Abstract
The transcriptional activity of the androgen receptor (AR) modulated by positive or negative regulators plays a critical role in controlling the growth and survival of prostate cancer cells. Although numerous positive regulators have been identified, negative regulators of AR are less well understood. We report here that Daxx functions as a negative AR coregulator through direct protein-protein interactions. Overexpression of Daxx suppressed AR-mediated promoter activity in COS-1 and LNCaP cells and AR-mediated prostate-specific antigen expression in LNCaP cells. Conversely, downregulation of endogenous Daxx expression by RNA interference enhances androgen-induced prostate-specific antigen expression in LNCaP cells. In vitro and in vivo interaction studies revealed that Daxx binds to both the amino-terminal and the DNA-binding domain of the AR. Daxx proteins interfere with the AR DNA-binding activity both in vitro and in vivo. Moreover, sumoylation of AR at its amino-terminal domain is involved in Daxx interaction and trans-repression. Together, these findings not only provide a novel role of Daxx in controlling AR transactivation activity but also uncover the mechanism underlying sumoylation-dependent transcriptional repression of the AR.
Prostate cancer (PCa) is the most frequently diagnosed malignancy and the second leading cause of cancer deaths in men in the United States and many other western countries. Several advances have been made recently to elucidate the mechanism by which androgens regulate PCa growth through the androgen receptor (AR) (14, 16, 28). The AR is found to be expressed in the majority of PCa samples, both at the primary and metastatic sites and including those with androgen-refractory status. Aberrant regulation of AR activity by positive and negative coregulators is likely to play key roles in PCa progression. A number of studies revealed that AR-interacting proteins are involved in positively or negatively regulating AR-mediated gene activation (20, 22, 27). For instance, several coactivators have been identified that bind to the AR and enhance AR transcriptional activation, including ARA24, ARA54, ARA55, ARA70, ARA160, SNURF, p/CIP/ACTR/AIB1, Rb, RIP140, SRC-1, FHL2, TIF-2/GRIP1, Tip60, BRCA1, cyclin E, PGC-1, TFIIH/CAK, PIAS1, ARIP3, Ubc9, and β-catenin (for a review, see reference 22). According to the studies of the other nuclear receptors, some of these factors (CBP/p300, PCAF/GCN5, p160 family, and TRAP/DRIP) appear to be organized in multiprotein complexes to facilitate the access of nuclear receptors and the RNA polymerase II core machinery to their target DNA by chromatin remodeling and histone modification (58).
In addition to coactivators, there are also corepressors that block AR-mediated gene activation. For instance, calreticulin has been reported to inhibit binding of the AR to androgen response element via interaction with the AR DNA-binding domain (8). HBO1, a protein initially identified as a protein that associates with the origin recognition complex, has been found to negatively regulate transcription from AR-dependent promoters (52). AES (named for amino-terminal enhancer of split), as a member of the highly conserved Groucho/TLE family of corepressors, represses AR-driven transcription by targeting the basal transcriptional machinery (possibly TFII E) (61). More recently, p53 and SMRT have been reported to repress androgen-induced gene expression through disrupting AR amino-terminal-to-carboxy-terminal interaction (40, 53).
Besides the protein-protein interactions, recent reports indicated that the AR could be regulated by SUMO-1 (named for small ubiquitin-like modifier protein) modification (4, 45). The SUMO-conjugation sites were mapped at Lys 386 and 520 at AR NH2-terminal domain. Mutation of both sumoylation sites results in enhancing AR transcriptional potential (4, 45), indicating that SUMO-1 modification plays a negatively regulatory role in modulating AR activity. Recently, SUMO modification has been reported to modulate the molecular interaction properties and/or the subcompartmentalization of its targets (17, 50, 51). It has been reported that increasing sumoylation of AR by SUMO E3 ligases PIAS1 and PIASxα does not change the subcellular localization of AR (36). In addition, Callewaert et al. showed that mutation of AR sumoylation sites does not alter its DNA-binding activity (4). The underlying mechanism as to how the sumoylation negatively modulates AR transcriptional activity is currently unclear.
Daxx, initially identified as a cytoplasmic signaling molecule linking Fas receptor to Jun N-terminal kinase in Fas-mediated apoptosis (60), has recently been demonstrated to have a nuclear role in the regulation of gene expression. Daxx can inhibit the transcriptional potential of several transcription factors, such as ETS1 (39), Pax3 (24, 37), glucocorticoid receptor (41, 42), p53 family proteins (33), and mineralocorticoid receptor (43) through direct protein-protein interactions and suppress several transcription factor-responsive reporter activities, including reporters of CRE, E2F1, Sp1, and NF-κB (12). It thus seems that Daxx can function as a corepressor or coactivator. Indeed, Emelyanov et al. reported that Daxx acting as a transcriptional coactivator or corepressor of Pax5 protein depends on the cellular contexts (13). More recently, Daxx was shown to interact with heat shock factor 1 and enhance its transcriptional activity (3).
In the present study, we show that Daxx is involved in the regulation of AR transcriptional activity in PCa cells. Overexpression of Daxx specifically represses AR-mediated transcription both in COS-1 and LNCaP cells. In contrast, depletion of Daxx protein level results in increment of prostate-specific antigen expression in LNCaP cells in response to androgen. This regulation is mediated by the direct interaction of Daxx with both the AR N-terminal and DNA-binding domains. Daxx is able to inhibit the DNA-binding activity of AR through interaction with AR DNA-binding domain. In addition, Daxx is capable of binding to the AR-N-terminal domain through a sumoylation-dependent manner. Sumoylation of AR-N-terminal domain enhances its association with Daxx. Accordingly, mutation of SUMO-conjugated sites in AR resulted in a loss of Daxx interaction and an increase of AR transcriptional activity. Together, our results provide an important role of Daxx on androgen-mediated gene regulation in PCa cells.
MATERIALS AND METHODS
Plasmid construction.
Constructs expressing LexA-Daxx, GST-Daxx, and HA-Daxx have been described in our recent studies (41). The constructs pSG5-hAR and pMMTV-Luc were kindly provided by Chawnshang Chang. For yeast and mammalian expression constructs of AR wild-type and domain deletion mutants, the corresponding cDNA fragments generated by PCR were subcloned into the prey vector pACT2 (Clontech) for Gal4 activation domain fusion and pCMV-Tag3 vector (Stratagene) for expressing Myc-tagged AR proteins in mammalian cells. pCMV-Flag-ARIP3 was constructed by subcloning a PCR fragment of full-length ARIP3 from human testis cDNA library (Clontech) into pCMV-Tag2 expression vector. pGal4AD-ARFL-ΔSUMO converting arginine-to-lysine mutation at amino acid positions 386 and 520 was generated by using a QuikChange site-directed mutagenesis kit according to the manufacturer's instructions (Stratagene). The AR amino-terminal fragment containing point mutations K386R and K520R was introduced into the pACT2 and pCMX Gal4 vectors to create pGal4AD-ARN-ΔSUMO and pCMX Gal4-ARN-ΔSUMO, respectively. pSUPER-Daxx was engineered by inserting an oligonucleotide corresponding to Daxx sequence 46GAT GAA GCA GCT GCT CAG C64 into the pSUPER vector (a generous gift from Reuven Agami). All constructs were verified by DNA sequencing.
Two-hybrid assay and β-Gal assay.
Yeast two-hybrid assays were performed as described previously (41). Briefly, L40 yeast strain was first transformed with individual bait, and this was followed by the prey construct transformation. Yeast transformants were selected on medium lacking histidine, leucine, and tryptophan for 3 days. His+ colonies were further analyzed for β-galactosidase (β-Gal) activity. Quantitative β-Gal assays were performed with yeasts containing pairs of bait and prey plasmids as indicated. The β-Gal activities were determined from three separate liquid yeast cultures according to the instructions of the Galacto-light Plus kit (Tropix, Inc., Bedford, Mass.). For ligand treatment, the culture was incubated in the presence of 10 nM R1881 or solvent as a control for 24 h before we measured the β-Gal activity. The relative β-Gal activity was determined after being normalized according to cell density (A600).
Cell culture, transient-transfection, and reporter gene assays.
All mammalian cell lines were obtained from the American Type Culture Collection. COS-1 cells were maintained in Dulbecco minimal essential medium supplemented with 10% fetal bovine serum (FBS) and antibiotics. LNCaP cells were maintained in RPMI medium supplemented with 10% FBS and antibiotics. Cells were seeded into 10-cm plates the day before transfection. Transient transfections were carried out by using a Lipofectamine transfection kit (Invitrogen). After 36 h, cells were harvested for coimmunoprecipitation assays and Western blot analysis. For the reporter gene assay, 2 × 105 to 3 × 105 cells were seeded on six-well plates for transfection. At 4 h prior to transfection, cells received fresh medium with 10% dextran-coated charcoal-stripped FBS. DNA constructs were transfected, including 50 ng of β-Gal expression vector as an indicator for the normalization of transfection efficiency. The total amount of plasmid transfected per well was kept constant by adding empty pcDNA3 vector as needed. After transfection, cells were cultured in the medium containing charcoal-stripped 2% (vol/vol) FBS with or without treatment with 10 nM R1881. Cell lysates were harvested 24 h later and assayed for relative activity (firefly luciferase for the reporter and β-Gal activity for the indicator) as described by the manufacturer (Packard, Meriden, Conn.). For stable Daxx transfectant in LNCaP cells, the plasmid construct pcDNA3-HA-Daxx was transfected into LNCaP cells and selected and maintained in 500 μg of G418 (Invitrogen)/ml. Individual colonies of stable clones were isolated for analyses of prostate-specific antigen (PSA) expression upon ligand (see below). For the pSUPER-Daxx experiments, 5 × 106 LNCaP cells were transfected with the pSUPER-Daxx construct by electroporation (Gene Pulser II; Bio-Rad). Approximately 80% of the LNCaP cells were transfected under this condition. After 48 h, cells were treated with or without 10 nM R1881 for further 48 h, and this was followed by PSA expression analyses.
