Abstract
Telomere length is negatively regulated by proteins of the telomeric DNA-protein complex. Rap1p in Saccharomyces cerevisiae binds the telomeric TG1-3 repeat DNA, and the Rap1p C terminus interacts with Rif1p and Rif2p. We investigated how these three proteins negatively regulate telomere length. We show that direct tethering of each Rif protein to a telomere shortens that telomere proportionally to the number of tethered molecules, similar to previously reported counting of Rap1p. Surprisingly, Rif proteins could also regulate telomere length even when the Rap1p C terminus was absent, and tethered Rap1p counting was completely dependent on the Rif proteins. Thus, Rap1p counting is in fact Rif protein counting. In genetic settings that cause telomeres to be abnormally long, tethering even a single Rif2p molecule was sufficient for maximal effectiveness in preventing the telomere overelongation. We show that a heterologous protein oligomerization domain, the mammalian PDZ domain, when fused to Rap1p can confer telomere length control. We propose that a nucleation and spreading mechanism is involved in forming the higher-order telomere structure that regulates telomere length.
Essential for faithful chromosome maintenance and replication, the ends of eukaryotic chromosomes, telomeres, are dynamic entities whose structures are regulated (26, 43). In the budding yeast Saccharomyces cerevisiae, each telomeric terminal DNA region consists of a tract of irregular TG1-3 repeat sequences onto which sequence-specific DNA binding proteins and associated proteins assemble. Telomeres in dividing yeast cells are maintained between 250 to 350 bp through a dynamic balance of lengthening and shortening activities. Telomeric DNA shortens as a result of the inability of the general DNA replication machinery to fully replicate the ends of linear DNA molecules (the “end replication problem”) and nuclease action (31, 51). Telomere lengthening is primarily mediated by telomerase, a cellular reverse transcriptase that catalyzes the de novo addition of telomeric DNA to chromosome ends, using a sequence within its intrinsic RNA subunit as a template (3).
How telomere structure is modulated by the telomeric DNA tract length and how length-dependent structural changes regulate the lengthening and shortening activities at the telomere are central but unresolved questions. Available data support the proposal that long telomeres assume an as-yet-unknown structural state that is inhibitory for telomerase-mediated telomere elongation, while short telomeres can undergo a structural change that results in either recruitment or activation of telomerase (4, 46). Physical models for higher-order telomere structure have been proposed. A “fold-back” model places the end of the telomere inward toward the subtelomeric region, preventing action by telomerase (11, 33, 45). In a related model, suggested by the clustering of telomeres at the nuclear periphery (17), telomere-telomere interactions restrict accessibility to telomerase. Another idea is based on T-loop DNA structures that have been isolated from human cell telomeres, in which the single-stranded 3′ end of the telomeric DNA invades and base pairs with more internal telomeric repeat sequences (19).
Rap1p is implicated in regulating telomere length in yeast. Rap1p binds duplex telomeric DNA via its DNA binding domain (DBD) on average every 18 bp (15). Several lines of evidence had suggested that Rap1p (which is also an essential transcriptional regulator of many genes) is a direct negative regulator of telomere length. Overexpression of the C terminus of Rap1p lacking the DBD or introduction of extra telomeric DNA sequence on plasmids into yeast causes average telomere lengths to increase, consistent with titration of negative length regulators off telomeres (10, 39). Various rap1t alleles cause elongated telomeres, ranging from ∼0.8 to ∼3 kb; these alleles encode mutant proteins that retain the Rap1p DBD and essential transcriptional functions, but lack the C-terminal domain, which interacts with other telomeric proteins (25). Disrupting the binding of Rap1p to telomeric DNA by introducing mutant telomeric repeats can also result in telomere elongation; for several such mutant telomeric sequences, the lengthening was proportional to their loss of Rap1p binding affinity in vitro (24, 37). Finally, targeting the C-terminal domain of Rap1p to the immediate subtelomere (i.e., immediately adjacent to the telomeric repeat sequence) as a Gal4p DBD fusion causes telomere shortening proportional to the number of tethered Rap1p C termini (32). Hence a Rap1p C-terminus counting model was proposed to explain how Rap1p negatively regulates telomere length. By this model, the property of a telomere that is sensed is not the length per se of its telomeric DNA, but rather the number of Rap1p C termini bound to it, which would serve as a readout of telomere length.
Length sensing and regulation are likely intimately related to the telomere structure formed by Rap1p and associated proteins bound to telomeric DNA. In vitro, Rap1p bends telomeric DNA (35, 49), implying that Rap1p molecules bound along a series of successive binding sites will fold the DNA into an overall trajectory of as-yet-unknown form. The importance of telomere structure for length regulation is highlighted by the observation that the spacing between consecutive Rap1p binding sites determines whether or not those sites are “seen” by the Rap1p counting mechanism; while 15/20-, 22-, and 27-bp spacings are counted, 17- and 31-bp spacings are not (20). Additionally, placing a 138-bp stretch of nontelomeric DNA between consensus Rap1p binding sites disrupts such Rap1p counting (38).
In yeast two-hybrid experiments, Rif1p and Rif2p interact with the C terminus of Rap1p and with each other (21, 50). Like Rap1p, both Rif proteins localize to yeast telomeres by a one-hybrid assay (5) and by chromatin immunoprecipitation (29, 43). Also like Rap1p, Rif1p and Rif2p negatively regulate telomere length; telomeres in rif1Δ strains lengthen to ∼0.5 to 1 kb and those in rif2Δ strains lengthen to ∼0.35 to 0.5 kb. rif1Δ rif2Δ has a synergistic effect, with telomere lengths ranging between ∼0.8 and 3 kb (21, 50). Conversely, Rif1p or Rif2p overexpression causes telomere shortening (50). Telomere lengthening by Rif protein deletion is telomerase dependent and RAD52 independent (47).
We investigated the mechanisms by which these telomeric proteins negatively regulate telomere length. We show that Rif proteins are directly counted and that Rap1p counting is primarily a consequence of Rif1p and Rif2p recruitment to the telomeres. Furthermore, even in the complete absence of the Rap1p C terminus, Rif proteins can prevent overelongation of telomeres. We conclude that telomere length regulation by Rap1p counting is in fact Rif protein counting. In certain genetic settings in which telomeres are long, a single targeted Rif2p is sufficient for a maximal effect in curtailing telomere overlengthening, while tethered Rif1p still shows a counting trend. Finally, simply targeting heterologous PDZ protein-protein interaction domains to the telomere is sufficient to mimic the cis-acting, negative telomere length regulation by Rif proteins. We propose that Rif1p and Rif2p regulate telomere length through distinct protein counting mechanisms that involve protein-protein interactions.
MATERIALS AND METHODS
Plasmids.
Plasmids used to generate yeast strains with Gal4p upstream activating sequence (UAS) sites at the immediate subtelomeric region of chromosome VIIL were derived from sp59 (32). First the BamHI/NotI fragment of sp59 was replaced with an oligonucleotide cassette composed of ODL23 and ODL24 to generate pDL1. (The plasmids and oligonucleotides used in this study are in Tables S1 and S2 in supplemental material.) Next, the SacI/NotI fragment of pDL1 was replaced with an oligonucleotide cassette composed of ODL68 and ODL69 to make pDL1*. pDL1* was digested with BamHI, and Gal4p UAS sites were cloned in as an oligonucleotide cassette composed of ODL95 and ODL96. The integrating plasmids expressing Gal4p DBD (GBD) fusions were as follows: pRS403 GBD only under the RIF1 promoter (pDL116), pRS403 GBD-Rif1p under the RIF1 promoter (pDL120), pRS403 GBD only under the RIF2 promoter (pDL114), pRS403 GBD-Rif2p under the RIF2 promoter (pDL115), pRS403 GBD only under the RAP1 promoter (pDL125), and pRS403 GBD-Rap1p C terminus (amino acids [aa] 653 to 827) under the RAP1 promoter (pDL124). Similar constructs cloned into CEN ARS plasmids were also made (9): pRS413 GBD only under the RIF1 promoter (pEHB11094), pRS413 GBD-Rif1p under the RIF1 promoter (pEHB11088), pRS414 GBD only under the RIF2 promoter (pDL56), and pRS414 GBD-Rif2p under the RIF2 promoter (pDL47). Plasmids pDL88 and pDL134 were used for making C-terminal fusions of PDZ to RAP1 and rap1ΔC where PDZ is aa 471 to 753 from rat GRIP1 (12). For overexpression of the free PDZ domain, plasmid pDL142 was used and pDL141 served as the vector-only control in such experiments. Additional information about these plasmids and others is listed in supplemental information (see Table S1 in the supplemental material), and details about their construction are available upon request.
