectional study of asthma prevalence in two regions of Peru with disparate degrees of urbanization [18]. The first site was Pampas de San Juan de Miraflores, a peri-urban community in Lima (latitude 12.0°S), the highly urbanized capital of Peru located at sea level. The second site was rural Tumbes (latitude 3°S), also at sea level and located in northern Peru. Average ambient temperature in Lima ranged between 17°C and 30°C year-round, and relative humidity ranged between 55% and 80%. Annual precipitation in Lima was 50 mm per year. Average ambient temperature in Tumbes ranged between 25°C and 33°C, relative humidity ranged between 55% and 80% and precipitation was much higher, with annual rainfall up to 2200 mm per year.
Study design
The study design was described in detail elsewhere [21, 22]. Briefly, a random sample of children aged 13–15 years were selected from community censuses using household level data in both Pampas de San Juan de Miraflores and Tumbes. A total of 1441 children were enrolled over the same time period at both sites. Only one child per household was enrolled. Children and their caregivers were surveyed on sociodemographics and environmental exposures and asked about asthma and allergy symptoms using a previously validated Spanish questionnaire [23]. The questionnaire was administered by trained field workers, who were residents of the respective local communities, and the interview was conducted in the presence of the study participant and his or her parent or legal guardian. Exhaled nitric oxide was measured using a handheld chemiluminescence analyser (NIOXMINO, Aerocrine, Solna, Sweden). Allergy skin testing was performed with the Multi-Test II (Lincoln Diagnostics, Decatur, USA) using 10 common household allergens (cockroach, a mix of 2 dust mites, cat hair, dog epithelium, mouse epithelium and a mix of 4 moulds from ALK-Abello, Round Rock, TX, USA). Serum samples were processed for 25OH vitamin D levels in duplicate using the LIAISON 25OH vitamin D total assay (DiaSorin Inc., Stillwater, MN). Serum specimens were analysed for total serum IgE using an US FDA cleared fluorescent enzyme immunoassay (ImmunoCAP250, Thermo Fisher Scientific, Kalamazoo, USA). The Institutional Review Boards of A.B. PRISMA (Lima, Peru) and Johns Hopkins Bloomberg School of Public Health (Baltimore, USA) approved the conduct of this study. Informed consent was obtained from parents or guardians of study subjects, and assent was obtained from the child participant.
Definitions
We defined allergic rhinitis as having a self-report of sneezing, or a runny, or blocked nose without having a cold or the flu in the past 12 months or a physician diagnosis of allergic rhinitis; eczema as a self-report of an intermittent itchy rash in the past 12 months; asthma as a history of wheezing or use of asthma medications in the past 12 months [22]; allergic sensitization as a positive skin response to one or more of the common household aeroallergens as previously described [22, 24]; being stunted if height was below the age- and sex-specific lower limit of normal of an international reference [25]; and being overweight if body mass index was above the age- and sex-specific international cut-off [26]. We categorized exhaled nitric oxide using cut-off values ≥20 ppb [27].
Biostatistical methods
The primary objectives were to determine the prevalence of allergic rhinitis and identify risk factors for this condition. The following a priori risk factors were considered: history of parental rhinitis, allergic sensitization to common household aeroallergens, exhaled nitric oxide level >20 ppb, total serum IgE levels, serum 25OH vitamin D levels, age, sex, personal history of smoking, second hand smoke exposure, being overweight, being stunted, living in rural (Tumbes) vs. urban setting (Lima), years of maternal education (>6 vs. fewer), household floor material (concrete vs. other), household income (<175 USD/month vs. more) and number of people per household (≥6 vs. fewer). We compared continuous variables between two subgroups with t-tests or Wilcoxon’s rank-sum tests, as appropriate, and compared categorical variables between two subgroups with chi-squared tests or Fisher’s exact tests, as appropriate. All a priori selected factors were included in a multivariable logistic regression with allergic rhinitis as the outcome. We then calculated population attributable risk (PAR) of selected risk factors for allergic rhinitis with the following formula: [28], where p is the prevalence of the risk factor in our study sample, and OR is the adjusted odds ratio (as an approximation to the relative risk) obtained from multivariable logistic regression. We used R (www.r-project.org) for statistical analyses.