Quantification of PSA expression level in Daxx stable clones and pSUPER-Daxx-transfected LNCaP cells.
Total RNA was extracted from Daxx stable or pSUPER-Daxx-transfected LNCaP cells by using TRIzol reagent (Invitrogen) and then reverse transcribed by using the ThermoScript RT-PCR system (Invitrogen). Quantitative PCR were performed on an Applied Biosystems Prism 7700 sequence detector by using PSA primers (forward primer, 5′-AGACACTCACAGCAAGGATGGA-3′; reverse primer, 5′-CTCCTTGGCTCACAGCCTTCT-3′) and SYBR Green dye (Applied Biosystems) as detailed in the manufacturer's guidelines. An aliquot of each sample was analyzed by quantitative PCR for β-actin to normalize for inefficiencies in cDNA synthesis and RNA input amounts. For each sample, the average threshold (Ct) value was resulted from quadruplicate assays, and the ΔCt value was determined by subtracting the average β-actin Ct value from the average PSA Ct value. Three independent experiments were performed for measuring PSA levels of Daxx stable clones and pSUPER-Daxx-transfected LNCaP cells.
Immunoprecipitation and Western blotting assays.
To test associations in mammalian cells, various AR deletion mutants or wild-type AR, along the with hemagglutinin (HA)-tagged Daxx expression construct, was transfected into COS-1 cells by the lipofection method. At 48 h after cotransfection, cells were solubilized in 1 ml of modified radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.8], 150 mM NaCl, 5 mM EDTA, 0.5% Triton X-100, 0.5% Nonidet P-40, 0.1% sodium deoxycholate) and protease inhibitor mixture (Complete; Roche Molecular Biochemicals). Cell lysates were mixed with antiserum against HA (BAbCO, Richmond, Calif.), Daxx (sc-7152; Santa Cruz Biotech), AR (PG-21) (catalog no. 06-680; Upstate Biotechnology) or AR (C-19; sc-815; Santa Cruz Biotech), and the immunocomplexes were collected on protein A-Sepharose beads (Amersham Pharmacia Biotech). Immunoblot analyses of precipitated proteins were performed as described previously (52). Antibodies to Myc-tag and Gal4-DBD were purchased from BAbCO and Santa Cruz Biotech, respectively. For endogenous protein interactions, LNCaP cells were also subjected to the coimmunoprecipitation assay.
GST pull-down assay.
For purification of GST and GST-Daxx fusion proteins, 293T cells were transfected with pDEST26 (Life Technologies) or pDEST26-Daxx. Cells were lysed 48 h later in buffer EM2 containing 50 nM HEPES (pH 7.6), 50 mM NaCl, 1% NP-40, 5 mM EDTA, 10% glycerol, and AEBSF [4-(2-aminoethyl)-benzenesulfonyl fluoride; Sigma] and centrifuged at 17,000 × g for 10 min at 4°C. Supernatants were incubated with glutathione-agarose beads for 1 h at 4°C. The bound proteins were then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) electrophoresis, followed by Western blotting with antibody to glutathione S-transferase (GST) protein. [35S]methionine-labeled AR proteins were made with the TNT reticulocyte lysate system (Promega). 35S-labeled AR proteins were incubated with GST or GST-Daxx agarose beads, which contained equal amounts of protein, in 0.2 ml of binding buffer (10 mM HEPES [pH 7.5], 50 mM NaCl, 0.1% Nonidet P-40, 0.5 mM dithiothreitol, and 0.5 mM EDTA) for 2 h, washed four times, and analyzed by SDS-PAGE and autoradiography.
Immunofluorescence.
LNCaP cells were plated onto collagen-coated (10 μM) coverslips for 1 day, and this was followed by transient transfection of HA-tagged Daxx expression vector. At 24 h after transfection, the cells received fresh stripped 2% FBS and were cultured for an additional 2 h in the presence 10 nM R1881. Cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) and permeabilized with 0.4% Triton X-100. The permeabilized cells were then incubated with anti-HA monoclonal antibody and anti-AR polyclonal antibody for 1 h at room temperature. After this incubation, cells were washed three times for 10 min with PBS at room temperature and then incubated with fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (IgG) and Texas red-conjugated anti-rabbit IgG (Dako) for 1 h at 20°C. Nuclei were visualized by DAPI (4′,6′-diamidino-2-phenylindole) staining (10 μg/ml). The stained cells were observed by a Leica TCS NT confocal microscope.
EMSA.
The GST and GST-ARD fusion proteins (GST fused with the DNA-binding domain [amino acids 528 to 627] of AR) was expressed and purified from Escherichia coli BL21(λDE3) for electrophoretic mobility shift assay (EMSA). The AR response element (ARE) oligonucleotide (5′-CGCGTGCAGAACAGCAAGTGCTAGCA) was synthesized and annealed to its complementary oligonucleotide to form a double strand. EMSA was performed according to the procedures described by Phan et al. (44). Briefly, affinity-purified AR fusion proteins (0.1 μg) were incubated with 10,000 cpm (∼1.5 fmol) labeled ARE probe in 20 μl of DNA-binding buffer containing 20 mM HEPES (pH 7.9), 20% (vol/vol) glycerol, 100 mM KCl, 0.2 mM EDTA, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride. For competition experiments, the proteins were preincubated for 10 min at 4°C with unlabeled ARE or unrelated oligonucleotide (100-fold excess) before a 30-min incubation with the ARE probe. For Daxx in EMSA, GST-Daxx proteins prepared as described above were preincubated with GST or GST-ARD for 20 min, and this was followed by adding ARE probe. For supershift assay, the ARD fusion proteins were preincubated with anti-GST antibody for 1 h before addition of ARE probe. Protein-DNA complexes were resolved by electrophoresis on 5% nondenaturing polyacrylamide gels in 0.5× TBE (0.089 M Tris-borate, 2 mM EDTA [pH 8.0]) at 150 V for 3 h. Autoradiograms prepared from the dried gels were analyzed by densitometry.
In vitro sumoylation and protein interaction assays.
The Myc-tagged ARN proteins subjected to in vitro sumoylation assays were prepared from mammalian cells. Expression construct of Myc-tagged ARN was transiently transfected into COS-1 cells. Cell extracts were harvested 48 h later for immunoprecipitation with anti-Myc antibody and followed by in vitro sumoylation assays. Briefly, the precipitated ARN proteins were divided equally into two portions for sumoylation reactions with or without SUMO-1 proteins. A typical sumoylation reaction was performed in a total volume of 20 μl with 150 ng of SUMO E1 recombinant proteins (LAE Biotechnology), 1 μg of Ubc9, 1 μg of SUMO-1 and Myc-ARN proteins bound to beads in a reaction buffer of 2 mM ATP, 20 mM HEPES (pH 7.5), and 5 mM MgCl2. Reactions were carried out at 37°C for 2 h and then washed extensively with PBS. Half of each sample was examined for sumoylation by SDS-PAGE separation and Western blot analysis with anti-Myc antibody. Another half amount of each sample was further incubated with cell lysates of COS-1 overexpressing HA-Daxx proteins at 4°C for overnight. Beads of samples were collected by centrifugation and washed with PBS, and bound proteins were fractionated by SDS-PAGE, followed by Western blotting with anti-HA antibody.
ChIP analysis.