Yeast strains and methods.
Standard methods of yeast genetics and molecular biology were used. The yeast strains used in this study are listed in Table 1. To generate strains (“counting-test strains”) with subtelomeric Gal4p UAS sites at chromosome VIIL, S288C-BY4705a (EHB11114) (6) was transformed with SalI/NotI-digested pDL1*, pDL55, pDL49, pDL52, pDL61, and pDL72 and selected for Ura+. Telomeric integrants were verified by Southern blot. For Rif1p tethering experiments, pDL116 and pDL120 were digested with Bsu36I to loop in at the RIF1 promoter. For Rif2p tethering, pDL114 and pDL115 were integrated into the RIF2 3′ untranslated region (UTR) by digesting with AgeI. Counting of Rap1p C termini was done by transforming Eco47III-digested pDL125 and pDL124 to integrate at his3Δ200. In all cases, His+ integrants were verified by diagnostic colony PCR. Test strains for examining the effect of tethered Rif1p, Rif2p, or Rap1p C termini on telomere length were passaged on plates or in liquid culture prior to measuring telomere length by URA3 teloblot as described below (see the supplemental material for a comparison of results obtained by passaging strains on plates versus in liquid culture). For experiments in which GBD fusions were expressed off CEN ARS plasmids, pDL47, pDL56, pEHB11088, or pEHB11094 was transformed into counting-test strains and transformants were passaged in selective media either on plates or in liquid culture.
TABLE 1.
S. cerevisiae strains used in this study
| EHB11114 | MATaade2Δ::hisG his3Δ200 leu2Δ0 lys2Δ0 met15Δ0 trp1Δ63 ura3Δ0 |
| EHB11125 | EHB11114 except adh4::URA3-TEL-VIIL |
| EHB11126 | EHB11114 except adh4::URA3-(UASG)1-TEL-VIIL |
| EHB11127 | EHB11114 except adh4::URA3-(UASG)2-TEL-VIIL |
| EHB11128 | EHB11114 except adh4::URA3-(UASG)3-TEL-VIIL |
| EHB11129 | EHB11114 except adh4::URA3-(UASG)4-TEL-VIIL |
| EHB11130 | EHB11114 except adh4::URA3-(UASG)6-TEL-VIIL |
| EHB11188 | MATa/α ade2Δ::hisG/ade2Δ::hisG his3Δ200/his3Δ200 leu2Δ0/leu2Δ0lys2Δ0/lys2Δ0 met15Δ0/met15Δ0 trp1Δ63/trp1Δ63 ura3Δ0/ura3Δ0 RIF1/rif1::TRP1 RIF2/rif2::kanMX6 |
| EHB11200 | EHB11188 except RAP1/RAP1-PDZ-HIS3 |
| EHB11201 | EHB11188 except RAP1/RAP1-HIS3 |
| EHB11205 | EHB11188 except RAP1/RAP1::pDL88(PmlI)-RAP1 |
| EHB11256 | MATa/α ade2Δ::hisG/ade2Δ::hisG his3Δ200/his3Δ200 leu2Δ0/leu2Δ0lys2Δ0/lys2Δ0 met15Δ0/met15Δ0 trp1Δ63/trp1Δ63 ura3Δ0/ura3Δ0 RIF1/rif1::TRF1 RIF2/rif2::kanMX6 RAP1/rap1ΔC-LEU2 |
| EHB11258 | MATα ade2Δ::hisG his3Δ200 leu2Δ0 lys2Δ0 met15Δ0 trp1Δ63 ura3Δ0 rif1::TRP1 rif2::kanMX6 rap1ΔC-LEU2 |
| EHB11259 | MATaade2Δ::hisG his3Δ200 leu2Δ0 lys2Δ0 met15Δ0 trp1Δ63 ura3Δ0 rif1::TRP1 rif2::kanMX6 rap1ΔC-LEU2 |
| EHB11261 | EHB11125 except RIF2::RIF2-pDL114(AgeI) |
| EHB11262 | EHB11126 except RIF2::RIF2-pDL114(AgeI) |
| EHB11263 | EHB11127 except RIF2::RIF2-pDL114(AgeI) |
| EHB11264 | EHB11128 except RIF2::RIF2-pDL114(AgeI) |
| EHB11265 | EHB11129 except RIF2::RIF2-pDL114(AgeI) |
| EHB11266 | EHB11130 except RIF2::RIF2-pDL114(AgeI) |
| EHB11267 | EHB11125 except RIF2::RIF2-pDL115(AgeI) |
| EHB11268 | EHB11126 except RIF2::RIF2-pDL115(AgeI) |
| EHB11269 | EHB11127 except RIF2::RIF2-pDL115(AgeI) |
| EHB11270 | EHB11128 except RIF2::RIF2-pDL115(AgeI) |
| EHB11271 | EHB11129 except RIF2::RIF2-pDL115(AgeI) |
| EHB11272 | EHB11130 except RIF2::RIF2-pDL115(AgeI) |
| EHB11273 | EHB11125 except RIF1::pDL116(Bsu361)-RIF1 |
| EHB11274 | EHB11126 except RIF1::pDL116(Bsu361)-RIF1 |
| EHB11275 | EHB11127 except RIF1::pDL116(Bsu361)-RIF1 |
| EHB11276 | EHB11128 except RIF1::pDL116(Bsu361)-RIF1 |
| EHB11277 | EHB11129 except RIF1::pDL116(Bsu361)-RIF1 |
| EHB11278 | EHB11130 except RIF1::pDL116(Bsu361)-RIF1 |
| EHB11279 | EHB11125 except RIF1::pDL120(Bsu361)-RIF1 |
| EHB11280 | EHB11126 except RIF1::pDL120(Bsu361)-RIF1 |
| EHB11281 | EHB11127 except RIF1::pDL120(Bsu361)-RIF1 |
| EHB11282 | EHB11128 except RIF1::pDL120(Bsu361)-RIF1 |
| EHB11283 | EHB11129 except RIF1::pDL120(Bsu361)-RIF1 |
| EHB11284 | EHB11130 except RIF1::pDL120(Bsu361)-RIF1 |
| EHB11298 | EHB11256 except HIS3-PGALI-RIF1/rif1::TRP1 |
| EHB11300 | EHB11256 except HIS3-PGALI-RIF2/rif2::kanMX6 |
| EHB11308 | EHB11125 except his3Δ200::pDL125(Eco47III)-his3Δ200 |
| EHB11309 | EHB11126 except his3Δ200::pDL125(Eco47III)-his3Δ200 |
| EHB11310 | EHB11127 except his3Δ200::pDL125(Eco47III)-his3Δ200 |
| EHB11311 | EHB11128 except his3Δ200::pDL125(Eco47III)-his3Δ200 |
| EHB11312 | EHB11129 except his3Δ200::pDL125(Eco47III)-his3Δ200 |
| EHB11313 | EHB11130 except his3Δ200::pDL125(Eco47III)-his3Δ200 |
| EHB11314 | EHB11125 except his3Δ200::pDL124(Eco47III)-his3Δ200 |
| EHB11315 | EHB11126 except his3Δ200::pDL124(Eco47III)-his3Δ200 |
| EHB11316 | EHB11127 except his3Δ200::pDL124(Eco47III)-his3Δ200 |
| EHB11317 | EHB11128 except his3Δ200::pDL124(Eco47III)-his3Δ200 |
| EHB11318 | EHB11129 except his3Δ200::pDL124(Eco47III)-his3Δ200 |
| EHB11319 | EHB11130 except his3Δ200::pDL124(Eco47III)-his3Δ200 |
| EHB11330 | EHB11188 except RAP1/rap1ΔC-PDZ-LEU2 |
| EHB11345 | EHB11256 except RAD52/rad52::natMX4 |
| EHB11349 | EHB11330 except RAD52/rad52::natMX4 |
| EHB11351 | EHB11261 × EHB11258 except RAD52/rad52::natMX4 |
| EHB11352 | EHB11262 × EHB11258 except RAD52/rad52::natMX4 |
| EHB11353 | EHB11265 × EHB11258 except RAD52/rad52::natMX4 |
| EHB11354 | EHB11267 × EHB11258 except RAD52/rad52::natMX4 |
| EHB11355 | EHB11268 × EHB11258 except RAD52/rad52::natMX4 |
| EHB11356 | EHB11271 × EHB11258 except RAD52/rad52::natMX4 |
| EHB11357 | EHB11273 × EHB11258 except RAD52/rad52::natMX4 |
| EHB11358 | EHB11274 × EHB11258 except RAD52/rad52::natMX4 |
| EHB11359 | EHB11277 × EHB11258 except RAD52/rad52::natMX4 |
| EHB11360 | EHB11279 × EHB11258 except RAD52/rad52::natMX4 |
| EHB11361 | EHB11280 × EHB11258 except RAD52/rad52::natMX4 |
| EHB11362 | EHB11283 × EHB11258 except RAD52/rad52::natMX4 |
Gene deletions were done as described previously (30). Deletion of RIF1 was done by transformation with PCR product templated off pFA6a-TRP1 with oligonucleotides ODL143 and ODL144. RIF2 disruption was with PCR product made off pFA6a-kanMX6 with ODL131 and ODL132. Deletion of RAD52 was done by transformation with PCR product templated off pFA6a-natMX4 (pAG25) (16) with oligonucleotides o13029 and o13030. To introduce the rap1ΔC mutation (deletion of nucleotides 1990 to 2481; deletion of aa 664 to 827), PCR product was made with ODL109 and ODL141 templated off pDL106. Leu+ transformants were selected, and the mutation was verified by diagnostic colony PCR and sequencing of the PCR product. The rap1ΔC allele introduces P662L and deletes aa 664 to 827 followed by TGA stop, 236-bp RAP1 3′ UTR, and LEU2; this allele is analogous to rap1-17 but deletes the Rap1p C terminus encoding DNA (18, 25). rap1-17 was originally identified as an intragenic suppressor of the temperature-sensitive rap1-5 allele, which encodes a mutant Rap1p, with a point mutation in the C terminus. Since the rap1-17 allele has a premature stop codon preceding the rap1-5 lesion, rap1-17 encodes a C-terminally truncated protein lacking the rap1-5 mutation (25). The original rap1-17 allele and our rap1ΔC allele encode the same truncated Rap1p. They differ only in the sequence downstream of the stop codon: while rap1ΔC coding sequence is followed immediately by endogenous RAP1 3′ UTR sequence, rap1-17 coding sequence is followed by rap1-5 C-terminus-encoding DNA. Hence, rap1ΔC cells express precisely the same truncated version of Rap1p that is expressed in rap1-17 cells.