Results
Characteristics of the study population
One thousand four hundred and forty-one children were enrolled into the study: 725 (50%) in Lima and 716 (50%) in Tumbes. A total of 1271 (88%) underwent spirometry, 1244 (86%) had skin allergy testing, 1199 (83%) had eNO testing, and 1134 (79%) had a blood sample available for measurement of total serum IgE and 25OH vitamin D levels. Mean age at enrollment was 14.9 years (SD = 0.9), 51% were boys, average height was 153.9 cm (SD = 5.7) in boys and 162.5 cm (SD = 7.9) in girls and average BMI was 21.7 kg/m2 (SD=3.4) in boys and 20.7 kg/m2 (SD = 3.1) in girls. The prevalences of asthma and allergic sensitization to common household aeroallergens were 12% vs. 3% and 56% vs. 38% in Lima vs. Tumbes, respectively.
Prevalence and severity of allergic rhinitis
A total of 261 children had allergic rhinitis for an overall prevalence of 18% (95% CI 16–20%). When stratified by site, the prevalence of allergic rhinitis was 23% (166/725) Lima and 13% (95/716) in Tumbes (P < 0.001). Among those with allergic rhinitis, 15% (39/261) reported symptoms every month of the year, 44% (116/261) of participants reported that their rhinitis symptoms negatively affected their daily activities and 7% (18/261) reported that their activities were severely affected. Children in Lima were more likely to report symptoms every month of the year than those in Tumbes (22% vs. 2%; P < 0.001). We found a strong association between asthma and allergic rhinitis in our study sample: 53% (56/106) of children with asthma had comorbid allergic rhinitis compared with only 15% (205/1335) in those without asthma (P < 0.001). We also found a similar association with eczema: 42% (39/93) of children with eczema also had comorbid allergic rhinitis vs. 17% (222/1348) in those without eczema (P < 0.001).
Risk factors for allergic rhinitis
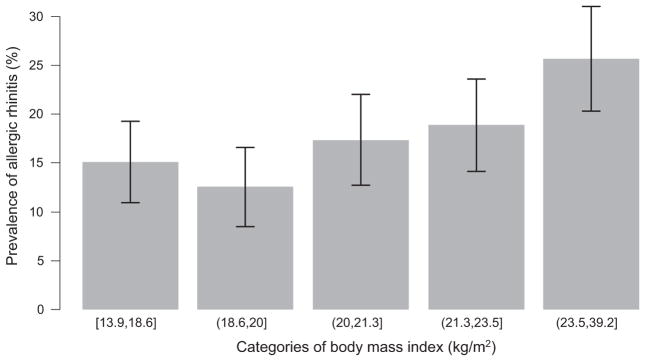
We compared the distribution of selected risk factors for allergic rhinitis in Table 1. There were no differences in age, sex, socioeconomic status, exposure to cigarette smoke and 25OH vitamin D levels between children with and without allergic rhinitis. Risk factors that were related to allergic rhinitis in single variable analysis were as follows: being overweight, allergic sensitization to common household aeroallergens, having parents with allergic rhinitis, having a higher exhaled nitric oxide (≥20 ppb cutoff) and total serum IgE levels (in the upper 95th percentile), and living in urban setting (Lima vs. Tumbes). The prevalence of allergic rhinitis increased with categories of body mass index (Fig. 1). Children with a parental history of allergic rhinitis had three times the odds of having the disease than those born to healthy parents (adjusted OR = 3.1, 95% CI 2.0–4.9) when adjusting for other factors. Most of the important variables in single variable analyses remained important in multivariable regression (Table 2). An exception was living in an urban environment, which became non-significant after controlling for other subject-specific risk factors. There was a gradient for higher odds of allergic rhinitis in children with two parents who also had allergic rhinitis vs. none when compared against the odds of at least one parent vs. none (Table 2). PARs for allergic rhinitis due to selected risk factors, in order of relative importance, were as follows: having an exhaled nitric oxide ≥20 ppb (25%), allergic sensitization to common household aeroallergens (22%), having a parental history of rhinitis (22%), being overweight (10%) and a total serum IgE ≥1778 kU/L (7%).
Table 1.
