Abstract
Human progesterone receptors (PR) are phosphorylated by cyclin-dependent protein kinase 2 (CDK2) at multiple sites, including Ser400. Herein, we have addressed the significance of phosphorylation of this residue. PR phospho-Ser400-specific antibodies revealed regulated phosphorylation of Ser400 in response to progestins and mitogens, and this correlated with increased CDK2 levels and activity. Expression of cyclin E elevated CDK2 activity and downregulated PR independently of ligand. Similarly, overexpression of activated mutant CDK2 increased PR transcriptional activity in the absence and presence of progestin. Mutation of PR Ser400 to alanine (S400A) blocked CDK2-induced PR activity in the absence, but not in the presence, of progestin. PR was unresponsive to activated CDK2 in breast cancer cells with elevated p27, and RNA interference knock-down of p27 partially restored CDK2-induced ligand-independent PR activation. Similarly, in p27−/− mouse embryonic fibroblasts, elevated CDK2 activity increased wild-type (wt) but not S400A PR transcriptional activity in the absence of progestin. CDK2 induced nuclear localization of unliganded wt but not S400A PR; liganded S400A PR exhibited delayed nuclear accumulation. These studies demonstrate that CDK2 regulates PR in the absence of progestins via phosphorylation of Ser400, thus revealing a novel mechanism for upregulated PR transcriptional activity in human breast cancer cells expressing altered cell cycle regulatory molecules.
The steroid hormones estrogen and progesterone regulate breast development (29, 89) and contribute to breast cancer progression (29, 54). Breast cancer cell lines are used to model the effects of steroid hormones on cell proliferation and survival. Steroidal control of cell cycle progression takes place at defined points in the G1 phase of the cell cycle (52). Progesterone has either stimulatory (25, 47) or biphasic (11, 23, 53) effects on human T47D breast cancer cell growth, dependent in part upon cell culture conditions and the presence of estrogenic stimuli. Cell cycle analyses of biphasic cell growth patterns indicate that following a single dose of progesterone, cyclin D1 and cyclin E levels initially increase as cells undergo S-phase entry. Cyclin-dependent protein kinase 2 (CDK2) activity peaks at approximately 16 h. Coincident with increased CDK2 activity, progesterone receptor (PR) protein levels begin to decline. The CDK inhibitors p21 and p27 are then induced after this early proliferative phase, leading to G1 arrest, and PR levels slowly recover as cells exit the mitotic cell cycle. Cells are further growth inhibited in the presence of additional progesterone treatments (23, 53), but progestin-primed cells can be induced to grow by administration of growth factors (23). These studies demonstrate a complex interplay between PR and cell cycle regulators.
Several studies have demonstrated that cyclin D1 and p27 play important roles in normal mammary gland development (14, 49, 81, 82). Cyclin D1−/− mice have a deficiency in pregnancy-associated mammary gland development (16, 80). In addition, overexpression of cyclins D1 and E and decreased expression of the CDK inhibitor p27 are associated with the high growth rates seen in human breast cancers. For example, approximately 45 to 50% of breast cancers overexpress cyclin D1 (5, 21). Furthermore, progression from normal breast tissue through invasive ductal carcinoma (77), high-grade ductal carcinoma relative to low grade (77), and late-stage lesions (34) are all associated with increased expression of cyclin E. In addition, decreased expression of p27 occurs in 30% of breast cancers and is correlated with poor prognosis in primary breast cancers (7, 63, 87). Mouse models of breast cancer support a role for alterations in cell cycle molecules in progression of mammary epithelial cells to preneoplastic stages (69). Deregulated cell cycle molecules are predicted to augment breast cancer progression in part as a result of increased CDK activity. The relevant CDK targets in breast cancers remain unknown.
The PR is highly phosphorylated, primarily on serine residues, by multiple kinases in a manner similar to other steroid hormone receptor family members (41, 85, 93). While the role of phosphorylation of steroid receptors is not fully understood, phosphorylation may influence promoter specificity (65), cofactor interaction (19), ligand-dependent (78) and ligand-independent (39) transcriptional activities, receptor turnover (43), and nuclear association (66). In addition, steroid hormone receptor phosphorylation may serve to integrate signals initiated by growth factors in tissues under steroidal control. A number of endogenously regulated phosphorylation sites on human PR have been well defined (41, 93). For example, serines at positions 294 and 345 in PR are predominantly phosphorylated following treatment of cells with progestin (96). Ser400 is both basally phosphorylated and regulated by ligand in cells; Ser400 is a basally phosphorylated site in vivo (96, 97) and phosphorylated by CDK2 in vitro (95). Of the 14 identified phosphorylation sites, 8 are known to be phosphorylated by CDK2 in vitro (36, 95). The consequence of PR phosphorylation by CDK2 is unknown but suggests a mechanism for cell cycle-dependent regulation of PR. We therefore investigated the role of direct regulation of PR by CDK2 in breast cancer cells by mitogenic stimuli, including progestins.
We seek to better understand how phosphorylation of PR in response to elevated CDK2 activity serves to link cell cycle progression to steroid hormone responsiveness. Our data indicate that phosphorylation of PR Ser400 is regulated by CDK2 in response to ligand and peptide growth factors. Transcriptional activity of PR is increased by activated CDK2 in both the presence and absence of progestin. Unliganded PR are well activated by CDK2 only in the absence of high levels of p27 protein, and Ser400 is required for ligand-independent CDK2-induced PR nuclear localization and transcriptional activity. These data have important implications for breast cancers with upregulated CDK2 activity and/or functional loss of p27 (1, 7, 14, 63, 87).
MATERIALS AND METHODS
PR antibodies.
A polyclonal antibody that recognizes the phosphorylated form of human PR Ser400 was produced using the peptide sequence EASAR(pS)PRSYLV (phosphorylated at Ser400) as an antigen (commissioned from Biosource International, Hopkinton, Mass.). Total PR was measured with a monoclonal antibody (Ab-8; NeoMarkers, Fremont, Calif.).
Cell lines and reagents.
T47Dco breast epithelial cells constitutively expressing both endogenous PR-A and PR-B isoforms of PR have been described elsewhere (27). T47D-YA and T47D-YB cells were created from PR-negative T47D-Y cells by stably reintroducing either the PR-A or PR-B isoform as described previously (27, 76). T47D-Y, YA, and YB cell stocks were maintained in minimal essential medium (MEM) containing phenol red and supplemented with 5% fetal bovine serum (FBS). For experiments, plated cells were placed in MEM starvation medium (unsupplemented serum-free medium containing phenol red) overnight prior to R5020 treatment. Omission of phenol red from the culture medium had no effect on R5020-induced changes in cell cycle regulation or growth of T47D variant cell lines (data not shown). PR-null T47D42W cells (50) were provided by V. Craig Jordan (Northwestern University, Chicago, Ill.), and wild-type (wt) and p27−/− mouse embryo fibroblast (MEF) cells containing a targeted deletion of the p27 gene (12) were obtained from Robert Sheaff (University of Minnesota) and maintained in Dulbecco's modified Eagle's medium containing 10% FBS. HeLa cervical carcinoma cells were maintained in MEM supplemented with 5% FBS. The CDK inhibitor roscovatine (used at 700 nM) was purchased from Calbiochem (La Jolla, Calif.). The CDK2-selective inhibitor CDK2 inhibitor II (used at 70 nM) was purchased from Calbiochem. The progestin agonist R5020 (Promegestone) was obtained from DuPont-New England Nuclear (Boston, Mass.). The adenovirus cyclin E viral expression vector (Ad-cyclin E) was a gift from James DeGregori (University of Colorado Health Sciences Center, Denver). P27 and p27 RNA interference (RNAi) plasmid expression vectors and active cyclin E/CDK2 were gifts from Robert Sheaff (University of Minnesota).
Immunoblotting.
For detection of total PRs, phospho-Ser400 PR, p27, and cyclin E, cells grown in 100-mm dishes were washed twice with phosphate-buffered saline (PBS). The cells were collected and lysed in RIPA buffer (10 mM sodium phosphate [pH 7.0], 150 mM NaCl, 2 mM EDTA, 1% [vol/vol] Nonidet P-40, 0.1% [wt/vol] sodium dodecyl sulfate [SDS], 1% sodium deoxycholate, 20 μg of aprotinin/ml, 50 mM sodium fluoride, 200 μM Na3VO4, 0.1% [vol/vol] β-mercaptoethanol, and 1 mM phenylmethylsulfonyl fluoride). Lysates were clarified by centrifugation for 10 min at 21,910 × g at 4°C. Soluble proteins were quantified by the Bradford method (Life Technologies, Inc.), and equal amounts of protein were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE; 7.5% for PR and 10% for cyclin E and p27). The proteins were transferred to nitrocellulose and immunoblotted with specific antibodies as described elsewhere (23). The Western blots shown are representative of a minimum of three separate experiments.
CDK2 activity assay.
To measure CDK2 activity, T47D-YB cells were plated at 800,000 cells per 100-mm cell culture dish in medium with 5% FBS. After 24 h, the cells were incubated in basal medium for 24 h and then treated with R5020 for various times (indicated in the figure legends). The cells were washed twice in PBS and collected in RIPA buffer as described above. A 75-μg aliquot of lysate was incubated with 200 ng of anti-CDK2 antibody (M2-G; Santa Cruz Biotechnology, Santa Cruz, Calif.) for 1 h at 4°C. The antibody-lysate mixture was then incubated with 10 μl of protein G-agarose (Roche Diagnostics, Indianapolis, Ind.) in a final volume of 500 to 600 μl for 1 h at 4°C. The immune complexes were washed three times with RIPA buffer, three times with kinase buffer (50 mM Tris-Cl [pH 7.5], 10 mM MgCl2, 5 mM dithiothreitol, 0.1 mg of bovine serum albumin [BSA]/ml) and incubated in a kinase reaction mixture containing 2 μg of histone H1 (Sigma) as a substrate and 5 μCi of γ-32P for 15 min at 37°C. The reaction was stopped by adding sample buffer, boiled for 2 min, and electrophoresed on an SDS-12.5% PAGE gel. As a negative control, a nonspecific antibody of similar isotype was incubated with the lysate and protein G-agarose beads before incubating the beads in the kinase reaction mixture. As a positive control, purified active cyclin E/CDK2 complex (provided by Robert Sheaff, University of Minnesota) was added to the kinase reaction mixture. Determination of the CDK2 activity in wt p27−/− MEF cells was performed using the same assay conditions above except the cells were cultured in 10% FBS. Phosphorylated purified histone H1 substrate proteins were visualized by autoradiography. Data shown are representative of typical results from three separate experiments.
Transcription assays.
HeLa, MDA-MB-435, wt p27+/+ MEFs, or p27−/− MEFs were plated at 250,000 cells per well in six-well dishes. After 24 h, cells were transiently transfected with various combinations of either wt PR or S400A PR (0.01 to 1.0 μg each), CDK2 (1 μg), and PRE-2x-TATA-luc reporter plasmid (1 μg) along with Renilla plasmid (10 ng) as a control for transfection efficiency. Cells were transfected with Effectene transfection reagent according to the manufacturer's instructions (QIAGEN Inc., Valencia, Calif.). After 24 h of incubation, the transfection reaction mixture was replaced with either unsupplemented serum-free phenol red containing Dulbecco's modified Eagle's medium or MEM for 8 h prior to treatment. Cells were collected following treatment with 10 nM R5020 or ethanol (EtOH) vehicle control for 18 h. Luciferase and Renilla activities were measured according to the manufacturer's protocol (Promega Corp., Madison, Wis.). The data are representative of typical results from a minimum of three separate experiments. The data are presented as means ± standard deviations of three replicates for each data point.
Viral infection of T47D-YB cells.
Aliquots of 400,000 T47D-YB cells were plated and grown overnight in 60-mm dishes. The cells were then incubated with 400 PFU of Ad-cyclin E, or control empty vector virus, for 48 h in growth medium containing 5% FBS. The cells were then washed, incubated overnight in nonsupplemented MEM, and treated with R5020 or vehicle control for 1 h. Cell lysate was collected with RIPA buffer, separated by SDS-PAGE, and subjected to Western blotting.
Immunohistochemistry and confocal microscopy.
The immunohistochemistry and confocal microscopy protocols used for these studies have been previously described (66). In brief, cells were seeded onto coverslips in six-well multidishes. After the appropriate treatments, the cells were washed, fixed by incubation in 1 ml of 3.7% paraformaldehyde in PBS for 10 min at room temperature (RT), and permeabilized by incubation in 1 ml of PBS containing 0.5% Triton X-100 for 5 min at RT. Cells on coverslips were incubated with primary antibody against PR (Ab-8; NeoMarkers) diluted 1:100 in 100 μl of PBS containing 1% BSA for 1 h at RT. After five washes in PBS, coverslips were incubated in secondary antibody (fluorescein isothiocyanate-conjugated goat anti-mouse; Santa Cruz Biotechnology, Inc.) diluted 1:100 in 100 μl of PBS containing 1% BSA for 1 h at RT. Cells on coverslips were subjected to direct fluorescence imaging with a Zeiss Atto Arc HBO 110W upright microscope (Carl Zeiss, Inc.). Samples were excited at 488 nm and analyzed at an emission of 522 nm. The data are representative of typical results from three separate experiments.