Modification of the genetic background of counting-test strains (i.e., strains with tethered Rif1p, Rif2p, or Rap1p C termini) was done by mating to EHB11258 followed by sporulation of the heterozygous diploid, dissection of tetrads, and selection of strains with the desired genotypes. Diploid strains EHB11351 to -11362 were made by mating EHB11261, -11262, -11265, -11267, -11268, -11271, -11273, -11274, -11277, -11279, -11280, and -11283 to EHB11258, respectively, and then selecting diploids, deleting RAD52 as described above to make RAD52/rad52::natMX4, and verifying the deletion and overall genotype by diagnostic colony PCR and checking all markers.
Strains overexpressing RIF1 and RIF2 were generated as described previously (30). The PCR product was made with ODL213 and ODL214 (for Gal Rif1p) and ODL215 and ODL216 (for Gal Rif2p) templated off pFA6a-His3MX6-PGAL1. The PCR product was transformed into EHB11256. Integration of the Gal promoter was verified for His+ colonies by diagnostic colony PCR and sequencing of the PCR product. EHB11298 and EHB11300 were sporulated and dissected on yeast extract-peptone-galactose (YP-gal) plates. Spores were genotyped and passaged on YP-gal plates prior to examination of telomere lengths by TG1-3 teloblots (see below).
EHB11200 was made by transforming EHB11188 with PCR product generated off pDL88 with ODL184 and ODL141. This fuses PDZ to the C terminus of RAP1 followed by two stop codons, CYC1 terminator sequence, and HIS3. EHB11201 was a control strain consisting of RAP1 followed by two stop codons, the CYC1 terminator, and HIS3; it was made by transforming EHB11188 with a PCR product made from pDL88 with ODL142 and ODL141. EHB11205 was another control strain that expressed free PDZ domain only from the RAP1 promoter and was made by digesting pDL88 with PmlI and looping the plasmid into a RAP1 promoter in EHB11188. For each strain, His+ integrants were selected and verified by diagnostic yeast colony PCR and sequencing of the PCR product as necessary. EHB11330 replaced the C terminus of RAP1 with PDZ followed by two stop codons, RAP1 3′ UTR sequence, and LEU2. This strain was made by transforming EHB11188 with PCR product made from pDL134 with ODL109 and ODL141. Leu+ transformants were selected and verified by diagnostic yeast colony PCR and sequencing of the PCR product. To make EHB11345 and -11349, EHB11256 and -11330, respectively, were made RAD52/rad52::natMX4 by deleting RAD52 as described above; the deletion and genotype were verified by diagnostic colony PCR and by checking all markers. Each diploid strain was sporulated and dissected at 23°C; spores with the desired genotype were passaged on plates at 23°C prior to determining bulk telomere lengths by TG1-3 teloblot.
Telomere Southern blots.
To measure length of the URA3-marked chromosome VIIL telomere, an EcoRV digestion of genomic DNA was done to generate a telomeric restriction fragment (Fig. 1a). A second, separate digestion of a portion of the DNA sample was done with EcoRV and BamHI to excise a fragment containing the Gal4p UAS sites, and BamHI was inactivated with EDTA. The BamHI/EcoRV fragment served to verify that the expected number of Gal4p UAS sites was present and that none had been lost through recombination (Fig. 1a). These digests were mixed and run together in the same lane of a 0.9% agarose gel along with a 607-bp fragment of URA3 generated by PCR (ODL185 and ODL186 templated off pDL1*). DNA was transferred to Hybond N+ (Amersham Biosciences) and probed for URA3 with a random prime-labeled URA3 PCR product (ODL185 and ODL186 templated off pDL1*). Telomere lengths were measured with ImageQuant (Molecular Dynamics). The peak position for each band was determined and standardized across a given gel using the 607-bp URA3 band as a size standard. Next, relative DNA lengths were converted to absolute lengths with a 1-kb DNA ladder (Invitrogen), end labeled with 32P, and run on the same gel. Finally, to obtain a peak telomere length value (i.e., length of TG1-3 repeat tract), the size of the EcoRV/BamHI band was subtracted from the size of the EcoRV band. Telomeres in which Gal4p UAS sites had been lost as evidenced by a smaller-than-expected BamHI/EcoRV fragment were excluded from these calculations. In some cases with URA3-marked telomeres in long-telomere genetic backgrounds, splitting was observed where lengths broke out into two distinct populations, one population comparable in length to genetically similar isolates and one population that was much shorter (Fig. 1m, lanes marked with †). The frequency of such split telomeres was not dependent on the number of Gal4p UAS sites or on expression of any GBD fusion protein. These telomeres likely arose through the process of telomeric rapid deletion (27). For the purpose of quantification, the band corresponding to the longer population was used.
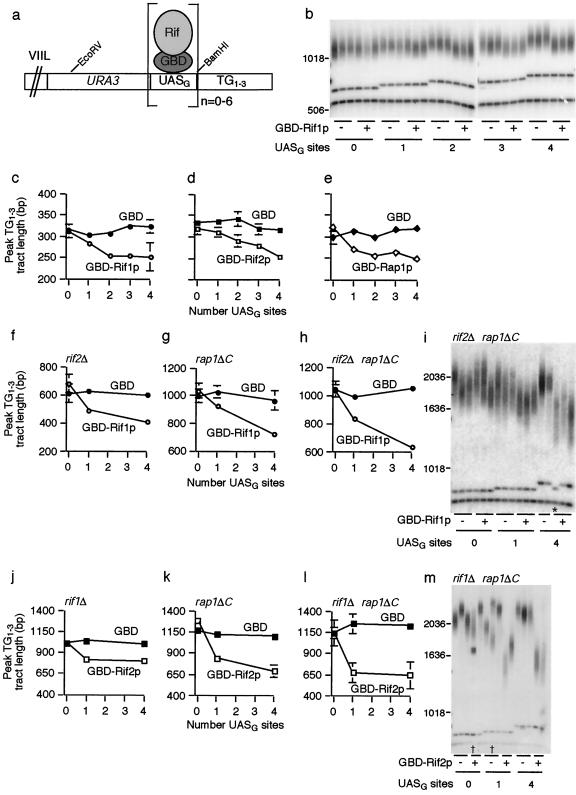
FIG. 1.