Participant characteristics stratified by allergic rhinitis status
| Variable | No allergic rhinitis (n = 1180) | Allergic rhinitis (n = 261) | P |
|---|---|---|---|
| Demographics | |||
| Male, % (n) | 47% (560) | 51% (134) | 0.26 |
| Age, mean (SD) | 14.9 (0.9) | 14.9 (0.9) | 0.29 |
| % Living in Tumbes (n) | 53% (625) | 35% (91) | <0.001 |
| BMI in kg/m2, mean (SD) | 21.0 (3.2) | 21.9 (3.6) | <0.001 |
| HAZ < –2 Z-scores, % (n) | 15 (157) | 14 (32) | 0.77 |
| Socioeconomic status, % (n) | |||
| Monthly income <175 USD) | 45% (531) | 39% (102) | 0.08 |
| Maternal education >6 years | 73% (846) | 75% (196) | 0.47 |
| 6 or more household members | 39% (456) | 43% (111) | 0.25 |
| Concrete floor | 47% (561) | 42% (110) | 0.11 |
| Allergic conditions | |||
| Atopy, % (n) | 44% (450) | 61% (134) | <0.001 |
| Exhaled nitric oxide in ppb, mean (SD) | 17.0 (16.9) | 31.4 (35.9) | <0.001 |
| Total serum IgE in kU/L, geometric mean (geometric SD) | 211.1 (3.6) | 290.6 (4.2) | <0.001 |
| 25OH vitamin D in ng/dL, mean (SD) | 25.7 (8.9) | 24.5 (8.7) | 0.07 |
| Current asthma, % (n) | 4% (50) | 22% (56) | <0.001 |
| Eczema in last 12 months, % (n) | 5% (54) | 15% (39) | <0.001 |
| History of parental rhinitis, % (n) | |||
| None | 90% (1061) | 72% (189) | <0.001 |
| 1 parent | 9% (106) | 24% (63) | |
| Both parents | 1% (13) | 3% (9) | |
| Exposures | |||
| Personal history of smoking, % (n) | 4% (43) | 3% (15) | 0.13 |
| Second hand smoke, % (n) | 20% (235) | 16% (43) | 0.20 |
Fig. 1.
Prevalence of allergic rhinitis (%) by categories of body mass index (kg/m2).
Table 2.
Single and multivariable analysis of factors associated with allergic rhinitis
| Single variable OR (95% CI) | P | Multivariable OR (95% CI) | P | |
|---|---|---|---|---|
| History of parental rhinitis | ||||
| One parent | 3.34 (2.36–4.73) | <0.001 | 2.97 (1.86–4.73) | <0.001 |
| Both parents | 3.89 (1.64–1.22) | 0.002 | 4.44 (1.48–13.69) | 0.01 |
| Exhaled nitric oxide > 20 ppb | 2.76 (2.04–3.74) | <0.001 | 1.86 (1.27–2.72) | <0.001 |
| Atopy | 2.01 (1.49–2.71) | <0.001 | 1.61 (1.11–2.33) | 0.01 |
| Being overweight | 1.69 (1.23–2.32) | <0.001 | 1.53 (1.01–2.31) | 0.04 |
| Total serum IgE level at the 95th percentile | 3.26 (1.88–5.65) | <0.001 | 2.44 (1.23–4.84) | 0.01 |
| Living in Tumbes | 0.48 (0.36–0.63) | <0.001 | 1.05 (0.65–1.71) | 0.85 |
| Personal history of smoking | 1.60 (0.87–2.93) | 0.13 | 1.11 (0.47–2.60) | 0.81 |
| Second hand smoke exposure | 0.79 (0.56–1.13) | 0.20 | 0.71 (0.44–1.15) | 0.17 |
| 25OH vitamin D levels (ng/mL) | 0.98 (0.97–1.00) | 0.07 | 0.99 (0.97–1.02) | 0.58 |
| Height for age < –2 Z-scores | 0.94 (0.62–1.42) | 0.77 | 0.87 (0.51–1.47) | 0.61 |
| Concrete floor | 0.80 (0.61–1.05) | 0.11 | 0.87 (0.61–1.25) | 0.46 |
| Maternal education >6 years | 1.12 (0.82–1.53) | 0.47 | 0.90 (0.60–1.36) | 0.62 |
| Sex (boys are reference) | 1.17 (0.89–1.53) | 0.26 | 1.02 (0.70–1.48) | 0.91 |
| Age | 1.09 (0.93–1.27) | 0.29 | 1.03 (0.84–1.26) | 0.79 |
| 6 or more household members | 1.17 (0.90–1.54) | 0.25 | 0.96 (0.67–1.39) | 0.85 |
| Income < 175 USD/month | 0.78 (0.60–1.03) | 0.08 | 1.04 (0.70–1.53) | 0.86 |
Discussion
Allergic rhinitis was prevalent in both settings and important subject-specific risk factors include parental history of rhinitis, allergic sensitization to common household aeroallergens, having a high total serum IgE as a biomarker for allergic sensitization, exhaled nitric oxide as a proxy for airways inflammation and being overweight. There was also an important association between allergic rhinitis and other allergic diseases such as asthma and eczema. A higher prevalence of allergic rhinitis was seen in the urbanized setting of Lima vs. rural Tumbes; however, this difference became non-significant when we accounted for the risk factors described above. Of these variables, those with the highest percentages of population attributable risk for allergic rhinitis were allergic sensitization to common household aeroallergens, presence or airway inflammation (elevated exhaled nitric oxide), and parental rhinitis.