RESULTS
Regulation of PR Ser400 by progestins, growth factors, and CDK2.
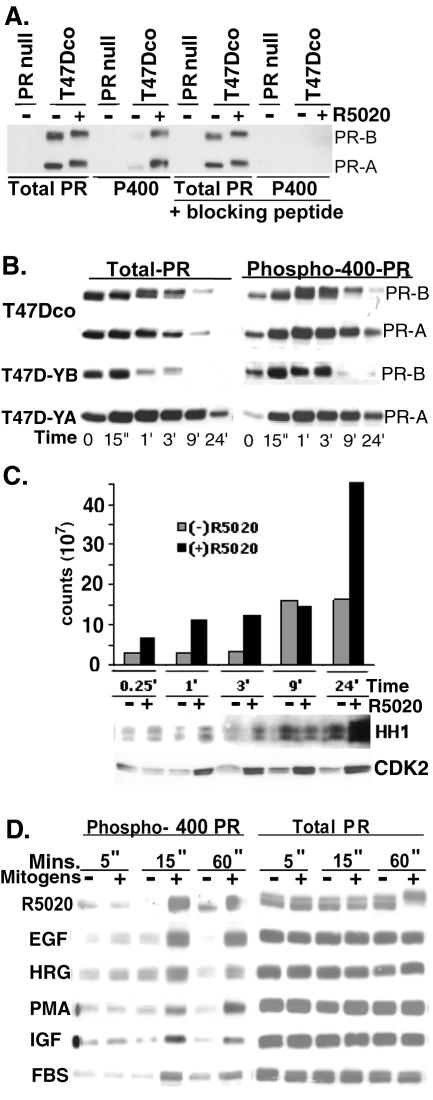
PR Ser400 was previously identified as a site that is basally phosphorylated in vivo (97) and phosphorylated by CDK2 in vitro (95). To investigate the regulation of PR phosphorylation at Ser400, a phospho-specific antibody was produced. Affinity-purified PR phospho-Ser400 antibodies were then used to blot cell lysates from T47Dco cells expressing two isoforms of PR (97-kDa PR-A and 120-kDa PR-B) independently of exogenously added estrogen (27, 76). PR null T47D cells served as negative controls (Fig. 1A). PR isoforms underwent a characteristic mobility upshift in the presence of the synthetic progestin R5020, indicative of multisite phosphorylation following ligand binding (79). A faint band of phospho-Ser400 PR was detected in the absence R5020, confirming basal phosphorylation of this residue (97). There was a dramatic increase in PR-A and PR-B Ser400 phosphorylation following R5020 addition, while no phosphorylated bands were visible in PR null T47D cells. To confirm the specificity of PR phospho-Ser400 antibodies, the peptide antigen used to generate the antibody was added to the blotting mixture. The phospho-Ser400 peptide specifically blocked PR phospho-Ser400 antibody binding to PR phosphorylated at Ser400 (Fig. 1A, far right lanes) but not binding of the total PR antibody to PR-A and PR-B. These data demonstrate that phospho-Ser400 PR antibodies specifically recognize the phosphorylated form of PR Ser400 and that Ser400 is phosphorylated in response to ligand.
FIG. 1.
Regulation of PR Ser400 phosphorylation. (A) Characterization of PR phospho-Ser400-specific antibodies. T47Dco cells expressing endogenous PR-A and PR-B isoforms or PR-null T47D cells were treated with R5020 (+) or EtOH vehicle control (−) for 1 h. Equal amounts of cell lysate (100 μg) were electrophoresed on SDS-7.5% PAGE gels, transferred to nitrocellulose, and blotted with antibodies specific for either total PR protein or PR phospho-Ser400(P400). As a control for the specificity of PR phospho-Ser400 antibodies, blots were incubated with antibodies in the presence or absence of a blocking peptide representing the peptide used to make the phospho-specific antibodies. (B) Time course of PR Ser400 phosphorylation. T47Dco, T47D-YA, and T47D-YB cells were treated with R5020 for 0 to 24 h, and Western blotting was performed as for panel A to detect either total PR or phospho-Ser400 PR. (C) CDK2 activity in R5020-treated T47D-YB cells. T47D-YB cells were treated for 0 to 24 h with either R5020 (+) or EtOH vehicle control (−), and CDK2 immune complexes were purified and subjected to in vitro kinase assays with histone H1 (HH1) as a substrate. Phosphorylation of HH1 was detected by autoradiography and quantified by phosphorimaging, and data are presented as a bar graph. Western blotting was performed to detect total levels of CDK2 in each cell lysate. (D) Mitogen-induced PR Ser400 phosphorylation. T47D-YB cells were treated with various mitogens (R5020, 10 nM; EGF, 30 ng/ml; heregulin [HRG], 10 ng/ml; PMA, 10 nM; IGF, 30 ng/ml; FBS, 10%) or vehicle control for 5 to 60 min, and cell lysates were subjected to SDS-PAGE and immunoblotting with antibodies specific for either total PR or phospho-Ser400 PR. Results are representative of two to three independent experiments.
To characterize the kinetics of PR Ser400 phosphorylation in breast cancer cells, time courses of R5020 treatment were performed in T47Dco or T47D-YA and T47D-YB cells (76) engineered to stably express only PR-A or PR-B (Fig. 1B). Cells were treated with R5020 for 0 to 24 h and harvested at various time points. As expected, PR was downregulated in response to ligand binding (56). Ligand-induced downregulation of PR-A occurs over a time course similar to that of PR-B when both isoforms are concurrently expressed in T47Dco cells. However, the time course of ligand-induced downregulation of total PR-A in T47D-YA cells was prolonged relative to that in T47Dco cells, while downregulation of PR-B in T47D-YB cells was more rapid relative to T47Dco cells expressing both isoforms, suggesting that PR-A may partially stabilize PR-B in the heterodimer when coexpressed. Phosphorylation of PR Ser400 increased after 15 min of R5020 treatment and was sustained over the course of PR downregulation. Since Ser400 is a CDK2 site and CDK2 activity is known to be regulated by progestins (23), CDK2 activity was measured following R5020 treatment in T47D-YB cells (Fig. 1C). R5020-stimulated CDK2 activity increased at 15 min and continued to rise for up to 24 h, with the largest increases in CDK2 activity occurring at 1, 3, and 24 h relative to basal activity in vehicle-treated controls. The rise in CDK2 activity at early time points (15 min) following progestin treatment was consistent with the time course of PR Ser400 phosphorylation. Additionally, R5020 treatment induced an increase in CDK2 protein levels over time beginning at 1 h (Fig. 1C), suggesting that increased CDK2 activity was in part due to stabilization of cyclin-CDK2 complexes in response to upregulation of G1-phase cyclins by progestins (23, 84).
To determine if stimulation of mitogenic signaling pathways by peptide growth factors can regulate the phosphorylation of PR-Ser400 independently of progestins, T47D-YB cells were treated with either R5020 or various mitogens (Fig. 1D). Total PR levels remained constant up to 1 h following mitogen treatment. Basal levels of PR Ser400 phosphorylation varied somewhat between cultures. However, several mitogens (epidermal growth factor [EGF], heregulin, phorbol myristate acetate [PMA], insulin-like growth factor [IGF], and FBS) induced PR Ser400 phosphorylation and mimicked the time course and intensity observed with R5020. The similar pattern of PR-Ser400 phosphorylation in response to these agents suggests a convergence of signaling pathways leading to the phosphorylation of PR Ser400 in response to progestins and mitogenic growth factors.
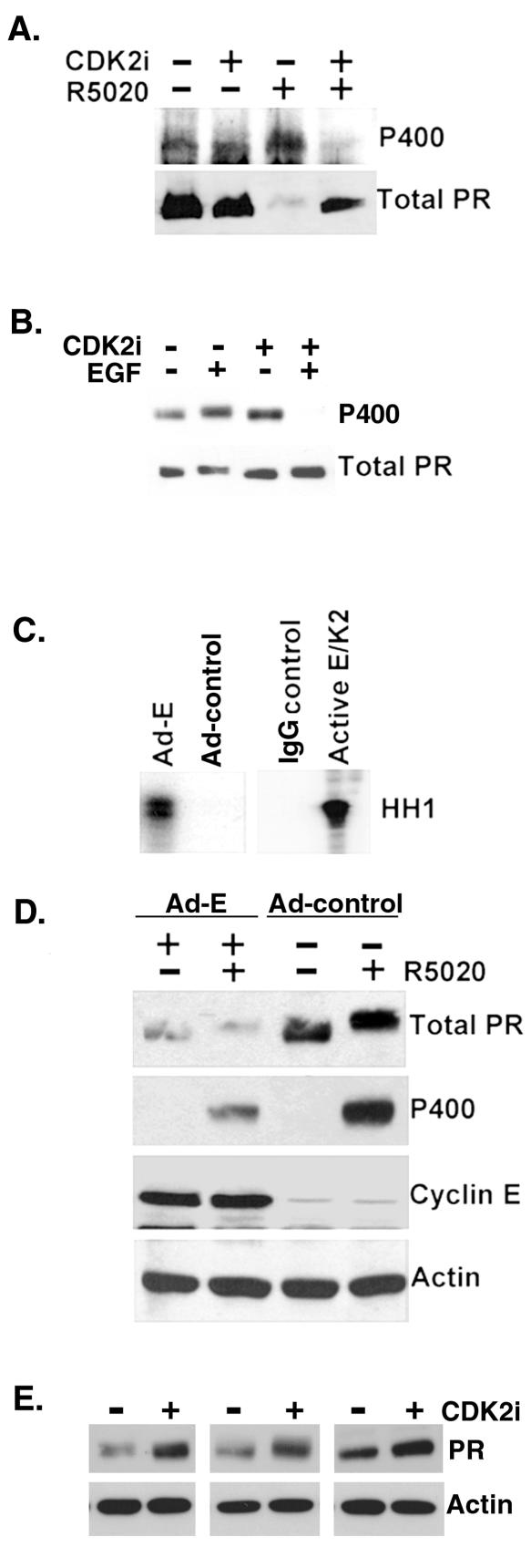
To test the CDK2 dependence of PR Ser400 phosphorylation in response to R5020, cells were treated with a CDK2-specific inhibitor prior to addition of R5020 (Fig. 2A). T47D-YB cells that expressed only the B isoform of PR were blotted for total and phospho-Ser400 PR, following long-term R5020 treatment, to induce PR downregulation. Cells were pretreated with or without a CDK2-specific inhibitor that interferes with the ATP-binding site of CDK2 (13). The R5020-induced phosphorylation of PR Ser400 was blocked by the CDK2 inhibitor, suggesting that phosphorylation of this site is indeed regulated by CDK2 in vivo. In addition, the CDK2 inhibitor blocked ligand-induced PR downregulation, indicating a role for CDK2 in this process. To test the CDK2 dependence of PR Ser400 phosphorylation in response to EGF treatment, cells were pretreated with or without a CDK inhibitor followed by EGF for 1 h (Fig. 2B). EGF stimulated increased PR Ser400 phosphorylation relative to basal levels. However, similar to the results with R5020 (Fig. 2A), phosphorylation of PR Ser400 was blocked by the CDK inhibitor. Interestingly, blockade of CDK2 activity in the presence of EGF reduced the level of phospho-Ser400 PR to below basal levels, suggesting that regulation of this site is highly dynamic. These data (Fig. 2A and B) indicate that phosphorylation of this site is regulated by CDK2 in vivo.
FIG. 2.
CDK2 activity decreases PR protein levels. (A) CDK2 inhibitors block ligand-dependent PR Ser 400 phosphorylation and PR downregulation. T47D-YB cells were preincubated with either vehiclecontrol or a CDK2-specific inhibitor (CDK2 inhibitor II) for 1 h andthen incubated with or without R5020 for an additional 11 h. Cell lysates were blotted for either total PR or phospho-Ser400 PR (P400). (B) CDK2 inhibitors block mitogen-induced phosphorylation of PR Ser400. T47D-YB cells were pretreated with vector control (dimethyl sulfoxide [DMSO]) or the CDK inhibitor roscovatine (700 nM) for 30 min and then treated with R5020 or EGF for 1 h. Cell lysates were blotted for total PR or phospho-Ser400 PR (P400). (C) Adenoviral expression of cyclin E stimulates CDK2 activity. HeLa cells stably expressing PR-B were infected with Ad-cyclin E (Ad-E) or a control adenovirus (Ad-control) for 48 h. CDK2 activity in immunoglobulin G (IgG-control) or CDK2 immune complexes was measured as described above (Fig. 1 legend) with histone H1 (HH1) as a substrate. Purified active cyclin E/CDK2 complexes (1 to 3 ng) were added to the reaction mixture as a positive control for CDK2 activity. (D) Ad-cyclin E induces loss of PR protein. Western blotting for total PR, phospho-Ser400 PR, cyclin E, and β-actin was performed on cell lysates from HeLa cells stably expressing PR-B and transduced with either control adenovirus (Ad-control) or Ad-cyclin E (Ad-E) in the presence and absence of R5020 for 1 h. (E) Inhibition of CDK2 activity stabilizes PR protein. T47D-YB cells were treated with a CDK2-specific inhibitor (CDK2 inhibitor II) or vehicle control (DMSO) for 12 h. Western blotting assays for total PR and actin were performed on cell lysates. Data from three separate experiments are shown.