Rif1p and Rif2p are counted differently and independently of the C terminus of Rap1p to regulate telomere length. (a) Diagram of URA3-marked chromosome VIIL. UASG represents 0, 1, 2, 3, 4, or 6 Gal4p UAS sites (UASG) that bind GBD alone or GBD fused to Rif1p, Rif2p, or the Rap1p C terminus. (b) Southern blot probed for URA3 to assess counting of Rif1p. GBD only (−) or GBD-Rif1p (+) was expressed in strains with zero to four Gal4p UAS sites. Each group of lanes represents three independent strain isolates. The upper band in each lane is the EcoRV telomeric restriction fragment. The second band is the EcoRV/BamHI fragment that contains the UASG sites and part of the URA3 gene. The bottom band is a 607-bp fragment of URA3 included as a size standard. Marker sizes (base pairs) are shown to the left of the gel. (c, d, and e) URA3-marked telomere lengths were measured as described in Materials and Methods, and the peak TG1-3 tract length was plotted. Each datapoint represents the average peak telomere length from three to six independent experiments. Standard deviations are displayed and were less than 11 bp where not visible. (f, g, and h) Each data point represents the average peak telomere length from two to five experiments. Standard deviations are displayed and were less than 30 bp where not visible. (i) Rif1p counting Southern blot in a rif2Δ rap1ΔC background. Symbols and bands are as described for panel b. The lane marked with an asterisk represents a strain in which two Gal4p UAS sites recombined out. Recombinational loss of UASG sites was periodically observed in all experiments, and such telomeres were excluded from length calculations. (j, k, and l) Each data point represents the average peak telomere length from two to four experiments, and standard deviations were less than 12 bp (j), 70 bp (k), or 34 bp (l) where not visible. (m) Southern blot examining Rif2p counting in a rif1Δ rap1ΔC background. GBD only (−) or GBD-Rif2p (+) was expressed in rif1Δ rap1ΔC strains with zero, one, or four UASG sites. Bands are as described for panel b. Lanes marked with † are examples of “split” telomeres arising from telomeric rapid deletion (27); the upper band was used for quantification in each case.
For Fig. 1b, EHB11273 to -11284 were passaged in liquid culture for 15 days in log phase prior to determining the length of the URA3-marked telomere. In Fig. 1c, GBD only or GBD-Rif1p was expressed from the RIF1 promoter. Constructs were integrated as a single copy at the RIF1 locus. Strains were passaged for 15 days in liquid culture prior to performing URA3 teloblots. For Fig. 1d, GBD only or GBD-Rif2p was expressed from the RIF2 promoter on a CEN ARS plasmid (pDL56 and pDL47, respectively) and strains were passaged for 15 days in liquid synthetic dropout medium (SD-Trp). For Fig. 1e, GBD only or GBD-Rap1p C terminus was expressed from the RAP1 promoter. Constructs were integrated as a single copy at his3Δ200, and strains were passaged for 15 days in liquid culture. Additional information on experiments similar to those presented in Fig. 1b to e but conducted by passaging strains on plates instead of in liquid culture is presented in supplemental information.
In Fig. 1f, strains expressing GBD only or GBD-Rif1p under the RIF1 promoter off a CEN ARS plasmid (pEHB11094 and pEHB11088, respectively) were made rif2Δ by transformation. Strains were passaged three streaks on SD-His plates. Data for Fig. 1g to i were obtained similarly to those for Fig. 1c, except that the genetic background of these strains was altered as indicated and as described above (i.e., by mating to EHB11258, sporulating, and dissecting) and strains were passaged three streaks on plates rather than in liquid culture. Data for Fig. 1j to k were obtained similarly to Fig. 1d, except that the genetic background of these strains was altered as indicated, and strains were passaged three streaks on SD-Trp plates rather than in liquid culture. For Fig. 1l to m, constructs expressing GBD only or GBD-Rif2p from the RIF2 promoter were integrated as a single copy at the RIF2 locus. The genetic background of these strains was made rif1Δ rap1ΔC, and strains were passaged three streaks on plates. Data for Fig. 4 were obtained similarly to Fig. 1e, except that the genetic background of these strains was altered as indicated, and strains were passaged five to six streaks on plates rather than in liquid culture.
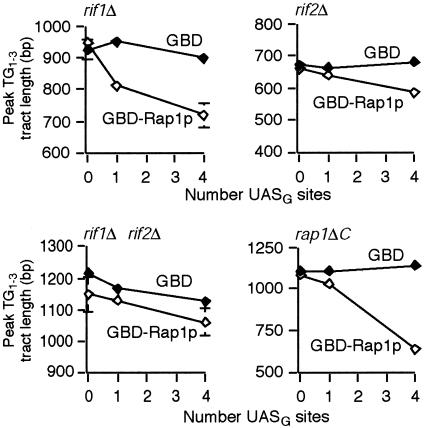
FIG. 4.
Rap1p C-terminus counting is dependent on Rif1p and Rif2p. Southern blots were probed for URA3 examining Rap1p C-terminus counting in different genetic backgrounds. Each data point represents the average peak telomere length from two to three independent experiments. Standard deviations are displayed and were less than 26 bp where not visible for rif1Δ, rif2Δ, and rif1Δ rif2Δ cells and less than 55 bp for rap1ΔC cells.
To measure bulk telomere lengths, genomic DNA was prepared, digested with XhoI, run on a 0.8% agarose gel, transferred to Hybond N+, and probed for TG1-3 with 32P-end-labeled ODL107.
Chromosome spreads.
Chromosome spreads were prepared and Rap1p was visualized by immunofluorescence with a rabbit anti-Rap1p antibody as previously described (14, 43). Slides were examined with a DeltaVision microscope system (Applied Precision) with a 60× lens and 1.5× magnifier. Fields were pseudo-colored blue for 4′,6′-diamidino-2-phenylindole (DAPI) and green for fluorescein isothiocyanate (FITC; anti-Rap1p). At least 50 spread nuclei were scored for each strain, and the total number of Rap1p foci per nucleus was counted.
RESULTS
Counting of Rif1p and Rif2p.
It was previously shown that telomere length is inversely proportional to the number of tethered Rap1p C termini (32). Since Rif1p and Rif2p interact with the C terminus of Rap1p (21, 50), we tested whether these Rif proteins are also counted. We targeted Rif1p or Rif2p, as GBD fusion proteins, to Gal4p UAS sites (UASG) inserted at the immediate subtelomere of a single telomere, directly adjacent to the telomeric repeat sequence (Fig. 1a). In a wild-type background, counting of tethered Rif1p was similar in trend and overall magnitude to that of Rap1p C termini. The greater the number of tandem UASG sites, and presumably of tethered Rif1p molecules, the shorter the telomere became (Fig. 1b and c). As previously reported for Rap1p, the magnitude of additional shortening with each added tethering site became progressively less as the number of tethered Rif1p molecules increased (Fig. 1b, c, and e) (32). Tethered Rif2p also was counted (Fig. 1d). Four targeted Rif1p or Rif2p molecules caused about 70 bp of shortening overall, an effect similar to that reported with tethered Rap1p C termini (32) (Fig. 1c to e). Results with a telomere bearing six UASG sites were indistinguishable from those with four sites (data not shown). Targeting Rif1p, Rif2p, or Rap1p C termini to UASG sites embedded within the telomeric repeat tract effected shortening similar to that when the same fusions were tethered at the immediate subtelomere (data not shown). In summary, in cells with initially wild-type-length telomeres, direct tethering of either Rif1p or Rif2p caused the targeted telomere to become shorter and the extent of shortening was proportional to the number of tethered molecules, consistent with a Rif protein counting mechanism.
Rif protein counting through distinct mechanisms.
We examined the genetic dependencies of Rif1p and Rif2p counting. We modified the genetic background of counting-test strains by mating them to a rif1Δ rif2Δ rap1ΔC strain, sporulating, and selecting spores with the desired genotypes (or directly by transformation in some cases where noted). The resulting strains were serially passaged either in liquid culture or on plates to allow the URA3-marked test telomere to elongate from the initially wild-type length to equilibrium length, and this length was then measured by Southern blotting.
In the absence of Rif2p, tethered Rif1p was still counted. In such rif2Δ cells, when one Rif1p molecule was tethered to the URA3-marked telomere, that telomere elongated and equilibrated at an average length 204 bp shorter than the zero-UASG-site control telomere; when four UASG sites were present, the final telomere length attained was 56 bp shorter still (Fig. 1f). Strikingly, in rap1ΔC cells, again generated by sporulation of heterozygous diploid strains so that the test telomere started at wild-type length, tethering of Rif1p to the test telomere prevented it from lengthening as much as the control zero-tethering-site telomere (Fig. 1g). The ability of tethered Rif1p to exert negative-telomere-length control in the absence of the C terminus of Rap1p was even more pronounced when Rif2p was also deleted. In rif2Δ rap1ΔC cells, one tethered Rif1p molecule prevented that telomere from full lengthening such that, once elongated to its equilibrium length, it was shorter than the zero-site telomere by 143 bp, and the telomere with four tethering sites was a further 259 bp shorter (Fig. 1h and i). Thus, tethered Rif1p is still counted in the absence of Rif2p and/or the Rap1p C terminus.
