Introduction
Lymphedema is a chronic, debilitating disorder characterized by abnormal tissue swelling, adipose deposition, tissue fibrosis, and edema resulting from disruption, blockage, or genetic abnormalities of the lymphatic system.[1] It arises most commonly as a consequence of cancer or the treatments of cancer. Upper extremity lymphedema is commonly associated with the treatment of breast cancer. Lower extremity lymphedema is seen in patients who have been treated for gynecologic malignancy and prostate cancer, as well as melanoma and lymphoma.[2,3] The damage of the lymph circulation results in an overload of lymph fluid that accumulates in the subcutaneous space, causing increased limb weight, decreased limb function, increased infection risk, decreased quality of life, and interference with body appearance.[4]
The lymphatic vascular system is a one-way transport system responsible for the maintenance of fluid homeostasis. The lymphatic vasculature is organized into a network of lymphatic vessels that collect extra tissue fluid from the interstitial space and drain it back to the venous circulation. Recent advances in indocyanine green lymphography, magnetic resonance imaging (MRI), and lymphoscintigraphy have led to the improved assessment of lymphatic vessels in the patients with extremities of lymphedema.[5] However, the assessment of central lymphatic vessels, such as the thoracic duct (TD), is uncommon.
The TD is difficult to be visualized because of its hidden course in the dorsal mediastinum; the combination of its shortness in diameter and the proximity to the lung impedes direct visualization.[6] Some imaging techniques presently available to visualize the entire TD include MRI, scintigraphy, and computed tomography (CT). However, these methods cannot illustrate the dynamic changes of the TD including lymphatic vascular walls, lymphatic valves, and lymph flows. Besides, the relatively low resolution of these methods also restricts the image of detailed anatomy and local characteristics of TD.[7,8]
The high-resolution ultrasonographic imaging has been reported to assess the diameters, structures, and chyle flows of normal TD. The ultrasound technology provides advantages such as the assessment of the inner structure of the TD and its relation to the adjacent tissues and vessel changes.[9] The aim of this study was to find the morphological and functional changes of the cervical part of the TD in patients with lymphedema, by utilizing the ultrasonographic imaging technique.
Methods
Study populations
Thirty-two patients with lymphedema, who were hospitalized at the Department of Plastic and Reconstructive Surgery, Peking Union Medical College Hospital, from June 2014 to December 2015, were enrolled in this study. The patients with right upper extremity lymphedema were excluded from the study due to anatomy and lymphatic drainage factors. This study also included twenty healthy volunteers who received sonographic examination in our department. This study was approved by the Ethics Committee of Peking Union Medical College Hospital. Written informed consent was obtained from each participator.
The diagnosis of lymphedema was generated based on patients’ medical history, physical examination, and indirect radionuclide lymphoscintigraphy. The circumferences of the bilateral forearms or ankles were measured in all patients, and any ≥3 cm increase in circumference when compared to the healthy side was regarded as positive. The results of lymphoscintigraphy were critical for diagnosis; the abnormal imaging characteristics included delayed radiotracer transport, cutaneous flare, dermal infiltration or backflow, or poorly visualized lymphatic collectors and lymph nodes.[10]
Sonographic methods
The ultrasonographic examination of all TDs was performed by one radiologist, with the same commercially available scanner (Philips, iU22, Bothell, WA, USA) equipped with an L12-5 transducer for real-time gray scale and color Doppler ultrasound. Each patient was examined in the supine position and the fasting state with the neck slightly hyperextended and rotated to the right.
The left internal jugular and subclavian veins were visualized with L12-5 transducer for real-time gray scale ultrasound in both transversal and oblique sections, the distal end of the TD appeared as a tubular structure, with highly reflective hyperechoic walls, draining into the left venous angle or in one of the internal jugular and subclavian veins. Color Doppler ultrasonography was also performed to differentiate the TD from the adjacent vessels, such as the vertebral vein. All the terminal TDs were clearly visualized and stored as both freeze images and videos. The images and videos were then reviewed by another radiologist with no awareness of the patient's symptoms and history. The diameter of the terminal TD was measured in the longitudinal section at 1 cm proximal to its junction with the venous angle. This site allowed a much more reproducible quantification of the TD diameter than the terminal part, where an ampullary structure is usually formed with variable ductal size. It has been reported the ampullar enlargement is nearly 4.8 ± 1.4 mm, thus producing additive errors, and reducing measurement accuracy.[11] The anterior and posterior walls of TD should be clearly visualized for the purpose of precise measurement, and each reported value of the duct diameter in this study was the mean of three consecutive measurements.