To confirm that CDK2 activation can contribute to PR downregulation in response to ligand, HeLa cells stably expressing PR-B were infected with Ad-cyclin E. Adenoviral expression of cyclin E led to an increase in CDK2 activity relative to adenoviral expression of control plasmid (Fig. 2C). Following adenoviral transduction, cells were then treated with or without R5020 for 1 h (Fig. 2D). Again, R5020 increased the phosphorylation of Ser400 in the absence of Ad-cyclin E as expected (Fig. 2D). Phospho-Ser400 PR-B was also detected in R5020-treated cells transduced with Ad-cyclin E. However, PR-B levels were greatly diminished in the presence of cyclin E overexpression, regardless of R5020 treatment, suggesting that elevated CDK2 activity, stimulated by cyclin E overexpression, may alter PR protein synthesis or folding or bypass the requirement for ligand in PR downregulation. Actin controls demonstrated the specificity of Ad-cyclin E effects on PR-B. PR levels decline at the peak of CDK2 activity following progestin treatment of human breast cancer cells (23). To determine if CDK2 activity mediates the regulation of basal levels of wt PR protein, T47D-YB cells were treated for 12 h with and without a CDK2 inhibitor in three separate experiments (Fig. 2E). PR levels were slightly elevated in cells treated with a CDK2 inhibitor, suggesting that the relative levels of CDK2 activity can regulate basal levels of PR in the absence of receptor ligands.
Activation of PR transcriptional activity by CDK2: role of PR Ser400.
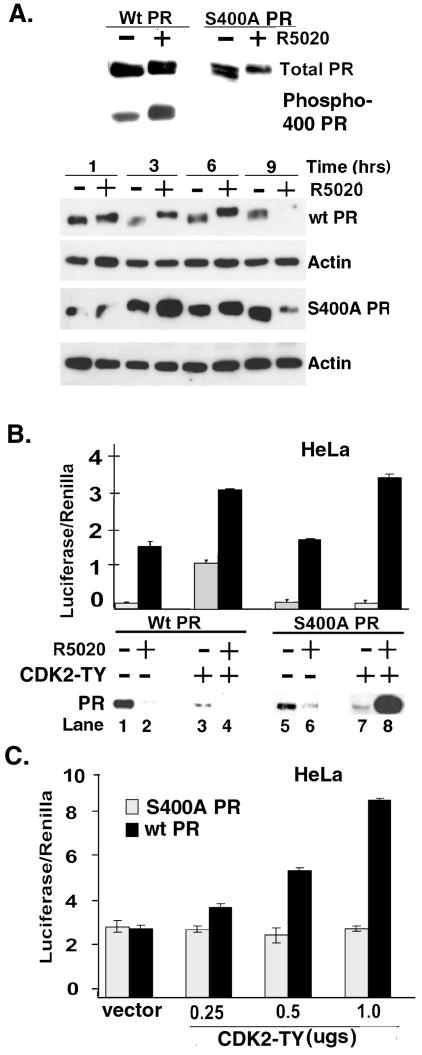
To further study the functional significance of PR Ser400 phosphorylation by CDK2, a mutant form of PR-B was produced that has a serine-to-alanine point mutation at position 400 (S400A PR-B). Western blotting for total PR (Fig. 3A, top) demonstrated that wt and S400A mutant PR-B comigrated and responded to the synthetic progestin, R5020, similarly (by gel upshift) when transiently expressed in HeLa cells. Importantly, the PR phospho-Ser400 antibody did not recognize S400A mutant PR. We then performed a time course of R5020 treatment to determine if wt and S400A PR-B undergo similar rates of ligand-dependent downregulation (Fig. 3A, bottom). HeLa cells were transiently transfected with wt or S400A PR-B and treated without or with R5020 for 1 to 9 h (Fig. 3A). Both wt and S400A PR were significantly downregulated following 9 h of R5020 treatment, although some S400A PR-B remained at this time point.
FIG. 3.
CDK2 increases PR transcriptional activity in the presence and absence of progestin. (A) Expression of S400A PR-B and time course of ligand-dependent wt and S400A PR downregulation. HeLa cells were transiently transfected with either wt or S400A mutant PR-B (1 μg each) and treated with R5020 (+) or ETOH vehicle control (−) for 1 h (upper panel) or for 1 to 9 h (lower panels). Equal amounts of cell lysates (100 μg) were probed for total PR, phospho-Ser400 PR, or β-actin by Western blotting. (B) CDK2-induced transcriptional activity of wt and S400A PR. HeLa cells were transiently cotransfected withPRE-driven luciferase and constitutive Renilla reporter plasmids, either wt or S400A PR-B (100 ng each), and either mutant activated CDK2 (CDK2-TY) or its parental control vector. Following treatment of cell cultures with R5020 for 18 h, PRE-luciferase activity was measured and normalized to that in the Renilla controls. Cell lysates were also immunoblotted for total PR levels at the same time point. (C) CDK2 dose dependence of PR transcriptional activity. HeLa cells were transiently cotransfected with wt or S400A PR-B (100 ng each) and PRE-luciferase and Renilla reporter plasmids, along with increasing amounts of mutant CDK2-TY cDNA. PRE-luciferase activity was measured and normalized to that of Renilla controls. Control cultures (vector) received 1.0 μg of parental vector control (for CDK2-TY). Results are representative of three independent experiments. The data are presented as means ± standard deviations of three replicates for each data point.
To determine the influence of CDK2 on PR transcriptional activity and protein levels, HeLa cells were transiently cotransfected with a PRE-luc reporter, a Renilla reporter construct as a transfection control, either wt PR-B or S400A mutant PR-B, and control parental vector or a vector encoding an active CDK2 double mutant (CDK2-TY) in which Thr14 has been mutated to Ala and Tyr15 has been mutated to Phe (38, 57) (Fig. 3B). Mutant CDK2-TY is resistant to inactivation by Wee-1 kinase-regulated phosphorylation at Thr-14 and Tyr-15 (30, 43). The transcriptional activity of both wt PR and S400A PR-B increased to a similar extent (10- to 15-fold) in the presence of R5020 (Fig. 3B, compare lanes 1 and 2 with lanes 5 and 6). Addition of CDK2-TY enhanced the activity of liganded wt and S400A PR receptors (compare lanes 2 and 4 with lanes 6 and 8). These data suggest that CDK2 enhances PR transcriptional activity in response to R5020 and that PR Ser400 is not required for induction of transcriptional activity by liganded PR. Surprisingly, however, CDK2-TY significantly increased the transcriptional activity of wt PR-B, but not S400A PR-B, in the complete absence of ligand (lanes 3 and 7), suggesting that CDK2-mediated phosphorylation of Ser400 is required for this ligand-independent effect. Remarkably, the magnitude of CDK2-TY-induced activation of wt PR-B approached that induced in response to R5020 alone (compare lanes 2 and 3). Western blotting of the cell lysates from each condition showed that both wt and S400A PR-B are downregulated in response to R5020 (lanes 1, 2, 5, and 6) as expected (Fig. 3A). In addition, CDK2-TY appeared to downregulate wt PR-B in the absence of R5020 (compare lanes 1 and 3). This effect was similar to the downregulation of PR by cyclin E overexpression and CDK2 activation shown in Fig. 2C. CDK2-TY also diminished the levels of S400A PR-B in the absence of ligand (Fig. 3B, compare lanes 5 and 7). However, in sharp contrast to wt PR-B, S400A PR-B protein accumulated following R5020 treatment in the presence of CDK2-TY (lane 8). Taken together (Fig. 2 and 3), these data suggest that CDK2 can regulate PR protein levels in a complex manner. In the absence of ligand, CDK2 activation mediates loss of PR protein, which is independent of Ser400 (Fig. 3B, lanes 3 and 7 relative to lanes 1 and 5). However, PR Ser400 is absolutely required for CDK2-induced turnover of liganded PR (compare lanes 4 and 8). The mechanism to explain these effects is unknown but may involve differential effects of CDK2 on PR synthesis or folding and turnover (see Discussion).
Ligand-independent regulation of human PR transcriptional activity is seldom reported (4, 39). To verify that the increase in ligand-independent transcriptional activity of wt PR was due to activated CDK2, PRE-luciferase activity was measured in HeLa cells expressing either wt or S400A mutant PR and increasing concentrations of CDK2-TY (Fig. 3C). A dose-dependent increase in PRE-luciferase activity occurred with increasing concentrations of mutant CDK2-TY, suggesting that stimulation of wt PR activity in the absence of ligand was due to activated CDK2. In contrast, CDK2-TY failed to increase S400A PR-B transcriptional activity under the same conditions, confirming that PR Ser400 is required for this ligand-independent PR response. Cells lacking PR or cells containing PR and a reporter construct in which the progesterone response element (PRE) was mutated to an estrogen-responsive element failed to respond to CDK2-TY (data not shown), demonstrating that the effect of CDK2-TY on PRE-driven transcription is not due to a nonspecific increase in overall or basal transcription. Furthermore, CDK2-TY induction of PR transcriptional activity was blocked by RU486 (data not shown; see Fig. 8D, below). These data demonstrate that PR Ser400 mediates ligand-independent PR transcriptional activity in response to elevated CDK2 activity.
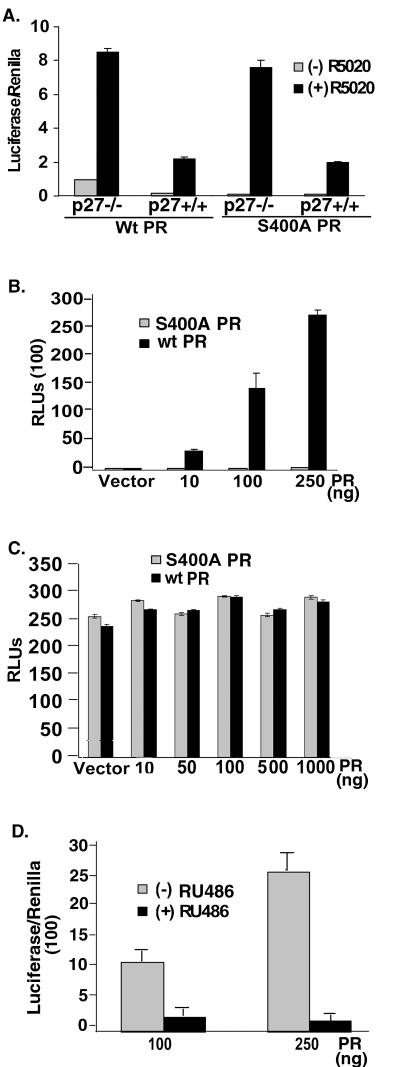
FIG. 8.
PR Ser400 is required for ligand-independent PR transcriptional activity in p27−/− MEF cells. (A) Increased PR transcriptional activity in p27−/− MEF cells. P27+/+ or p27−/− MEF cells were transiently transfected with PRE-luciferase and Renilla reporter plasmids and either wt or S400A PR-B (100 ng) and, 48 h after transfection, cell cultures were treated without or with R5020 for 18 h. PRE-luciferase activity in cell lysates was measured and normalized to that of Renilla controls. (B) PR dose dependence of PR transcriptionalactivity in p27−/− MEF cells. p27−/− cells were transiently transfected with increasing concentrations (10 to 250 ng) of vectors expressing either wt or S400A PR-B, control empty vectors to normalize total cDNA concentrations, and a constant amount of PRE-luciferase reporter plasmid. PRE-luciferase activity in lysates from unstimulated cells was measured and is reported as relative luminometer units (RLUs). (C) Absence of ligand-independent PR transcriptional activity in p27+/+ cells. P27+/+ MEF cells were transiently transfected with increasing concentrations of either wt or S400A PR-B (10 to 1,000 ng), control empty vector as appropriate, and a constant amount of PRE-luciferase reporter plasmid as for panel B, and PRE-luciferase activity in lysates from unstimulated cells was measured. (Note the difference in scales between Fig. 7B and C.) (D) RU486 blocks ligand-independent PR transcriptional activity in p27−/− cells. p27−/− cells were transiently transfected with a PRE-luc reporter along with either 100 or 250 ng wt PR. 48 h following transfection, cultures were treated with 10−7 M RU486 or EtOH vehicle control, and PRE-luciferase activity was measured and normalized to that of Renilla controls. The data are presented as means ± standard deviations of three replicates for each data point. Results are representative of three to four independent experiments.
P27 limits CDK2-induced PR transcriptional activity.