LNCaP parental cells and Daxx stable clones were plated on 150-mm dishes and grown to 70 to 90% confluence in RPMI 1640 (Gibco-BRL) supplemented with 10% FBS, at which point cells were hormone deprived in phenol-red-free RPMI 1640 supplemented with 5% charcoal-dextran-stripped FBS for 3 days, followed by serum starvation overnight. Cells were treated with 10 nM dihydroxytestosterone (DHT) for 30 min or 2 h before being cross-linked for 8 min with a final concentration of 1% formaldehyde in 10 mM NaCl, 0.1 mM EDTA, and 5 mM HEPES (pH 7.5). Cells were washed with ice-cold PBS, harvested with a cell scraper, and pelleted. Cell pellets (200 μl) were resuspended in 1 ml of ice-cold lysis-sonication buffer (10 mM EDTA, 50 mM Tris-HCl [pH 8], and 0.5% SDS supplemented with Complete protease inhibitor cocktail [Roche] and 0.2 mg of AEBSF/ml) and sonicated by using a Macrotip at power 1.6, with a duty cycle of 33%, for 30 pulses, three times, with 2 min on ice between each cycle. Cell debris was removed by centrifugation, the chromatin solutions were diluted fivefold with ice-cold dilution buffer (150 mM NaCl, 2 mM EDTA, 20 mM Tris-HCl [pH 8], 1% Triton X-100, Complete protease inhibitor cocktail, 0.2 mg of AEBSF/ml), and 200 μl was removed for input control. Chromatin fragments were immunoprecipitated with anti-AR antibody overnight at 4°C. For a 5-ml diluted chromatin solution, 5 μg of α-AR, PG-21 (Upstate Biotechnology, Lake Placid, N.Y.), was used. Immunocomplexes were captured by adding protein A-Sepharose 4B beads (Zymed) and incubating them for 2 h at 4°C. Beads were pelleted and resuspended in TSE (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8]) plus 150 mM NaCl. Beads were washed in succession with TSE plus 150 mM NaCl, TSE plus 500 mM NaCl, buffer III (0.25 M LiCl, 1% Nonidet P-40, 1% sodium deoxycholate, 1 mM EDTA, 10 mM Tris-HCl [pH 8]), and then Tris-EDTA buffer. Complexes were eluted with 1% SDS-0.1 M NaHCO3 and reverse cross-linked at 65°C overnight. DNA fragments were purified by using the QIAquick PCR purification kit (Qiagen) and eluted with 100 μl of TE buffer (pH 8). A total of 10% of the ChIP product or 2% of the input control was used in each PCR. PCR was performed with the following parameters: 1 min at 95°C; 30 cycles of 1 min at 95°C, 1 min at 55°C, and 1 min at 72°C; and then 5 min at 72°C. The primers spanning the ARE region include the forward primer 5′-CATGTTCACATTAGTACACCTTGCC-3′ and the reverse primer 5′-TCTCAGATCCAGGCTTGCTTACTGTC-3′. For the ChIP analyses of Gal4 fusion proteins with the Gal4 fusion-driven reporter, 5 × 106 LNCaP cells were electroporated with 5 μg of TK-MH-Luc and 10 μg of Gal4 or Gal4-fusion proteins and cultured in RPMI 1640 (Gibco-BRL) supplemented with 10% FBS. After 48 h, cells were subjected to ChIP procedure as described above except with anti-Gal4 antibody (Santa Cruz) for chromatin fragment precipitation. The primers spanning the TK promoter containing Gal4 binding sites were the forward primer 5′-AGCGTCTTGTCATTGGCG-3′ and the reverse primer 5′-GTTAAGCGGGTCGCTGCA-3′. In re-chromatin immunoprecipitation (Re-ChIP) experiments, complexes were eluted by incubation for 30 min at 37°C in 25 μl of 10 mM dithiothreitol. The eluted samples were diluted 20 times with Re-ChIP buffer (1% Triton X-100, 2 mM EDTA, 150 mM NaCl, 20 mM Tris-HCl [pH 8]) and subjected again to the ChIP procedure with specific antibodies.
RESULTS
Daxx is an AR-interacting protein.
To examine whether the AR associates with Daxx in mammalian cells, we performed coimmunoprecipitation experiments. COS-1 cells were cotransfected with constructs expressing HA-tagged Daxx and AR proteins. Cell lysates were separately subjected to immunoprecipitation assay with anti-HA or anti-AR antibody. AR protein was detected in the anti-HA immunoprecipitates from cells cotransfected with HA-Daxx but not from cells cotransfected with empty vector (Fig. 1A). This interaction was also confirmed in a reciprocal coimmunoprecipitation assay shown in the middle panel of Fig. 1A. These results provided the first evidence of the interaction between the AR and Daxx proteins. We next investigated whether endogenous AR and Daxx would associate together in LNCaP PCa cells. As shown in Fig. 1B, endogenous AR and Daxx proteins could be reciprocally coimmunoprecipitated in LNCaP cells by anti-AR or anti-Daxx but not by an anti-HA control antibody. Notably, the Daxx-AR interaction in LNCaP cells was not significantly altered by treatment of the synthetic androgen, R1881 (lane 1 versus lane 2). Taken together, our findings suggest that the AR and Daxx can specifically form a complex in cells and that this interaction is hormone independent.
FIG. 1.
Daxx binds to AR both in vivo and in vitro. (A) COS-1 cells were transiently transfected with the indicated combinations of expression constructs encoding the AR and HA-tagged Daxx, respectively. After 48 h of transfection, cell lysates were immunoprecipitated (IP), followed by Western blot analysis with antibodies as indicated (top panels). The bottom panels show the expression levels of the AR and Daxx from the transfected cell lysates. (B) Cell lysates from LNCaP cells treated with or without 10 nM R1881 for 24 h were subjected to immunoprecipitation experiments, followed by Western blot analysis with the indicated antibodies. The bottom panel shows the expression levels of the AR and Daxx from the cell lysates used for coimmunoprecipitation experiments. (C) Yeast cotransformed with bait (LexA fusion protein) and prey (Gal4 AD fusion protein) as indicated were treated with or without 10 nM R1881 and subsequently subjected to quantitative β-Gal assays. The bar graph shows the average activity observed from three independent experiments. (D) GST pull-down assay. In vitro translated 35S-labeled AR protein was incubated with GST or GST-Daxx protein. Input represents a 20% amount of 35S-labeled AR subjected to the GST pull-down assay. Immunoblot analyses of GST and GST-Daxx proteins with antibody to GST were aligned to show protein levels (bottom panel).
To further confirm the interaction between Daxx and AR, we used two additional independent approaches: yeast two-hybrid assay and GST-pull down assay of in vitro-translated product. Yeast strain L40 transformed with the pLexA-Daxx or pLexA-lamin was cotransformed with either pGal-AD alone or pGal-AD-AR fusion constructs and then grown in selective medium with or without R1881. The yeast extracts from these transformants were subjected to the β-Gal assay. As shown in Fig. 1C, the AR can interact with Daxx in yeast, and this AR-Daxx interaction is not affected by ligand R1881 treatment. In contrast, LexA-lamin/pGal-AD-AR produced only basal levels of β-galactosidase activity. These results suggest that the AR may bind to Daxx directly. To further verify this interaction, GST pull-down assay was carried out. In vitro transcriptional and translational lysates containing [35S]methionine-labeled full-length AR protein were incubated with the immobilized GST-Daxx fusion protein or GST protein, analyzed by SDS-PAGE, and detected by autoradiography. As seen in Fig. 1D, a strong retention of AR protein was obtained only in the sample containing immobilized GST-Daxx. Furthermore, adding R1881 in this assay did not significantly alter the binding of the AR with GST-Daxx (data not shown). These results are consistent with those of the yeast two-hybrid experiments, suggesting that Daxx is an AR-binding protein and that its binding is not affected by the agonist R1881.
Daxx represses AR-mediated transactivation.
Given the previous demonstration of a role for Daxx in transcriptional repression, we further examined the possible effect of Daxx on the transcriptional activity of the AR. Transient transfections of AR-mediated reporter gene assay were performed. AR, Daxx expression constructs, and a luciferase reporter plasmid driven by the steroid hormone responsive elements in the mouse mammary tumor virus (MMTV) long terminal repeat (pMMTV-Luc) were transfected into COS-1 cells. After the transfection, cells were treated with or without R1881, and cell lysates were assayed for luciferase activity. In the absence of Daxx transfection, an ∼30-fold induction of AR-mediated transcriptional activity was observed in the presence of 10 nM R1881 (Fig. 2A). Overexpression of Daxx itself did not affect the reporter gene activity. Notably, the ligand-dependent AR transactivation was suppressed in a dose-dependent manner by ∼80% when the Daxx expression vector was cotransfected. In contrast, cotransfection with ARIP3, an AR coactivator, caused a potentiation of the activity of AR transactivation. The repression by Daxx was not associated with a change in the expression level of AR protein (data not shown), suggesting that the repression results from direct effects on AR transactivation rather than downregulation of AR expression.
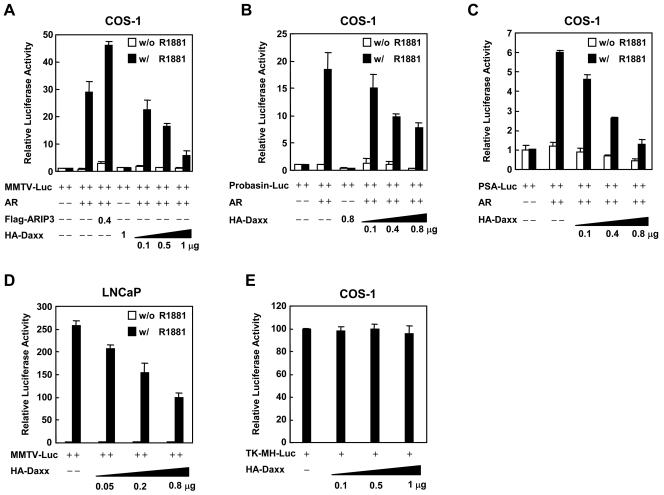
FIG. 2.
Daxx suppresses AR-mediated gene activation. COS-1 cells were transiently transfected with 600 ng of pMMTV-Luc (A), Probasin-Luc (B), or PSA (−630/+12)-Luc (C) reporter, along with 200 ng of pSG5-AR and increasing amounts of HA-Daxx expression plasmid or pCMV-Flag-ARIP3 as indicated. Transfected cells were incubated in complete medium for 18 h and then washed and treated with or without 10 nM R1881 for another 24 h. (D) The relative luciferase activities are represented as the means ± the standard deviations (i.e., luciferase light units/β-galactosidase). LNCaP cells were transiently transfected with pMMTV-Luc reporter and increasing amounts of HA-Daxx expression plasmid as indicated. Transfected cells were treated with or without R1881 and subsequently subjected to the analysis of relative luciferase activity as described above. (E) COS-1 cells were transfected with 900 ng of TK-MH-Luc reporter construct and increasing amounts of HA-Daxx expression plasmid as indicated. The relative luciferase activity of each sample was determined as described above.
