Abstract
Background:
Cryopyrin-associated periodic syndrome (CAPS) is a group of rare, heterogeneous autoinflammatory disease characterized by interleukin (IL)-1β-mediated systemic inflammation and clinical symptoms involving skin, joints, central nervous system, and eyes. It encompasses a spectrum of three clinically overlapping autoinflammatory syndromes including familial cold autoinflammatory syndrome, Muckle-Wells syndrome (MWS), and neonatal-onset multisystem inflammatory disease. CAPS is associated with gain-of-function missense mutations in NOD-like receptor family pyrin domain-containing protein 3 (NLRP3), the gene encoding NLRP3. Moreover, most mutations leading to MWS occurred in exon 3 of NLRP3 gene. Here, we reported a novel mutation occurred in exon 1 of NLRP3 gene in an MWS patient and attempted to explore the pathogenic mechanism.
Methods:
Genetic sequence analysis of NLRP3 was performed in an MWS patient who presented with periodic fever, arthralgia, and multiform skin lesions. NLRP3 was also analyzed in this patient's parents and 50 healthy individuals. Clinical examinations including X-ray examination, skin biopsy, bone marrow aspiration smear, and blood test of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum levels of IL-1β, immunoglobulin E (IgE), antineutrophil cytoplasmic antibodies, antinuclear antibodies, and extractable nuclear antigen were also analyzed. The protein structure of mutant NLRP3 inflammasome was calculated by SWISS-MODEL software. Proteins of wild type and mutant components of NLRP3 inflammasome were expressed and purified, and the interaction abilities between these proteins were tested by surface plasmon resonance (SPR) assay.
Results:
X-ray examination showed no abnormality in the patient's knees. Laboratory tests indicated an elevation of CRP (233.24 mg/L) and ESR (67 mm/h) when the patient had fever. Serum IL-1β increased to 24.37 pg/ml, and serum IgE was higher than 2500.00 IU/ml. Other blood tests were normal. Bone marrow aspiration smear was normal. A novel point mutation c.92A>T in exon 1 of NLRP3 gene was identified, which caused a p.D31V mutation in pyrin domain (PYD) of NLRP3. SPR assay showed that this point mutation may strengthen the interaction between the PYD of NLRP3 and the PYD of the apoptosis-associated speck-like protein. The mutation c.92A>T in exon 1 of the NLRP3 gene was not found in the patient's parents and 50 healthy individuals.
Conclusions:
The mutation c.92A>T in exon 1 of the NLRP3 gene is a novel mutation associated with MWS. The p.D31V mutation might promote the activation of NLRP3 inflammasome and induce MWS in this patient.
Keywords: Muckle-Wells Syndrome, Mutation, NOD-like Receptor Family Pyrin Domain-containing Protein 3, Pyrin Domain
Introduction
Cryopyrin-associated periodic syndrome (CAPS) is a group of rare, heterogeneous autoinflammatory disease characterized by interleukin (IL)-1β-mediated systemic inflammation and clinical symptoms involving skin, joints, central nervous system, and eyes. It encompasses a spectrum of three clinically overlapping autoinflammatory syndromes including familial cold autoinflammatory syndrome, Muckle-Wells syndrome (MWS), and neonatal-onset multisystem inflammatory disease, which were initially thought to be distinct entities, but in fact share a single genetic mutation and pathogenic pathway.[1]
MWS, which usually present in childhood, is characterized by episodic attacks of inflammation associated with fever, urticaria, arthralgia, and conjunctivitis.[2] Later in the course, MWS is typically accompanied by progressive sensorineural hearing loss and multi-organ AA-type amyloidosis.[3,4] Laboratory findings of MWS show abnormal leukocytosis with neutrophilia, thrombocytosis, anemia, and increased erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP).[3]
In recent years, it has been demonstrated that the majority of MWS cases are closely related to point mutations in the NOD-like receptor family pyrin domain-containing protein 3 (NLRP3) gene (OMIM number *606416). NLRP3 gene is located in the chromosomal region 1q44 and encodes NLRP3. NLRP3, also designated as NACHT-, LRR-, and pyrin-domain (PYD)-containing protein 3, is the key component of the NLRP3 inflammasome.[5] Mutations of NLRP3 lead to enhanced activity of NLRP3 inflammasome and overproduction of IL-1 β. The disorder caused by NLRP3 mutations has an extraordinary response to IL-1 inhibitors.[6] It seems that the effective treatment options are very limited merely targeting at IL-1 receptor or IL-1β including anakinra which is a short-acting IL-1 receptor antagonist, canakinumab which is a fully human monoclonal antibody providing selective and prolonged IL-1β blockade, and rilonacept which is an IL-1 trap fusion protein.[7]
However, up to now, few studies have investigated the mechanism of the functional changes of NLRP3 inflammasome which should also be a target for the treatment. In our study, we identified a novel mutation in PYD of NLRP3 rather than other sites as reported before. The aim of this study was to investigate the mechanism of the enhanced function of NLRP3 inflammasome which causes the increased production of IL-1β, and results might serve as a foundation to investigate new options for the treatment of MWS in the future.