Statistical analysis
The data were expressed as mean ± standard deviation (SD). All analyses were conducted by SPSS software version 23.0 (IBM, Chicago, IL, USA). The differences in mean diameter of the TD between the healthy volunteers and the patients with lymphedema were evaluated by unpaired Student's t-test. Differences were considered statistically significant at P < 0.05.
Results
Subject characteristics
The 32 patients comprised 30 females and 2 males, with a mean age of 49.9 ± 13.1 years (ranging from 19 years to 70 years). All the patients were diagnosed with lymphedema in Peking Union Medical College Hospital, including 12 patients with left upper limb lymphedema and 20 with lower extremity lymphedema. Among 12 patients with left upper limb lymphedema, 11 patients had a prior history of breast cancer surgery, while another patient had a prior history of infected left upper limb. Among 20 patients with lower extremity lymphedema, 15 patients had prior operation on pelvis (due to cervical cancer), 4 had combined injuries, and the other patient had a prior history of infected lower limb. The patient's clinical characteristics are shown in Table 1. The 20 volunteers included 15 females and 5 males, with a mean age of 38.7 ± 8.1 years (ranging from 20 years to 59 years).
Table 1.
Characteristics of all patients with lymphedema in this study
| Characteristics | Values |
|---|---|
| Age (years), mean ± SD | 49.9 ± 13.1 |
| Duration of lymph edema (months), mean ± SD | 70.0 ± 20.1 |
| Left upper limb involved | 12 |
| Left lower limb involved | 9 |
| Right lower limb involved | 11 |
| Gender, n | |
| Male | 2 |
| Female | 30 |
| Primary disease, n | |
| Breast cancer | 11 |
| Cervical cancer | 15 |
| Injury | 4 |
| Infection | 2 |
| Lymph node dissection, n | |
| Yes | 26 |
| No | 6 |
| Radiation treatment, n | |
| Yes | 14 |
| No | 18 |
SD: Standard deviation.
Ultrasound outcomes
The terminal TDs for all the patients and healthy volunteers had been visualized. The TD was located between the internal jugular vein (ventral) and the vertebral vein (dorsal), the ultrasonographic visible part of the TD ranged 3–5 cm distal from the venous angle, where the jugular vein and subclavian vein join as innominate vein. In healthy volunteers, the TD walls were visualized clearly and the lymph walls were seen to be smooth and continuous. The valves were found located directly at the entrance. In some cases, we were even able to visualize the valves opening and closing together with lymph flow [Figure 1]. The diameter of the TD in healthy volunteers was 2.65 ± 0.20 mm.
Figure 1.