To further confirm the repression of AR activity by Daxx, transient-transfection experiments were performed in COS-1 cells with a luciferase reporter driven by the human PSA promoter or rat probasin promoter, which are regulated by the AR and have widely been used to analyze AR-mediated transcriptional activity (31). In agreement with the above findings, the luciferase activity induced by the AR could significantly be repressed by Daxx in a dose-dependent manner (Fig. 2B and C). It should be noted that the basal activity of both probasin and PSA promoters is also affected by increasing amounts of Daxx. To further demonstrate the Daxx repressive effect in cells expressing endogenous AR, we performed transient-transfection experiments with LNCaP cells. As shown in Fig. 2D, the ligand-dependent induction by the endogenous AR was also repressed in a dose-dependent manner by cotransfection of Daxx. As a control for the specificity of Daxx repression on AR-mediated transactivation, a luciferase reporter construct TK-MH-Luc that contains four copies of Gal4-binding site linked to the TK promoter (11) was assayed in the transient-transfection experiments. Overexpression of Daxx had no suppressive effect on this reporter activity (Fig. 2E). Taken together, our findings indicate that Daxx functions as a transcriptional repressor in regulating the AR-mediated gene activation.
Daxx suppresses the androgen-induced PSA expression in LNCaP cells.
To establish the functional significance of Daxx in modulating AR-mediated gene expression, we examined the expression of endogenous PSA in several Daxx stably transfected LNCaP cells. Overexpression of Daxx to about three- to fivefold higher than the endogenous level was seen in these stable clones (Fig. 3A, right panel). These clones display a decreased level of PSA transcript, in response to R1881, as measured by quantitative-PCR analyses of these LNCaP cell lines (Fig. 3A, left panel). These results further substantiate the notion that Daxx functions as a negative AR coregulator in downregulating AR target gene expression. Furthermore, the level of AR in each of these clones does not vary much from the parental LNCaP, indicating that the diminution of AR activity by Daxx is not due to destabilization of AR.
FIG. 3.
Daxx suppresses androgen-induced PSA expression. (A) Daxx stably transfected LNCaP cells were treated with or without 10 nM R1881 for 48 h. Total RNA from cells was subjected to reverse transcription, and this was followed by quantitative SYBR Green PCR analysis of PSA expression. The left panel represents the relative expression of PSA in three independent experiments. The right panel indicates the expression levels of Daxx and AR in these stable cell lines. (B) LNCaP cells transfected with pSUPER or pSUPER-Daxx construct were harvested at 48 or 72 h for Western blot analysis of endogenous Daxx, AR, and actin. The arrow and asterisk indicate the Daxx protein and a nonspecific band, respectively. (C) LNCaP cells were transfected with pSUPER or pSUPER-Daxx for 48 h, followed by R1881 stimulation for an additional 48 h. The expression of PSA from these transfected cells was analyzed as described for panel A.
To further investigate the physiological role of Daxx in regulating AR-dependent PSA expression, we used RNA interference approach to knock down endogenous Daxx protein expression in LNCaP cells. A Daxx oligonucleotide was engineered into the pSUPER vector for generating small interfering RNA. This construct, pSUPER-Daxx, was transfected into LNCaP cells, and cell lysates were prepared 2 or 3 days later for immunoblotting analysis. As shown in Fig. 3B, transfection of pSUPER-Daxx resulted in a decrease of endogenous Daxx without affecting the protein levels of AR and actin. Next, we determined the effect of pSUPER-Daxx on PSA expression in LNCaP cells induced by AR agonist. Knocking down the expression of Daxx caused a significant increase in PSA expression after exposure to R1881 (Fig. 3C), further confirming the role of Daxx in controlling AR-mediated gene expression.
Daxx does not alter the nuclear translocation of AR.
To further explore the molecular mechanism by which Daxx suppresses ligand-dependent activation of AR activity, we examined whether overexpression of Daxx influences the nuclear translocation of the AR upon ligand stimulation. LNCaP cells were transfected with HA-tagged Daxx construct, followed by treatment with or without R1881 for 2 h. The subcellular localization of the AR and Daxx was examined by laser-scanning confocal microscopy with anti-AR or anti-HA antibodies. Without ligand treatment, endogenous AR was distributed in both cytoplasmic and nuclear compartments, whereas Daxx was localized in the nucleus (Fig. 4a and b). Upon ligand stimulation, AR was concentrated in the nucleus, and overexpressed Daxx did not alter the nuclear staining of AR (Fig. 4e), suggesting that the Daxx repressive effect does not result from alteration of AR nuclear translocation. The same observation was obtained when COS-1 cells were transfected with AR and Daxx expression constructs (data not shown).
FIG. 4.
Daxx does not alter the nuclear shuttling of AR induced by R1881. LNCaP cells were transiently transfected with HA-Daxx expression plasmid. After 24 h, cells were treated with or without R1881 for 2 h and then subjected to immunostaining analyses with anti-HA monoclonal antibody and anti-AR rabbit antibody. The secondary antibodies for these studies were fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G and Texas red-conjugated anti-rabbit immunoglobulin G. After immunostaining and washing procedures, the samples were analyzed by confocal immunofluorescence microscopy. DAPI staining revealed the positions of the nucleus. The open arrowhead indicates the HA-Daxx-transfected cells.
Mapping of Daxx interaction domains in the AR.
To further investigate the mechanism underlying the role of Daxx on AR-mediated transcriptional activity, we first delineated the domain(s) of the AR required for its binding to Daxx. Various AR deletion mutants were generated and analyzed by coimmunoprecipitation experiments. Full-length AR and various deletion mutants, including ARND(1-663), ARN(1-560), ARDL(486-918), and ARL(663-918) (Fig. 5A), were expressed together with HA-tagged Daxx protein in COS-1 cells. The protein expression levels of each mutant and HA-Daxx are shown in Fig. 5B. ARND and ARN have higher expression levels than the wild type, whereas ARL and ARDL have a comparable expression level. It is noteworthy that ARN gave a pattern of doublet protein bands, which is likely due to posttranslational modification(s) (29). Cell lysates were immunoprecipitated with anti-HA antibody, and Western blot analysis was performed with anti-AR NH2- or COOH-terminus antibody. The ARN, ARND, and ARDL domains, like the wild-type AR, retained the capacity to interact with Daxx, whereas the ARL failed to do so (Fig. 5B), indicating that the N and D domains of the AR are involved in Daxx binding. To further substantiate these interactions, a yeast two-hybrid assay was performed with each subdomain of the AR fused to the Gal4 activation domain, including the N-terminal domain of the AR (ARN), the DBD of the AR (ARD), and the ligand-binding domain of the AR (ARL) (Fig. 5A). Gal4AD-ARN and Gal4AD-ARD were able to interact with LexA-Daxx but not LexA-lamin, as indicated by the quantitative β-Gal assay (Fig. 5C). Notably, ARN conferred stronger interaction than the full-length AR. In contrast, no appreciable interaction was detected between Gal4AD-ARL and LexA-Daxx. These results suggest that the AR binds to Daxx through its N-terminal and DNA-binding domains.
FIG. 5.
The NH2 domain and DNA-binding domain of AR mediate Daxx interaction. (A) Schematic presentation of AR wild-type and different deletion mutants tested in mammalian transfection and yeast two-hybrid assays. N, DBD, and LBD represent the subdomains of the amino-terminal domain, DNA-binding domain, and ligand-binding domain of the AR, respectively. (B) COS-1 cells transfected with HA-Daxx and full-length or various deletion mutants of AR expression constructs as indicated were subjected to immunoprecipitation with anti-HA antibody, and this was followed by immunoblotting with anti-AR antibodies PG-21 and C-19, which are specifically against with the N-terminal and C-terminal domains of AR, respectively. The top-right and bottom-right panels show immunoblotting of whole-cell lysates to indicate the expression levels of AR wild-type and mutants and HA-Daxx from the transfected cells used for coimmunoprecipitation experiments. The asterisks depict full-length and deleted forms of AR proteins. The open arrowhead indicates the position of ARL protein if it is precipitated by HA-Daxx. (C) Yeast cotransformed with bait and prey as indicated were analyzed by quantitative β-Gal assays. The data represent the means ± the standard deviations of three separate experiments.
Daxx prevents AR from binding to its target DNA.