Methods
Patient
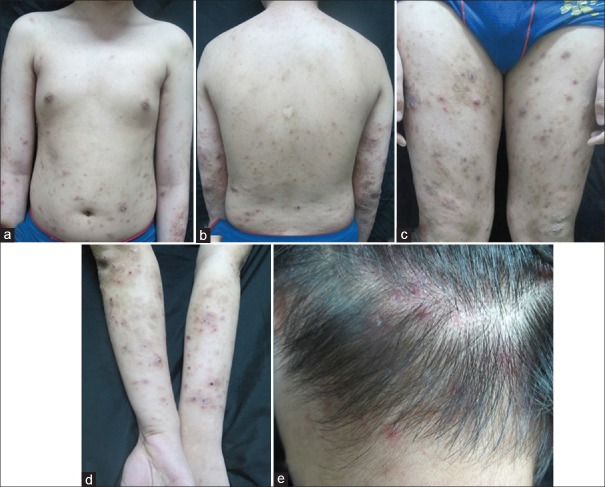
A 15-year-old male was referred to the Department of Dermatology, Peking University People's Hospital with a 10-year history of periodic fever, which could peak at 40°C and occurred every other week lasing 4–5 days accompanied with arthralgia in his knees, fatigue, chills, anorexia, and occasional conjunctivitis. He also complained a 6-month history of multiform skin lesions including eczema-like rashes and prurigo-like rashes with intense itching on his trunk, limbs, and occipital scalp [Figure 1]. The rashes recurring pattern seems to overlap with the fever occurrences. The patient has no family history of hereditary diseases. There were no similar clinical manifestations in his family.
Figure 1.
Photos of the patient with Muckle-Wells syndrome. There were erythema, papules, vesicles, and nodules on the trunk (a and b) and limbs (c and d), and excoriation and crusts could also be seen; papules with erosion and crusts could be seen on the scalp (e).
The patient took methotrexate and etanercept for 1 month before visiting our hospital, which helped reduce the fever duration to 1–2 days and prolonged the interval to 15–30 days, and relieved the pains in his knees. Antihistamines were applied to alleviating the intense itching of the rashes. Because of the symptoms of fever and arthralgia in his knees, the patient was once diagnosed with rheumatic diseases and treated with nonsteroidal anti-inflammatory drugs and corticosteroids. The treatment was effective in reducing the duration of the fever but could not extend the interval of recurrence.
The study protocol was approved by the Ethics Committee of Peking University People's Hospital. The patient signed written informed consent.
Clinical examinations
A series of clinical examinations were carried out after he was accepted to our department including X-ray examination, skin biopsy, bone marrow aspiration smear, and blood test of CRP by automatic chemistry analyzer (Olympus, Japan), ESR by automated erythrocyte sedimentation rate analyzer (Perlong, Beijing, China), serum IL-1β, immunoglobulin E (IgE) by enzyme-linked immunosorbent assay (Rapidbio, USA), serum antineutrophil cytoplasmic antibodies, antinuclear antibodies, and extractable nuclear antigen by indirect immunofluorescence (Ek-Bioscience, Switzerland).
Genetic analysis
Genetic mutation analyses of exon 1 to exon 3 of NLRP3 gene were performed for the patient, the patient's parents, and fifty healthy individuals at Kangson Medical Inspection, Beijing, China. Genomic DNA was prepared from whole peripheral blood using Qiagen FlexiGene DNA Kit (Qiagen, Germany). For Sanger sequencing, DNA covering exon 1 to exon 3 of NLRP3 gene was amplified by polymerase chain reaction using the primers listed in Table 1.