Tethered Rif2p was also effective in exerting negative telomere length control on the targeted telomere in cells lacking Rif1p and/or Rap1p C termini. However, in the absence of Rif1p, rather than observing a counting trend, tethered Rif2p prevented telomere elongation to the same extent independently of the number of tethered molecules. Starting with diploid heterozygotes having wild-type-length URA3-marked test telomeres, in the rif1Δ spore progeny, the extent to which telomeres were shorter relative to the zero-UASG-site control was similar whether one or up to four Rif2p tethering sites were present (225 bp shorter with one site and 207 bp shorter with four sites; Fig. 1j). Similarly, in rif1Δ rap1ΔC cells, the same degree of negative length control was seen whether there were one or four tethering sites (571 bp with one site and 580 bp with four sites) (Fig. 1l and m). This potent ability of a single tethered Rif2p to prevent overelongation of the telomere was reproduced in four independent experiments, with each strain background represented by two or three different spore isolates in each experiment. Such a result was never obtained in multiple comparable Rif1p tethering experiments. Thus, Rif2p differs from Rif1p and the Rap1p C terminus in that, in genetic settings where telomeres are longer than usual, a single tethered Rif2p molecule is sufficient for a maximal effect on length control in the absence of the other two proteins.
We repeated a subset of the experiments described above in rad52Δ cells and obtained results comparable to those in RAD52 cells (see Fig. S1 in the supplemental material). Thus, the major recombination pathway is not involved in the length regulatory effects of tethered Rap1p and the Rif proteins. Experiments with control telomeres lacking UASG sites showed that tethering of the Rif proteins is necessary for their length control effects (Fig. 1). Also, bulk telomere lengths for telomeres lacking UASG sites remained unaffected in these tethering experiments (data not shown). Hence the Rif proteins act in cis to regulate telomere length.
Rif proteins can act independently of the Rap1p C terminus and of each other.
It was reported previously that telomeres in cells expressing C-terminal deletions of Rap1p (e.g., rap1-17) are comparable in length to those in rif1Δ rif2Δ double and rif1Δ rif2Δ rap1-17 triple mutants (50). This suggested that the negative length regulatory functions of Rif1p and Rif2p are mediated solely through the C terminus of Rap1p. However, our finding that tethered Rif proteins can potently block overelongation of the test telomere in rap1ΔC cells suggested that the Rif proteins can perform this function independently of the C terminus of Rap1p. We therefore tested whether Rif protein overexpression, rather than telomeric tethering, can block bulk telomere lengthening in rap1ΔC cells. These rap1ΔC cells express a truncated Rap1p constructed with the same amino acids deleted as in the original Rap1-17p. Heterozygous diploid strains with wild-type-length telomeres were sporulated and dissected on plates containing galactose to drive overexpression of RIF1 or RIF2 from a galactose-inducible promoter. Spore products with the desired genotypes were serially passaged on galactose plates to allow telomeres to elongate to their equilibrium lengths. Bulk telomere lengths were compared between rap1ΔC strains expressing either RIF1 or RIF2 from its endogenous promoter or the galactose-inducible promoter.
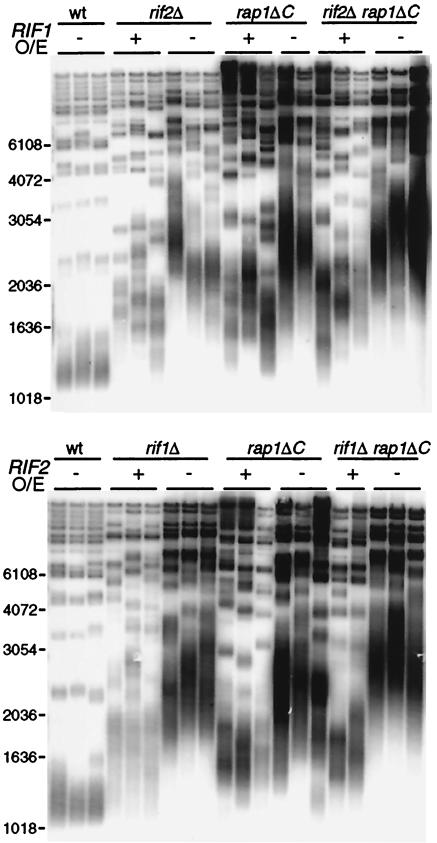
If Rif1p and Rif2p act only through binding the C terminus of Rap1p, then when that domain is deleted, Rif protein overexpression should have no effect on telomere length. Contrary to this prediction, overexpressing either Rif1p or Rif2p in rap1ΔC cells prevented much of the lengthening seen in the control rap1ΔC strains (mean telomere length was at least 450 bp shorter; Fig. 2). (See Fig. S2 and the other supplemental material for further discussion of these results in relation to the previous genetic study using rap1-17 [50].) Rif1p overexpression in rif2Δ cells and Rif2p overexpression in rif1Δ cells each partially suppressed the overlengthening of telomeres that is characteristic of each of these single-deletion strains (Fig. 2). This result is consistent with the previous observation that Rif1p and Rif2p can each partially regulate telomere length without the other, since rif1Δ and rif2Δ single-mutant telomeres are shorter than those in a rif1Δ rif2Δ double mutant (50). Furthermore, even in the absence of the C-terminal domain of Rap1p, Rif1p overexpression could still negatively control telomere overelongation independently of Rif2p and vice versa (Fig. 2). In summary, Rif1p and Rif2p not only can act independently of each other, but also can substantially regulate telomere length without any requirement for the Rap1p C terminus.
FIG. 2.
Rif1p and Rif2p can act independently of each other and the C terminus of Rap1p. Shown is a Southern blot examining changes in bulk telomere length when Rif1p or Rif2p is overexpressed (O/E) in different genetic backgrounds. EHB11256, EHB11298, and EHB11300 (Table 1) were sporulated, dissected on YP-gal plates, and passaged three streaks on YP-gal plates. Strains express RIF1 or RIF2 either under their endogenous promoters (−) or under the GAL1 promoter (+). Each set of lanes represents two or three independent spores. Marker sizes (base pairs) are shown to the left of each gel. wt, wild type.
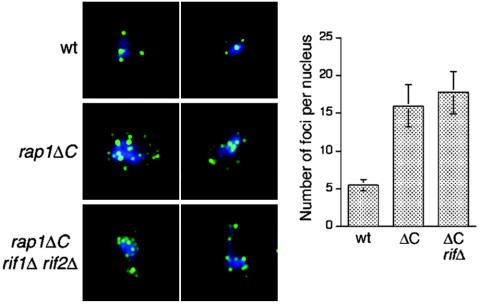
Sir3p and Sir4p are chromatin silencing proteins that also interact with the Rap1p C terminus (22, 34). Since the C-terminal region of Rap1p that interacts with Sir proteins overlaps with the domain that interacts with Rif proteins in vivo and since we had shown that Rif proteins can act independently of this Rap1p C-terminal region, it was conceivable that the Sir proteins might also retain some function in rap1Δ cells. We therefore asked whether, like the Rif proteins, Sir3p and Sir4p might also be able to act at telomeres independently of the Rap1p C terminus by examining an aspect of telomere behavior in which these Sir proteins are known to function: telomere clustering (22, 34). In wild-type cells, immunofluorescence against Rap1p reveals four to six foci at the nuclear periphery and deletion of Sir proteins leads to telomere unclustering and release from the nuclear periphery (22). If Sir proteins could act independently of the Rap1p C terminus, then telomeres might still cluster in rap1ΔC cells. However, in rap1ΔC chromosome spreads stained for Rap1p, we observed loss of clustering (Fig. 3). Hence, Rap1p clustering requires the C terminus of Rap1p. The degree of unclustering was the same in rap1ΔC rif1Δ rif2Δ as in rap1ΔC cells, showing the Rif proteins do not act independently of the C terminus of Rap1p to promote clustering (Fig. 3). In fact, deletion of Rif proteins slightly reduces the average number of Rap1p foci per spread nucleus: approximately three to four foci in rif1Δ cells but only approximately three foci in rif2Δ and rif1Δ rif2Δ cells (D. L. Smith and E. H. Blackburn, unpublished data). Rif and Sir proteins may compete for binding the Rap1p C terminus (50); thus, more Sir proteins might bind Rap1p in the absence of Rif proteins, thereby enhancing clustering.
FIG. 3.