Given Daxx's ability to bind the AR DNA-binding domain, one potential that Daxx can repress AR activity is through direct interference with the AR's DNA-binding capacity. To test this possibility, EMSAs were performed. Affinity-purified GST-AR DNA-binding domain (GST-ARD) fusion protein was incubated with the 32P-labeled ARE. The protein-DNA complexes were resolved by native PAGE. Clearly, GST-ARD recombinant protein and ARE probe formed a protein-DNA complex, whereas GST protein could not (Fig. 6A, lane 2 versus lane 12). This protein-DNA complex is specific since the formation of a complex can be abolished by a 100-fold excess of unlabeled ARE oligonucleotide but not by an unrelated oligonucleotide (Fig. 6A, lanes 2 to 4). Moreover, this complex can be further supershifted when an anti-GST antibody was added (lane 5), indicating the specific complex formation between GST-ARD and the ARE. We next tested the effect of Daxx on this complex formation. Remarkably, preincubation of the ARD reaction mixture with Daxx prevented the complex formation of GST-ARD with ARE probe in a dose-dependent fashion (Fig. 6A, lanes 9 to 11), whereas addition of GST protein did not affect the complex formation (lanes 6 to 8). Furthermore, no DNA-protein complex formation between Daxx and ARE probe occurred (lane 13), indicating that prevention of ARD-ARE complex formation by Daxx is not due to competition for ARE binding.
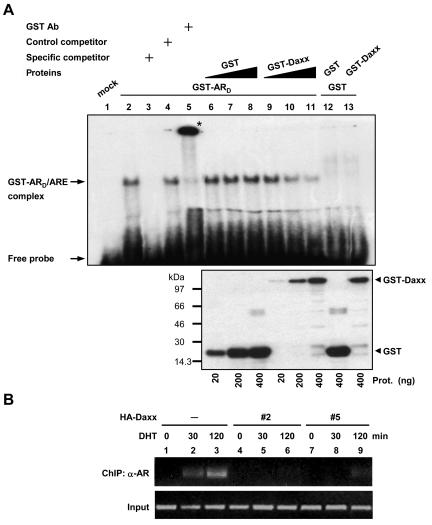
FIG. 6.
Daxx inhibits AR DNA-binding activity. A, Recombinant GST-ARD fusion protein was incubated with 32P-labeled ARE oligonucleotide (lane 2), along with increasing amounts of purified GST (lanes 6 to 8) or GST-Daxx (lanes 9 to 11) and further analyzed by EMSA as described in Materials and Methods. Excess amounts of unlabeled ARE oligonucleotide (specific competitor) or unrelated oligonucleotide (control competitor) and anti-GST antibody were added to the binding reactions for competitive assays (lanes 3 and 4) and supershift assays (lane 5), respectively. The supershifted DNA-protein complex is indicated by an asterisk. Arrows indicate the positions of the specific protein-DNA complexes and free probe. Indicated amounts of the GST and GST-Daxx proteins used in the EMSA are indicated by immunoblotting with an anti-GST antibody (bottom panel). (B) LNCaP parental and HA-Daxx stable cells were treated with 10 nM DHT for 30 min or 2 h, and ChIP assays were performed with anti-AR antibody as described in Materials and Methods. Immunoprecipitated chromatin was amplified by using primers encompassing the PSA promoter region containing ARE (top panel). Input chromatin DNA taken before immunoprecipitation from different samples was also analyzed by PCR (bottom panel).
Next, we examined whether the expression of Daxx prevents the ARE-binding activity of endogenous AR in LNCaP cells. LNCaP cells and Daxx stable clones were treated with or without DHT and then subjected to ChIP analysis. After formaldehyde cross-linking and precipitation of the chromatin with anti-AR antibody, the precipitated DNA was PCR amplified with a set of specific primers flanking around the ARE in the PSA enhancer region. As a control, androgen treatment stimulated the association of AR with the endogenous ARE in a time-dependent manner (Fig. 6B, lane 1 to 3). Notably, the amount of ARE bound by AR was significantly less in Daxx stable clones (clones 2 and 5) compared to parental LNCaP cells (lanes 4 to 9). It should be noted that Daxx stable clone 2 seems to confer more inhibition of AR DNA binding than clone 5 at the 120-min time point (lane 6 versus lane 9), although both clones give a similar PSA expression induced by AR ligand (Fig. 3A), implying the mechanism other than inhibition of AR DNA binding also operates by Daxx. Together, these results suggest that Daxx inhibits AR activity by physical interaction, displacing AR from ARE. These findings point to a novel mechanism whereby Daxx represses AR-mediated transactivation.
SUMO modification of AR contributes to Daxx interaction and transcriptional repression.
Recent studies indicated that the AR is covalently modified by SUMO-1 (45). The sumoylation sites of the human AR are K386 and K520 in the NH2-terminal domain. Substitution of both lysine residues to arginine enhanced the transcriptional activity of the AR, suggesting that sumoylation of the AR may suppress its transcriptional activity. Since we and others (47; unpublished results) have isolated SUMO-1 as a Daxx interacting protein, it is possible that the SUMO moieties of the AR serve as the anchor sites for Daxx. If this is true, mutation of both sumoylation sites should diminish the interaction between the AR and Daxx. To test this possibility, we constructed the K386/520R mutant (ΔSUMO) in the context of the full length and the N-terminal domain of the AR (Fig. 7A). The interactions of these mutants with LexA-Daxx were assayed in a yeast two-hybrid assay, with β-Gal as a reporter. All of the proteins were expressed at equal levels as judged by Western blot analysis (data not shown). In the context of the full-length AR, the removal of sumoylation sites (Fig. 7B, compare Gal4AD-ARFL and Gal4AD-ARFL-ΔSUMO) reduced the interaction between Daxx and AR by ∼30%. This suggests that the two lysine residues contribute to the interaction, but they are not the sole Daxx anchor sites on the AR, a finding consistent with earlier data showing that Daxx interacts with multiple domains of the AR. Removal of the sumoylation sites has a much more dramatic effect on the interaction of the N-terminal domain of the AR (Gal4AD-ARN) with Daxx. In this case, nearly all of the interactions were abolished when the two lysines were mutated (compare Gal4AD-ARN-ΔSUMO and Gal4AD-ARN, Fig. 7B). Together, these findings implicate that the two sumoylation sites of the AR are largely responsible for the binding of Daxx to the N-terminal domain of AR.
FIG. 7.
SUMO modification of AR mediates Daxx interaction. (A) Schematic representation of the human AR and its sumoylation mutants used in the yeast two-hybrid and mammalian reporter gene assays. Two sumoylation acceptor lysine residues (386 and 520) or mutants with changes from Lys to Arg of hAR are shown. (B) Yeast cotransformed with the plasmid constructs as indicated were subjected to quantitative β-Gal assays. The data represent the means ± standard deviations of three independent experiments. (C) Myc-tagged ARN proteins prepared from pCMV-ARN transfected COS-1 cells were subjected to an in vitro sumoylation assay as described in Materials and Methods. Half amount of the SUMO-1 modified and unmodified ARN proteins were analyzed by immunoblotting with anti-Myc antibody (left panel). Another portion of modified and unmodified proteins on agarose beads was incubated with the cell lysates expressing HA-Daxx. After an extensive washing, bound proteins were analyzed by SDS-PAGE and Western blotting with anti-HA antibody (right panel).
To further substantiate that the sumoylation of the AR is involved in Daxx-AR interaction, we modified the N-terminal domain of the AR (ARN) with SUMO-1 in vitro and tested its ability for Daxx interaction. ARN protein was subjected to an in vitro sumoylation assay. Several SUMO-1-conjugated species of Myc-tagged ARN were generated by an in vitro sumoylation reaction (Fig. 7C, left panel, lane 2). The resulting products were further subjected to the binding assay with Daxx. Significantly, Daxx was precipitated by SUMO-1-modified ARN but not by ARN protein (right panel), indicating that ARN-Daxx interaction is SUMO conjugation dependent. These findings suggest that sumoylation of the AR may help recruit Daxx, leading to AR transcriptional repression.
To establish the functional relationship between the Daxx-sumoylated AR interaction and transcriptional repression, we next examined whether the transcriptional activity of AR-ΔSUMO is no longer repressed by Daxx. To avoid the complication of Daxx inhibitory effect through the interaction with AR DNA-binding domain, we used a modified mammalian one-hybrid system to analyze the transcriptional activity of ARN or ARN-ΔSUMO fused to Gal4 DNA-binding domain. Expression vector of Gal4-ARN or Gal4-ARN-ΔSUMO was cotransfected with a 4X Gal4-binding site containing reporter gene (TK-MH-Luc) and Daxx expression construct into COS-1 cells, and the activity of the luciferase reporter was measured. In accordance with previous studies (30), the Gal4-ARN is capable of activating reporter expression (Fig. 8A, lane 5). Notably, the transactivation of Gal4-ARN-ΔSUMO was twofold higher than that of Gal4-ARN (lane 5 versus lane 8). The higher transcriptional activity of the Gal4-ARN-ΔSUMO mutant cannot be explained by fusion protein levels because, if anything, the expression level of the double mutant was lower than that of wild-type ARN fusion assessed by immunoblotting (Fig. 8A, bottom panel). As expected, coexpression of Daxx no longer repressed Gal4-ARN-ΔSUMO-mediated transactivation (lanes 8 to 10), whereas Daxx diminished the activity of the Gal4-ARN in a dose-dependent manner (lanes 5 to 7), indicating the involvement of Daxx in sumoylation-mediated transcriptional repression.