Table 1.
Primers used in Sanger sequencing performed in a patient with Muckle-Wells syndrome
| NLRP3 Exons | Region of Sequence | Primers | Sequences |
|---|---|---|---|
| Exon 1 | 2652-3256 | Ex1-F | 5’-CCGGCCTTGGCTAACTTTTC-3’ |
| Ex1-R | 5’-CCTGGGCAACAAGAGCAACT-3’ | ||
| Exon 2 | 7109-7532 | Ex2-F | 5’-GCAGAAATGCTCCCAACCAG-3’ |
| Ex2-R | 5’- TGGAATGCAAAGGCCTGTCT -3’ | ||
| Exon 3 | 7797-8522 | Ex3-1F | 5’-TTCTCCATTCCGGTTACCAC-3’ |
| Ex3-1R | 5’-AAGCAGCTTCTTTCTGATGAGG-3’ | ||
| 8231-9045 | Ex3-2F | 5’-GTCGGGGACACTCTACCAAG-3’ | |
| Ex3-2R | 5’-TCTCGCAGTCCACTTCCTTT-3’ | ||
| 8678-9512 | Ex3-3F | 5’-GGCAGCCTTCAGTCTGATTC-3’ | |
| Ex3-3R | 5’-ACTCCACCCGATGACAGTTC-3’ | ||
| 9292-9849 | Ex3-4F | 5’-GCAAGATCTCTCAGCAAATCA-3’ | |
| Ex3-4R | 5’-CCTCATCCCATTTATCCACCT-3’ |
Ex: Exon; F: Forward; R: Reverse; NLRP3: NOD-like receptor family pyrin domain-containing protein 3.
Comparison of amino acid variation of pyrin domain
Sequence alignment of wild type as well as mutant NLRP3 PYD and other PYDs including NLRP1, apoptosis-associated speck-like protein (ASC), and POP1 was performed by ClustalW2 server software (European Bioinformatics Institute, London, UK).[8] Amino acid sequences of these proteins were compared, and conserved and nonconserved sites were analyzed. The amino acid types on the same site with residue D31 of wild-type NLRP3 were compared.
Bioinformatical and structural analysis of wild type, mutant NOD-like receptor family pyrin domain-containing protein 3 pyrin domain and apoptosis-associated speck-like protein pyrin domain
The structural-based alignment result was modified by online ESPript program software (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi).[9] The structures of NLRP3 PYD and ASC PYD were acquired from Protein Data Bank server (Research Collaboratory for Structural Bioinformatics, USA) with ID code 3QF2 and 2KN6. The p.D31V mutant structure of NLRP3 PYD was calculated by SWISS-MODEL Server (Swiss Institute of Bioinformatics, Lausanne, Switzerland) using its wild-type structure as the starting template.[10] Residues for interactions between proteins were analyzed.
Protein expression and purification
DNA fragments encoding human wild-type NLRP3 PYD, D31V mutant NLRP3 PYD (amino acids 1-112), and human ASC PYD (amino acids 1-111) were synthesized by BGI company, Beijing, China. Then, the sequence was subcloned into the pET vector (Novagen, USA), and all proteins were expressed in Escherichia coli BL21 by overnight induction at 20°C. All three proteins containing an N-terminal His tag were first purified by nickel affinity chromatography and then gel filtration chromatography with Superdex-75 gel filtration column (GE Healthcare, Piscataway, NJ, USA) preequilibrated with 50 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer at pH 7.4 and 150 mmol/L NaCl. Each protein was concentrated to a final concentration of 1 mg/ml.
Surface plasmon resonance assay
The binding affinity of ASC PYD to NLRP3 PYD wild type or its p.D31V mutant was determined by surface plasmon resonance (SPR) using the Biacore system (GE Healthcare, Piscataway, NJ, USA). The CM5 sensor chip (GE Healthcare, Piscataway, NJ, USA) was immobilized, and the NLRP3 PYD and its D31V mutant were immobilized in separate channel, then ASC PYD at different concentrations (2.5 μmol/L, 5.0 μmol/L, 10.0 μmol/L, and 20.0 μmol/L) were injected to these two channels simultaneously. The unreacted sites and the control channel were blocked with ethanolamine. The association reaction was carried out by injecting ASC PYD at different concentrations in HEPES buffer (pH 7.4) with 0.05% Tween-20.