Rap1p foci no longer cluster in the absence of the C terminus of Rap1p. Chromosome spreads were prepared for wild-type (wt), rap1ΔC, and rap1ΔC rif1Δ rif2Δ cells, and Rap1p was visualized by immunofluorescence. DAPI is pseudo-colored in blue and Rap1p is in green. Two representative spreads for each strain are shown. The number of Rap1p foci per spread nucleus was counted for approximately 50 nuclei per strain, and the average number of foci with standard deviation is plotted.
Rap1p counting is mediated predominantly through the Rif proteins.
A prediction of the hypothesis that Rap1p counting is mediated entirely through Rif1p and Rif2p is that Rap1p counting should depend on one or both Rif proteins. In the absence of Rif1p, tethered GBD-Rap1p C termini still were able to control against telomere overelongation: one targeted Rap1p C terminus resulted in the test telomere attaining a final equilibrium length 160 bp shorter than the control zero-site telomere, and the telomere with four sites was 42 bp shorter still (Fig. 4). However, tethered Rap1p C termini had much less of an effect on telomere length in rif2Δ cells and little or no effect in a rif1Δ rif2Δ background (Fig. 4). We further predicted that tethered Rap1p C termini would be counted in a rap1ΔC background, but only in the presence of Rif proteins. Indeed, in rap1ΔC cells, one tethered Rap1p C terminus kept the telomere shorter than the control zero-site telomere by 53 bp and four kept it 422 bp shorter; however, tethered Rap1p C termini were ineffective in the rif2Δ rap1ΔC and rif1Δ rif2Δ rap1ΔC backgrounds (Fig. 4) (data not shown). Strikingly, in rap1ΔC cells, such Rap1p counting absolutely depended on Rif2p but not on Rif1p, consistent with the results in RAP1 cells. Hence, Rap1p counting is mediated substantially through the Rif proteins, with a greater dependence on Rif2p than Rif1p.
A tethered heterologous protein oligomerization domain confers telomere length control.
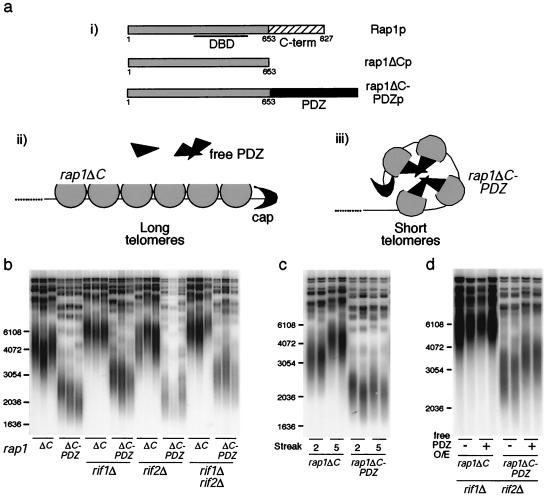
Current information on how telomere length is regulated suggests that Rap1p-DNA and protein-protein interactions act together to fold the telomere into a structure that limits telomerase action or access. To investigate the role of protein-protein interactions in length regulation, we engineered a heterologous protein-protein interaction domain into Rap1p and tested whether this was sufficient to confer telomere length control. We used the PDZ domain, a modular protein-protein interaction motif common in many different mammalian proteins but apparently absent from S. cerevisiae. PDZ domains cluster mammalian cell membrane receptors, thereby effecting localized signaling reactions (41). Since PDZ domains homomultimerize, they have been used in other studies as generic protein-protein interaction domains (13, 36). Specifically, we replaced the C terminus of Rap1p at the endogenous RAP1 locus with PDZ456 of rat GRIP1, which interacts with itself in coimmunoprecipitation and yeast two-hybrid experiments (12). Thus, the only form of Rap1p in the cell was the resulting rap1ΔC-PDZp (Fig. 5a). This fusion protein was expressed at levels comparable to that of rap1ΔCp (see Fig. S3 in the supplemental material), and growth rates of rap1ΔC and rap1ΔC-PDZ strains were similar.
FIG. 5.
PDZ-mediated protein interactions at the telomere confer telomere length regulation. (ai) The upper diagram depicts wild-type Rap1p with its central DBD and a C-terminal domain (aa 653 to 827) that interacts with the Rif proteins. Shown below are rap1ΔCp and rap1ΔC-PDZp, in which the C terminus of Rap1p was replaced with PDZ456 from rat GRIP1. (aii and aiii) Speculative model of the result. rap1ΔCp, partial circles; telomeric cap, crescents; PDZ domain, triangles. In rap1ΔC cells, telomeres are long and expression of free PDZ has no effect on length. Fusion of PDZ to rap1ΔCp may cause the telomere to assume a closed structure mediated by homomeric PDZ interactions, and telomeres equilibrate around a shorter length. (b, c, and d) Southern blots probed for bulk telomere lengths. Marker sizes (base pairs) are shown to the left of each gel. Wild-type Y′ telomeres run as 1.2-kb XhoI fragments on these teloblots. For panel b, heterozygous diploid strains expressing rap1ΔC (EHB11256) or rap1ΔC-PDZ (EHB11330) were sporulated and dissected and the resulting strains were passaged five streaks at 23°C. Each set of lanes represents three independent isolates. In panel c, two rap1ΔC and rap1ΔC-PDZ strains each were passaged two or five streaks. For panel d, one isolate each of rap1ΔC and rap1ΔC-PDZ strains in a rif1Δ rif2Δ background was transformed with a plasmid overexpressing free PDZ domain (pDL142) or a control plasmid (pDL141) and selected on SD−ura plates. Two transformants for each were passaged five streaks on selective medium plates.
Diploid strains heterozygous for rap1ΔC or rap1ΔC-PDZ were sporulated and dissected, and spore products were successively streaked on plates to allow telomeres to elongate from wild type up to their new equilibrium lengths. Bulk telomere lengths were then examined by Southern blotting. In rap1ΔC-PDZ strains, telomeres were kept dramatically shorter than in the rap1ΔC control strains, although they were slightly longer than wild type (Fig. 5b; wild-type length Y′ telomeres are ∼1.2-kb XhoI fragments in the Southern blots shown). This blocking of elongation did not require Rif1p and Rif2p (Fig. 5b). The rap1ΔC-PDZ telomere lengths equilibrated within two passages on plates and were then stable (Fig. 5c). Finally, PDZ fused to the C terminus of full-length Rap1p was also competent in regulating telomere length in the absence of Rif1p and Rif2p (see Fig. S4 in the supplemental material). A subset of these experiments was repeated in rad52 cells, and the same results were obtained (see Fig. S1 in the supplemental material). Hence, PDZ can at least partially substitute for the telomere length regulatory role of the C terminus of Rap1p, without any Rif1p or Rif2p, and not via a recombination-based mechanism.
We tested whether disrupting the PDZ-PDZ interactions at telomeres would cause telomere lengthening. To achieve this, we overexpressed free PDZ in cells expressing rap1ΔC-PDZp; free PDZ was expressed approximately 30-fold over rap1ΔC-PDZp levels (data not shown). Indeed, this caused significant telomere lengthening in rap1ΔC-PDZ cells, but not in control rap1ΔC cells (Fig. 5d; see Fig. S4 in the supplemental material). Such overexpression was unlikely to have titrated the rap1ΔC-PDZp off the telomeres because the fusion protein contains a full Rap1p DBD, and the strength of the Rap1p-DNA interaction (dissociation constant [kD] < 10−11 M) (8, 48) is much greater than that of the PDZ-PDZ interaction (kD ∼10−8 to 10−6 M) (41). The fact that overexpression of free PDZ alone did not cause telomere shortening showed that the PDZ domain itself does not simply inhibit telomerase activity directly nor interact independently with telomeres (Fig. 5d; see Fig. S4 in the supplemental material). Furthermore, because fusion of other protein domains such as GBD or GFP to RAP1 does not lead to shorter telomeres (see the supplemental material), it is unlikely that the PDZ fusion is acting nonspecifically. Taken together, these data show that PDZ domains targeted to telomeres are sufficient to confer negative length regulation, most likely via oligomerization of PDZ domains.
DISCUSSION
A complete understanding of telomere length regulation will ultimately require a mechanistic explanation of how telomere length affects telomere structure and how the telomere length sensing mechanism interfaces with telomerase activity. Here we have shown that Rif proteins on telomeres can be directly counted and that Rap1p C-terminus counting is entirely dependent on Rif1p and Rif2p. Hence, Rap1p counting is actually a Rif protein counting mechanism.