The importance of parental rhinitis as a risk factor for allergic rhinitis in their offspring is well understood [13, 14], and the results of our study further emphasize this relationship. Children with at least one parent who suffered from allergic rhinitis were more likely to have the disease themselves. The odds were even greater in children for whom both parents had allergic rhinitis, even though this was a less common scenario. Our data support the theory of a genetic component in the aetiology of allergic rhinitis in addition to environmental factors. Furthermore, our study also reinforced the association of allergic rhinitis with other allergic diseases. Children suffering from asthma had significantly higher rates of allergic rhinitis, as well as an increased likelihood of developing the disease was seen amongst atopic participants.
As the prevalence of being overweight has increased children worldwide, investigators are paying closer attention to the relationship between weight status and allergic disease. A positive association has been shown between central obesity and allergic rhinitis, and waist circumference is an isolated risk factor for asthma independent of body mass index [17, 29]. In addition, higher prevalence of rhinoconjunctivitis and asthma has been demonstrated in overweight children [30]. Our results also support these findings, as we saw a significant increase in the likelihood of having allergic rhinitis with greater body mass index. Moreover, children who were classified as overweight had a higher prevalence of the disease.
While many studies have focused on the association of exhaled nitric oxide with asthma, the relationship between airway inflammation and allergic rhinitis has not been well studied [31–33]. Increased oxidative stress has been shown in the airways of children with allergic rhinitis, as demonstrated by higher nasal and oral levels of malondialdehyde, and lower levels of glutathione [34]. Higher exhaled nitric oxide levels were also seen in children with allergic rhinitis compared to their healthy counterparts [35]. However, others have failed to elucidate any relationship between exhaled nitric oxide and allergic rhinitis [36]. Our study found a strong association between airway inflammation and allergic rhinitis, and children with higher exhaled nitric oxide levels (≥20 ppb) had twice the odds of having the condition.
The relationship between vitamin D and allergic rhinitis is conflicting as that with other allergic diseases such as asthma [19, 37, 38]. A study conducted in Iran found an increased prevalence of allergic rhinitis in patients with vitamin D deficiency [39]. In contrast, another study using NHANES III data found higher prevalence of allergic rhinitis amongst those with increased serum levels of vitamin D [40]; however, this study is admittedly open to potential confounding. Others still found no statistical relationship between allergic rhinitis and vitamin D levels [41, 42]. A recent study of Norwegian adults found that lower levels of vitamin D was associated with a higher prevalence of allergic rhinitis in men, whereas it was associated with a lower prevalence of allergic rhinitis in women [43] The results of our study in children are consistent with other studies that show no relationship between serum 25OH vitamin D levels and allergic rhinitis [44]. While vitamin D deficiency continues to be a problem in low- to middle-income countries, its association with allergic rhinitis is not supported by our findings.
An important result of our study is that while there was a difference in allergic rhinitis prevalence between children living in an urban vs. rural setting, this difference was no longer significant after controlling for subject- specific risk factors such as parental history of rhinitis and atopy. This finding is consistent with global data from the ISAAC study, which demonstrated a large variation in allergic rhinitis prevalence around the world, with no apparent distribution between developing countries and industrialized nations [45]. This pattern is different than what is traditionally seen with allergic disease, particularly asthma, where the prevalence is greater in high-income countries [45, 46]. Some have hypothesized that this variation may be due to language differences around the globe and the lack of proper vocabulary for medical terms in some versions of the ISAAC questionnaire [47, 48]. Consistent with ISAAC findings, we found higher prevalence of asthma seen in Lima vs. Tumbes even after controlling for multiple risk factors [19]; however, the difference in allergic rhinitis between sites was explained by markers of other allergic conditions. We believe that this pattern instead is attributable to a higher prevalence of risk factors present in urbanized areas, such as being overweight, atopy and parental rhinitis.