Results
The patient was diagnosed as Muckle-Wells syndrome
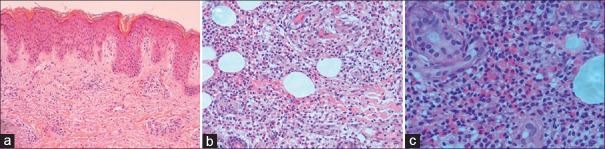
The results of laboratory tests indicated an elevation of CRP (233.24 mg/L) and ESR (67 mm/h) when the patient had fever. The serum IL-1β was increased to 24.37 pg/ml, and serum IgE was higher than 2500.00 U/ml. Other blood test results were normal. Bone marrow aspiration smear was normal. The X-ray examination showed no abnormality in his knees. The patient had no hearing loss and the intelligence was normal. There was no family history of similar conditions. The skin biopsy showed slightly hyperkeratosis and slightly acanthosis of the epidermis, and infiltration of lymphocytes and eosinophils in the dermis and the subcutaneous tissue [Figure 2].
Figure 2.
The pathological feature of Chinese patient with Muckle -Wells syndrome. The skin biopsy (H and E staining) was taken from a papule of the extension part of the forearm. (a) Slightly hyperkeratosis and slightly acanthosis of the epidermis; infiltration of lymphocytes around vessels, and scattered eosinophils in the upper dermis (×40); (b and c) abundant lymphocytes and eosinophils in the deep dermis and the subcutaneous tissue, respectively. Lymphocytes and eosinophils gradually increased from the upper dermis to the subcutaneous tissue (b: ×100, c: ×200).
According to the clinical manifestations of a history of periodic fever overlapping with the rashes, accompanying symptoms such as arthralgia in knees and occasional conjunctivitis, as well as the clinical examination of an elevation of CRP, ESR, and serum IL-1β, this patient was diagnosed as MWS.
Mutation in NOD-like receptor family pyrin domain- containing protein 3 gene
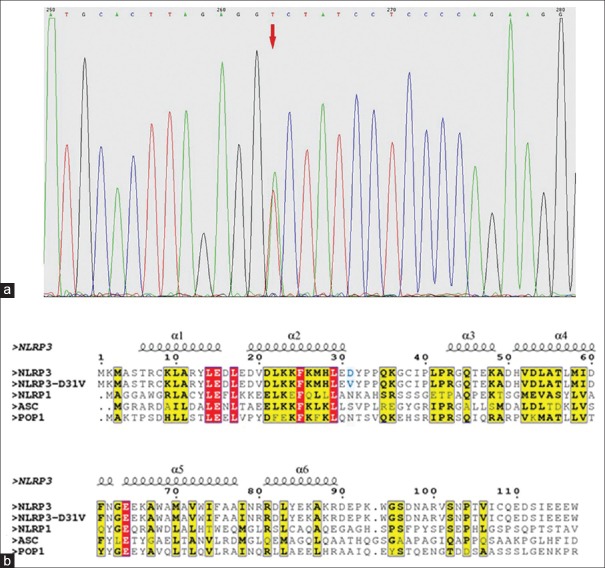
A heterozygous point mutation of A92T (chromosome 1: 247582188) in exon 1 of NLRP3 gene was identified in the patient with MWS [Figure 3a]. The mutation caused a nonsynonymous amino acid change of D31V, which is an amino acid site in PYD of NLRP3, a component of NLRP3 inflammasome. No mutation of NLRP3 gene was found in the patient's parents, and this mutation of A92T was not found in the 50 healthy individuals either.
Figure 3.
The pyrin domain of p.D31V mutation of NOD-like receptor family pyrin domain-containing protein 3 in Muckle-Wells syndrome patient. (a) Gene sequencing in NLRP3. A heterozygous mutation of c.92A>T in exon 1 of NLRP3 was detected. (b) Structural based sequence alignment of NLRP3 PYD with other PYDs. Secondary structures (helices α1 to α6) are shown above the sequences according to the crystal structure of NLRP3 PYD. Red arrow: p.D31V mutation. Yellow and red frame: conserved residues. NLRP3: NOD-like receptor family pyrin domain-containing protein 3; PYD: Pyrin domain, ASC: Apoptosis-associated speck-like protein.