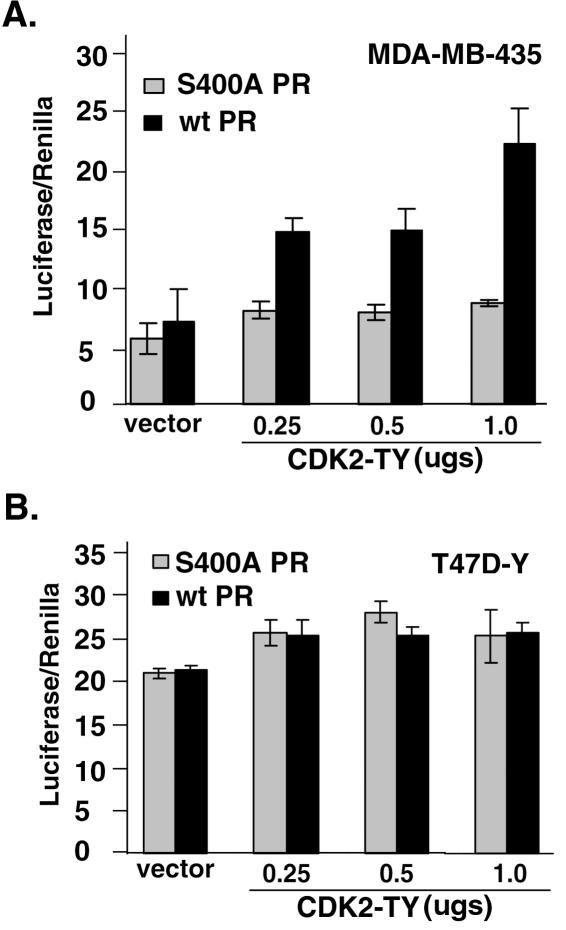
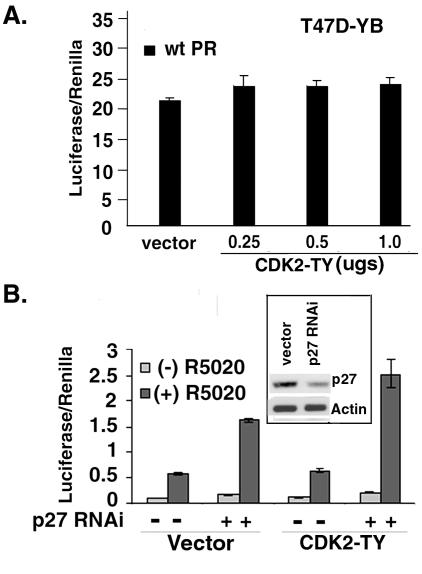
To confirm that the effect of activated CDK2 on ligand-independent PR-B transcriptional activity occurs in breast cancer cells, the above experiments (Fig. 3C) were performed in PR-null MDA-MB-435 (Fig. 4A) and PR-null T47D-Y (Fig. 4B) cell lines. Cells were transfected with wt or S400A PR-B, a PRE-luciferase reporter construct, a constitutive Renilla reporter construct, and increasing concentrations CDK2-TY cDNA. Similar to the results in HeLa cells (Fig. 3C), there was a dose-dependent effect of CDK2-TY on wt PR but not S400A PR transcriptional activity in MDA-MB-435 cells (Fig. 4A). However, in contrast to these results, CDK2-TY caused only a slight increase in wt and S400A PR-B transcriptional activity in T47D-Y cells relative to vector controls, and increasing the dose of CDK2-TY was without effect (Fig. 4B).
FIG. 4.
Activated CDK2 induces ligand-independent PR transcriptional activity in PR-null MDA-MB-435 (A) but not T47D-Y (B) breast cancer cells. (A) PR-B activity in MDA-MD-435 cells. MDA-MB-435 cells were transiently transfected with wt or S400A PR-B along with PRE-luciferase and Renilla reporter plasmids and increasing concentrations of mutant CDK2-TY or its empty parental vector as a control (vector). PRE-luciferase activity was measured and is reported in arbitrary units as the luciferase/Renilla ratio. (B) PR-B activity in T47D-Y cells. T47D-Y PR-null cells were transiently transfected with either wt or S400A PR along with PRE-luciferase and Renilla reporter plasmids and increasing concentrations of CDK2-TY or its empty parental vector as a control (vector). PRE-luciferase activity was measured and is reported in arbitrary units as the luciferase/Renilla ratio. The data are presented as means ± standard deviations of three replicates for each data point. Results are representative of two to three independent experiments.
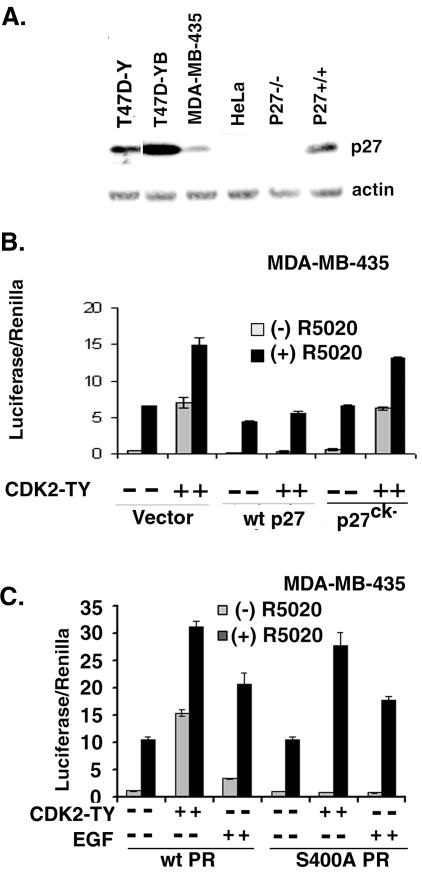
Since the absolute levels of CDK2 activity are determined by the relative presence of CDK2 inhibitory molecules and breast cancers frequently display loss of p27 (7, 63, 86), we postulated that these cell type differences may reflect differences in p27 expression between breast cancer cell lines (Fig. 4) and HeLa cells (Fig. 3C). Therefore, we performed Western blotting of p27 expression levels in these cell lines (Fig. 5A). Lysates of wt (p27+/+) and p27−/− MEFs were included as positive and negative controls, respectively. P27−/− cells are immortalized MEFs derived from knockout mice with a targeted deletion of the CDK2 inhibitor p27. T47D-Y PR-null cells and T47D-YB cells express elevated levels of p27 relative to HeLa and MDA-MB-435 cells, suggesting that p27 may block the stimulation of PR activity by activated CDK2 in T47D breast cancer cell lines (Fig. 4B). We reasoned that if the increase in PR transcriptional activity were due to elevated CDK2 activity, then addition of p27 should block ligand-independent PR activation. P27, a control p27 mutant (p27ck−) that is unable to bind and inhibit CDK2 activity (91), or a parental control vector were therefore cotransfected into MDA-MB-435 cells along with either wt or S400A PR-B, CDK2-TY or its parental control vector, and PRE-luciferase and Renilla reporters. Cells were then treated without or with R5020 for 18 h (Fig. 5B). As in HeLa cells (Fig. 3B), CDK2-TY increased PR-B transcriptional activity in both the absence and presence of R5020 in MDA-MB-435 cells. Wild-type P27, but not p27ck−, slightly diminished PR-B activity in the absence of CDK2-TY but blocked the CDK2-TY-induced increase in PR activity observed in both the absence and presence of ligand, suggesting that the increase in PR transcriptional activity induced by CDK2-TY overexpression (Fig. 3 and 4) was due to elevated CDK2 activity.
FIG. 5.
CDK2-induced PR transcriptional activity is inhibited by the CDK2 inhibitor p27. (A) p27 expression in cell line models. Cell lysates from T47D-Y, T47D-YB, MDA-MB-435, HeLa, and p27−/− and p27+/+ MEF cells were electrophoresed on 10% gels and blotted for p27; β-actin served as a loading control. (B) Reversal of CDK2-induced PR activity by p27. MDA-MB-435 cells were transiently cotransfected with wt PR (100 ng), mutant CDK2-TY in the presence and absence of either p27 or mutant p27ck−, and the appropriate vector controls along with the PRE-luciferase and Renilla reporter plasmids. PRE-luciferase activity was measured following treatment with either EtOH vehicle control or R5020 for 18 h. (C) PR transcriptional activity is enhanced by ligand and EGF in MDA-MB-435 breast cancer cells. MDA-MB-435 cells were transiently transfected with a PRE-luciferase reporter, Renilla reporter, with wt or S400A PR (100 ng) with or without CDK2-TY. Following treatment with R5020 (10 nM) or EGF (30 ng/ml) for 18 h, PRE-luciferase activity was measured and normalized to that of Renilla controls. The data are presented as means ± standard deviations of three replicates for each data point. Results are representative of three independent experiments.
Regulation of PR Ser400 phosphorylation and activity in response to mitogens was also investigated in low-p27 MDA-MB-435 cells (Fig. 5C). MDA-MB-435 cells were transiently cotransfected with a PRE-luciferase reporter, a Renilla reporter construct as a transfection control with either wt or S400A PR, and treated with R5020 or EGF. In the absence of EGF or CDK-TY, the basal and R5020-induced transcriptional activities of both wt and S400A PR were similar. As in previous experiments (Fig. 3B and 5B), CDK2-TY greatly enhanced wt PR transcriptional activity in both the presence and absence of R5020, and the unliganded activity mapped to PR Ser400. Similarly, EGF enhanced ligand-dependent (40 to 50%) and ligand-independent (three- to fourfold) wt PR transcriptional activity, while only the ligand-independent transcriptional activity mapped to Ser400. These data suggest that EGF, which induces phosphorylation of PR Ser400 (Fig. 1D), can also induce ligand-independent PR transcriptional activity, presumably via CDK2 activation in breast cancer cells expressing relatively low levels of p27, and that PR Ser400 is required for this effect.
To test the effects of elevated CDK2 activity on endogenously expressed PR in cells that also express elevated levels of p27 (Fig. 5A), we measured PR transcriptional activity in T47D-YB cells (Fig. 6A). T47D-YB cells were cotransfected with PRE-luc and Renilla reporter plasmids and either control vector or increasing concentrations of CDK2-TY. Similar to the results with PR-null T47D-Y cells transiently expressing PR (Fig. 4B), there was only a slight increase in PR transcriptional activity in T47D-YB cells cotransfected with CDK2-TY and no effect of increasing the dose of this kinase (Fig. 6A). If elevated p27 in the T47D-derived cell lines functions to limit the transcriptional activity of PR, then knock-down of p27 protein expression by p27 RNAi would be predicted to increase PR transcriptional activity in response to CDK2-TY and R5020. To demonstrate that p27 RNAi targets p27 expression, T47D-YB cells were transfected with expression vectors containing p27 RNAi and cell lysates were blotted with p27-specific antibodies (Fig. 6B, insert). Knock-down of p27 resulted in a 50 to 60% reduction of p27 relative to controls and was limited to some extent by the efficiency of transient transfection (i.e., every cell contains p27). We then overexpressed p27 RNAi or control RNAi by transient transfection of the appropriate pSHAG expression vectors in T47D-YB cells along with PRE-luciferase and Renilla reporter plasmids and either CDK2-TY or its parental control vector (Fig. 6B). As in Fig. 6A, CDK2-TY alone had no effect on PR transcriptional activity in these cells. P27 RNAi enhanced PR transcriptional activity in both the absence and presence of R5020 and partially restored the ability of CDK2-TY to increase PR transcriptional activities. Unliganded PR underwent a twofold induction in the presence of p27 RNAi, and this increased further in the presence of CDK2-TY (two- to threefold). These data suggest that p27 acts to repress PR transcriptional activity in the presence and absence of progestins and that p27 levels may serve to limit PR action in breast cancer cells.
FIG. 6.
Ligand and mitogen-induced PR transcriptional activity is enhanced in cells with low levels of p27. (A) Absence of CDK2-induced PR activity in p27-rich T47D cells. T47D-YB cells were transiently transfected with a PRE-luciferase reporter along with increasing concentrations of CDK2-TY. PRE-luciferase activity was measured and normalized to that of Renilla controls. Vector control cultures (vector) received 1.0 μg of parental vector control (for CDK2-TY). (B) p27 RNAi restores CDK2-induced PR transcriptional activity in T47D-YB cells. T47D-YB cells were transiently transfected with PRE-luciferase and Renilla reporter plasmids, CDK2-TY (+) or its parental control vector (−), and pSHAG vectors expressing either control RNAi (−) or p27 RNAi (+). Following treatment with R5020 (10 nM) for 18 h, PRE-luciferase activity was measured and normalized to that of Renilla controls. (Inset) p27 RNAi knock-down of p27. P27 RNAi or control RNAi was cotransfected into T47D-YB cells, and cell lysates were blotted for p27 and β-actin. The data are presented as means ± standard deviations of three replicates for each data point. Results are representative of two to three independent experiments.
PR Ser400 mediates ligand-independent PR action in p27−/− MEFs.
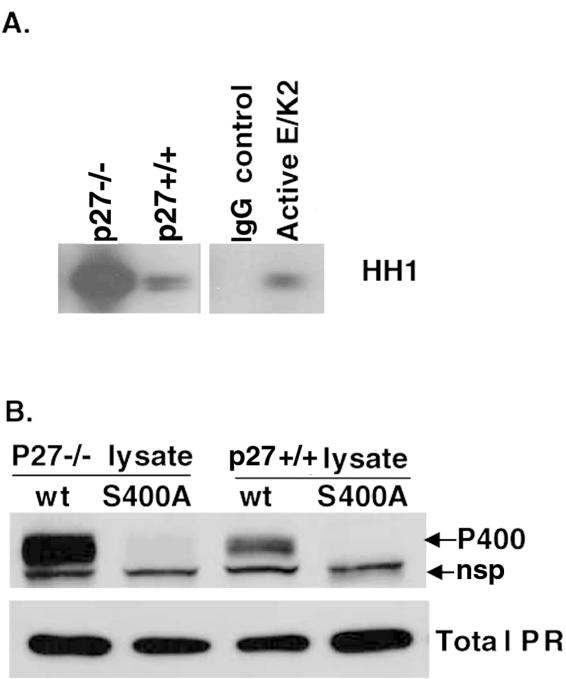
To confirm a repressive role for p27 on the Ser400-mediated regulation of ligand-independent PR transcriptional activity, we again utilized p27−/− and p27+/+ MEF cell line models. Endogenous CDK2 activity was measured in unstimulated p27+/+ and p27−/− cells in an in vitro kinase assay with histone H1 as a substrate. p27−/− MEF cells had greatly elevated CDK2 activity compared to wt p27+/+ cells (Fig. 7A). We then transiently expressed either wt or S400A PR-B in these cells (Fig. 7B). Similar levels of both wt and S400A PR-B were expressed in both cell lines (lower panel). However, wt PR was more highly phosphorylated on Ser400 in the p27−/− cells compared to p27+/+ cells, suggesting that the phosphorylation status of PR Ser400 is inversely related to the level of p27 CDK2 inhibitor and directly related to the level of CDK2 activity.