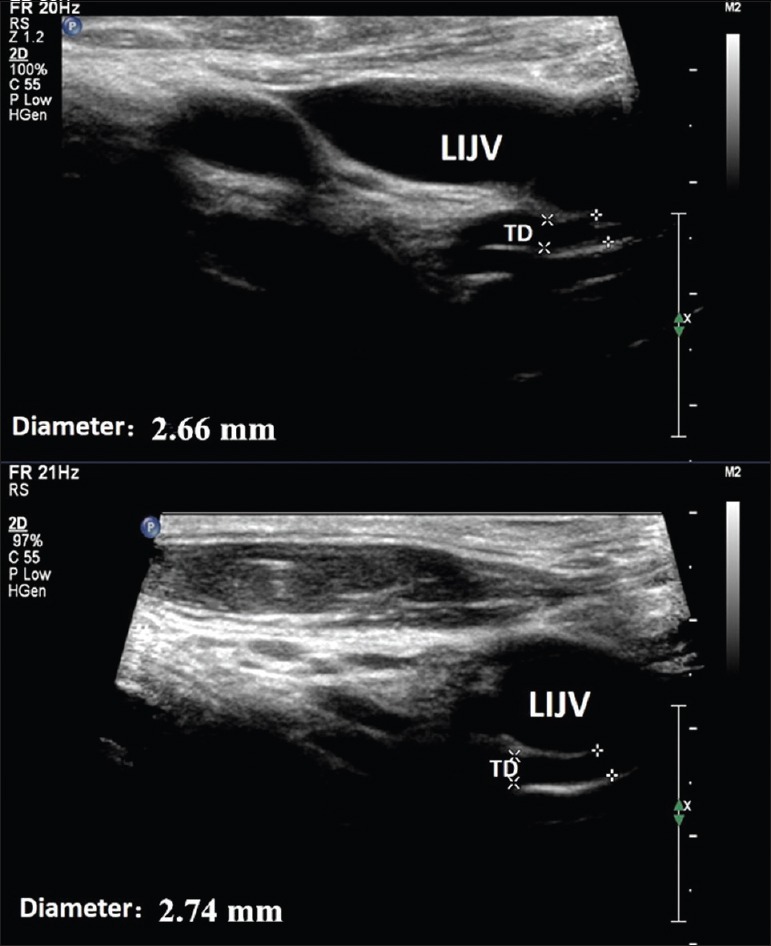
The ultrasound results of healthy volunteers. The TD wall was continuous and the inner structure appears anechoic. The diameter of the terminal TD is shown. LIJV: Left internal jugular vein; TD: Thoracic duct.
The examination results for most of the patients with lymphedema were abnormal (24 of 32 patients). The TD walls were thickened, the lymph vessels were irregularly narrow, and the valves were difficult to detect [Figure 2]. Furthermore, we were able to locate lymph deposits in the TD lumen, which might have induced slower lymph flow and faster adipose accumulation. Compared with the healthy volunteers’ group, the diameter of the TD in patients with lymphedema was significantly decreased (2.65 ± 0.20 mm vs. 2.20 ± 0.50 mm, P = 0.01). Meanwhile, there was no difference in the diameter of the TD between patients with the left upper limb lymphedema and with the lower extremity lymphedema (2.10 ± 0.50 mm vs. 2.10 ± 0.60 mm, P = 0.44).
Figure 2.
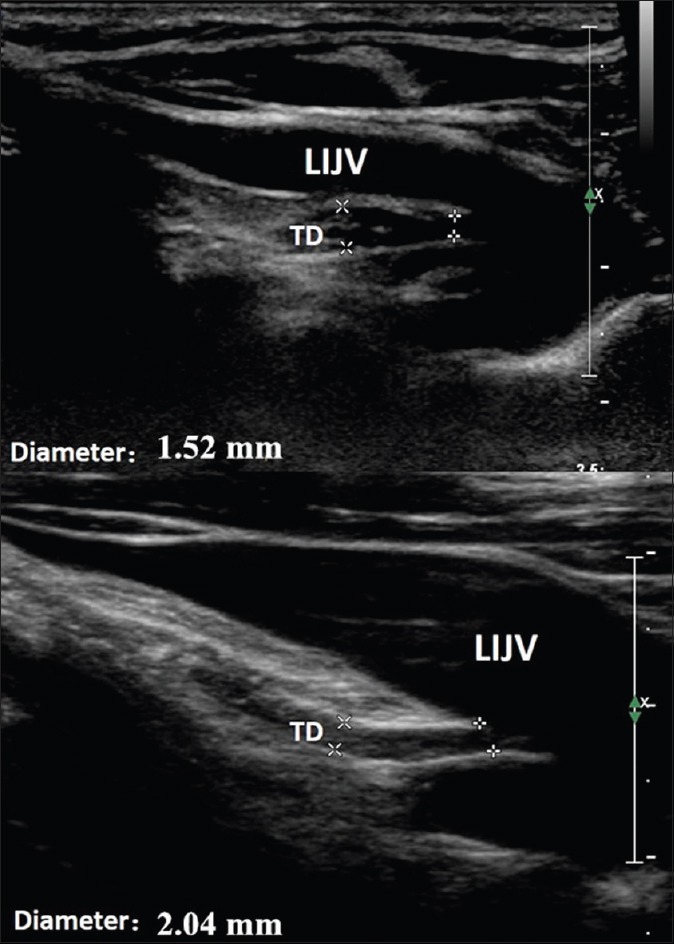
The ultrasound results of patients with lymphedema. The TD walls were thickened, and the inner structure appears hypoechoic and heterogeneous. The diameter of the terminal TD is shown. LIJV: Left internal jugular vein; TD: Thoracic duct.