D31 of pyrin domain is a nonconserved residue
The predicated and the secondary structure of D31V PYD based on the crystal structure of NLPR3 PYD (Protein Data Bank ID code: 3QF2), and the comparison among the amino acid sequence of PYD of wild type NLRP3, mutant NLRP3, and other PYD-containing proteins including NLPR1, ASC, and POP1 are shown in Figure 3b. PYD of wild type NLRP3, NLPR1, ASC, and POP1 has similar structures and amino sequences. There are six α helixes in the PYD of these proteins. All helices have conserved residues, and conserved sites of α1 to α6 helix are shown in yellow and red. The residue D31 of NLRP3 PYD on the edge of α2 helix is not a conserved residue.
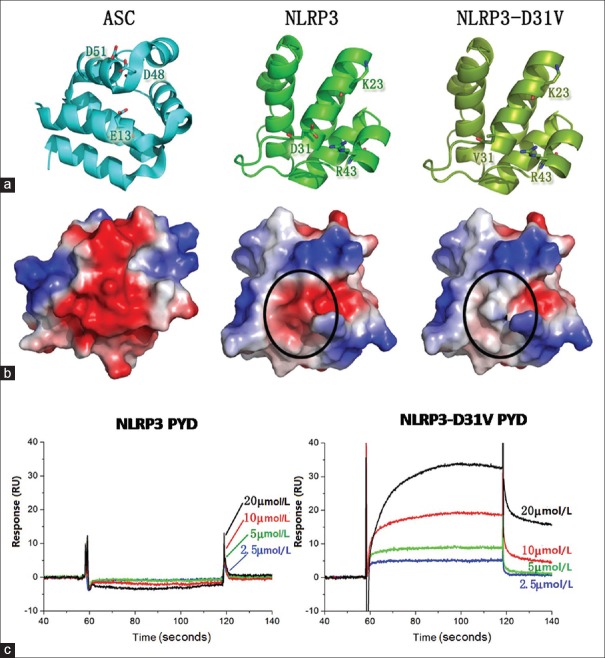
The p.D31V mutation could increase binding between NOD-like receptor family pyrin domain-containing protein 3 and apoptosis-associated speck-like protein
In the ASC PYD [Figure 4a], residue E13, D48, and D51 form a strong negatively charged surface, while in the NLPR3 PYD, residue K23 and R43 form a positively charged surface. These two opposing charged surfaces mediate the interaction between NLRP3 and ASC. The interaction between the negatively charged surface of ASC PYD with the positively charged surface of NLPR3 PYD could be largely enhanced by the D31V mutation [Figure 4b]. The results of SPR assay also showed that ASC PYD could bind to NLRP3 PYD D31V and the affinity is increased in a dose-dependent manner. However, the ASC PYD had much higher affinity for the D31V mutant than for the wild-type NLRP3 PYD [Figure 4c].
Figure 4.
The p.D31V single mutation has enhanced the interaction between pyrin domains of NOD-like receptor family pyrin domain-containing protein 3 and apoptosis-associated speck-like protein. (a) Structures of the ASC PYD, NLRP3 PYD and NLRP3-D31V PYD were shown as cartoon and colored in cyan, green and split pea. The important residues for interactions are shown as sticks. (b) Surface electrostatic potential of the PYDs of ASC, NLRP3, and NLRP3-D31V are shown in the same orientation as (a). The surface change location is highlighted with black circle. (c) SPR assay for the binding affinity of ASC PYD to either NLRP3 PYD or NLRP3-D31V PYD. ASC PYD concentrations are indicated. NLRP3: NOD-like receptor family pyrin domain-containing protein 3; PYD: Pyrin domain; ASC: Apoptosis-associated speck-like protein; SPR: Surface plasmon resonance; RU: Response unit.