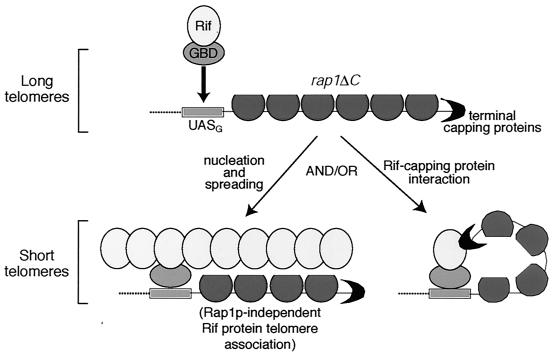
The results reported here also reveal new properties of Rif proteins. The overexpression and tethering experiments show that Rif1p and Rif2p are able to partially negatively regulate telomere length even in the absence of the C terminus of Rap1p, the only protein-protein interaction domain for the Rif proteins known. Although rap1ΔC might conceivably retain residual binding affinity for Rif1p and Rif2p through other domains of Rap1p, in coimmunoprecipitation experiments Rif1p and Rif2p interact with wild-type Rap1p, but not with rap1ΔCp (unpublished data). While normally, in the presence of wild-type Rap1p, the Rif proteins likely are brought to the telomere by interacting with the Rap1p C terminus, our results also provide evidence that Rif proteins can be brought to telomeres by binding other telomeric factors in addition to Rap1p (Fig. 6). Accordingly, we propose a Rif protein counting mechanism, not solely dependent on Rap1p, by which telomere length can be sensed and negatively regulated predominantly by the amounts of Rif1p and Rif2p that are present at the telomere. Consistent with such a model, first, two chromosome ends in S. cerevisiae lack detectable Rap1p by chromatin immunoprecipitation, yet Rif1p is associated with at least one of these ends in vivo (29, 43). Second, telomeres composed of a tract of vertebrate telomeric repeat sequence are maintained slightly shorter than wild type in yeast, yet are not detectably bound by Rap1p, as measured by chromatin immunoprecipitation (1, 7), although Tbf1p may also play a role in telomere length control in this context. Third, in a telomerase template mutant strain with mutant sequence telomeres longer than that in a rif1Δ rif2Δ strain and having a >300-fold reduction in Rap1p binding affinity (37), even a slight increase in cellular Rif2p levels was sufficient to dramatically curtail telomere overelongation (J. Lin, D. L. Levy, and E. H. Blackburn, unpublished data).
FIG. 6.
Models for the mechanism of Rif protein action. rap1ΔCp, partial circles; telomeric cap, crescents. UASG represents a single Gal4p UAS site bound by GBD fused to an oval Rif protein. The oval represents either a single Rif protein or a Rif molecule associated with additional proteins. rap1ΔC telomeres are long, but tethering a single Rif protein dramatically curtails lengthening. One model is that the single tethered Rif molecule recruits additional Rif proteins and/or other telomeric factors and the protein complex nucleated by the tethered Rif protein spreads throughout the chromosome end. Also depicted is the idea that Rif proteins, with or without additional factors, engage in Rap1p-independent associations with the telomere. A second model is that the single tethered Rif protein associates with the cap. This generates a structure that blocks telomerase access or activity, and telomere lengths therefore equilibrate shorter.
We found that a small number of Rif protein molecules (and even a single Rif2p molecule) targeted to a telomere potently suppress overlengthening of that telomere in cis (Fig. 1). We propose that tethered Rif proteins can nucleate the formation of a higher-order complex by initiating the recruitment of additional Rif and/or other telomeric proteins. This allows a structure to form that spreads throughout the telomeric region and blocks elongation by telomerase (Fig. 6). This mechanism is analogous to models for nucleation and spreading of Sir proteins to establish transcriptionally silenced domains (40). It is consistent with Rif1p chromatin immunoprecipitation studies which indicate that Rif1p spreads several kilobases into the subtelomeric region (43). Rif1p spreading even occurs on one of the Rap1p-free telomeres and on other telomeres, Rif1p also spreads into particular subtelomeric regions where Rap1p is not bound (29, 43). A previous model proposed that Rif proteins may be located only on the outer portion of the telomeric repeat tract (50). In contrast, in the model in Fig. 6, the Rif proteins can localize to a telomere and exert their length regulatory effects even in the complete absence of any Rap1p C-terminal domain. An alternative mechanism, by which a very few tethered Rif protein molecules could also cause the observed extreme telomere shortening, is by interaction of Rif proteins with telomere end-binding proteins. Subtelomerically tethered Rif1p and Rif2p would thus generate a fold-back structure or possibly promote formation of a T-loop (Fig. 6).
Higher-order chromatin structure appears to be regulated by mechanisms that ensure domains of chromatin structure are stably established in a switch-like manner. Our data support a model in which telomeric chromatin is formed by a nucleation and spreading mechanism reminiscent of the establishment of silenced heterochromatin initiated by yeast Sir proteins (40). Rif proteins may play a comparable role at telomeres to influence telomerase access (Fig. 6). The similarity between the modes of length regulation in mammals (2, 44) and yeasts and the existence of Rap1p and Rif1p homologs in Schizosaccharomyces pombe (23) and human cells (28, 42; L. Xu and E. H. Blackburn, in press) suggest that a similar nucleation and spreading mechanism may operate at telomeres in higher eukaryotes.
Supplementary Material
Acknowledgments
We thank Carol Anderson for oligonucleotides to delete RAD52, Judith Berman for the anti-Rap1p antibody, Jennifer Fung and Wallace Marshall for assistance with microscopy and for use of the DeltaVision microscope, Richard Huganir for a rat GRIP1 PDZ456 plasmid, Arthur Lustig for the rap1-17 strain, and Stéphane Marcand for plasmid sp59. We thank Maria Enquist-Newman, Jue Lin, David Morgan, Jeffrey Seidel, and Tanya Williams for critical reading of the manuscript and members of the Blackburn lab and Maria Enquist-Newman for helpful advice and discussions.
D.L.L. was supported by a predoctoral fellowship from the Howard Hughes Medical Institute. This work was supported by grant GM26259 from the National Institutes of Health to E.H.B.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org.
REFERENCES
- 1.Alexander, M. K., and V. A. Zakian. 2003. Rap1p telomere association is not required for mitotic stability of a C(3)TA(2) telomere in yeast. EMBO J. 22:1688-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancelin, K., M. Brunori, S. Bauwens, C.-E. Koering, C. Brun, M. Ricoul, J. P. Pommier, L. Sabatier, and E. Gilson. 2002. Targeting assay to study the cis functions of human telomeric proteins: evidence for inhibition of telomerase by TRF1 and for activation of telomere degradation by TRF2. Mol. Cell. Biol. 22:3474-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackburn, E. H. 2000. The end of the (DNA) line. Nat. Struct. Biol. 7:847-850. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn, E. H. 2000. Telomere states and cell fates. Nature 408:53-56. [DOI] [PubMed] [Google Scholar]
- 5.Bourns, B. D., M. K. Alexander, A. M. Smith, and V. A. Zakian. 1998. Sir proteins, Rif proteins, and Cdc13p bind Saccharomyces telomeres in vivo. Mol. Cell. Biol. 18:5600-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li, P. Hieter, and J. D. Boeke. 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115-132. [DOI] [PubMed] [Google Scholar]
- 7.Brevet, V., A. S. Berthiau, L. Civitelli, P. Donini, V. Schramke, V. Geli, F. Ascenzioni, and E. Gilson. 2003. The number of vertebrate repeats can be regulated at yeast telomeres by Rap1-independent mechanisms. EMBO J. 22:1697-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchman, A. R., W. J. Kimmerly, J. Rine, and R. D. Kornberg. 1988. Two DNA-binding factors recognize specific sequences at silencers, upstream activating sequences, autonomously replicating sequences, and telomeres in Saccharomyces cerevisiae. Mol. Cell. Biol. 8:210-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christianson, T. W., R. S. Sikorski, M. Dante, J. H. Shero, and P. Hieter. 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119-122. [DOI] [PubMed] [Google Scholar]
- 10.Conrad, M. N., J. H. Wright, A. J. Wolf, and V. A. Zakian. 1990. RAP1 protein interacts with yeast telomeres in vivo: overproduction alters telomere structure and decreases chromosome stability. Cell 63:739-750. [DOI] [PubMed] [Google Scholar]