FIG. 7.
PR Ser400 is highly phosphorylated in p27−/− relative to wt p27+/+ MEF cells. (A) Endogenous CDK2 activity in MEF cells. Endogenous CDK2 in lysates from unstimulated wt p27+/+ and knockout p27−/− MEFs was purified by immunoprecipitation with CDK2-specific antibodies, and immune complexes were assayed in in vitro kinase assays with histone H1 (HH1) as a substrate. Controls included nonspecific immunoglobulin G immunoprecipitates (IgG control) and purified recombinant active cyclinE/CDK2 as a positive control for CDK2 activity. (B) Increased PR Ser400 phosphorylation in p27−/− MEF cells. wt PR or S400A PR was transiently transfected into p27+/+ or p27−/− MEF cells. Cell lysates were electrophoresed on SDS-PAGE gels and subjected to Western blotting with either total PR or phospho-Ser400 PR antibodies (P400). The PR phospho-Ser400 antibody recognized a nonspecific protein (nsp), which migrated just under PR in MEF cells, that is not present in breast epithelial cells or HeLa cells.
To examine the effect of elevated CDK2 activity on PR transcriptional activity in MEF cells, either wt or S400A PR-B was transiently cotransfected with both PRE-luciferase and Renilla reporter plasmids, and cells were treated without or with R5020 (Fig. 8A). Similar to the results with HeLa cells (Fig. 3B) and MDA-MD-435 cells (Fig. 4A and 5B and C), there was significantly greater R5020-induced PR transcriptional activity in p27−/− MEF cells with elevated CDK2 activity (Fig. 8A) relative to wt p27+/+ cells expressing either wt or S400A PR. In addition, wt and S400A PR reached a similar maximum level of transcriptional activity in both cell types, indicating that Ser400 is not required for PR transcriptional activity in response to progestins. However, the transcriptional activity of unliganded wt PR-B, but not S400A PR-B, was elevated ninefold in p27−/− cells relative to wt p27+/+ cells, confirming that elevated CDK2 activity induces increased PR transcriptional activity independently of progestin binding and that Ser400 is required for this effect.
To test the PR dependence of ligand-independent PR activity in p27−/− MEF cells, PR concentration curves were evaluated. P27−/− cells were transfected with increasing levels of either wt or S400A PR-B, and PRE-driven luciferase activity was measured as described above (Fig. 8B). Ligand-independent PR transcriptional activity increased with increasing levels of wt PR, but not S400A PR, indicating that the transcriptional response is PR dependent and requires PR Ser400. PRE-luc activity was similarly measured in p27+/+ MEF cells transiently expressing either S400A mutant or wt PR-B (Fig. 8C). As shown for T47D cells with high levels of p27 (Fig. 4B and 6A), increasing levels of either wt or S400A PR-B failed to induce PRE-driven luciferase promoter activity in cells containing functional p27, suggesting that the ligand-independent response of PR is a result of p27 deletion and consequent elevated CDK2 activity (note the difference in scales between Fig. 8B and C). The PR antagonist RU486 abolished ligand-independent PRE-luc activity in p27−/− cells expressing wt PR (Fig. 8D). Additionally, no transcriptional activity was detected in the absence of transfected wt PR, indicating that endogenous glucocorticoid receptor (GR) was not responsible for these effects (data not shown). These results suggest that when CDK2 is not under the negative control of the CDK2 inhibitor p27, there is a subsequent increase in PR transcriptional activity both in the absence and presence of ligand. Furthermore, PR Ser400 is required for ligand-independent activation of PR in response to elevated CDK2 activity.
PR Ser400 mediates CDK2-induced PR nuclear translocation.
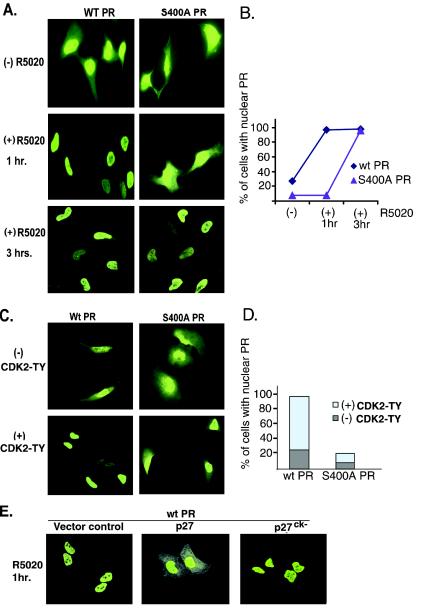
Unliganded PR are located in the cytoplasm and nucleus and have been shown to rapidly shuttle between these compartments (24). Following treatment with progestins, liganded PR is entirely nuclear within 30 to 60 min. To visualize the subcellular location of S400A PR-B relative to wt PR-B, imaging experiments were performed in intact cells (Fig. 9A). HeLa cells were transfected with either wt or S400A mutant PR-B and treated with or without R5020 for 1 and 3 h. Cells were fixed, permeabilized, and immunostained with an antibody specific for total PR. wt PR localized to the nucleus within 1 h of R5020 treatment. In contrast, S400A PR-B remained cytoplasmic following 1 h of exposure to R5020 but was predominantly found in the nucleus after at least 3 h of R5020 treatment. To confirm the differential kinetics of wt and S400A PR nuclear accumulation, cells expressing either PR protein were counted (100 each) and scored for exclusive nuclear PR staining at both 1 and 3 h post-progestin addition (Fig. 9B). Prior to R5020 treatment, approximately 75% of the cells expressing wt PR contained both nuclear and cytoplasmic receptors, while wt PR was exclusively in the nucleus of 25% of the transfected cells. R5020 treatment caused nuclear translocation in almost 100% of cells containing wt PR by 1 h. In contrast, few S400A PR-transfected cells contained exclusively nuclear PR before R5020 treatment and following a 1-h exposure to this ligand (Fig. 9B). However, S400A PR-B was localized in the nucleus of almost 100% of transfected cells after 3 h of R5020 treatment, indicating that S400A PR undergoes delayed nuclear translocation in response to progestins.
FIG. 9.
Confocal microscopy showing the subcellular localization of wt and S400A PR. (A) Delayed ligand-induced nuclear translocation of S400A PR. HeLa cells growing on coverslips in six-well dishes were transiently transfected with wt or S400A PR-B and treated with EtOH vehicle control or R5020 for either 1 or 3 h. The cells were fixed and subjected to IHC with total PR monoclonal antibodies followed by fluorescein isothiocyanate (FITC)-conjugated secondary antibodies, and PR-stained cells were visualized by confocal microscopy. Controls for the primary andsecondary antibodies demonstrated the specificity of PR staining (data not shown). (B) Percentage of transfected cells containing exclusively nuclear PR. Transfected HeLa cells containing wt or S400A PR were prepared as for panel A and counted (100 each), and the data are expressed graphically as the percentage of transfected cells with PR exclusively in the nucleus relative to PR staining in both compartments. (C) Wild-type but not S400A PR nuclear association induced by activated CDK2. HeLa cells growing on coverslips in six-well dishes were transiently cotransfected with either wt or S400A PR-B along with mutant CDK2-TY or empty vector control. PR in transfected cells was immunostained as above with total PR monoclonal antibodies followed by FITC-conjugated secondary antibodies. PR-transfected cells were visualized by confocal microscopy. (D) Percentage of cells with exclusively nuclear PR. HeLa cells transfected with either wt or S400A PR were prepared as for panel C and counted (100 each), and the data are expressed graphically as the percentage of cells with PR exclusively in the nucleus relative to those with PR in both cytoplasmic and nuclear compartments. (E) p27 and CDK2-dependent cytoplasmic retention of liganded PR. HeLa cells growing on coverslips in six-well dishes were transiently transfected with wt PR-B and either empty vector control or vectors encoding wt p27 or a p27ck− mutant unable to bind CDK2 and, 48 h later, transfected cells were treated with R5020 for 1 h. Cells were stained with PR-specific monoclonal antibodies as described above and visualized by confocal microscopy. These experiments were repeated two times in HeLa cells and one time in MDA-MB-435 cells with similar results.
CDK2 may induce ligand-independent PR transcriptional activity in part by increasing PR access to the nucleus, perhaps via phosphorylation of Ser400. We therefore performed additional imaging experiments in intact cells to visualize the subcellular location of wt and S400A PR in the absence and presence of activated CDK2 (Fig. 9C). HeLa cells were cotransfected with either wt or S400A PR and mutant CDK2-TY or its parental control vector. In the absence of progestin, CDK2-TY induced the nuclear localization of wt PR in nearly 100% of transfected cells (Fig. 9C and D). In contrast, S400A PR was resistant to CDK2-induced nuclear localization relative to wt PR. Quantification of transfected cells revealed nuclear localization of S400A PR in a minority of cells (Fig. 9D), indicating that PR Ser400 mediates PR nuclear localization in response to activated CDK2. Imaging studies were also performed in PR- and CDK2-TY-cotransfected MDA-MB-435 cells with similar results (data not shown).
Finally, to determine if p27 could also alter PR subcellular localization via regulation of CDK2 activity, imaging studies were repeated in HeLa cells by cotransfection of wt PR-B and either wt p27 or the CDK2 binding mutant of p27 (p27ck−) (Fig. 9E). Transiently transfected HeLa cells were treated with R5020 for 1 h to induce nuclear localization of wt PR. Overexpression of wt p27, but not the CDK2 binding mutant (p27ck−), resulted in cytoplasmic PR localization in at least 80% of cotransfected cells. Taken together, these data confirm that PR translocation to the nucleus is mediated by multiple mechanisms (66). The classical ligand-dependent mechanism (24) does not require PR Ser400 phosphorylation but occurs more efficiently (i.e., in under 1 h) when Ser400 is phosphorylated (Fig. 9A). An alternate mechanism of PR nuclear localization occurs independently of ligand, is induced by activated CDK2, and requires PR Ser400 phosphorylation (Fig. 9C).
DISCUSSION
CDK2 regulates PR transcriptional activity.
Our results define a novel role for elevated CDK2 activity in the regulation of PR transcriptional activity and subcellular localization. Furthermore, CDK2 phosphorylation of PR Ser400 is required for increased PR nuclear localization and transcriptional activity in the absence of added progestins. Studies using PR phospho-Ser400 antibodies confirmed previous reports (95) that PR Ser400 is basally phosphorylated in resting cells and that progestin treatment also increases phosphorylation at this site (Fig. 1). Additionally, several mitogens predicted to activate CDK2 rapidly induce PR Ser400 phosphorylation in the absence of steroid hormones. Although PR Ser400 can be phosphorylated in vitro by purified cyclin A/CDK2 (95), the inhibition of Ser400 phosphorylation by CDK2-specific inhibitors (Fig. 2A) in intact cells suggests that CDK2 or CDK2-dependent downstream kinases phosphorylate endogenous PR at this site.
Progestins are known to regulate cell cycle molecules (23) and induce CDK2 activity (23). Herein, we found that progestins induced increased CDK2 activity as early as 15 min. Interestingly, however, CDK2 protein levels were also increased in response to R5020 (Fig. 1C). R5020 rapidly upregulates cyclin D1 and cyclin E protein levels (23) in a mitogen-activated protein kinase (MAPK)-dependent manner (42). Similarly, MEK inhibitors block CDK2 protein upregulation in response to R5020 (E. Faivre and C. A. Lange, unpublished results), suggesting that progestin-mediated activation of MAPK and upregulation of G1-phase cyclins may stabilize cyclin-CDK complexes and thus increase CDK2 protein levels and activity. Phosphorylation of PR by CDK2 occurs on at least eight sites (36, 95-97) and may provide a highly sensitive means of positive feedback or “feed-forward” regulation by steroid hormones. Similar to progestins, a variety of mitogens, including peptide growth factors, also stimulate rapid phosphorylation of PR Ser400, suggesting that MAPK activation leading to a rise in CDK2 activity is an important input to PR regulation (Fig. 1).
Activated CDK2 increased the transcriptional activity of both wt and S400A mutant PR in the presence of progestin, indicating that CDK2 activity is able to positively regulate liganded PR and that this regulation does not require Ser400 (Fig. 3B). Recent data indicate that increased phosphorylation of PR in S phase corresponds to an increase in PR transcriptional activity and that overexpression of either cyclin A or CDK2 enhances both PR and androgen receptor activity in transiently transfected COS cells (55). Those authors found that CDK2 altered PR function by increasing the recruitment of the SRC-1 coactivator to liganded PR. In agreement with our studies (Fig. 3B and 8A), CDK2 regulation of liganded PR did not map to any known PR phosphorylation sites (55). These studies demonstrate that phosphorylation events can regulate PR action at multiple levels.