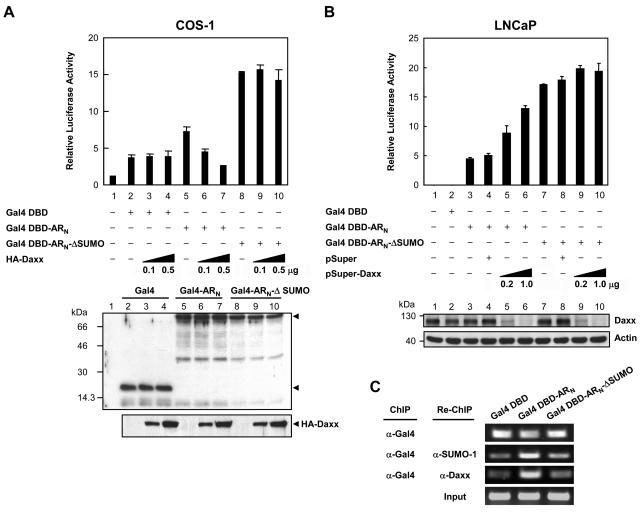
FIG. 8.
SUMO modification of AR mediates Daxx transrepression. (A) COS-1 cells transfected with the TK-MH-Luc reporter and the indicated plasmid constructs were incubated in complete medium for 48 h and then harvested for luciferase assay. Relative luciferase activity was determined as described above in Fig. 2. Bar graph represents the mean ± standard deviations of three independent experiments. The bottom panel shows the expression levels of Gal4 DBD and fusion constructs, as well as the HA-Daxx in each sample, by immunoblotting of transfected cell extracts with anti-Gal4-DBD and anti-HA antibodies. (B) LNCaP cells transfected with the indicated plasmid constructs and increasing amounts of pSUPER or pSUPER-Daxx were cultured in complete medium for 48 h and then harvested for luciferase assays. The relative luciferase activity was determined as described above. The protein levels of endogenous Daxx in transfected cells are shown (bottom panel). (C) LNCaP cells transfected with constructs expressing Gal4, Gal-ARN, or Gal-ARN-ΔSUMO, along with the TK-MH-Luc, were incubated in complete medium for 48 h and then subjected to the ChIP and Re-ChIP procedure as described in Materials and Methods with anti-Gal4 antibody, followed by anti-SUMO-1 or anti-Daxx antibody. Immunoprecipitated DNA was amplified in PCRs with primers encompassing the promoter region containing Gal4-binding sites.
To further verify the endogenous Daxx involved in sumoylation-mediated repression, the pSUPER-Daxx was transiently transfected into the LNCaP cells, along with the TK-MH-Luc reporter and Gal4-ARN or Gal4-ARN-ΔSUMO constructs. Transfection of increasing amount of pSUPER-Daxx resulted in a markedly elevated reporter activity in the Gal4-ARN-transfected cells than in the Gal4-ARN-ΔSUMO-transfected cells (Fig. 8B, lanes 3 to 6 versus lanes 7 to 10), albeit the endogenous protein levels of Daxx in both transfected cells were downregulated to a similar extent (Fig. 8B, bottom panel). These results strongly implicate the involvement of endogenous Daxx in AR sumoylation-mediated transcriptional repression.
We lastly demonstrated this sumoylation-dependent interaction in vivo by ChIP analysis. The Gal4-ARN wild type and ΔSUMO mutant were transfected into LNCaP cells, along with the TK-MH-Luc reporter construct. The transfected cells were then chromatin immunoprecipitated with anti-Gal4 antibody first, followed by Re-ChIP with anti-SUMO-1 or anti-Daxx antibody. As shown in Fig. 8C, less SUMO-1 associated with the promoter was found in the ARN-ΔSUMO mutant (second panel, lane 3 versus lane 2). Importantly, less Daxx was detected to associate with this promoter in the Gal4-ARN-ΔSUMO-transfected cells compared to the wild-type-transfected cells (third panel, lane 3 versus lane 2). As controls, the extent of the promoter region bound by Gal4 DNA-binding domain, Gal4-ARN wild type, or ΔSUMO mutant was comparable (top panel), indicating that the differential recruitment of Daxx resulted from the sumoylation of the AR rather from the distinct expression levels of transfected Gal4-ARN wild type and ΔSUMO mutant in LNCaP cells. Taken together, these findings provide a nice correlation between the Daxx-sumoylated AR interaction and transcriptional repression.
DISCUSSION
Sumoylation has emerged as an important mechanism in controlling gene expression. Studies of a number of SUMO-targeted transcription factors revealed two possible categories of molecular mechanisms of protein sumoylation involved in negatively regulating transcriptional activity (19, 32). First, sumoylation antagonizes other posttranslational modifications, such as ubiquitination and acetylation, by targeting a common acceptor lysine residue. The prototypical example is that SUMO conjugation of IκBα at lysine 21 stabilizes this protein by blocking ubiquitination at the same site (9). Second, SUMO modification alters the molecular interaction properties that are important for transcription factor activity and/or subcellular localization. In the case of heat shock transcription factor 2, sumoylation results in a significant increase in its DNA-binding activity (21). Sumoylation of p300 has recently been shown to mediate the recruitment of HDAC6, leading to SUMO-dependent transcriptional repression (18), whereas sumoylation of Sp3 results in its translocation away from the transcriptional locale to nuclear domain PML oncogenic domains (46, 49). What is the molecular mechanism of sumoylation in regulating AR transcriptional potential? Pestell and coworkers demonstrated that a conserved KLKK motif localized at the hinge region of the AR is responsible for acetylation by p300 and P/CAF and that the extent of AR sumoylation is independent of its acetylation status (15), excluding the possibility of sumoylation in antagonizing the same lysine residues for acetylation. Furthermore, Kotaja et al. reported that PIAS family proteins PIAS1 and ARIP3 (PIASxα) could enhance AR sumoylation but could not alter the subnuclear localization of the AR (36), indicating that sumoylation of the AR is irrelevant to AR subnuclear distribution. In addition, Callewaert et al. (4) showed that sumoylation does not affect its DNA-binding capability. In the present study, we demonstrated that Daxx is a SUMO-dependently recruited negative repressor in mediating AR sumoylation-dependent transcriptional repression. Our findings that (i) wild-type but not the ΔSUMO mutants of the N-terminal domain ARN can associate with Daxx in yeast (Fig. 7B), (ii) Daxx binds to sumoylated molecules of the ARN in vitro and in vivo (Fig. 7C and 8C), (iii) Daxx can suppress the transcriptional activity of the ARN wild type but not ΔSUMO mutant (Fig. 8A), and (iv) depletion of Daxx proteins significantly increases the transcriptional activity of ARN wild-type but not ΔSUMO mutant (Fig. 8B) strongly suggest that sumoylation of AR is involved in the Daxx interaction and transcriptional repression. Collectively, our findings provide the mechanistic insight underlying SUMO-dependent transcriptional repression of the AR.
Another important issue worth mentioning is that the sumoylation acceptor sites of the AR have also been mapped to a previously identified inhibitory motif called the synergy control (SC) motif (25). The SC motif has also been found within the negative regulatory regions of many different transcription factors, including GR, MR, ETS1, PR, C/EBPα, ɛ, c-Myb, SREBP-1, SF-1, and Sp3 (25). Recently, the SUMO acceptor sites of these factors (GR, PR, C/EBPα, ɛ, c-Myb, SREBP-1, Sp3, and SF-1) have been defined within these SC motifs (1, 2, 5, 6, 34, 35, 46, 49, 55). Mutation of these SUMO acceptor sites in these factors leads to an increase in the transcriptional activation. These findings suggested that sumoylation-dependent repression is a common regulatory mechanism in transcriptional control. Our current findings indicate that Daxx mediates SUMO-dependent transcriptional repression of AR. Furthermore, we have also found that Daxx could regulate the GR activity through sumoylation at the SC motif of the GR (unpublished data), suggesting that Daxx may be a common factor in mediating SUMO-dependent repression of aforementioned sumoylated transcription factors.
Baniahmad and coworkers recently reported that the transcriptional corepressor SMRT suppressed the AR transactivation through the AR NH2-terminal domain in the presence of the partial agonist cyproterone acetate (10). By using a modified mammalian two-hybrid system on the MMTV promoter, these researchers mapped the first 328 amino acid residues of the hAR critical for SMRT transrepression. In addition, mutation of AR sumoylation acceptor Lys residues to Glu also attenuated SMRT-mediated transrepression, suggesting that sumoylation sites may contribute to the transcriptional repression by SMRT as well. In contrast, Chen and coworkers independently demonstrated that SMRT inhibited ligand-dependent transcriptional activity by the AR largely through the ligand-binding domain (40). This study group also provided evidence to suggest that the mechanisms of SMRT-mediated AR transrepression occur through disruption of AR NH2-COOH interaction and competition with the p160 coactivators (40). Thus, it remains to be clarified whether SMRT indeed directly binds to sumoylated AR and inhibits AR transcriptional activity.
Although Daxx functions as a transcriptional corepressor, the molecular mechanism for how it suppresses the transcriptional activation remains largely unclear. A previous study demonstrated that the treatment with a deacetylase inhibitor, trichostatin A, efficiently reversed the repressive effect of Daxx (38), suggesting that histone deacetylation may be involved in Daxx-mediated transcriptional repression. In support of this model, interaction between the Daxx and HDAC1 has been demonstrated by in vitro pull-down assay and by in vivo coimmunoprecipitation experiments (38). Furthermore, Hollenbach et al. have recently shown that endogenous Daxx associates with multiple proteins that are critical for transcriptional repression, such as histone deacetylase II; components of chromatin such as core histones H2A, H2B, H3, and H4; and a chromatin-associated protein Dek (23). More recently, Daxx has been shown to associate with ATRX, a protein binding to heterochromatin protein HP1 and functioning as part of a chromatin-remodeling complex (54, 59). Its association with condensed chromatin in cells lacking promyelocytic leukemia protein has also been reported (26), indicating a role of Daxx in association with a transcriptionally silenced chromatin structure. Although not mutually exclusive, our finding that Daxx inhibits the DNA-binding activity of AR adds a new mode of repression mechanism mediated by Daxx.