One limitation to our study is that it is a cross-sectional study, which limits our ability to determine causation. However, our study is population-based, which is a major strength and allows for the estimation of population attributable risk of multiple factors. Another limitation is the lack of data on parent history of other allergic disease such as asthma or eczema. In addition, symptoms of allergic rhinitis were self-reported in the questionnaire; therefore, we could not conduct a sensitivity analysis of these findings. Finally, we did not use a validated scale to assess the quality of life related to allergic rhinitis and were not able to more adequately differentiate persistent from intermittent symptoms.
In summary, the prevalence of allergic rhinitis in two disparate regions of Peru demonstrated a distribution different from what is commonly seen in asthma, with similar rates to those seen in high-income countries. This pattern may be explained by subject-specific risk factors, such as being overweight, atopy and parental rhinitis. In addition, our data found a strong association between allergic rhinitis and elevated exhaled nitric oxide. Thus, the use of exhaled nitric oxide as biomarker for the assessment and management of allergic rhinitis needs to be better characterized.
Acknowledgments
Sources of funding: This study was supported by the Johns Hopkins Center for Global Health and the Fogarty International Center (R24TW007988). Lauren Baumann was supported by a pre-doctoral NIH T35 Training Grant (T35AI065385). William Checkley was also supported by a K99/R00 Pathway to Independence Award (R00HL096955). Nadia Hansel and William Checkley were further supported by a R01 grant from the National Institutes of Environmental Health Sciences (R01ES018845). Colin Robinson was a Fogarty International Clinical Research Scholar during the time of this work and was further supported by Tufts University School of Medicine. John Lima was supported by a grant from the Nemours Biomedical Research. Karina Romero were further supported by a Fogarty International Center Grant (5R25TW009340) from the UJMT Consortium and the National Institutes of Health Study sponsors played no role in the study design, data collection, data analysis, data interpretation or the decision to submit the article for publication.
Contributions: All authors were involved in the study design and writing of the manuscript. Lauren Baumann, Karina Romero and William Checkley conducted statistical analysis and interpretation and drafted the manuscript. Colin Robinson and Lauren Baumann share responsibilities in study design and conduct, in data management and participated in the writing of manuscript. Karina Romero was responsible for the study conduct and data management and also participated in the writing of manuscript. Nadia Hansel, Robert Gilman, Robert Wise and Robert Hamilton contributed to study design, interpretation of results and also participated in writing of this manuscript. John Lima was responsible for vitamin D analysis and laboratory quality control and also participated in writing of the manuscript. William Checkley had ultimate oversight over study design and conduct, analysis and writing of this manuscript.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. 2011;378:2112–22. doi: 10.1016/S0140-6736(11)60130-X. [DOI] [PubMed] [Google Scholar]
- 2.Pawankar R, Bunnag C, Khaltaev N, Bousquet J. Allergic rhinitis and its impact on asthma in Asia Pacific and the ARIA update 2008. World Allergy Organ J. 2012;5(Suppl 3):S212–7. doi: 10.1097/WOX.0b013e318201d831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canonica GW, Bousquet J, Mullol J, Scadding GK, Virchow JC. A survey of the burden of allergic rhinitis in Europe. Allergy. 2007;62(Suppl 85):17–25. doi: 10.1111/j.1398-9995.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 4.Valovirta E, Pawankar R. Survey on the impact of comorbid allergic rhinitis in patients with asthma. BMC Pulm Med. 2006;6(Suppl 1):S3. doi: 10.1186/1471-2466-6-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong S, Son DK, Lim WR, et al. The prevalence of atopic dermatitis, asthma, and allergic rhinitis and the comorbidity of allergic diseases in children. Environ Health Toxicol. 2012;27:e2012006. doi: 10.5620/eht.2012.27.e2012006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang WC, Chen YM, Tan HK, et al. Allergic rhinitis and non-allergic rhinitis in children in the tropics: prevalence and risk associations. Pediatr Pulmonol. 2012;47:1026–33. doi: 10.1002/ppul.22554. [DOI] [PubMed] [Google Scholar]