Discussion
The TD usually arises from the cisterna chyli and ascends to the thorax. It generally locates to the right of the aorta and exits the thorax between the esophagus and the left subclavian artery. The lymph fluids from the lower half of the body and the abdominal cavity join the chyle, which is derived from the intestinal trunk, and frequently drain into the junction of the left jugular and subclavian veins. The right lymphatic duct drains fluid from the right arm, right side of the head, and right thoracic cavity, and then empties into the right subclavian vein.[11] Therefore, patients with right upper limb lymphedema were excluded from our study. As a connecting terminal between the lymph circulation and the venous system, the terminal TD provides a great deal of influence on the whole lymph circulation. Some studies reported that many diseases such as chylothorax, lymphoma, and portal hypertension could lead to changes in the morphology and function of the terminal TD. Moreover, the pathological changes of the end TD could in turn aggravate the primary disease.[12,13,14]
With the advancement in ultrasound technologies, detailed structure and function of the terminal TD can be further assessed through analyzing the sonographic images for all the patients and healthy volunteers. The results of our study showed that the diameter of the TD in healthy volunteers was 2.65 ± 0.20 mm, which was consistent with the past studies.[9]
Our results showed that patients with lymphedema presented abnormal changes in the terminal TD, which was frequently ignored in clinical evaluation because lymphedema was often regarded as the destruction in the local lymph circulation. Our ultrasound examination showed the presence of TD stenosis and deposits in the lymph lumen, which were both considered as the characteristic manifestations of lymphedema. Furthermore, examination methods such as lymphoscintigraphy had also detected abnormal accumulation of lymphatic products in some of the patients with lymphedema. Unfortunately, we were not able to obtain more pathological results to confirm our hypothesis due to the lack of experience in TD biopsy under ultrasound guidance.
The ultrasound examination could provide us with more detailed information on the terminal TD than CT or MRI. The advantages of ultrasonography include the convenience, the relatively low examination cost, and the lack of contrast agent requirements. However, ultrasound cannot substitute for CT or MRI because ultrasonography cannot illustrate the entire lymph circulation. Through our study, the findings in the changes of terminal TD in patients with lymphedema might assist physicians in evaluating the progression and severity of lymphedema and might also be an independent risk factor for patients with lymphedema. In the future, long-term follow-up is essential for further analysis of the changes in terminal TD.
In summary, this study found ultrasonic examination as a convenient and accurate method to assess the morphological and functional changes of TD in patients with lymphedema. The findings of the terminal TD in patients with lymphedema included lymphatic stenosis, lymph deposits, and the thickening of lymphatic vessel walls. We believed that ultrasonography may be advantageous in the prognosis, evaluation, and treatment of lymphedema.