- 11.de Bruin, D., S. M. Kantrow, R. A. Liberatore, and V. A. Zakian. 2000. Telomere folding is required for the stable maintenance of telomere position effects in yeast. Mol. Cell. Biol. 20:7991-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong, H., P. Zhang, I. Song, R. S. Petralia, D. Liao, and R. L. Huganir. 1999. Characterization of the glutamate receptor-interacting proteins GRIP1 and GRIP2. J. Neurosci. 19:6930-6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dueber, J. E., B. J. Yeh, K. Chak, and W. A. Lim. 2003. Reprogramming control of an allosteric signaling switch through modular recombination. Science 301:1904-1908. [DOI] [PubMed] [Google Scholar]
- 14.Enomoto, S., P. D. McCune-Zierath, M. Gerami-Nejad, M. A. Sanders, and J. Berman. 1997. RLF2, a subunit of yeast chromatin assembly factor-I, is required for telomeric chromatin function in vivo. Genes Dev. 11:358-370. [DOI] [PubMed] [Google Scholar]
- 15.Gilson, E., M. Roberge, R. Giraldo, D. Rhodes, and S. M. Gasser. 1993. Distortion of the DNA double helix by RAP1 at silencers and multiple telomeric binding sites. J. Mol. Biol. 231:293-310. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein, A. L., and J. H. McCusker. 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15:1541-1553. [DOI] [PubMed] [Google Scholar]
- 17.Gotta, M., T. Laroche, A. Formenton, L. Maillet, H. Scherthan, and S. M. Gasser. 1996. The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. J. Cell Biol. 134:1349-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham, I. R., R. A. Haw, K. G. Spink, K. A. Halden, and A. Chambers. 1999. In vivo analysis of functional regions within yeast Rap1p. Mol. Cell. Biol. 19:7481-7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffith, J. D., L. Comeau, S. Rosenfield, R. M. Stansel, A. Bianchi, H. Moss, and T. de Lange. 1999. Mammalian telomeres end in a large duplex loop. Cell 97:503-514. [DOI] [PubMed] [Google Scholar]
- 20.Grossi, S., A. Bianchi, P. Damay, and D. Shore. 2001. Telomere formation by Rap1p binding site arrays reveals end-specific length regulation requirements and active telomeric recombination. Mol. Cell. Biol. 21:8117-8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardy, C. F., L. Sussel, and D. Shore. 1992. A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev. 6:801-814. [DOI] [PubMed] [Google Scholar]
- 22.Hediger, F., F. R. Neumann, G. Van Houwe, K. Dubrana, and S. M. Gasser. 2002. Live imaging of telomeres: yKu and Sir proteins define redundant telomere-anchoring pathways in yeast. Curr. Biol. 12:2076-2089. [DOI] [PubMed] [Google Scholar]
- 23.Kanoh, J., and F. Ishikawa. 2001. spRap1 and spRif1, recruited to telomeres by Taz1, are essential for telomere function in fission yeast. Curr. Biol. 11:1624-1630. [DOI] [PubMed] [Google Scholar]
- 24.Krauskopf, A., and E. H. Blackburn. 1996. Control of telomere growth by interactions of RAP1 with the most distal telomeric repeats. Nature 383:354-357. [DOI] [PubMed] [Google Scholar]
- 25.Kyrion, G., K. A. Boakye, and A. J. Lustig. 1992. C-terminal truncation of RAP1 results in the deregulation of telomere size, stability, and function in Saccharomyces cerevisiae. Mol. Cell. Biol. 12:5159-5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laroche, T., S. G. Martin, M. Tsai-Pflugfelder, and S. M. Gasser. 2000. The dynamics of yeast telomeres and silencing proteins through the cell cycle. J. Struct. Biol. 129:159-174. [DOI] [PubMed] [Google Scholar]
- 27.Li, B., and A. J. Lustig. 1996. A novel mechanism for telomere size control in Saccharomyces cerevisiae. Genes Dev. 10:1310-1326. [DOI] [PubMed] [Google Scholar]
- 28.Li, B., S. Oestreich, and T. de Lange. 2000. Identification of human Rap1: implications for telomere evolution. Cell 101:471-483. [DOI] [PubMed] [Google Scholar]
- 29.Lieb, J. D., X. Liu, D. Botstein, and P. O. Brown. 2001. Promoter-specific binding of Rap1 revealed by genome-wide maps of protein-DNA association. Nat. Genet. 28:327-334. [DOI] [PubMed] [Google Scholar]
- 30.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 31.Lydall, D. 2003. Hiding at the ends of yeast chromosomes: telomeres, nucleases and checkpoint pathways. J. Cell Sci. 116:4057-4065. [DOI] [PubMed] [Google Scholar]
- 32.Marcand, S., E. Gilson, and D. Shore. 1997. A protein-counting mechanism for telomere length regulation in yeast. Science 275:986-990. [DOI] [PubMed] [Google Scholar]
- 33.McEachern, M. J., and E. H. Blackburn. 1995. Runaway telomere elongation caused by telomerase RNA gene mutations. Nature 376:403-409. [DOI] [PubMed] [Google Scholar]
- 34.Moretti, P., K. Freeman, L. Coodly, and D. Shore. 1994. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes Dev. 8:2257-2269. [DOI] [PubMed] [Google Scholar]
- 35.Muller, T., E. Gilson, R. Schmidt, R. Giraldo, J. Sogo, H. Gross, and S. M. Gasser. 1994. Imaging the asymmetrical DNA bend induced by repressor activator protein 1 with scanning tunneling microscopy. J. Struct. Biol. 113:1-12. [DOI] [PubMed] [Google Scholar]
- 36.Park, S. H., A. Zarrinpar, and W. A. Lim. 2003. Rewiring MAP kinase pathways using alternative scaffold assembly mechanisms. Science 299:1061-1064. [DOI] [PubMed] [Google Scholar]
- 37.Prescott, J. C., and E. H. Blackburn. 2000. Telomerase RNA template mutations reveal sequence-specific requirements for the activation and repression of telomerase action at telomeres. Mol. Cell. Biol. 20:2941-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray, A., and K. W. Runge. 1999. The yeast telomere length counting machinery is sensitive to sequences at the telomere-nontelomere junction. Mol. Cell. Biol. 19:31-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Runge, K. W., and V. A. Zakian. 1989. Introduction of extra telomeric DNA sequences into Saccharomyces cerevisiae results in telomere elongation. Mol. Cell. Biol. 9:1488-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rusche, L. N., A. L. Kirchmaier, and J. Rine. 2003. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu. Rev. Biochem. 72:481-516. [DOI] [PubMed] [Google Scholar]
- 41.Sheng, M., and C. Sala. 2001. PDZ domains and the organization of supramolecular complexes. Annu. Rev. Neurosci. 24:1-29. [DOI] [PubMed] [Google Scholar]
- 42.Silverman, J., H. Takai, S. B. C. Buonomo, F. Eisenhaber, and T. de Lange. 2004. Human Rif1, ortholog of a yeast telomeric protein, is regulated by ATM and 53BP1 and functions in the S-phase checkpoint. Genes Dev. 18:2108-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith, C. D., D. L. Smith, J. L. DeRisi, and E. H. Blackburn. 2003. Telomeric protein distributions and remodeling through the cell cycle in Saccharomyces cerevisiae. Mol. Biol. Cell 14:556-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smogorzewska, A., B. van Steensel, A. Bianchi, S. Oelmann, M. R. Schaefer, G. Schnapp, and T. de Lange. 2000. Control of human telomere length by TRF1 and TRF2. Mol. Cell. Biol. 20:1659-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strahl-Bolsinger, S., A. Hecht, K. Luo, and M. Grunstein. 1997. SIR2 and SIR4 interactions differ in core and extended telomeric heterochromatin in yeast. Genes Dev. 11:83-93. [DOI] [PubMed] [Google Scholar]
- 46.Teixeira, M. T., M. Arneric, P. Sperisen, and J. Lingner. 2004. Telomere length homeostasis is achieved via a switch between telomerase-extendible and -nonextendible states. Cell 117:323-335. [DOI] [PubMed] [Google Scholar]
- 47.Teng, S. C., J. Chang, B. McCowan, and V. A. Zakian. 2000. Telomerase-independent lengthening of yeast telomeres occurs by an abrupt Rad50p-dependent, Rif-inhibited recombinational process. Mol. Cell 6:947-952. [DOI] [PubMed] [Google Scholar]
- 48.Vignais, M. L., J. Huet, J. M. Buhler, and A. Sentenac. 1990. Contacts between the factor TUF and RPG sequences. J. Biol. Chem. 265:14669-14674. [PubMed] [Google Scholar]
- 49.Vignais, M. L., and A. Sentenac. 1989. Asymmetric DNA bending induced by the yeast multifunctional factor TUF. J. Biol. Chem. 264:8463-8466. [PubMed] [Google Scholar]
- 50.Wotton, D., and D. Shore. 1997. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev. 11:748-760. [DOI] [PubMed] [Google Scholar]
- 51.Zakian, V. A. 1996. Structure, function, and replication of Saccharomyces cerevisiae telomeres. Annu. Rev. Genet. 30:141-172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.