Notably, our studies show that CDK2 or EGF induces a significant increase in ligand-independent PR transcriptional activity that maps to PR Ser400 (Fig. 3B, 4A, 5C, and 8). Thus, phosphorylation of PR Ser400 by CDK2 may provide a mechanism for setting the basal level of PR transcriptional activity in response to changes in CDK2 activity during normal cell cycle progression or in breast cancer. To date, there are few examples of ligand-independent activation of human PR. Bamberger et al. (4) demonstrated ligand-independent PR transcriptional activity with an activating protein 1 (AP-1)-driven promoter in human endometrial adenocarcinoma cells. Interestingly, addition of progestin dampened PR-dependent AP-1 activity. Most recently, Labriola et al. (39) reported that heregulin activation of c-erbB2 receptors mediated a modest increase in ligand-independent PR transcriptional activity in T47D breast cancer cells (39). Similarly, in MDA-MB-435 cells, EGF stimulated a three- to fourfold induction of PR transcriptional activity in the absence of ligand (Fig. 5C). In contrast, many pathways leading to ligand-independent regulation of estrogen receptor alpha (ER-α) have been reported (51), and growth factors are now recognized as an important input to ER-α action.
PR transcriptional activity and turnover are inversely related.
The inhibition of ligand-dependent PR downregulation by the CDK2 inhibitor suggests a role for CDK2 activity in this process (Fig. 2A). In support of this interpretation, cyclin E overexpression and hyperactivation of CDK2 stimulated PR downregulation in both the absence and presence of ligand (Fig. 2C), and these conditions resulted in increased PR transcriptional activity (Fig. 3B). In addition, inhibition of CDK2 activity elevated PR protein levels in the absence of ligand (Fig. 2D). These observations are consistent with recent studies suggesting that there is a positive relationship between the rate of transcription factor protein turnover and transcriptional activity (72, 73). Although many sites on PR are known to be phosphorylated, Ser294 is the only other site that has been functionally well characterized (43, 66, 78). Increased PR transcriptional activity following ligand-induced phosphorylation of Ser294, located within a “destruction box” motif, is coupled to rapid cytoplasmic degradation of PR by the ubiquitin proteosome pathway (43, 66). Similarly, the location of Ser400 adjacent to an additional destruction box motif in the N terminus of PR and upstream of AF-1 may suggest a role for regulation of PR ubiquitination by phosphorylation of Ser400. Recent evidence suggests transcriptional activation domains can be regulated by ubiquitination, a required event for the transcriptional activation of multiple transcription factors, from yeast VP16 (71) to human ER-α (67). Additionally, in contrast to wt PR, S400A PR protein accumulates in the presence of progestin when CDK2 activity is high (Fig. 3, lane 8), perhaps due to CDK2-induced nuclear sequestration (Fig. 9C). Alternatively, Ser400 may play a role in PR protein production or the stepwise ATP-dependent process of PR folding in the absence of progestins (64) and/or PR protein turnover in their presence. Interestingly, PR Ser400 is predicted to be exposed on the surface of native PR and is located in an accessible “hot spot” region for cleavage by proteases (3). The ability of CDK2 to regulate PR protein synthesis or folding, ubiquitination, and targeting to the 26S proteasome via phosphorylation of Ser400 is the subject of a separate study.
P27 is a transcriptional repressor of PR.
P27 limits the activity of unliganded PR (Fig. 5 and 6), presumably due to its ability to inhibit CDK2 activity. However, CDK inhibitors appear to have novel functions independently of their kinase subunits (45). For example, although the significance is unknown, cytoplasmic p27 can bind to and sequester GRB-2 from SOS/Ras complexes in mouse fibroblast cells and in breast cancer cells (45). Our studies in HeLa cells, breast cancer cells, and p27−/− MEF cells provide strong evidence that CDK2 is a positive regulator of PR action in the absence of progestins and that p27 may act as a transcriptional repressor of PR, possibly due in part to retention of PR in the cytoplasm, an effect that requires p27 binding to CDK2 (Fig. 9E). During these studies, another group of investigators demonstrated that p27 acts as a transcriptional repressor of the GR by investigating the phosphorylation and activation of GR in p27−/− MEFs (92). However, whether increased GR transcriptional activity in p27−/− cells relative to that in wt MEF cells was dependent on altered CDK2 activity or direct binding of CDK subunits or p27 to GR was not determined. Cell cycle regulatory molecules have been reported to interact with and regulate other transcription factors. In addition, the transcription factor B-Myb has been demonstrated to be a direct target of cyclin A/CDK2 (68). The transcriptional activity of B-Myb is enhanced by cyclin A/CDK2 phosphorylation (2, 33, 40, 68, 70, 99), and poly(ADP-ribose) polymerase may function as a cofactor that promotes cyclin/CDK2-dependent phosphorylation of B-Myb (75). Cyclin A1 binds directly to B-Myb in vitro and enhances its activity by phosphorylation (48). In contrast, cyclin D1 binding to B-Myb does not require enzymatic activity (26), and there appears to be an inverse correlation between cyclin D1/B-Myb association and transcriptional activity (8). Cyclin E has been reported to activate transcription when bound to DNA via a heterologous DNA binding domain and in a cell cycle-dependent manner; this requires both CDK2-cyclin E association and CDK2 kinase activity (35). Cyclins and CDKs have also been shown to regulate the transcriptional activity of ER-α (90, 100). For example, overexpression of cyclin A increased ER-α transcriptional activity in both the presence and absence of estrogen in a variety of cell types, and this effect required CDK2 kinase activity (90). However, cyclin A was not shown to associate with ER-α in these studies. In contrast, cyclin D1 increased ER-α transcriptional activity by direct association with ER-α, and this effect appeared to be independent of CDK kinase activity (100). Similarly, cyclinA/CDK2 has been reported to bind to PR (55), and we have recently noted cyclinE/CDK2 binding to PR (L. Pierson-Mullany and C. A. Lange, unpublished results). Direct binding of cyclin, CDK, or CDK inhibitory molecules to PR may explain the relative resistance of wt unliganded PR to CDK2-dependent activation in T47D cells (Fig. 4 and 6). We are currently examining the ability of cell cycle regulatory molecules to bind to and modulate PR action.
CDK2 regulates nuclear localization of PR via Ser400 phosphorylation.
PR is located in both the cytoplasm and the nucleus in the absence of progestins and accumulates in the nucleus in their presence. Imaging studies of wt and mutant PR indicate that Ser400 is important for efficient (i.e., timely) progestin-induced nuclear localization of PR and required for nuclear translocation induced by activated-CDK2 in the absence of steroid hormone ligand (Fig. 9). Since PRs are located in both the cytoplasmic and nuclear compartments of unstimulated cells, it is unknown where in the cell CDK2 is able to phosphorylate PR. Although the CDK2 partners cyclin A and cyclin E are nuclear proteins, recent cell imaging data demonstrated that like PRs (24), cyclin E and cyclin A/CDK2 complexes dynamically shuttle between the nucleus and cytoplasm, suggesting that CDK2 may phosphorylate both cytoplasmic and nuclear substrates (30). Indeed, CDK2 has both nuclear and cytoplasmic targets, including E2F (37, 94), NF-κB (61), the NPAT regulator of S-phase-specific histone transcription (44, 98), p300/CBP coactivators (17), PRC1, a regulator of cytokinesis (32), and proteins involved in centrosome duplication, including nucleophosmin NPM/B23 (58, 88), mouse Mps1/TTK (18), and CP110 (9). According to our imaging data (Fig. 9), a possible consequence of CDK2-regulated phosphorylation of PR Ser400 is to regulate nuclear accumulation and/or shuttling between the cytoplasm and nuclear compartments. Our investigators reported a similar role for phosphorylation of PR Ser294 in response to progestins or EGF (66). Phosphorylation regulates nuclear translocation of a number of transcription factors, including NF-κB, c-rel, Dorsal, and yeast SWI5 (as reviewed by Jans et al. [31]). Similarly, CDK sites regulate maximal levels of nuclear accumulation of simian virus 40 large tumor antigen (31) and other cell cycle-regulated molecules, such as CDC6, a regulator of initiation of DNA replication (62). Similar to CDK2 regulation of unliganded PR nuclear localization, studies from this lab have demonstrated that MAPKs induce PR nuclear localization in the absence of progestin via phosphorylation of Ser294, but they are not required in the presence of ligand (66). Unlike CDK2-induced PR transcriptional activation of PRE-driven promoters in the absence of progestins (Fig. 3 to 5), MAPK activation alone was unable to induce transcriptional activation of unliganded PRs measured on PRE-driven promoters (43) but was required for PR-dependent expression of IRS-1 (65), suggesting that different kinase inputs to PR can mediate changes in promoter specificity independently of ligand binding.
Role of cell cycle regulation of PR in breast development and cancer.
Surprisingly, studies in knock-out mice have shown that cyclin E and CDK2 are not required for cell proliferation or mouse development (6, 20, 59, 60). However, cyclin E is required for cells to exit the G0 quiescent state and thereby enter the cell cycle and for the process of endoreduplication in which cells replicate their DNA without cell division, such as trophoblasts in the placenta (20). In addition, cells from cyclin E1 and E2 knockout mice are resistant to oncogenesis (20). Thus, although cyclin E and CDK2 are not necessary for the process of mitosis in otherwise normal cells, there are correlations between aberrant overexpression of cyclin E and D-type cyclins and/or loss of p27 and tumorigenesis, including poor patient prognosis and resistance to chemotherapy in breast cancer patients (5, 7, 21, 77).
Cells systematically progress through the cell cycle in part due to the orderly synthesis of cyclins. Cyclins in turn modulate the progression of the cell cycle by binding to their catalytic CDK partners. For example, during the G1 phase of the cell cycle, cyclin D forms a complex with either CDK4 or CDK6 followed by cyclin E complex formation with CDK2. Cyclins play a critical role in regulating cell proliferation by responding to extracellular signals. Deregulation of cyclins or CDK activity could lead to oncogenesis by making the cells less dependent on growth factors (28). Studies with PR knockout mice indicate that PR plays a key role in lobulo-alveolar development, and progesterone's proliferative signal is required to induce a high incidence of mammary tumors (29). Similarly, cyclin D1 is expressed in normal mammary epithelium during lobulo-alveolar development (81), and mice lacking cyclin D1 resemble PR knockout mice in that lobulo-alveolar development is blocked (16, 80). In addition, the importance of the suppressive role of P27 is apparent in p27−/− mammary epithelial cells, where elevated CDK2 activity positively regulates both cell proliferation and survival (14). Another recent study implicates p27 as important for normal mammary development (49), while loss of p27 is a frequent occurrence in breast cancer (7, 63, 87). Interestingly, steroid hormones and peptide growth factors share common targets in mammary epithelial cells, such as c-myc, c-fos, cyclin D1 and D2, CDK2, pRb, and CDK inhibitors (15, 83). In fact, growth factor signaling pathways have been shown to profoundly regulate PR independently of (39) or in combination with (39, 41, 43) progestins. Taken together, these data emphasize the intimate connection between cell cycle control and regulation of PR action during normal mammary gland development and in breast cancer.
In summary, these studies indicate that CDK2 activity is able to regulate liganded and unliganded PR transcriptional activity and PR nuclear localization. In the absence of progestins, CDK2-induced PR transcriptional activity and nuclear translocation requires PR Ser400 and p27 limits PR action. Cell cycle regulation of PR transcriptional activity may occur in breast cancers with a deregulated cell cycle resulting from upregulated cyclins and/or reduced levels of cyclin/CDK inhibitors and thereby contribute to breast cancer progression (10, 22). Our results have uncovered a mechanism for hormone-independent and cell cycle-dependent regulation of PR in breast cancer cells and suggest that patients with steroid receptor-positive breast cancer may benefit from endocrine therapies that target both estrogen and progesterone receptors (46) as well as growth-regulating kinase pathways (74).
Acknowledgments
We thank Robert Sheaff (University of Minnesota) for numerous helpful comments and suggestions in addition to the p27 and activated CDK2 expression vectors, activated cyclin E/CDK2 complex, and the wt and p27−/− MEFs and James DeGregori (University of Colorado Health Sciences Center) for cyclin E adenoviruses. We are grateful to Craig Jordan (Northwestern University) for PR-null T47D42W cells and to Kathryn B. Horwitz (University of Colorado Health Sciences Center) for T47Dco, T47D-YB, and YA breast cancer cell lines. We are grateful to Ming Qiu (University of Minnesota) and Jim McCabe and Jerry Sedgewick (University of Minnesota Bio-Imaging Processing Lab Core Facility) for excellent confocal microscopy technical assistance.
This work was supported by Department of Defense Breast Cancer Research Program DAMD17-02-1-0495 (to L. Pierson-Mullany) and NIH R01-DK053825 (to C. Lange).