The p.D31V mutation may also impair NOD-like receptor family pyrin domain-containing protein 3 pyrin domain dimeric interaction
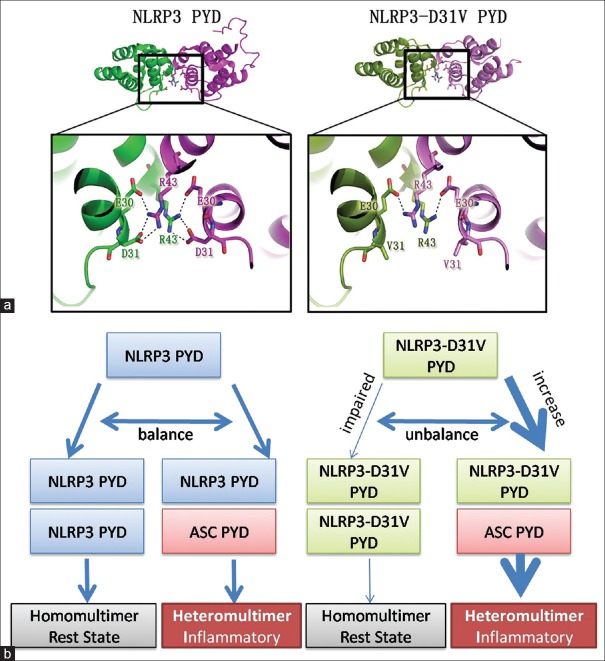
In the crystal structure of NLRP3 PYD homodimer, residue E30 and D31 were on the same protein, and these two residues could interact with residue R43 on another protein. The six salt bridges between two NLRP3 proteins form a strong interaction network in this narrow interface. However, in the established structure of p.D31V mutant PYD, unlike D31, residue V31 could not help form a strong linking. Hence, this interaction network was totally abolished. Since no interaction could be formed between R43 and V31, the formation of NLRP3 homodimer has been largely compromised by this p.D31V mutation [Figure 5a].
Figure 5.
The predicted mechanism of p.D31V in NLRP3 induced inflammatory response. (a) p.D31V single mutation induced dimeric interface change of NLRP3 PYD. The close-up view shows the interacting residues in the binding surface between two dimers. NLRP3 PYDs are colored in green and magentas, and p.D31V mutants are colored in split pea and violet. Salt bridges are shown as dashed lines. (b) The predicted mechanism of p.D31V induced NLRP3 inflammasome enhancement. NLRP3: NOD-like receptor family pyrin domain-containing protein 3; PYD: Pyrin domain; ASC: Apoptosis-associated speck-like protein.
Discussion
CAPS are associated with a gain-of-function missense mutation predominantly occurred in exon 3 of NLRP3 gene encoding NLRP3 inflammasome. Intracellular formation and interaction of components of NLRP3 inflammasome complex such as NLRP3 and ASC leads to the activation of this inflammasome and then caused the production of IL-1β and IL-18. The mutation in NLRP3 gene leads to an aberrant formation of components of NLRP3 inflammasome and subsequently caused unregulated production of IL-1β. Up to now, 180 heterogeneous mutations in NLRP3 gene associated with CAPS have been identified.[11] It is suggested that rare mutations are more frequently associated with severe phenotype, and some mutations are associated with distinct phenotype, and these may probably reflect that different mutations have distinct impact on the activities of the NLRP3 inflammasome in the context of an individual genetic background of the patient.[12]
Instead of those common mutations found in exon 3 of NLRP3 gene which encodes the central nucleotide-biding site (NOD) domain,[1] we identified a novel mutation of c.92A>T in exon 1 of NLRP3 gene in our patient, which were verified by three authoritative databases: Exome Aggregation Consortium Browser (http://exac.broadinstitute.org/), 1000genomes Browser (http://www.internationalgenome.org/), and Exome Variant Server (http://evs.gs.washington.edu/EVS/). This novel mutation causes a nonsynonymous amino acid change of p. D31V in the PYD of NLRP3. Does this novel mutation make any clinical significance and how can this mutation affect the function of NLRP3 protein? Our study has suggested a new mechanism of this novel mutation in MWS.
NLRP3 protein is a component of NLRP3 inflammasome and is composed of NOD domain and PYD. PYD is an important pivot for linking between different components of NLRP3 inflammasome complex including the interaction between NLRP3 and ASC or between NLRP3 and NLRP3 which form a homodimer.[13] Different PYD-containing proteins have similar amino acid sequence and structures of PYD. The interactions of PYDs between NLRP3 and ASC or NLRP3 and NLRP3 are through series of important residues.[13,14] In the mutation we identified, residue D31 at the end of the α2 helix of NLRP3 PYD mutated to a nonconserved residue V31. This variation of residue may lead to an alteration in the distribution of surface charge.