- 7.Wright AL, Holberg CJ, Martinez FD, Halonen M, Morgan W, Taussig LM. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatr. 1994;94:895–901. [PubMed] [Google Scholar]
- 8.Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225–32. [PubMed] [Google Scholar]
- 9.Sultesz M, Katona G, Hirschberg A, Galffy G. Prevalence and risk factors for allergic rhinitis in primary schoolchildren in Budapest. Int J Pediatr Otorhinolaryngol. 2010;74:503–9. doi: 10.1016/j.ijporl.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Zhang YM, Zhang J, Liu SL, et al. Prevalence and associated risk factors of allergic rhinitis in preschool children in Beijing. Laryngoscope. 2013;123:28–35. doi: 10.1002/lary.23573. [DOI] [PubMed] [Google Scholar]
- 11.Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(1 Suppl):S2–8. doi: 10.1067/mai.2001.115569. [DOI] [PubMed] [Google Scholar]
- 12.Bedolla-Barajas M, Morales-Romero J, Robles-Figueroa M, Fregoso-Fregoso M. Asthma in late adolescents of Western Mexico: prevalence and associated factors. Arch Bronconeumol. 2013;49:47–53. doi: 10.1016/j.arbres.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Wang QP, Wu KM, Li ZQ, et al. Association between maternal allergic rhinitis and asthma on the prevalence of atopic disease in offspring. Int Arch Allergy Immunol. 2012;157:379–86. doi: 10.1159/000328789. [DOI] [PubMed] [Google Scholar]
- 14.Westman M, Kull I, Lind T, et al. The link between parental allergy and offspring allergic and nonallergic rhinitis. Allergy. 2013;68:1571–8. doi: 10.1111/all.12267. [DOI] [PubMed] [Google Scholar]
- 15.Dold S, Wjst M, von Mutius E, Reitmeir P, Stiepel E. Genetic risk for asthma, allergic rhinitis, and atopic dermatitis. Arch Dis Child. 1992;67:1018–22. doi: 10.1136/adc.67.8.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamay Z, Akcay A, Ergin A, Guler N. Effects of dietary habits and risk factors on allergic rhinitis prevalence among Turkish adolescents. Int J Pediatr Otorhinolaryngol. 2013;77:1416–23. doi: 10.1016/j.ijporl.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Musaad SM, Patterson T, Ericksen M, et al. Comparison of anthropometric measures of obesity in childhood allergic asthma: central obesity is most relevant. J Allergy Clin Immunol. 2009;123:1321–7. e12. doi: 10.1016/j.jaci.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Wjst M, Dold S. Genes, factor X, and allergens: what causes allergic diseases? Allergy. 1999;54:757–9. doi: 10.1034/j.1398-9995.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 21.Robinson CL, Baumann LM, Gilman RH, et al. The Peru Urban versus Rural Asthma (PURA) Study: methods and baseline quality control data from a cross-sectional investigation into the prevalence, severity, genetics, immunology and environmental factors affecting asthma in adolescence in Peru. BMJ Open. 2012;2:e000421. doi: 10.1136/bmjopen-2011-000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson CL, Baumann LM, Romero K, et al. Effect of urbanisation on asthma, allergy and airways inflammation in a developing country setting. Thorax. 2011;66:1051–7. doi: 10.1136/thx.2011.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mata Fernandez C, Fernandez-Benitez M, Perez Miranda M, Guillen Grima F. Validation of the Spanish version of the Phase III ISAAC questionnaire on asthma. J Investig Allergol Clin Immunol. 2005;15:201–10. [PubMed] [Google Scholar]
- 24.Checkley W, Robinson CL, Baumann LM, et al. Effect of urbanization on the relation of total serum immunoglobulin E to asthma. Eur Respir J. 2013;41:1074–81. doi: 10.1183/09031936.00025512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 26.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin ML. The occurrence of lung cancer in man. Acta - Unio Internationalis Contra Cancrum. 1953;9:531–41. [PubMed] [Google Scholar]
- 29.Leone N, Courbon D, Berr C, et al. Abdominal obesity and late-onset asthma: cross-sectional and longitudinal results: the 3C study. Obesity. 2012;20:628–35. doi: 10.1038/oby.2011.308. [DOI] [PubMed] [Google Scholar]