REFERENCES
- 1.Alkarain, A., R. Jordan, and J. Slingerland. 2004. p27 deregulation in breast cancer: prognostic significance and implications for therapy. J. Mammary Gland Biol. Neoplasia 9:67-80. [DOI] [PubMed] [Google Scholar]
- 2.Ansieau, S., E. Kowenz-Leutz, R. Dechend, and A. Leutz. 1997. B-Myb, a repressed trans-activating protein. J. Mol. Med. 75:815-819. [DOI] [PubMed] [Google Scholar]
- 3.Bain, D. L., M. A. Franden, J. L. McManaman, G. S. Takimoto, and K. B. Horwitz. 2000. The N-terminal region of the human progesterone A-receptor. Structural analysis and the influence of the DNA binding domain. J. Biol. Chem. 275:7313-7320. [DOI] [PubMed] [Google Scholar]
- 4.Bamberger, A. M., C. M. Bamberger, B. Gellersen, and H. M. Schulte. 1996. Modulation of AP-1 activity by the human progesterone receptor in endometrial adenocarcinoma cells. Proc. Natl. Acad. Sci. USA 93:6169-6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartkova, J., J. Lukas, H. Muller, D. Lutzhoft, M. Strauss, and J. Bartek. 1994. Cyclin D1 protein expression and function in human breast cancer. Int. J. Cancer 57:353-361. [DOI] [PubMed] [Google Scholar]
- 6.Berthet, C., E. Aleem, V. Coppola, L. Tessarollo, and P. Kaldis. 2003. Cdk2 knockout mice are viable. Curr. Biol. 13:1775-1785. [DOI] [PubMed] [Google Scholar]
- 7.Catzavelos, C., N. Bhattacharya, Y. C. Ung, J. A. Wilson, L. Roncari, C. Sandhu, P. Shaw, H. Yeger, I. Morava-Protzner, L. Kapusta, E. Franssen, K. I. Pritchard, and J. M. Slingerland. 1997. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat. Med. 3:227-230. [DOI] [PubMed] [Google Scholar]
- 8.Cesi, V., B. Tanno, R. Vitali, C. Mancini, M. L. Giuffrida, B. Calabretta, and G. Raschella. 2002. Cyclin D1-dependent regulation of B-myb activity in early stages of neuroblastoma differentiation. Cell Death Differ. 9:1232-1239. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Z., V. B. Indjeian, M. McManus, L. Wang, and B. D. Dynlacht. 2002. CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev. Cell 3:339-350. [DOI] [PubMed] [Google Scholar]
- 10.Chlebowski, R. T., S. L. Hendrix, R. D. Langer, M. L. Stefanick, M. Gass, D. Lane, R. J. Rodabough, M. A. Gilligan, M. G. Cyr, C. A. Thomson, J. Khandekar, H. Petrovitch, and A. McTiernan. 2003. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative randomized trial. JAMA 289:3243-3253. [DOI] [PubMed] [Google Scholar]
- 11.Clarke, C. L., and R. L. Sutherland. 1990. Progestin regulation of cellular proliferation. Endocr. Rev. 11:266-301. [DOI] [PubMed] [Google Scholar]
- 12.Coats, S., P. Whyte, M. L. Fero, S. Lacy, G. Chung, E. Randel, E. Firpo, and J. M. Roberts. 1999. A new pathway for mitogen-dependent cdk2 regulation uncovered in p27(Kip1)-deficient cells. Curr. Biol. 9:163-173. [DOI] [PubMed] [Google Scholar]
- 13.Davis, S. T., B. G. Benson, H. N. Bramson, D. E. Chapman, S. H. Dickerson, K. M. Dold, D. J. Eberwein, M. Edelstein, S. V. Frye, R. T. Gampe, Jr., R. J. Griffin, P. A. Harris, A. M. Hassell, W. D. Holmes, R. N. Hunter, V. B. Knick, K. Lackey, B. Lovejoy, M. J. Luzzio, D. Murray, P. Parker, W. J. Rocque, L. Shewchuk, J. M. Veal, D. H. Walker, and L. F. Kuyper. 2001. Prevention of chemotherapy-induced alopecia in rats by CDK inhibitors. Science 291:134-137. [DOI] [PubMed] [Google Scholar]
- 14.Davison, E. A., C. S. Lee, M. J. Naylor, S. R. Oakes, R. L. Sutherland, L. Hennighausen, C. J. Ormandy, and E. A. Musgrove. 2003. The cyclin-dependent kinase inhibitor p27 (Kip1) regulates both DNA synthesis and apoptosis in mammary epithelium but is not required for its functional development during pregnancy. Mol. Endocrinol. 17:2436-2447. [DOI] [PubMed] [Google Scholar]
- 15.Doisneau-Sixou, S. F., C. M. Sergio, J. S. Carroll, R. Hui, E. A. Musgrove, and R. L. Sutherland. 2003. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr. Relat. Cancer 10:179-186. [DOI] [PubMed] [Google Scholar]
- 16.Fantl, V., G. Stamp, A. Andrews, I. Rosewell, and C. Dickson. 1995. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 9:2364-2372. [DOI] [PubMed] [Google Scholar]
- 17.Felzien, L. K., S. Farrell, J. C. Betts, R. Mosavin, and G. J. Nabel. 1999. Specificity of cyclin E-Cdk2, TFIIB, and E1A interactions with a common domain of the p300 coactivator. Mol. Cell. Biol. 19:4241-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisk, H. A., and M. Winey. 2001. The mouse Mps1p-like kinase regulates centrosome duplication. Cell 106:95-104. [DOI] [PubMed] [Google Scholar]
- 19.Font de Mora, J., and M. Brown. 2000. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol. Cell. Biol. 20:5041-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geng, Y., Q. Yu, E. Sicinska, M. Das, J. E. Schneider, S. Bhattacharya, W. M. Rideout, R. T. Bronson, H. Gardner, and P. Sicinski. 2003. Cyclin E ablation in the mouse. Cell 114:431-443. [DOI] [PubMed] [Google Scholar]
- 21.Gillett, C., V. Fantl, R. Smith, C. Fisher, J. Bartek, C. Dickson, D. Barnes, and G. Peters. 1994. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 54:1812-1817. [PubMed] [Google Scholar]
- 22.Gray, S. 2003. Breast cancer and hormone-replacement therapy: the Million Women Study. Lancet 362:1332. [DOI] [PubMed] [Google Scholar]
- 23.Groshong, S. D., G. I. Owen, B. Grimison, I. E. Schauer, M. C. Todd, T. A. Langan, R. A. Sclafani, C. A. Lange, and K. B. Horwitz. 1997. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1). Mol. Endocrinol. 11:1593-1607. [DOI] [PubMed] [Google Scholar]
- 24.Guiochon-Mantel, A., P. Lescop, S. Christin-Maitre, H. Loosfelt, M. Perrot-Applanat, and E. Milgrom. 1991. Nucleocytoplasmic shuttling of the progesterone receptor. EMBO J. 10:3851-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hissom, J. R., and M. R. Moore. 1987. Progestin effects on growth in the human breast cancer cell line T-47D—possible therapeutic implications. Biochem. Biophys. Res. Commun. 145:706-711. [DOI] [PubMed] [Google Scholar]
- 26.Horstmann, S., S. Ferrari, and K. H. Klempnauer. 2000. Regulation of B-Myb activity by cyclin D1. Oncogene 19:298-306. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz, K. B., M. B. Mockus, and B. A. Lessey. 1982. Variant T47D human breast cancer cells with high progesterone-receptor levels despite estrogen and antiestrogen resistance. Cell 28:633-642. [DOI] [PubMed] [Google Scholar]
- 28.Hunter, T., and J. Pines. 1994. Cyclins and cancer. II: cyclin D and CDK inhibitors come of age. Cell 79:573-582. [DOI] [PubMed] [Google Scholar]
- 29.Ismail, P. M., P. Amato, S. M. Soyal, F. J. DeMayo, O. M. Conneely, B. W. O'Malley, and J. P. Lydon. 2003. Progesterone involvement in breast development and tumorigenesis—as revealed by progesterone receptor “knockout” and “knockin” mouse models. Steroids 68:779-787. [DOI] [PubMed] [Google Scholar]
- 30.Jackman, M., Y. Kubota, N. den Elzen, A. Hagting, and J. Pines. 2002. Cyclin A- and cyclin E-Cdk complexes shuttle between the nucleus and the cytoplasm. Mol. Biol. Cell 13:1030-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jans, D. A., and S. Hubner. 1996. Regulation of protein transport to the nucleus: central role of phosphorylation. Physiol. Rev. 76:651-685. [DOI] [PubMed] [Google Scholar]
- 32.Jiang, W., G. Jimenez, N. J. Wells, T. J. Hope, G. M. Wahl, T. Hunter, and R. Fukunaga. 1998. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol. Cell 2:877-885. [DOI] [PubMed] [Google Scholar]
- 33.Johnson, T. K., R. E. Schweppe, J. Septer, and R. E. Lewis. 1999. Phosphorylation of B-Myb regulates its transactivation potential and DNA binding. J. Biol. Chem. 274:36741-36749. [DOI] [PubMed] [Google Scholar]
- 34.Keyomarsi, K., N. O'Leary, G. Molnar, E. Lees, H. J. Fingert, and A. B. Pardee. 1994. Cyclin E, a potential prognostic marker for breast cancer. Cancer Res. 54:380-385. [PubMed] [Google Scholar]
- 35.Kim, T. Y., and W. G. Kaelin, Jr. 2001. Differential control of transcription by DNA-bound cyclins. Mol. Biol. Cell 12:2207-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knotts, T. A., R. S. Orkiszewski, R. G. Cook, D. P. Edwards, and N. L. Weigel. 2001. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J. Biol. Chem. 276:8475-8483. [DOI] [PubMed] [Google Scholar]
- 37.Krek, W., M. E. Ewen, S. Shirodkar, Z. Arany, W. G. Kaelin, Jr., and D. M. Livingston. 1994. Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell 78:161-172. [DOI] [PubMed] [Google Scholar]
- 38.Krek, W., and E. A. Nigg. 1991. Mutations of p34cdc2 phosphorylation sites induce premature mitotic events in HeLa cells: evidence for a double block to p34cdc2 kinase activation in vertebrates. EMBO J. 10:3331-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labriola, L., M. Salatino, C. J. Proietti, A. Pecci, O. A. Coso, A. R. Kornblihtt, E. H. Charreau, and P. V. Elizalde. 2003. Heregulin induces transcriptional activation of the progesterone receptor by a mechanism that requires functional ErbB-2 and mitogen-activated protein kinase activation in breast cancer cells. Mol. Cell. Biol. 23:1095-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lane, S., P. Farlie, and R. Watson. 1997. B-Myb function can be markedly enhanced by cyclin A-dependent kinase and protein truncation. Oncogene 14:2445-2453. [DOI] [PubMed] [Google Scholar]
- 41.Lange, C. A. 2004. Making sense of cross-talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol. Endocrinol. 18:269-278. [DOI] [PubMed] [Google Scholar]
- 42.Lange, C. A., J. K. Richer, T. Shen, and K. B. Horwitz. 1998. Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways. J. Biol. Chem. 273:31308-31316. [DOI] [PubMed] [Google Scholar]
- 43.Lange, C. A., T. Shen, and K. B. Horwitz. 2000. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc. Natl. Acad. Sci. USA 97:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma, T., B. A. Van Tine, Y. Wei, M. D. Garrett, D. Nelson, P. D. Adams, J. Wang, J. Qin, L. T. Chow, and J. W. Harper. 2000. Cell cycle-regulated phosphorylation of p220(NPAT) by cyclin E/Cdk2 in Cajal bodies promotes histone gene transcription. Genes Dev. 14:2298-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moeller, S. J., E. D. Head, and R. J. Sheaff. 2003. p27Kip1 inhibition of GRB2-SOS formation can regulate Ras activation. Mol. Cell. Biol. 23:3735-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore, M. R. 2004. A rationale for inhibiting progesterone-related pathways to combat breast cancer. Curr. Cancer Drug Targets 4:183-189. [DOI] [PubMed] [Google Scholar]
- 47.Moore, M. R., J. L. Conover, and K. M. Franks. 2000. Progestin effects on long-term growth, death, and Bcl-xL in breast cancer cells. Biochem. Biophys. Res. Commun. 277:650-654. [DOI] [PubMed] [Google Scholar]
- 48.Muller-Tidow, C., W. Wang, G. E. Idos, S. Diederichs, R. Yang, C. Readhead, W. E. Berdel, H. Serve, M. Saville, R. Watson, and H. P. Koeffler. 2001. Cyclin A1 directly interacts with B-myb and cyclin A1/cdk2 phosphorylate B-myb at functionally important serine and threonine residues: tissue-specific regulation of B-myb function. Blood 97:2091-2097. [DOI] [PubMed] [Google Scholar]
- 49.Muraoka, R. S., A. E. Lenferink, J. Simpson, D. M. Brantley, L. R. Roebuck, F. M. Yakes, and C. L. Arteaga. 2001. Cyclin-dependent kinase inhibitor p27(Kip1) is required for mouse mammary gland morphogenesis and function. J. Cell Biol. 153:917-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy, C. S., J. J. Pink, and V. C. Jordan. 1990. Characterization of a receptor-negative, hormone-nonresponsive clone derived from a T47D human breast cancer cell line kept under estrogen-free conditions. Cancer Res. 50:7285-7292. [PubMed] [Google Scholar]
- 51.Murphy, L., T. Cherlet, A. Lewis, Y. Banu, and P. Watson. 2003. New insights into estrogen receptor function in human breast cancer. Ann. Med. 35:614-631. [DOI] [PubMed] [Google Scholar]
- 52.Musgrove, E. A., C. S. Lee, M. F. Buckley, R. L. Sutherland, T. C. Wang, R. D. Cardiff, L. Zukerberg, E. Lees, A. Arnold, and E. V. Schmidt. 1994. Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc. Natl. Acad. Sci. USA 91:8022-8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musgrove, E. A., C. S. Lee, and R. L. Sutherland. 1991. Progestins both stimulate and inhibit breast cancer cell cycle progression while increasing expression of transforming growth factor alpha, epidermal growth factor receptor, c-fos, and c-myc genes. Mol. Cell. Biol. 11:5032-5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nandi, S., R. C. Guzman, and J. Yang. 1995. Hormones and mammary carcinogenesis in mice, rats, and humans: a unifying hypothesis. Proc. Natl. Acad. Sci. USA 92:3650-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narayanan, R., A. A. Adigun, D. P. Edwards, and N. L. Weigel. 2003. Human progesterone receptor function is modulated by cyclin A/Cdk-2 and is impaired during the G2 phase of the cell cycle. Progr. Abstr. Endocr. Soc. 85th Annu. Meet. Endocr. Soc. Endo 2003, abstr. OR5-2, p. 65. The Endocrine Society, Chevy Chase, Md.