Financial support and sponsorship
This study was supported by grants from Beijing Nova Program from the Government of Beijing, China (No. Z131107000413063), and the National Natural Science Funds for Distinguished Young Scholar (No. 81301268).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
References
- 1.Cemal Y, Jewell S, Albornoz CR, Pusic A, Mehrara BJ. Systematic review of quality of life and patient reported outcomes in patients with oncologic related lower extremity lymphedema. Lymphat Res Biol. 2013;11:14–9. doi: 10.1089/lrb.2012.0015. doi: 10.1089/lrb.2012.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brayton KM, Hirsch AT, O Brien PJ, Cheville A, Karaca-Mandic P, Rockson SG. Lymphedema prevalence and treatment benefits in cancer: Impact of a therapeutic intervention on health outcomes and costs. PLoS One. 2014;9:e114597. doi: 10.1371/journal.pone.0114597. doi: 10.1371/journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tiwari P, Coriddi M, Salani R, Povoski SP. Breast and gynecologic cancer-related extremity lymphedema: A review of diagnostic modalities and management options. World J Surg Oncol. 2013;11:237. doi: 10.1186/1477-7819-11-237. doi: 10.1186/1477-7819-11-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson KC, DeSarno M, Ashikaga T, Dee J, Henry SM. Ultrasound and clinical measures for lymphedema. Lymphat Res Biol. 2016;14:8–17. doi: 10.1089/lrb.2015.0001. doi: 10.1089/lrb.2015.0001. [DOI] [PubMed] [Google Scholar]
- 5.Johnson KC, Kennedy AG, Henry SM. Clinical measurements of lymphedema. Lymphat Res Biol. 2014;12:216–21. doi: 10.1089/lrb.2014.0019. doi: 10.1089/lrb.2014.0019. [DOI] [PubMed] [Google Scholar]
- 6.Restrepo CS, Eraso A, Ocazionez D, Lemos J, Martinez S, Lemos DF. The diaphragmatic crura and retrocrural space: Normal imaging appearance, variants, and pathologic conditions. Radiographics. 2008;28:1289–305. doi: 10.1148/rg.285075187. doi: 10.1148/rg.285075187. [DOI] [PubMed] [Google Scholar]
- 7.Kiyonaga M, Mori H, Matsumoto S, Yamada Y, Sai M, Okada F. Thoracic duct and cisterna chyli: Evaluation with multidetector row CT. Br J Radiol. 2012;85:1052–8. doi: 10.1259/bjr/19379150. doi: 10.1259/bjr/19379150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okuda I, Udagawa H, Takahashi J, Yamase H, Kohno T, Nakajima Y. Magnetic resonance-thoracic ductography: Imaging aid for thoracic surgery and thoracic duct depiction based on embryological considerations. Gen Thorac Cardiovasc Surg. 2009;57:640–6. doi: 10.1007/s11748-009-0483-4. doi: 10.1007/s11748-009-0483-4. [DOI] [PubMed] [Google Scholar]
- 9.Seeger M, Bewig B, Günther R, Schafmayer C, Vollnberg B, Rubin D, et al. Terminal part of thoracic duct: High-resolution US imaging. Radiology. 2009;252:897–904. doi: 10.1148/radiol.2531082036. doi: 10.1148/radiol.2531082036. [DOI] [PubMed] [Google Scholar]
- 10.Witte CL, Witte MH, Unger EC, Williams WH, Bernas MJ, McNeill GC, et al. Advances in imaging of lymph flow disorders. Radiographics. 2000;20:1697–719. doi: 10.1148/radiographics.20.6.g00nv141697. doi: 10.1148/radiographics.20.6.g00nv141697. [DOI] [PubMed] [Google Scholar]
- 11.Johnson OW, Chick JF, Chauhan NR, Fairchild AH, Fan CM, Stecker MS, et al. The thoracic duct: Clinical importance, anatomic variation, imaging, and embolization. Eur Radiol. 2016;26:2482–93. doi: 10.1007/s00330-015-4112-6. doi: 10.1007/s00330-015-4112-6. [DOI] [PubMed] [Google Scholar]
- 12.Schild HH, Strassburg CP, Welz A, Kalff J. Treatment options in patients with chylothorax. Dtsch Arztebl Int. 2013;110:819–26. doi: 10.3238/arztebl.2013.0819. doi: 10.3238/arztebl.2013.0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito K, Shimizu A, Tanabe M, Matsunaga N. Cisterna chyli in patients with portal hypertension: Evaluation with MR imaging. J Magn Reson Imaging. 2012;35:624–8. doi: 10.1002/jmri.22875. doi: 10.1002/jmri.22875. [DOI] [PubMed] [Google Scholar]
- 14.Gerstein J, Kofahl-Krause D, Frühauf J, Bremer M. Complete remission of a lymphoma-associated chylothorax by radiotherapy of the celiac trunk and thoracic duct. Strahlenther Onkol. 2008;184:484–7. doi: 10.1007/s00066-008-1840-4. doi: 10.1007/s00066-008-1840-4. [DOI] [PubMed] [Google Scholar]