In view of Daxx in AR regulation, it would be interesting to know whether the expression of Daxx is associated with PCa formation. Recently, Waghray et al. showed that Daxx expression was stromal specific in human PCa and that its expression level was stronger in areas surrounding tumor glands than in areas surrounding normal glands by the serial analysis of gene expression analysis (57). Immunohistochemical studies demonstrated that the AR is expressed in both epithelial tumor cells and the stromal cell compartments of PCa, although the stromal expression is variable (48, 56). Although the role of androgen-regulated processes in the stromal cells associated with PCa cells is not well understood, it is possible that Daxx exerts some repressive effect, leading to cells insensitive to androgen stimulation. In a subpopulation of prostate carcinomas, mutations and deletions of the AR have been reported, which might account for androgen-independent tumor growth (7). However, no explanation has been found for the androgen insensitivity in the vast majority of prostate tumors, which still express wild-type AR. One hypothesis to explain this clinically disastrous phenomenon is an altered composition and/or regulation of AR-coregulator complexes. The findings that AR activity can be regulated by sumoylation and Daxx should provide more clues to evaluate the roles of these proteins in association with PCas.
Acknowledgments
We thank Ronald Evans and Chawshang Chang for plasmid constructs.
This study was supported by Intramural Funds of National Health Research Institutes MG-093-PP-03 to H.-M.S.
REFERENCES
- 1.Abdel-Hafiz, H., G. S. Takimoto, L. Tung, and K. B. Horwitz. 2002. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J. Biol. Chem. 277:33950-33956. [DOI] [PubMed] [Google Scholar]
- 2.Bies, J., J. Markus, and L. Wolff. 2002. Covalent attachment of the SUMO-1 protein to the negative regulatory domain of the c-Myb transcription factor modifies its stability and transactivation capacity. J. Biol. Chem. 277:8999-9009. [DOI] [PubMed] [Google Scholar]
- 3.Boellmann, F., T. Guettouche, Y. Guo, M. Fenna, L. Mnayer, and R. Voellmy. 2004. DAXX interacts with heat shock factor 1 during stress activation and enhances its transcriptional activity. Proc. Natl. Acad. Sci. USA 101:4100-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callewaert, L., G. Verrijdt, A. Haelens, and F. Claessens. 2004. Differential effect of small ubiquitin-like modifier (SUMO)-ylation of the androgen receptor in the control of cooperativity on selective versus canonical response elements. Mol. Endocrinol. 18:1438-1449. [DOI] [PubMed] [Google Scholar]
- 5.Chauchereau, A., L. Amazit, M. Quesne, A. Guiochon-Mantel, and E. Milgrom. 2003. Sumoylation of the progesterone receptor and of the steroid receptor coactivator SRC-1. J. Biol. Chem. 278:12335-12343. [DOI] [PubMed] [Google Scholar]
- 6.Chen, W. Y., W. C. Lee, N. C. Hsu, F. Huang, and B. C. Chung. 2004. SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1). J. Biol. Chem. 279:38730-38735. [DOI] [PubMed] [Google Scholar]
- 7.Culig, Z., A. Hobisch, A. Hittmair, H. Peterziel, A. C. Cato, G. Bartsch, and H. Klocker. 1998. Expression, structure, and function of androgen receptor in advanced prostatic carcinoma. Prostate 35:63-70. [DOI] [PubMed] [Google Scholar]
- 8.Dedhar, S., P. S. Rennie, M. Shago, C. Y. Hagesteijn, H. Yang, J. Filmus, R. G. Hawley, N. Bruchovsky, H. Cheng, R. J. Matusik, et al. 1994. Inhibition of nuclear hormone receptor activity by calreticulin. Nature 367:480-483. [DOI] [PubMed] [Google Scholar]
- 9.Desterro, J. M., M. S. Rodriguez, and R. T. Hay. 1998. SUMO-1 modification of IκBα inhibits NF-κB activation. Mol. Cell 2:233-239. [DOI] [PubMed] [Google Scholar]
- 10.Dotzlaw, H., U. Moehren, S. Mink, A. C. Cato, J. A. Iniguez Lluhi, and A. Baniahmad. 2002. The amino terminus of the human AR is target for corepressor action and antihormone agonism. Mol. Endocrinol. 16:661-673. [DOI] [PubMed] [Google Scholar]
- 11.Doucas, V., M. Tini, D. A. Egan, and R. M. Evans. 1999. Modulation of CREB binding protein function by the promyelocytic (PML) oncoprotein suggests a role for nuclear bodies in hormone signaling. Proc. Natl. Acad. Sci. USA 96:2627-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ecsedy, J. A., J. S. Michaelson, and P. Leder. 2003. Homeodomain-interacting protein kinase 1 modulates Daxx localization, phosphorylation, and transcriptional activity. Mol. Cell. Biol. 23:950-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emelyanov, A. V., C. R. Kovac, M. A. Sepulveda, and B. K. Birshtein. 2002. The interaction of Pax5 (BSAP) with Daxx can result in transcriptional activation in B cells. J. Biol. Chem. 277:11156-11164. [DOI] [PubMed] [Google Scholar]
- 14.Feldman, B. J., and D. Feldman. 2001. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 1:34-45. [DOI] [PubMed] [Google Scholar]
- 15.Fu, M. F., C. G. Wang, J. Wang, Z. X. P., T. Sakamaki, Y. Y. G., C. S. Chang, T. Hopp, S. A. W. Fuqua, E. Jaffray, R. T. Hay, J. J. Palvimo, O. A. Janne, and R. G. Pestell. 2002. Androgen receptor acetylation governs trans-activation and MEKK1-induced apoptosis without affecting in vitro sumoylation and trans-repression function. Mol. Biol. Cell 22:3373-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelmann, E. P. 2002. Molecular biology of the androgen receptor. J. Clin. Oncol. 20:3001-3015. [DOI] [PubMed] [Google Scholar]
- 17.Gill, G. 2003. Post-translational modification by the small ubiquitin-related modifier SUMO has big effects on transcription factor activity. Curr. Opin. Genet. Dev. 13:108-113. [DOI] [PubMed] [Google Scholar]
- 18.Girdwood, D., D. Bumpass, O. A. Vaughan, A. Thain, L. A. Anderson, A. W. Snowden, E. Garcia-Wilson, N. D. Perkins, and R. T. Hay. 2003. P300 transcriptional repression is mediated by SUMO modification. Mol. Cell 11:1043-1054. [DOI] [PubMed] [Google Scholar]
- 19.Girdwood, D. W., M. H. Tatham, and R. T. Hay. 2004. SUMO and transcriptional regulation. Semin. Cell Dev. Biol. 15:201-210. [DOI] [PubMed] [Google Scholar]
- 20.Glass, C. K., and M. G. Rosenfeld. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14:121-141. [PubMed] [Google Scholar]
- 21.Goodson, M. L., Y. Hong, R. Rogers, M. J. Matunis, O. K. Park-Sarge, and K. D. Sarge. 2001. Sumo-1 modification regulates the DNA binding activity of heat shock transcription factor 2, a promyelocytic leukemia nuclear body associated transcription factor. J. Biol. Chem. 276:18513-18518. [DOI] [PubMed] [Google Scholar]
- 22.Heinlein, C. A., and C. Chang. 2002. Androgen receptor (AR) coregulators: an overview. Endocrinol. Rev. 23:175-200. [DOI] [PubMed] [Google Scholar]
- 23.Hollenbach, A. D., C. J. McPherson, E. J. Mientjes, R. Iyengar, and G. Grosveld. 2002. Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein Dek. J. Cell Sci. 115:3319-3330. [DOI] [PubMed] [Google Scholar]
- 24.Hollenbach, A. D., J. E. Sublett, C. J. McPherson, and G. Grosveld. 1999. The Pax3-FKHR oncoprotein is unresponsive to the Pax3-associated repressor hDaxx. EMBO J. 18:3702-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iniguez-Lluhi, J. A., and D. Pearce. 2000. A common motif within the negative regulatory regions of multiple factors inhibits their transcriptional synergy. Mol. Cell. Biol. 20:6040-6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishov, A. M., A. G. Sotnikov, D. Negorev, O. V. Vladimirova, N. Neff, T. Kamitani, E. T. Yeh, J. F. Strauss III, and G. G. Maul. 1999. PML is critical for ND10 formation and recruits the PML-interacting protein Daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147:221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janne, O. A., A. M. Moilanen, H. Poukka, N. Rouleau, U. Karvonen, N. Kotaja, M. Hakli, and J. J. Palvimo. 2000. Androgen-receptor-interacting nuclear proteins. Biochem. Soc. Trans. 28:401-405. [PubMed] [Google Scholar]