- 56.Nardulli, A. M., and B. S. Katzenellenbogen. 1988. Progesterone receptor regulation in T47D human breast cancer cells: analysis by density labeling of progesterone receptor synthesis and degradation and their modulation by progestin. Endocrinology 122:1532-1540. [DOI] [PubMed] [Google Scholar]
- 57.Norbury, C., M. MacFarlane, H. Fearnhead, and G. M. Cohen. 1994. Cdc2 activation is not required for thymocyte apoptosis. Biochem. Biophys. Res. Commun. 202:1400-1406. [DOI] [PubMed] [Google Scholar]
- 58.Okuda, M., H. F. Horn, P. Tarapore, Y. Tokuyama, A. G. Smulian, P. K. Chan, E. S. Knudsen, I. A. Hofmann, J. D. Snyder, K. E. Bove, and K. Fukasawa. 2000. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell 103:127-140. [DOI] [PubMed] [Google Scholar]
- 59.Ortega, S., I. Prieto, J. Odajima, A. Martin, P. Dubus, R. Sotillo, J. L. Barbero, M. Malumbres, and M. Barbacid. 2003. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 35:25-31. [DOI] [PubMed] [Google Scholar]
- 60.Parisi, T., A. R. Beck, N. Rougier, T. McNeil, L. Lucian, Z. Werb, and B. Amati. 2003. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO J. 22:4794-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perkins, N. D., L. K. Felzien, J. C. Betts, K. Leung, D. H. Beach, and G. J. Nabel. 1997. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science 275:523-527. [DOI] [PubMed] [Google Scholar]
- 62.Petersen, B. O., J. Lukas, C. S. Sorensen, J. Bartek, and K. Helin. 1999. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J. 18:396-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Porter, P. L., K. E. Malone, P. J. Heagerty, G. M. Alexander, L. A. Gatti, E. J. Firpo, J. R. Daling, and J. M. Roberts. 1997. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat. Med. 3:222-225. [DOI] [PubMed] [Google Scholar]
- 64.Pratt, W. B., and D. O. Toft. 2003. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 228:111-133. [DOI] [PubMed] [Google Scholar]
- 65.Qiu, M., and C. A. Lange. 2003. MAP kinases couple multiple functions of human progesterone receptors: degradation, transcriptional synergy, and nuclear association. J. Steroid Biochem. Mol. Biol. 85:147-157. [DOI] [PubMed] [Google Scholar]
- 66.Qiu, M., A. Olsen, E. Faivre, K. B. Horwitz, and C. A. Lange. 2003. Mitogen activated protein kinase regulates nuclear association of human progesterone receptors. Mol. Endocrinol. 17:628-642. [DOI] [PubMed] [Google Scholar]
- 67.Reid, G., M. R. Hubner, R. Metivier, H. Brand, S. Denger, D. Manu, J. Beaudouin, J. Ellenberg, and F. Gannon. 2003. Cyclic, proteasome-mediated turnover of unliganded and liganded ERα on responsive promoters is an integral feature of estrogen signaling. Mol. Cell 11:695-707. [DOI] [PubMed] [Google Scholar]
- 68.Robinson, C., Y. Light, R. Groves, D. Mann, R. Marias, and R. Watson. 1996. Cell-cycle regulation of B-Myb protein expression: specific phosphorylation during the S phase of the cell cycle. Oncogene 12:1855-1864. [PubMed] [Google Scholar]
- 69.Said, T. K., R. C. Moraes, U. Singh, F. S. Kittrell, and D. Medina. 2001. Cyclin-dependent kinase (cdk) inhibitors/cdk4/cdk2 complexes in early stages of mouse mammary preneoplasia. Cell Growth Differ. 12:285-295. [PubMed] [Google Scholar]
- 70.Sala, A., M. Kundu, I. Casella, A. Engelhard, B. Calabretta, L. Grasso, M. G. Paggi, A. Giordano, R. J. Watson, K. Khalili, and C. Peschle. 1997. Activation of human B-MYB by cyclins. Proc. Natl. Acad. Sci. USA 94:532-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salghetti, S. E., A. A. Caudy, J. G. Chenoweth, and W. P. Tansey. 2001. Regulation of transcriptional activation domain function by ubiquitin. Science 293:1651-1653. [DOI] [PubMed] [Google Scholar]
- 72.Salghetti, S. E., S. Y. Kim, and W. P. Tansey. 1999. Destruction of Myc by ubiquitin-mediated proteolysis: cancer-associated and transforming mutations stabilize Myc. EMBO J. 18:717-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salghetti, S. E., M. Muratani, H. Wijnen, B. Futcher, and W. P. Tansey. 2000. Functional overlap of sequences that activate transcription and signal ubiquitin-mediated proteolysis. Proc. Natl. Acad. Sci. USA 97:3118-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santen, R. J., R. X. Song, R. McPherson, R. Kumar, L. Adam, M. H. Jeng, and W. Yue. 2002. The role of mitogen-activated protein (MAP) kinase in breast cancer. J. Steroid Biochem. Mol. Biol. 80:239-256. [DOI] [PubMed] [Google Scholar]
- 75.Santilli, G., M. N. Cervellera, T. K. Johnson, R. E. Lewis, S. Iacobelli, and A. Sala. 2001. PARP co-activates B-MYB through enhanced phosphorylation at cyclin/cdk2 sites. Oncogene 20:8167-8174. [DOI] [PubMed] [Google Scholar]
- 76.Sartorius, C. A., S. D. Groshong, L. A. Miller, R. L. Powell, L. Tung, G. S. Takimoto, and K. B. Horwitz. 1994. New T47D breast cancer cell lines for the independent study of progesterone B- and A-receptors: only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 54:3868-3877. [PubMed] [Google Scholar]
- 77.Scott, K. A., and R. A. Walker. 1997. Lack of cyclin E immunoreactivity in non-malignant breast and association with proliferation in breast cancer. Br. J. Cancer 76:1288-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen, T., K. B. Horwitz, and C. A. Lange. 2001. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol. Cell. Biol. 21:6122-6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sheridan, P. L., R. M. Evans, and K. B. Horwitz. 1989. Phosphotryptic peptide analysis of human progesterone receptor. New phosphorylated sites formed in nuclei after hormone treatment. J. Biol. Chem. 264:6520-6528. [PubMed] [Google Scholar]
- 80.Sicinski, P., J. L. Donaher, S. B. Parker, T. Li, A. Fazeli, H. Gardner, S. Z. Haslam, R. T. Bronson, S. J. Elledge, and R. A. Weinberg. 1995. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82:621-630. [DOI] [PubMed] [Google Scholar]
- 81.Sicinski, P., and R. A. Weinberg. 1997. A specific role for cyclin D1 in mammary gland development. J. Mammary Gland Biol. Neoplasia 2:335-342. [DOI] [PubMed] [Google Scholar]
- 82.Sutherland, R. L., and E. A. Musgrove. 2002. Cyclin D1 and mammary carcinoma: new insights from transgenic mouse models. Breast Cancer Res. 4:14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sutherland, R. L., O. W. J. Prall, C. K. W. Watts, and E. A. Musgrove. 1998. Estrogen and progestin regulation of cell cycle progression. J. Mammary Gland Biol. Neoplasia 3:63-72. [DOI] [PubMed] [Google Scholar]
- 84.Sweeney, K. J., B. Sarcevic, R. L. Sutherland, and E. A. Musgrove. 1997. Cyclin D2 activates Cdk2 in preference to Cdk4 in human breast epithelial cells. Oncogene 14:1329-1340. [DOI] [PubMed] [Google Scholar]
- 85.Takimoto, G. S., D. M. Tasset, A. C. Eppert, and K. B. Horwitz. 1992. Hormone-induced progesterone receptor phosphorylation consists of sequential DNA-independent and DNA-dependent stages: analysis with zinc finger mutants and the progesterone antagonist ZK98299. Proc. Natl. Acad. Sci. USA 89:3050-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan, M., J. Yao, and D. Yu. 1997. Overexpression of the c-erbB-2 gene enhanced intrinsic metastasis potential in human breast cancer cells without increasing their transformation abilities. Cancer Res. 57:1199-1205. [PubMed] [Google Scholar]
- 87.Tan, P., B. Cady, M. Wanner, P. Worland, B. Cukor, C. Magi-Galluzzi, P. Lavin, G. Draetta, M. Pagano, and M. Loda. 1997. The cell cycle inhibitor p27 is an independent prognostic marker in small (T1a,b) invasive breast carcinomas. Cancer Res. 57:1259-1263. [PubMed] [Google Scholar]
- 88.Tokuyama, Y., H. F. Horn, K. Kawamura, P. Tarapore, and K. Fukasawa. 2001. Specific phosphorylation of nucleophosmin on Thr(199) by cyclin-dependent kinase 2-cyclin E and its role in centrosome duplication. J. Biol. Chem. 276:21529-21537. [DOI] [PubMed] [Google Scholar]
- 89.Topper, Y. J., and C. S. Freeman. 1980. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol. Rev. 60:1049-1106. [DOI] [PubMed] [Google Scholar]
- 90.Trowbridge, J. M., I. Rogatsky, and M. J. Garabedian. 1997. Regulation of estrogen receptor transcriptional enhancement by the cyclin A/Cdk2 complex. Proc. Natl. Acad. Sci. USA 94:10132-10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vlach, J., S. Hennecke, and B. Amati. 1997. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 16:5334-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang, Z., and M. J. Garabedian. 2003. Modulation of glucocorticoid receptor transcriptional activation, phosphorylation, and growth inhibition by p27Kip1. J. Biol. Chem. 278:50897-50901. [DOI] [PubMed] [Google Scholar]
- 93.Weigel, N. L. 1996. Steroid hormone receptors and their regulation by phosphorylation. Biochem. J. 319:657-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu, M., K. A. Sheppard, C. Y. Peng, A. S. Yee, and H. Piwnica-Worms. 1994. Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Mol. Cell. Biol. 14:8420-8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang, Y., C. A. Beck, A. Poletti, J. P. Clement, P. Prendergast, T. T. Yip, T. W. Hutchens, D. P. Edwards, and N. L. Weigel. 1997. Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol. Endocrinol. 11:823-832. [DOI] [PubMed] [Google Scholar]
- 96.Zhang, Y., C. A. Beck, A. Poletti, D. P. Edwards, and N. L. Weigel. 1995. Identification of a group of Ser-Pro motif hormone-inducible phosphorylation sites in the human progesterone receptor. Mol. Endocrinol. 9:1029-1040. [DOI] [PubMed] [Google Scholar]
- 97.Zhang, Y., C. A. Beck, A. Poletti, D. P. Edwards, and N. L. Weigel. 1994. Identification of phosphorylation sites unique to the B form of human progesterone receptor. In vitro phosphorylation by casein kinase II. J. Biol. Chem. 269:31034-31040. [PubMed] [Google Scholar]
- 98.Zhao, J., B. K. Kennedy, B. D. Lawrence, D. A. Barbie, A. G. Matera, J. A. Fletcher, and E. Harlow. 2000. NPAT links cyclin E-Cdk2 to the regulation of replication-dependent histone gene transcription. Genes Dev. 14:2283-2297. [PMC free article] [PubMed] [Google Scholar]
- 99.Ziebold, U., O. Bartsch, R. Marais, S. Ferrari, and K. H. Klempnauer. 1997. Phosphorylation and activation of B-Myb by cyclin A-Cdk2. Curr. Biol. 7:253-260. [DOI] [PubMed] [Google Scholar]
- 100.Zwijsen, R. M., E. Wientjens, R. Klompmaker, J. van der Sman, R. Bernards, and R. J. Michalides. 1997. CDK-independent activation of estrogen receptor by cyclin D1. Cell 88:405-415. [DOI] [PubMed] [Google Scholar]