NLRP3 PYD and ASC PYD share similar structures and conformations and interact with each other through contacts of surface charge.[15] Residue K23 in α2 helix and R43 α3 helix form a positively charged surface on NLRP3. Residue E13 in α1 helix, D48 in α3 helix, and D51 α4 helix form a strong negatively charged surface on ASC. Intriguingly, D31 in α2 helix located right between K23 and R43 which forms a positively charged surface as shown in Figure 4a. The strong negative charge of D31 side chain interferes with the uniformity of the charge of the K23-R43 surface. However, with D31 mutated to V31, this extra opposing charge disappears, and the K23-R43 positively charged surface became much stronger. Therefore, the p.D31V mutation would enhance the interaction between NLRP3 and ASC. Moreover, this prediction was confirmed by the SPR experiment. NLRP3-D31V PYD binds to ASC PYD in a dose-dependent manner, and with much stronger binding affinity than that of the NLRP3 wild-type PYD and ASC PYD [Figure 4c].
However, it is noteworthy that the binding between NLRP3-D31V PYD to ASC PYD in the SPR assay did not fit the 1:1 Langmuir binding model. This may be attributed to the fact that PYD could mediate self-association between the same proteins in solution. The recombinant ASC PYD and NLRP3 PYD may exist as monomer, dimer, trimer, or other higher-ordered polymers in buffer.[14] Therefore, we were not able to calculate the kinetic parameters, such as the association rate, dissociation rate, or equilibrium constant. Nevertheless, the qualitative analysis of the interactions indicated that the difference between NLRP3 and its mutant was statistically significant. Because the PYD could mediate self-association, we were also interested in understanding the change in the dimeric interface induced by p. D31V mutation. In the wild-type structure, NLRP3 PYD could interact with itself through residue R43, E30, and D31,[13] while D31 is directly involved. This kind of homodimer formation was also found in other DD superfamily proteins.[16] The p. D31V mutation disrupts the salt bridge formed between residue R43 and D31, which compromised the dimerization of NLRP3 PYD.
Our results showed that D31V mutation could directly influence the interaction between NLRP3 and ASC or NLRP3 and NLRP3 in homodimer. Based on these results, we established a possible molecular mechanism underlying the inflammation induced by NLRP3-D31V mutation as following [Figure 5b]. NLRP3 could form homodimer or interact with ASC through its PYD. Although the PYD homodimer between NLRP3 and NLRP3 could not transfer inflammatory signal to facilitate the formation of inflammasome, when NLRP3 binds to ASC, the heterodimer formed through the two PYDs induced inflammatory responses. In normal human body, the formation of heterodimer or homodimer is at equilibrium and being tightly controlled. The p.D31V mutation decreases the NLRP3-PYD homodimer formation while enhances interaction between NLRP3 and ASC, which tips the balance toward the formation of the NLRP3 inflammasome and an elevation of inflammatory signaling. This causes overproduction of IL-1β and excessive immune responses including periodic fever, arthralgia, and occasional conjunctivitis, which constitute the syndrome of MWS.
Besides, the patient showed multiform skin lesions including eczema-like rashes and prurigo-like rashes, but not urticaria which is the typical lesion of MWS. The skin biopsy showed infiltration of lymphocytes and eosinophils rather than neutrophils in the dermis and the subcutaneous tissue. It was compatible with the features of atopic dermatitis rather than typical lesions of MWS. Moreover, the patient once had allergies in spring. Mutations in FLG gene encoding filaggrin are a highly significant risk factor for atopic dermatitis.[17] We analyzed FLG to investigate whether coexisting mutations may be responsible for the new phenotype, but detected no functionally significant mutations. In addition, the patient presented no hearing loss which may due to his young age. The novel mutation of c.92A>T which caused a special mutation occurred in PYD may somewhat have an impact on this special phenotype, and further investigations are needed to clarify the causes of the unusual skin features.