- 28.Jenster, G. 1999. The role of the androgen receptor in the development and progression of prostate cancer. Semin. Oncol. 26:407-421. [PubMed] [Google Scholar]
- 29.Jenster, G., P. E. de Ruiter, H. A. van der Korput, G. G. Kuiper, J. Trapman, and A. O. Brinkmann. 1994. Changes in the abundance of androgen receptor isotypes: effects of ligand treatment, glutamine-stretch variation, and mutation of putative phosphorylation sites. Biochemistry 33:14064-14072. [DOI] [PubMed] [Google Scholar]
- 30.Jenster, G., H. A. van der Korput, J. Trapman, and A. O. Brinkmann. 1995. Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J. Biol. Chem. 270:7341-7346. [DOI] [PubMed] [Google Scholar]
- 31.Jenster, G., H. A. van der Korput, C. van Vroonhoven, T. H. van der Kwast, J. Trapman, and A. O. Brinkmann. 1991. Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol. Endocrinol. 5:1396-1404. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, E. S. 2004. Protein modification by sumo. Annu. Rev. Biochem. 73:355-382. [DOI] [PubMed] [Google Scholar]
- 33.Kim, E. J., J. S. Park, and S. J. Um. 2003. Identification of Daxx interacting with p73, one of the p53 family, and its regulation of p53 activity by competitive interaction with PML. Nucleic Acids Res. 31:5356-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim, J., C. A. Cantwell, P. F. Johnson, C. M. Pfarr, and S. C. Williams. 2002. Transcriptional activity of CCAAT/enhancer-binding proteins is controlled by a conserved inhibitory domain that is a target for sumoylation. J. Biol. Chem. 277:38037-38044. [DOI] [PubMed] [Google Scholar]
- 35.Komatsu, T., H. Mizusaki, T. Mukai, H. Ogawa, D. Baba, M. Shirakawa, S. Hatakeyama, K. I. Nakayama, H. Yamamoto, A. Kikuchi, and K. I. Morohashi. 2004. SUMO-1 modification of the synergy control motif of Ad4BP/SF-1 regulates synergistic transcription between Ad4BP/SF-1 and Sox9. Mol. Endocrinol. 18:2451-2462. [DOI] [PubMed] [Google Scholar]
- 36.Kotaja, N., U. Karvonen, O. A. Janne, and J. J. Palvimo. 2002. PIAS proteins modulate transcription factors by functioning as SUMO-1 ligases. Mol. Cell. Biol. 22:5222-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehembre, F., S. Muller, P. P. Pandolfi, and A. Dejean. 2001. Regulation of Pax3 transcriptional activity by SUMO-1-modified PML. Oncogene 20:1-9. [DOI] [PubMed] [Google Scholar]
- 38.Li, H., C. Leo, J. Zhu, X. Wu, J. O'Neil, E. J. Park, and J. D. Chen. 2000. Sequestration and inhibition of Daxx-mediated transcriptional repression by PML. Mol. Cell. Biol. 20:1784-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, R., H. Pei, D. K. Watson, and T. S. Papas. 2000. EAP1/Daxx interacts with ETS1 and represses transcriptional activation of ETS1 target genes. Oncogene 19:745-753. [DOI] [PubMed] [Google Scholar]
- 40.Liao, G., L. Y. Chen, A. Zhang, A. Godavarthy, F. Xia, J. C. Ghosh, H. Li, and J. D. Chen. 2003. Regulation of androgen receptor activity by the nuclear receptor corepressor SMRT. J. Biol. Chem. 278:5052-5061. [DOI] [PubMed] [Google Scholar]
- 41.Lin, D. Y., M. Z. Lai, D. K. Ann, and H. M. Shih. 2003. Promyelocytic leukemia protein (PML) functions as a glucocorticoid receptor co-activator by sequestering Daxx to the PML oncogenic domains (PODs) to enhance its transactivation potential. J. Biol. Chem. 278:15958-15965. [DOI] [PubMed] [Google Scholar]
- 42.Lin, D. Y., and H. M. Shih. 2002. Essential role of the 58-kDa microspherule protein in the modulation of Daxx-dependent transcriptional repression as revealed by nucleolar sequestration. J. Biol. Chem. 277:25446-25456. [DOI] [PubMed] [Google Scholar]
- 43.Obradovic, D., M. Tirard, Z. Nemethy, O. Hirsch, H. Gronemeyer, and O. F. Almeida. 2004. DAXX, FLASH, and FAF-1 modulate mineralocorticoid and glucocorticoid receptor-mediated transcription in hippocampal cells—toward a basis for the opposite actions elicited by two nuclear receptors? Mol. Pharmacol. 65:761-769. [DOI] [PubMed] [Google Scholar]
- 44.Phan, D., X. Sui, D. T. Chen, S. M. Najjar, G. Jenster, and S. H. Lin. 2001. Androgen regulation of the cell-cell adhesion molecule-1 (Ceacam1) gene. Mol. Cell Endocrinol. 184:115-123. [DOI] [PubMed] [Google Scholar]
- 45.Poukka, H., U. Karvonen, O. A. Janne, and J. J. Palvimo. 2000. Covalent modification of the androgen receptor by small ubiquitin-like modifier 1 (SUMO-1). Proc. Natl. Acad. Sci. USA 97:14145-14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross, S., J. L. Best, L. I. Zon, and G. Gill. 2002. SUMO-1 modification represses Sp3 transcriptional activation and modulates its subnuclear localization. Mol. Cell 10:831-842. [DOI] [PubMed] [Google Scholar]
- 47.Ryu, S. W., S. K. Chae, and E. Kim. 2000. Interaction of Daxx, a Fas binding protein, with sentrin and Ubc9. Biochem. Biophys. Res. Commun. 279:6-10. [DOI] [PubMed] [Google Scholar]
- 48.Sadi, M. V., P. C. Walsh, and E. R. Barrack. 1991. Immunohistochemical study of androgen receptors in metastatic prostate cancer: comparison of receptor content and response to hormonal therapy. Cancer 67:3057-3064. [DOI] [PubMed] [Google Scholar]
- 49.Sapetschnig, A., G. Rischitor, H. Braun, A. Doll, M. Schergaut, F. Melchior, and G. Suske. 2002. Transcription factor Sp3 is silenced through SUMO modification by PIAS1. EMBO J. 21:5206-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt, D., and S. Muller. 2003. PIAS/SUMO: new partners in transcriptional regulation. Cell Mol. Life Sci. 60:2561-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seeler, J. S., and A. Dejean. 2003. Nuclear and unclear functions of SUMO. Nat. Rev. Mol. Cell. Biol. 4:690-699. [DOI] [PubMed] [Google Scholar]
- 52.Sharma, M., M. Zarnegar, X. Li, B. Lim, and Z. Sun. 2000. Androgen receptor interacts with a novel MYST protein, HBO1. J. Biol. Chem. 275:35200-35208. [DOI] [PubMed] [Google Scholar]
- 53.Shenk, J. L., C. J. Fisher, S. Y. Chen, X. F. Zhou, K. Tillman, and L. Shemshedini. 2001. p53 represses androgen-induced transactivation of prostate-specific antigen by disrupting hAR amino- to carboxyl-terminal interaction. J. Biol. Chem. 276:38472-38479. [DOI] [PubMed] [Google Scholar]
- 54.Tang, J., S. Wu, H. Liu, R. Stratt, O. G. Barak, R. Shiekhattar, D. J. Picketts, and X. Yang. 2004. A novel transcription regulatory complex containing death domain-associated protein and the ATR-X syndrome protein. J. Biol. Chem. 279:20369-20377. [DOI] [PubMed] [Google Scholar]
- 55.Tian, S., H. Poukka, J. J. Palvimo, and O. A. Janne. 2002. Small ubiquitin-related modifier-1 (SUMO-1) modification of the glucocorticoid receptor. Biochem. J. 367:907-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tilley, W. D., S. S. Lim-Tio, D. J. Horsfall, J. O. Aspinall, V. R. Marshall, and J. M. Skinner. 1994. Detection of discrete androgen receptor epitopes in prostate cancer by immunostaining: measurement by color video image analysis. Cancer Res. 54:4096-4102. [PubMed] [Google Scholar]
- 57.Waghray, A., M. Schober, F. Feroze, F. Yao, J. Virgin, and Y. Q. Chen. 2001. Identification of differentially expressed genes by serial analysis of gene expression in human prostate cancer. Cancer Res. 61:4283-4286. [PubMed] [Google Scholar]
- 58.Xu, L., C. K. Glass, and M. G. Rosenfeld. 1999. Coactivator and corepressor complexes in nuclear receptor function. Curr. Opin. Genet. Dev. 9:140-147. [DOI] [PubMed] [Google Scholar]
- 59.Xue, Y., R. Gibbons, Z. Yan, D. Yang, T. L. McDowell, S. Sechi, J. Qin, S. Zhou, D. Higgs, and W. Wang. 2003. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc. Natl. Acad. Sci. USA 100:10635-10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang, X., R. Khosravi-Far, H. Y. Chang, and D. Baltimore. 1997. Daxx, a novel Fas-binding protein that activates JNK and apoptosis. Cell 89:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu, X., P. Li, R. G. Roeder, and Z. Wang. 2001. Inhibition of androgen receptor-mediated transcription by amino-terminal enhancer of split. Mol. Cell. Biol. 21:4614-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]