- 30.Cibella F, Cuttitta G, La Grutta S, Melis MR, Bucchieri S, Viegi G. A cross-sectional study assessing the relationship between BMI, asthma, atopy, and eNO among schoolchildren. Ann Allergy Asthma Immunol. 2011;107:330–6. doi: 10.1016/j.anai.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Barnes PJ, Dweik RA, Gelb AF, et al. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2010;138:682–92. doi: 10.1378/chest.09-2090. [DOI] [PubMed] [Google Scholar]
- 32.Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 33.Brussee JE, Smit HA, Kerkhof M, et al. Exhaled nitric oxide in 4-year-old children: relationship with asthma and atopy. Eur Respir J. 2005;25:455–61. doi: 10.1183/09031936.05.00079604. [DOI] [PubMed] [Google Scholar]
- 34.Celik M, Tuncer A, Soyer OU, Sackesen C, Tanju Besler H, Kalayci O. Oxidative stress in the airways of children with asthma and allergic rhinitis. Pediatr Allergy Immunol. 2012;23:556–61. doi: 10.1111/j.1399-3038.2012.01294.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee KJ, Cho SH, Lee SH, et al. Nasal and exhaled nitric oxide in allergic rhinitis. Clin Exp Otorhinolaryngol. 2012;5:228–33. doi: 10.3342/ceo.2012.5.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Bot CM, Moed H, Bindels PJ, et al. Exhaled nitric oxide measures allergy not symptoms in children with allergic rhinitis in primary care: a prospective cross-sectional and longitudinal cohort study. Prim Care Respir J. 2013;22:44–50. doi: 10.4104/pcrj.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul G, Brehm JM, Alcorn JF, Holguin F, Aujla SJ, Celedon JC. Vitamin D and asthma. Am J Respir Crit Care. 2012;185:124–32. doi: 10.1164/rccm.201108-1502CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown SD, Calvert HH, Fitzpatrick AM. Vitamin D and asthma. Dermatoendocrinol. 2012;4:137–45. doi: 10.4161/derm.20434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arshi S, Ghalehbaghi B, Kamrava SK, Aminlou M. Vitamin D serum levels in allergic rhinitis: any difference from normal population? Asia Pac Allergy. 2012;2:45–8. doi: 10.5415/apallergy.2012.2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wjst M, Hypponen E. Vitamin D serum levels and allergic rhinitis. Allergy. 2007;62:1085–6. doi: 10.1111/j.1398-9995.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 41.Ehlayel MS, Bener A, Sabbah A. Is high prevalence of vitamin D deficiency evidence for asthma and allergy risks? Eur Ann Allergy Clin Immunol. 2011;43:81–8. [PubMed] [Google Scholar]
- 42.Cheng HM, Kim S, Park GH, et al. Low vitamin D levels are associated with atopic dermatitis, but not allergic rhinitis, asthma, or IgE sensitization, in the adult Korean population. J Allergy Clin Immunol. 2014;133:1048–55. doi: 10.1016/j.jaci.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 43.Mai XM, Chen Y, Camargo CA, Jr, Langhammer A. Serum 25-hydroxyvitamin D levels and self-reported allergic rhinitis in Norwegian adults - The HUNT Study. Allergy. 2014;69:488–93. doi: 10.1111/all.12365. [DOI] [PubMed] [Google Scholar]
- 44.Goleva E, Searing DA, Jackson LP, Richers BN, Leung DY. Steroid requirements and immune associations with vitamin D are stronger in children than adults with asthma. J Allergy Clin Immunol. 2012;129:1243–51. doi: 10.1016/j.jaci.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beasley R, Clayton T, Crane J, et al. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6–7 years: analysis from Phase Three of the ISAAC programme. Lancet. 2008;372:1039–48. doi: 10.1016/S0140-6736(08)61445-2. [DOI] [PubMed] [Google Scholar]
- 46.Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma P. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–78. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 47.Strachan D, Sibbald B, Weiland S, et al. Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC) Pediatr Allergy Immunol. 1997;8:161–76. doi: 10.1111/j.1399-3038.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 48.Bjorksten B, Clayton T, Ellwood P, Stewart A, Strachan D, Group IPIS. Worldwide time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008;19:110–24. doi: 10.1111/j.1399-3038.2007.00601.x. [DOI] [PubMed] [Google Scholar]