Financial support and sponsorship
This work was supported by the grant from the National Natural Science Foundation of China (No. 81201267).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
References
- 1.Aksentijevich I, Putnam CD, Remmers EF, Mueller JL, Le J, Kolodner RD, et al. The clinical continuum of cryopyrinopathies: Novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum. 2007;56:1273–85. doi: 10.1002/art.22491. doi: 10.1002/art.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tassi S, Carta S, Delfino L, Caorsi R, Martini A, Gattorno M, et al. Altered redox state of monocytes from cryopyrin-associated periodic syndromes causes accelerated IL-1beta secretion. Proc Natl Acad Sci U S A. 2010;107:9789–94. doi: 10.1073/pnas.1000779107. doi: 10.1073/pnas.1000779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almeida de Jesus A, Goldbach-Mansky R. Monogenic autoinflammatory diseases: Concept and clinical manifestations. Clin Immunol. 2013;147:155–74. doi: 10.1016/j.clim.2013.03.016. doi: 10.1016/j.clim.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabroe RA, Stokes CA, Parker LC, Higgins K, Prince LR, Sabroe I. Muckle-Wells syndrome without mutation in exon 3 of the NLRP3 gene, identified by evidence of excessive monocyte production of functional interleukin 1ß and rapid response to anakinra. Clin Exp Dermatol. 2013;38:874–7. doi: 10.1111/ced.12186. doi: 10.1111/ced.12186. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa K, Gonzalez-Roca E, Souto A, Kawai T, Umebayashi H, Campistol JM, et al. Somatic NLRP3 mosaicism in Muckle-Wells syndrome. A genetic mechanism shared by different phenotypes of cryopyrin-associated periodic syndromes. Ann Rheum Dis. 2015;74:603–10. doi: 10.1136/annrheumdis-2013-204361. doi: 10.1136/annrheumdis-2013-204361. [DOI] [PubMed] [Google Scholar]
- 6.Murphy G, Daly M, O’ Sullivan M, Stack J, Rowczenio D, Lachmann H, et al. An unusual phenotype in Muckle-Wells syndrome associated with NLRP3 E311K. Rheumatology (Oxford) 2011;50:419–20. doi: 10.1093/rheumatology/keq280. doi: 10.1093/rheumatology/keq280. [DOI] [PubMed] [Google Scholar]
- 7.Kuemmerle-Deschner JB, Koitschev A, Tyrrell PN, Plontke SK, Deschner N, Hansmann S, et al. Early detection of sensorineural hearing loss in Muckle-Wells-syndrome. Pediatr Rheumatol Online J. 2015;13:43. doi: 10.1186/s12969-015-0041-9. doi: 10.1186/s12969-015-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8. doi: 10.1093/bioinformatics/btm404. doi: 10.1016/S0165-9936(03)00504-1. [DOI] [PubMed] [Google Scholar]
- 9.Robert X, Gouet P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014;42:W320–4. doi: 10.1093/nar/gku316. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–8. doi: 10.1093/nar/gku340. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Infevers Database. [Last accessed on 2017 Jan 07]. Available from: http://www.fmf.igh.cnrs.fr/infevers .
- 12.Tilson H, Primatesta P, Kim D, Rauer B, Hawkins PN, Hoffman HM, et al. Methodological challenges in monitoring new treatments for rare diseases: Lessons from the cryopyrin-associated periodic syndrome registry. Orphanet J Rare Dis. 2013;8:139. doi: 10.1186/1750-1172-8-139. doi: 10.1186/1750-1172-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae JY, Park HH. Crystal structure of NALP3 protein pyrin domain (PYD) and its implications in inflammasome assembly. J Biol Chem. 2011;286:39528–36. doi: 10.1074/jbc.M111.278812. doi: 10.1074/jbc.M111.278812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vajjhala PR, Mirams RE, Hill JM. Multiple binding sites on the pyrin domain of ASC protein allow self-association and interaction with NLRP3 protein. J Biol Chem. 2012;287:41732–43. doi: 10.1074/jbc.M112.381228. doi: 10.1074/jbc.M112.381228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Coussens NP, Mowers JC, McDonald C, Nuñez G, Ramaswamy S. Crystal structure of the Nod1 caspase activation and recruitment domain. Biochem Biophys Res Commun. 2007;353:1–5. doi: 10.1016/j.bbrc.2006.11.122. doi: 10.1016/j.bbrc.2006.11.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bin L, Leung DY. Genetic and epigenetic studies of atopic dermatitis. Allergy Asthma Clin Immunol. 2016;12:52. doi: 10.1186/s13223-016-0158-5. doi: 10.1186/s13223-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]